Abstract
Neuroinflammation induced by activated microglia and astrocytes can be elicited by drugs of abuse. Methamphetamine administration activates glial cells and increases proinflammatory cytokine production, and there is recent evidence of a linkage between glial cell activation and drug abuse-related behavior. We have previously reported that ibudilast (AV411; 3-isobutyryl-2-isopropylpyrazolo-[1,5-a]pyridine), which inhibits phosphodiesterase (PDE) and pro-inflammatory activity, blocks reinstatement of methamphetamine-maintained responding in rats, and that ibudilast and AV1013, an amino analog of ibudilast, which has similar glial-attenuating properties but limited PDE activity, attenuate methamphetamine-induced locomotor activity and sensitization in mice. The present study's objective was to determine whether co-administered ibudilast, AV1013, or minocycline, which is a tetracycline derivative that also suppresses methamphetamine-induced glial activation, would attenuate active methamphetamine i.v. self-administration in Long-Evans hooded rats. Rats were initially trained to press a lever for 0.1 mg/kg/inf methamphetamine according to a FR1 schedule during 2-h daily sessions. Once stable responding was obtained, twice daily ibudilast (1, 7.5, 10 mg/kg), AV1013 (1, 10, 30 mg/kg), or once daily minocycline (10, 30, 60 mg/kg), or their corresponding vehicles, were given i.p. for three consecutive days during methamphetamine (0.001, 0.03, 0.1 mg/kg/inf) self-administration. Ibudilast, AV1013, and minocycline all significantly (p<0.05) reduced responding maintained by 0.03 mg/kg/inf methamphetamine that had maintained the highest level of infusions under vehicle conditions. These results suggest that targeting glial cells may provide a novel approach to pharmacotherapy for treating methamphetamine abuse.
Keywords: ibudilast, AV411, AV1013, minocycline, methamphetamine, glial cell, self-administration
1. Introduction
While neuroinflammation is commonly associated with neurodegenerative conditions, decades of evidence indicate that some CNS-active drugs can induce neuroinflammatory processes via activation of glial cells as well. For example, activated microglia and astrocytes release pro-inflammatory cytokines following methamphetamine administration (Goncalves et al., 2008; Loftis et al., 2011; Nakajima et al., 2004; Yamaguchi et al., 1991). Drug-induced glial activation is involved with methamphetamine’s neurotoxicity and alterations of the blood brain barrier (Clark et al., 2012), and non-neurotoxic levels of methamphetamine-induced neuroinflammation can alter behavior (Miguel-Hidalgo, 2009).
Methamphetamine abuse cost society an estimated 23.4 billion dollars in 2005 (Nicosia et al., 2009; Watanabe-Galloway et al., 2009), and its chronic abuse can lead to hallucinations, pulmonary and cardiac deterioration, dental disease, and suppressed immune function (Hamamoto and Rhodus, 2009; Hauer, 2010; Srisurapanont et al., 2003). Nevertheless, there are currently no approved pharmacotherapies for directly treating methamphetamine abuse, nor any to complement the limited behavioral interventions (Obert et al., 2000; Rawson et al., 2002). Because methamphetamine is best known for disrupting the monoaminergic dopamine, serotonin, and norepinephrine neurotransmitter systems (Cho and Segal, 1994; Creese, 1983), most research has focused on conventional receptor-mediated approaches to develop useful pharmacotherapeutic agents, but heretofore without success (Karila et al., 2010). Furthermore, compounds with varied mechanistic actions such as bupropion, modafinil, and aripiprazole have all had inconsistent efficacy (Brackins et al., 2011). An expanded understanding of methamphetamine’s less studied mechanisms, such as glial cell activation, may lead to fresh approaches in drug development.
Developing evidence indicates that attenuating glial activation can reduce methamphetamine-induced behavioral effects (Fujita et al., 2012; Narita et al., 2006; Zhang et al., 2006). Correspondingly, an enhancement of neuroprotective growth factors, like glial cell derived neurotrophic factor (GDNF), blocks methamphetamine self-administration and vulnerability towards reinstatement (Yan et al., 2007). Previously our lab reported that the anti-inflammatory glial cell modulator, ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine), and its amino analogue, AV1013, attenuates methamphetamine-induced locomotor activity and its sensitization (Snider et al., 2012), and ibudilast attenuates prime- and cue-induced reinstatement of methamphetamine-maintained responding (Beardsley et al., 2010). Among ibudilast’s many actions, it is a non-selective phosphodiesterase (PDE) inhibitor (Gibson et al., 2006; Kishi et al., 2001), glial cell modulator, anti-inflammatory agent, and increases production of GDNF (Cho et al., 2010; Mizuno et al., 2004; Rolan et al., 2009; Suzumura et al., 1999). AV1013 exhibits similar glial attenuating actions as ibudilast, but is impotent at inhibiting PDE function (Cho et al., 2010). Other glial-mediated anti-inflammatory drugs attenuate methamphetamine's effects as well. For instance, minocycline hydrochloride significantly attenuates microglial activation (Sriram et al., 2006; Zhang et al., 2006), attenuates methamphetamine and cocaine-induced hyperlocomotion and sensitization (Chen et al., 2009; Zhang et al., 2006), and prevents methamphetamine conditioned place preference (Fujita et al., 2012). Given these reports implicating glial cell activity and methamphetamine's effects, further investigation of anti-inflammatory agents may provide new targets for affecting methamphetamine drug-abuse. In the present study, ibudilast, AV1013, and minocycline were evaluated for their ability to interrupt on-going methamphetamine self-administration in rats.
2. Materials and methods
2.1 Subjects
Adult male Long-Evans hooded rats (Harlan, Indianapolis, IN) weighing 275–300 g at the start of studies were acclimated to the vivarium for at least one week prior to catheter implantation. When not in testing, rats were individually housed in standard plastic rodent cages in a temperature-controlled (22° C), Association for the Assessment and Accreditation of Laboratory Animal Care International-accredited facility in which they had ad libitum access to water. The rats were allowed ad libitum rat chow (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories, Inc., Indianapolis, IN) for at least one week prior to commencement of training, after which they were maintained at 320 g by controlled feedings given after experimental sessions or at a comparable time of day if not tested. The rats were maintained on a reversed, 12 h/12 h light-dark cycle (0600-1800 h lights off) for the duration of the experiment, and they were trained and tested during the dark segment of this cycle.
All procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press, 1996) and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (IACUC Approval Number AM10032).
2.1.1 Infusion assembly system
Catheters were constructed from polyurethane tubing (Access Technologies, Skokie, IL; 0.044” O.D. X 0.025” I.D.). The proximal 3.2 cm of the catheter was tapered by stretching following immersion in hot sesame oil. The catheters were prepared with a retaining cuff approximately 3 cm from the proximal end of the catheter. A second larger retaining cuff was positioned approximately 3.4 cm from the proximal end of the catheter. Mid-scapula cannula connectors were obtained from Plastics One (Roanoke, VA). The cannula connectors consisted of a threaded plastic post through which passed an “L” shaped section of 22 gauge stainless steel needle tubing. The lower surface of the plastic post was affixed to a 2 cm diameter disc of Dacron mesh. During sessions the exposed threaded portion of the infusion cannula was connected to an infusion tether consisting of a 35 cm length of 0.40 mm i.d. polypropylene tubing encased within a 30 cm stainless steel spring to prevent damage. The upper portion of the 0.40 polypropylene tubing was connected to a fluid swivel (Lomir Biomedical, Inc, Quebec, Canada) that was, in turn, attached via 0.40 polypropylene tubing to the infusion syringe.
2.1.2 Surgical procedure
Following acclimation to the laboratory environment, indwelling venous catheters were implanted into the right external jugular vein. Rats were administered 5 mg/kg carprofen s.c. (Rimadyl, Pfizer Animal Health, New York, NY) before surgery. Surgical anesthesia was induced with a combination of 50 mg/kg ketamine (KetaThesia, Butler Animal Health Supply, Dublin, OH) and 8.7 mg/kg xylazine (X-Ject E, Butler Animal Health Supply, Dublin, OH). The ventral neck area and back of the rat were shaved and wiped with povidone-iodine, 7.5% (Betadine, Purdue Products L.P., Stamford, CT) and isopropyl alcohol. The rat was placed ventral side down on the surgical table and a 3 cm incision was made 1 cm lateral from mid-scapula. A second 0.5 cm incision was then made mid-scapula. The rat was then placed dorsal side down on the operating table and a 2.5 cm incision was made longitudinally through the skin above the jugular area. The underlying fascia was bluntly dissected and the right external jugular vein isolated and ligated. A small cut was made into the vein using an iris scissors and the catheter was introduced into the vein and inserted up to the level of the larger retaining cuff. The vein encircling the catheter between the two cuffs was then tied with silk suture. A second suture was then used to anchor the catheter to surrounding fascia. The distal end of the catheter was passed subcutaneously and attached to the cannula connector that was then inserted subcutaneously through the larger incision. The upper post portion of the cannula connector exited through the smaller mid-scapula incision. All incisions were then sprayed with a gentamicin sulfate/betamethasone valerate topical antibiotic (Betagen, Med-Pharmex, Inc., Pomona, CA) and the incisions were closed with Michel wound clips. Five mg/kg oral carprofen (Rimadyl, Bio-Serv, Frenchtown, NJ) was administered 24 h after surgery, and 8 mg/kg oral enrofloxacin (Baytril, Bio-Serv, Frenchtown, NJ) was administered daily for three days following surgery.
Rats were allowed to recover from surgery for at least 5 days before self-administration training began. Periodically throughout training, ketamine (5 mg/kg) (KetaThesia, Butler Animal Health Supply, Dublin, OH) was infused through the catheters to determine patency as inferred when immediate anesthesia was induced. Between sessions the catheters were flushed and filled with 0.1 ml of a 25% glycerol (Acros, New Jersey)/75% sterile saline locking solution containing: 250 units/ml heparin (Abraxis Pharmaceutical Products, Schaumburg, IL) and 250 mg/ml ticarcillin/9 mg/ml clavulanic acid (Timentin, GlaxoSmithKline, Research Triangle Park, NC). If during the experiment a catheter was determined to be in-patent, the left external jugular was then catheterized and the rat was returned to testing.
2.2 Apparatus
Commercially-obtained test chambers equipped with two retractable levers, a 5-w house light, and a Sonalert® tone generator (MED Associates, Inc., St. Albans, VT) were used. Positioned above each lever was a white cue light. A syringe pump (Model PHS-100; MED Associates, Inc., St. Albans, VT) when activated, delivered a 6-sec, 0.2 ml infusion. Recording of lever presses and activation of lights, shockers, pumps, and Sonalerts were accomplished by a microcomputer, interface, and associated software (MED-PC® IV, MED Associates, Inc., St. Albans, VT).
2.3 Self-administration procedure
Methamphetamine self-administration training sessions were conducted seven days per week for 2 h daily. Each response (fixed ratio 1, FR1) on the right-side lever resulted in delivery of a 0.1 mg/kg methamphetamine infusion (0.2 ml/6 sec) followed by a 14-s timeout period. At the start of an infusion the house light was extinguished, the Sonalert® was sounded, and the cue lights above each lever flashed at 3 Hz. The Sonalert® and cue lights remained activated during the 6 s infusion. Twenty seconds following the onset of the infusion the house light was re-illuminated, and the opportunity to self-administer methamphetamine was again made available (i.e., each methamphetamine infusion initiated a 20 s period during which lever presses were recorded but were without scheduled consequences and further infusions could not be obtained). Active (right-side) lever presses during the infusions as well as all inactive (left-side) lever presses were recorded but were without scheduled consequences.
Training sessions occurred until stability criteria were met. Stability criteria were defined in which during the first and last session of at least 3 consecutive sessions neither the highest nor lowest number of infusions were obtained, and the number of infusions during each session was ±20% from the mean. Following training, ibudilast (1, 7.5 and 10 mg/kg) or AV1013 (1, 10 and 30 mg/kg) was administered i.p. twice daily, or minocycline (10, 30 and 60 mg/kg) was administered i.p. once daily, or their corresponding vehicles were administered for three consecutive days during self-administration of each of three doses of methamphetamine (0.1, 0.03, and 0.001 mg/kg/inf). Thus, a total of nine consecutive days of b.i.d. or once daily dosing of vehicle or dose of test drug was necessary to complete testing at each self-administered dose of methamphetamine. Between tests of vehicle or dose of test drug, rats were maintained under 0.1 mg/kg methamphetamine self-administration conditions with i.p. injections of the test drug’s vehicle until training criteria were once again met.
2.3.1 Rate Dependency Analysis
Preliminary results had indicated that ibudilast reduced response rates maintained by 0.03 mg/kg/inf but not by 0.1 mg/kg/inf methamphetamine. In order to determine if the higher baseline response rates maintained by 0.03 mg/kg/inf methamphetamine were the sole determinants for the greater ibudilast-induced response rate reductions, relative to those maintained at 0.1 mg/kg/inf methamphetamine, response rates were matched across the two methamphetamine doses. Fixed-ratio requirements reinforced by 0.1 mg/kg/inf methamphetamine were increased to increase response rates, and ibudilast was then re-tested at its most influential dose of 10 mg/kg. Rats were trained to self-administer 0.1 mg/kg/inf methamphetamine reinforced according to a FR1 schedule, and they were required to complete the previously described stability criteria before proceeding. The fixed ratio requirement was then adjusted to between FR2- FR4 in individual rats in order to increase response rates reinforced by 0.1 mg/kg/inf methamphetamine to approximate, as a group mean response rate, those maintained by 0.03 mg/kg/inf methamphetamine. Once the response rates were stably matched between 0.03 and 0.1 mg/kg/inf methamphetamine groups, ibudilast (10 mg/kg) or its vehicle was then administered b.i.d. i.p. for three consecutive days. Between three-day sets of testing, animals were returned to training conditions and were required to meet stability criteria once again.
2.4 Drugs
(±)-Methamphetamine hydrochloride (National Institute on Drug Abuse, Rockville, MD) was prepared in sterile 0.9% saline. Methamphetamine stock solutions were sterilized by filtration through 0.2 μm filtration disks. Heparin (5 units/ml) was additionally added to methamphetamine and saline infusates. Ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) and AV1013 ((R)-2-amino-1-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)propan-1-one hydrochloride) were received as a gift from MediciNova, Inc. (San Diego, CA) and were dissolved in a 35% PEG400, 10% Cremophor® RH40 (BASF, Ludwigshafen, Germany) aqueous vehicle. Minocycline hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in saline and a few drops of 1 M hydrochloric acid. Immediately prior administration, the minocycline solution was adjusted to pH 3–4 using a few drops of sodium hydroxide. Ibudilast, AV1013, and minocycline were all administered i.p. in 1 ml/kg body weight volume, except for 60 mg/kg minocycline that was given at 2 ml/kg body weight volume due to insolubility at the lower volume.
2.5 Data Analysis
The number of infusions obtained on the third (and final) day of testing at each condition was used for data analysis because it was assumed it would most likely represent terminal behavior. Numbers of infusions comparing methamphetamine dose to saline under vehicle-treatment conditions were made with Dunnett’s Multiple Comparison posttests following a one-way within-subjects ANOVA to determine if a dose of methamphetamine served as a reinforcer. Additionally, infusion numbers were analyzed using a two-way repeated measures ANOVA (repeated measures on treatment dose and between comparisons on methamphetamine dose), and comparisons of ibudilast, AV1013, or minocycline treatment on methamphetamine self-administration were assessed using Bonferroni Multiple Comparisons tests. AD50 (CI) values for attenuating methamphetamine self-administration by 50% relative to vehicle control conditions were estimated by first converting total infusions obtained to percent of their respective vehicle control infusions, logarithmically transforming dose, and using nonlinear regression assuming a normalized response.
For Rate Dependency Analysis, "response rate" was defined as the: (number of presses of the right-side lever - those occurring during time-out periods) ÷ (total session duration in sec - cumulative duration of all time-out periods). Response rates maintained by 0.03 mg/kg/inf methamphetamine at FR1 were considered matched to response rates for 0.1 mg/kg/inf methamphetamine at FR2-4 when there were no significant differences between group mean rates when compared by an unpaired t-test. During 10 mg/kg ibudilast and vehicle treatment, response rates at 0.03 mg/kg/inf methamphetamine administration were compared to those at 0.1 mg/kg/inf methamphetamine using a two-way analysis of variance (ANOVA) with repeated measures on ibudilast "dose" (i.e., 10 mg/kg or vehicle) followed by Sidak's Multiple Comparisons tests.
All statistical tests were conducted using commercial computer software (Prism 5d for Macintosh, GraphPad Software, Inc., San Diego, CA), and all types of comparisons were considered statistically significant if p < 0.05.
3. Results
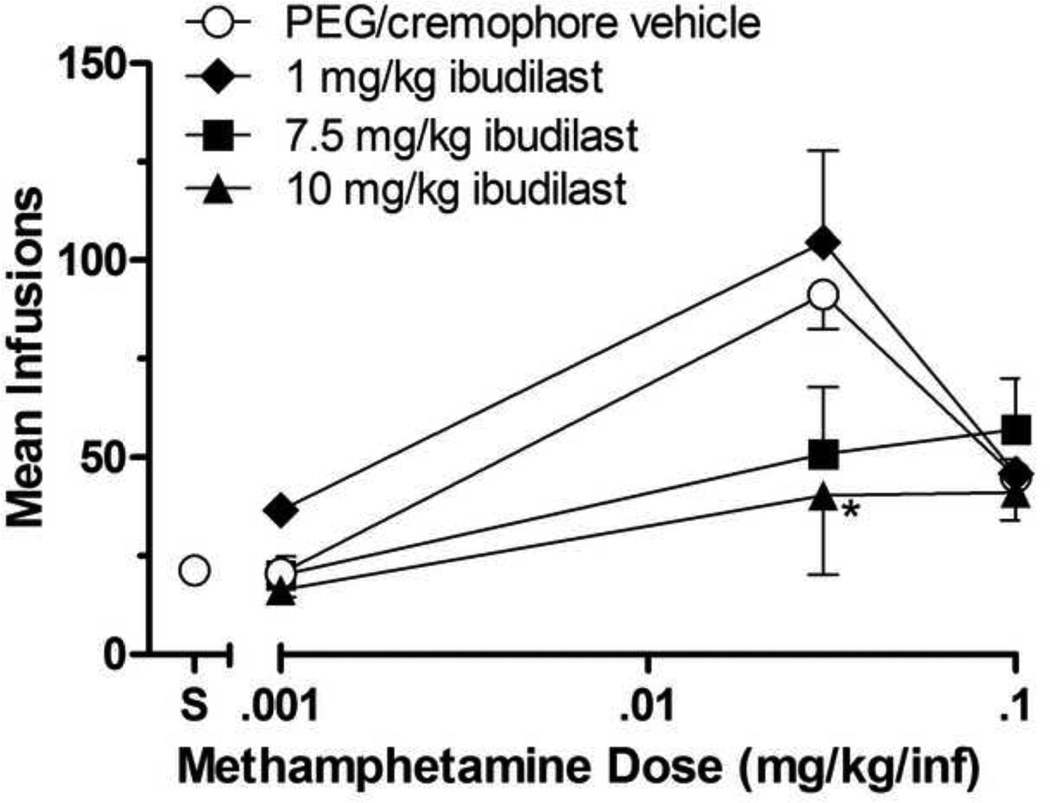
Figure 1 illustrates the effects of ibudilast on methamphetamine self-administration. Under b.i.d. vehicle conditions, methamphetamine was self-administered characterized by an inverted U-shaped curve relating infusion numbers to dose, and the one-way within-subjects ANOVA on infusion numbers was significant [F(3,15)=32.75; p<0.001]. Dunnett’s posttests revealed that infusions of 0.03 and 0.1 mg/kg/inf methamphetamine were self-administered significantly greater than those of saline (p<0.05) indicating that these doses were serving as positive reinforcers under baseline conditions.
Fig 1.
Effects of ibudilast or its vehicle on group mean infusions of methamphetamine (0.001, 0.03, and 0.1 mg/kg/inf) obtained during daily 2-h self-administration sessions. Ibudilast was administered at 1, 7.5, or 10 mg/kg i.p. b.i.d. for 3 consecutive days at each methamphetamine self-administered dose. Data points represent the group means of total infusions obtained during the third day of testing at each ibudilast dose. Bars through symbols indicate ±S.E.M. Data point above "S" on the abscissa indicates results when saline was self-administered when ibudilast's vehicle was given b.i.d. N = 4 rats. *p < 0.05 with respect to infusions obtained under ibudilast’s vehicle condition.
There was a significant effect of methamphetamine dose [F(2,27)=19.90; p= 0.0005] and ibudilast dose [F(3,27)=3.44; p=0.0308]. Ibudilast did not systematically affect the number of 0.001 mg/kg methamphetamine infusions, which had not served as a positive reinforcer under vehicle conditions, nor the number 0.1 mg/kg methamphetamine infusions, which had served as a positive reinforcer. At its two highest doses (7.5 and 10 mg/kg), ibudilast reduced the number of 0.03 mg/kg methamphetamine infusions, the methamphetamine dose that had maintained the greatest number of infusions above those of saline under baseline conditions, and significantly so at the 10 mg/kg ibudilast dose (p<0.05).
Response Rate Dependency analysis indicated that group mean response rate maintained by 0.03 mg/kg/inf methamphetamine at FR1 was not significantly different from the group mean response rate maintained by 0.1 mg/kg/inf methamphetamine in the matched response rate group (FR2-4) (t=0.1591, df=6, p=0.8788). Although 10 mg/kg ibudilast reduced infusion levels of 0.03 mg/kg/inf methamphetamine relative to vehicle control (t=3.998, df=6, p<0.05), and as described above, infusion rates of 0.01 mg/kg/inf methamphetamine in the matched response rate group were unaffected (t=1.324, df=6, p>0.05).
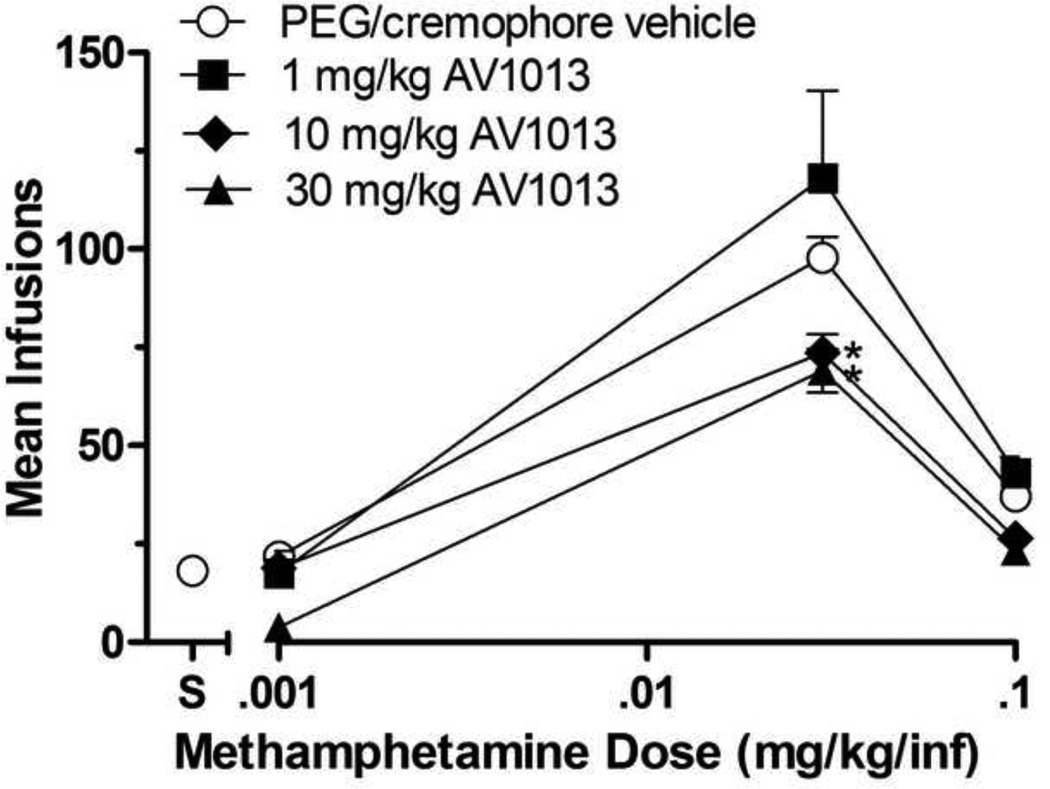
Figure 2 shows AV1013’s effects on methamphetamine self-administration. Under b.i.d. vehicle conditions, methamphetamine was self-administered characterized by an inverted U-shaped curve relating infusion numbers to dose. The one-way within subjects ANOVA on infusion numbers was significant [F(3,19)=214.9; p<0.0001]. Dunnett’s posttest results indicated that 0.03 and 0.1 mg/kg/inf methamphetamine doses were self-administered significantly above those of saline (p<0.05) indicating that these doses were serving as positive reinforcers.
Fig 2.
Effects of AV1013 or its vehicle on group mean infusions of methamphetamine (0.001, 0.03, and 0.1 mg/kg/inf) obtained during daily 2-h self-administration sessions. AV1013 was administered at 1, 10, or 30 mg/kg i.p. b.i.d. for 3 consecutive days at each methamphetamine self-administered dose. Data points represent the group means of total infusions obtained during the third day of testing at each AV1013 dose. Bars through symbols indicate ±S.E.M. Data point above "S" on the abscissa indicates results when saline was self-administered when AV1013s vehicle was given b.i.d. N = 5 rats. *p < 0.05 with respect to infusions obtained under AV1013s vehicle condition.
There was a significant effect of methamphetamine dose [F(2,36)=62.59; p<0.0001] and AV1013 dose [F(3,36)=10.59; p<0.0001]. As with ibudilast, AV1013, did not systematically affect the number of 0.001 mg/kg methamphetamine infusions, which had not served as a positive reinforcer under vehicle conditions, nor the number 0.1 mg/kg methamphetamine infusions, which had served as a positive reinforcer. AV1013 did, however, significantly reduce the number of 0.03 mg/kg methamphetamine infusions at the 10 and 30 mg/kg AV1013 doses (p<0.05).
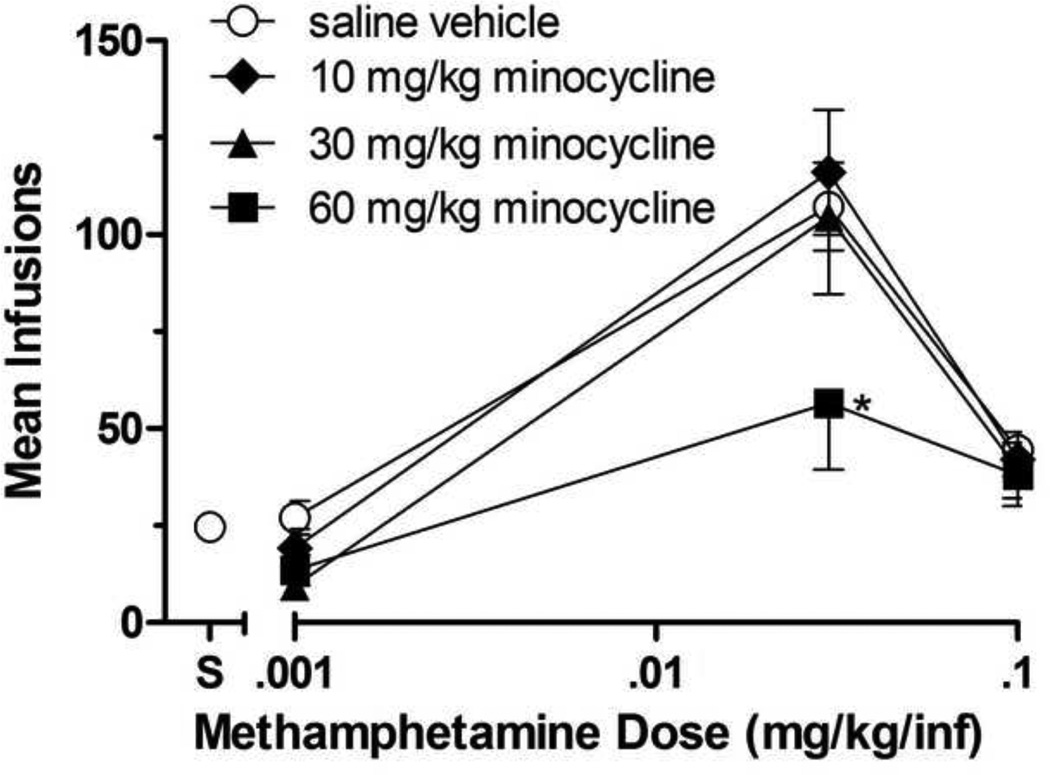
Minocycline’s effects on methamphetamine self-administration are shown in Figure 3. During daily dosing conditions with minocycline’s vehicle (saline), methamphetamine self-administration was characterized by an inverted U-shaped curve relating infusion numbers to dose. The oneway ANOVA on infusion numbers was significant [F(3,19)=34.07; p<0.0001]. Dunnett’s posttests indicated that the 0.03 mg/kg/inf methamphetamine dose (p<0.05), but neither the 0.001 mg/kg/inf nor the 0.1 mg/kg/inf methamphetamine doses, were self-administered significantly above those of saline (although the level of 0.1 mg/kg/inf methamphetamine self-administration infusions was similar to those obtained under baseline conditions during tests with ibudilast and AV1013, self-administered saline infusions were greater during minocycline testing).
Fig 3.
Effects of minocycline or its vehicle on group mean infusions of methamphetamine (0.001, 0.03, and 0.1 mg/kg/inf) obtained during daily 2-h self-administration sessions. Minocycline was administered at 10, 30, or 60 mg/kg i.p once daily for 3 consecutive days at each methamphetamine self-administered dose. Data points represent the group means of total infusions obtained during the third day of testing at each minocycline dose. Bars through symbols indicate ±S.E.M. Data point above "S" on the abscissa indicates results when saline was self-administered when minocycline's vehicle (saline) was given daily. N = 5 rats. *p < 0.05 with respect to infusions obtained under minocycline's vehicle condition.
There was a significant effect of methamphetamine dose [F(2,36)=23.09; p<0.0001] and minocycline dose [F(3,36)=6.907; p=0.0009]. Minocycline reduced infusion numbers of 0.03 mg/kg/inf methamphetamine, significantly so at the 60 mg/kg dose of minocycline (p<0.05), while infusion numbers of other self-administered doses of methamphetamine were nonsystematically affected.
The potency (AD50 value) relationship amongst the drugs for reducing total infusions obtained during 0.03 mg/kg/inf methamphetamine self-administration differed [F(2,53)=7.909; p=0.001], and resulted in ibudilast being the most potent, followed by AV1013, and then minocycline with respective AD50 (CI) values of 10.67 (3.86–29.47), 60.80 (23.26–158.9) and 128.8 (57.14–290.3).
4. Discussion
Methamphetamine was established as a positive reinforcer and was self-administered under vehicle pretreatment conditions characterized by an inverted U-shaped curve relating infusion numbers to dose, with significantly more methamphetamine infusions being obtained at the intermediate dose (0.03 mg/kg/inf) during testing of all drugs, and at the highest dose (0.1 mg/kg/inf) during testing of ibudilast and AV1013, relative to those obtained of saline. Ibudilast (10 mg/kg), AV1013 (10 and 30 mg/kg) and minocycline (60 mg/kg) significantly reduced total 0.03 mg/kg/inf methamphetamine infusions compared to vehicle pretreatment conditions. These results suggest that modulating glial cell activity, and consequent neuroinflammatory processes, can, in turn, modulate abuse-related effects of methamphetamine.
All three compounds, ibudilast, AV1013, and minocycline; reduced infusion rates for 0.03 mg/kg methamphetamine self-administration. None of the test drugs increased infusion rates of 0.001 mg/kg/inf, the lowest tested dose of methamphetamine that was not self-administered under vehicle conditions. These observations suggest that the infusion-rate reducing effects of these drugs at the 0.03 mg/kg/inf dose of methamphetamine was not attributable to the test drugs "enhancing" the effects 0.03 mg/kg/inf methamphetamine to be functionally experienced as a higher dose (and thus, advancing it along the descending limb of the dose-effect curve). Instead, the data suggest that the effects promoting methamphetamine self-administration were diminished at 0.03 mg/kg/inf by the test compounds. None of the compounds, however, affected infusions maintained by the highest self-administered methamphetamine dose (0.1 mg/kg/inf). There are several possible levels of explanation for the lack of effect on 0.1 mg/kg/inf methamphetamine-maintained behavior, the first being at the neurochemical level. Methamphetamine’s effects on glial cell activation and induction of pro-inflammatory signals have been well established (Goncalves et al., 2008; Nakajima et al., 2004; Yamaguchi et al., 1991). Additionally, methamphetamine’s glial cell activation is associated with changes in behavior (Miguel-Hidalgo, 2009). In the present study, rats self-administered methamphetamine at an average of 3.7–4.5 mg/kg/2-h session when given access to the 0.1 mg/kg/inf dose, and an average of 2.74–3.2 mg/kg/2-h session methamphetamine at the 0.03 mg/kg/inf dose. Self-administration of both doses are likely high enough to produce pro-inflammatory conditions, as it has been shown that a single dose of 1 mg/kg methamphetamine administered subcutaneously produces a significant enhancement of cytokine and chemokine induction in mice (Loftis et al., 2011). However, perhaps 0.1 mg/kg/inf methamphetamine produces a glial response "insurmountable" by the tested doses of ibudilast, AV1013, or minocycline. For instance, as the dose of methamphetamine increases, it may activate glial cells faster and promote more cytokine transcription to induce its neuroinflammatory effects.Thomas et al. (2004) noted that there was a dose-dependent effect of methamphetamine on microglial activation in the mouse striatum. Thus, the test compounds may not be effective against those processes recruited at higher doses.
How methamphetamine promotes neuroinflammation is not yet known. Reactive oxygen species and substance P contribute to methamphetamine-induced cellular damage and apoptosis (Fleckenstein et al., 1997; Zhu et al., 2006). As a result of the damaged cells and neurotoxicity, astrocytes and microglia become activated and elicit an immune response and increase pro-inflammatory cytokine production (Clark et al., 2012; Kita et al., 2008). While cell damage and death is a common catalyst for inflammation induction, methamphetamine’s effects on inflammatory pathways can also temporally occur before dopamine cell terminal pathology (LaVoie et al., 2004). Thus, methamphetamine-induced inflammation can occur at non-neurotoxic levels and independently of cell damage. A mechanism has been proposed for methamphetamine-induced inflammation via the nuclear transcription factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Shah et al., 2012). Methamphetamine’s release of excitatory neurotransmitters activates the metabotropic glutamate receptor, mGluR5. mGluR5 is described to activate the intracellular signaling pathway, AKT/PI3K, that downstream induces the release of NF-κB, which, in turn, translocates to the nucleus to promote transcription of inflammatory cytokine proteins such as TNFα, IL-6 and IL-8 (Shah et al., 2012).
Bacterial lipopolysaccharide (LPS) is a gram-negative endotoxin that stimulates inflammation via toll-like receptor-4 (TLR-4) (Chow et al., 1999). Methamphetamine and LPS both induce inflammation through the AKT/PI3K pathways and induce NF-κB to translocate to the nucleus and promote transcription of inflammatory cytokines (Ojaniemi et al., 2003; Shah et al., 2012). Methamphetamine exacerbates LPS’s inflammatory signal (Liu et al., 2012). These effects are likely attributable to both compounds acting via NF-κB, MAPK, and AKT/PI3K pathways (Liu et al., 2012). Ibudilast and AV1013 antagonize macrophage migration inhibitory factor (MIF) (Cho et al., 2010), a pro-inflammatory factor essential for TLR-4 function and inflammatory response (Roger et al., 2001). If LPS and methamphetamine’s inflammatory signals are similar, ibudilast and AV1013’s antagonism of the TLR-4 receptor via modulation of MIF may be one mechanism in which these compounds are reducing cytokine production and inflammation. Interestingly, morphine’s inflammatory response occurs when the glycoprotein, MD-2, forms a complex with TLR-4 and induces inflammation similar to LPS (Wang et al., 2012), thus providing evidence for ibudilast’s mechanism of action in reducing opioid-induced inflammation and behavior as well. Minocycline’s proposed mechanism also includes interaction with LPS and the NF-κB pathway. Minocycline prevents LPS induced degradation of Iκbα, an inhibitory factor, which ultimately prevents NF-κB translocation to the nucleus and induction of inflammatory cytokine production (Nikodemova et al., 2006). Minocycline also decreases binding of NF-κB to DNA which disrupts transcription (Bernardino et al., 2009). Thus, all three test compounds are hypothesized to inhibit inflammation and methamphetamine-induced behaviors via a similar neurochemical pathway.
Another possible explanation for the effectiveness of these compounds for reducing 0.03 but not 0.1 mg/kg/inf methamphetamine self-administration is at the behavioral level and recognizes that attenuation of total infusions obtained at the 0.03 mg/kg/inf methamphetamine dose, but not 0.1 mg/kg/inf, may represent a rate dependent effect. Dews (1955) reported that a dose of pentobarbital can increase low rates, but the same dose can decrease high rates of schedule controlled behavior. Thus, a drug’s effect on behavior is a function of the control response rate. In the present study, total responses (and infusions) emitted during the sessions in which responding was maintained by 0.03 mg/kg/inf methamphetamine were greater than that maintained by 0.1 mg/kg/inf methamphetamine. Thus, it may be argued that the treatment compounds differentially affected responding because baseline responding (i.e. total responses and infusions obtained under vehicle-treatment conditions) differed between methamphetamine doses to start. However, this explanation is unlikely because when response rates were specifically matched in rats maintained by 0.03 and 0.1 mg/kg/inf methamphetamine during Rate Dependency Analysis tests, 10 mg/kg ibudilast still did not reduce response rates for 0.1 mg/kg/inf methamphetamine. Furthermore, the response rate reductions at 0.03 mg/kg/inf methamphetamine were not likely a result of non-specific behavioral suppression because total infusions (and consequently, response rate) following pretreatment with the test compounds did not differ from baseline rates at either the lowest or highest tested methamphetamine doses in the present study (and they would have been if there was non-specific behavioral suppression) nor did ibudilast or AV1013 significantly suppress locomotor activity in mice when tested alone under similar dosage regimens (Snider et al., 2012). Ibudilast has been reported, however, to produce transient sedation and decreased reactivity to touch in the Irwin test in Wistar (Han) rats (Ledeboer et al., 2006).
Relative potency analysis revealed that ibudilast was ~6 times more potent than AV1013, and ~13 times more potent than minocycline in reducing self-administration of methamphetamine. These data are consistent with our previous reports that ibudilast is ~4–7 more potent in reducing methamphetamine-induced hyperlocomotion in mice than AV1013 (Snider et al., 2012). These findings also support that while glial cell modulation is sufficient to attenuate some methamphetamine-induced behaviors, ibudilast’s phosphodiesterase inhibition activity may have acted in combination with glial suppression. Just as other phosphodiesterase inhibitors have been shown to attenuate methamphetamine-induced behaviors such as locomotor activity, sensitization, and drug discrimination (Iyo et al., 1996a; Iyo et al., 1996b; Iyo et al., 1995; Mori et al., 2000; Yan et al., 2004; Yan et al., 2006), ibudilast’s combination of these two mechanisms (i.e., glial and PDE) may help explain its increased potency.
While the present study is the first example of ibudilast, AV1013, or minocycline being examined for their effects on ongoing self-administration of an abused drug, our findings are consistent with previous reports that glial cell modulation can attenuate other drug-induced behaviors. Ibudilast has been reported to decrease opioid dependence and withdrawal signs (Hutchinson et al., 2009; Ledeboer et al., 2007), attenuate morphine-induced dopamine release in the nucleus accumbens (Bland et al., 2009), and CPP reinstatement in rats (Schwarz et al., 2011). Furthermore, we have reported ibudilast attenuates prime- and stress-induced methamphetamine reinstatement in rats (Beardsley et al., 2010), and ibudilast and AV1013 reduces methamphetamine-induced locomotor activity and its sensitization in mice (Snider et al., 2012). Minocycline has been reported to attenuate cocaine-induced locomotor activity and its sensitization in mice (Chen et al., 2009; Zhang et al., 2006), as well as methamphetamine CPP (Fujita et al., 2012). Clearly, previously published data and the present report, strengthen the linkage between glial cell activation, neuroinflammation, and the behavioral effects of abused drugs.
5. Conclusions
In conclusion, the present study established methamphetamine self-administration at levels likely great enough to induce glial activation and pro-inflammatory signaling. Ibudilast, AV1013, and minocycline, three compounds that attenuate glial activity, all reduced self-administration of 0.03 mg/kg/inf methamphetamine. While the mechanism of these effects has yet to be definitively identified, the results suggest that glial cell modulation may provide a novel target for pharmacotherapeutics reducing methamphetamine drug-seeking behavior.
Acknowledgements
This research was supported by NIDA training grant (#2T32DA007027-36). The present study was completed in partial fulfillment of the doctoral requirements in Pharmacology & Toxicology for S. E. S. Some of these data were presented at the June, 2012 International Study Group Investigating Drugs as Reinforcers Meeting (ISGIDAR) held in Palm Springs, CA, under a travel fellowship by the Society for the Experimental Analysis of Behavior (SEAB) to S. E. S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
P. M. B. had served as a consultant to Avigen Inc., a company that at one time was involved with the pharmaceutical development of ibudilast and AV1013, prior to and terminating before the commencement of these studies.
References
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino AL, Kaushal D, Philipp MT. The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi. J Infect Dis. 2009;199:1379–1388. doi: 10.1086/597807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun. 2009;23:492–497. doi: 10.1016/j.bbi.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H. Minocycline affects cocaine sensitization in mice. Neurosci Lett. 2009;452:258–261. doi: 10.1016/j.neulet.2009.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Segal DS. Amphetamine and its analogs : psychopharmacology, toxicology, and abuse. San Diego: Academic Press; 1994. [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant Abuse and Neuroinflammation: Emerging Evidence of Their Interconnection. Neurotox Res. 2012 doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- Creese I. Stimulants, neurochemical, behavioral, and clinical perspectives. New York: Raven Press; 1983. [Google Scholar]
- Dews PB. Studies on behavior. I. Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharmacol Exp Ther. 1955;113:393–401. [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Fujita Y, Kunitachi S, Iyo M, Hashimoto K. The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol Biochem Behav. 2012;101:303–306. doi: 10.1016/j.pbb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann N Y Acad Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15:27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Hauer P. Systemic affects of methamphetamine use. S D Med. 2010;63:285–287. [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S. Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. Eur J Pharmacol. 1996a;312:163–170. doi: 10.1016/0014-2999(96)00479-7. [DOI] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Tomitaka SI, Inada T, Fukui S. Does an increase of cyclic AMP prevent methamphetamine-induced behavioral sensitization in rats? Ann N Y Acad Sci. 1996b;801:377–383. doi: 10.1111/j.1749-6632.1996.tb17458.x. [DOI] [PubMed] [Google Scholar]
- Iyo M, Maeda Y, Inada T, Kitao Y, Sasaki H, Fukui S. The effects of a selective cAMP phosphodiesterase inhibitor, rolipram, on methamphetamine-induced behavior. Neuropsychopharmacology. 1995;13:33–39. doi: 10.1016/0893-133X(94)00133-K. [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi Y, Ohta S, Kasuya N, Sakita S, Ashikaga T, Isobe M. Ibudilast: a nonselective PDE inhibitor with multiple actions on blood cells and the vascular wall. Cardiovasc Drug Rev. 2001;19:215–225. doi: 10.1111/j.1527-3466.2001.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Kita T, Takeshima M, Wagner GC, Hozumi H, Miyazaki I, Asanuma M. New perspectives on the mechanism of methamphetamine-induced neurotoxicity. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008;28:49–61. [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Silverstein PS, Singh V, Shah A, Qureshi N, Kumar A. Methamphetamine increases LPS-mediated expression of IL-8, TNF-alpha and IL-1beta in human macrophages through common signaling pathways. PLoS One. 2012;7:e33822. doi: 10.1371/journal.pone.0033822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. The Role of Glial Cells in Drug Abuse. Curr Drug Abuse Rev. 2009;2:76–82. doi: 10.2174/1874473710902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Laccardo Pacula R, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. Santa Monica, CA: RAND Corporation; 2009. p. 169. [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96:314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- Obert JL, McCann MJ, Marinelli-Casey P, Weiner A, Minsky S, Brethen P, Rawson R. The matrix model of outpatient stimulant abuse treatment: history and description. J Psychoactive Drugs. 2000;32:157–164. doi: 10.1080/02791072.2000.10400224. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. J Subst Abuse Treat. 2002;23:145–150. doi: 10.1016/s0740-5472(02)00256-8. [DOI] [PubMed] [Google Scholar]
- Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Silverstein PS, Singh DP, Kumar A. Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NF-kappaB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J Neuroinflammation. 2012;9:52. doi: 10.1186/1742-2094-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL, Beardsley PM. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol. 2012;679:75–80. doi: 10.1016/j.ejphar.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M. Psychotic symptoms in methamphetamine psychotic in-patients. Int J Neuropsychopharmacol. 2003;6:347–352. doi: 10.1017/S1461145703003675. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837:203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109:6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Galloway S, Ryan S, Hansen K, Hullsiek B, Muli V, Malone AC. Effects of methamphetamine abuse beyond individual users. J Psychoactive Drugs. 2009;41:241–248. doi: 10.1080/02791072.2009.10400534. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M. Methamphetamine-induced expression of interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;128:90–92. doi: 10.1016/0304-3940(91)90766-m. [DOI] [PubMed] [Google Scholar]
- Yan Y, Mizuno T, Nitta A, Yamada K, Nabeshima T. Nefiracetam attenuates methamphetamine-induced discriminative stimulus effects in rats. Ann N Y Acad Sci. 2004;1025:274–278. doi: 10.1196/annals.1316.034. [DOI] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizuno T, Nakajima A, Yamada K, Nabeshima T. Discriminative-stimulus effects of methamphetamine and morphine in rats are attenuated by cAMP-related compounds. Behav Brain Res. 2006;173:39–46. doi: 10.1016/j.bbr.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. Faseb J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance p in the striatum of mice. Ann N Y Acad Sci. 2006;1074:135–148. doi: 10.1196/annals.1369.013. [DOI] [PMC free article] [PubMed] [Google Scholar]