Abstract
MPYS (a.k.a STING, MITA, TMEM173) is a type I IFN stimulator that is essential for host defense against DNA virus infection and appears important in defense against certain bacteria. The in vivo significance and mechanisms by which MPYS mediates host defense against non-viral pathogens are unknown. Using a MPYS deficient mouse (Tmem173<tm1Camb>) we determined that, distinct from the IFNRA−/− mice, MPYS deficiency leads to increased bacterial burden in the liver upon Listeria monocytogenes infection. The increase was correlated with the diminished MCP-1 and MCP-3 chemokine production, and decreased blood and liver Ly6Chi monocytes frequency. We further demonstrate that MPYS deficient Ly6Chi monocytes are intrinsically defective in migration to the liver. Lastly, adoptive transfer of WT Ly6Chi monocyte into MPYS deficient mice decreases their liver bacterial burden. Our findings reveal a novel in vivo function of MPYS that is distinct from its role in activating type I IFN production.
Introduction
Listeria monocytogenes (L.M.) is a Gram-positive intracellular bacterium. Infection by L.M. is the cause of listeriosis, which primarily affects the elderly, pregnant women, newborns, and people with weakened immune systems. In the U.S., an estimated 1,600 people suffer serious illness from L.M. each year; approximately 16% of these illnesses result in death (1). The murine model of listeriosis has been used to investigate immune responses to human infection. These studies have shown that innate immunity controls the early response of the infection and is critical for survival (2).
Neutrophils were thought to be the primary cells responsible for killing L.M. during the innate immune response (3). This was supported by the observation that the depletion of neutrophils with Gr-1 mAb greatly enhances susceptibility of mice to infection with L.M (3). However, it has since been recognized that Gr-1 mAb reacts with both Ly6G on neutrophils, and Ly6C on monocytes and CD8+ memory T cells. Recent reports using mAb specific for Ly6G showed that neutrophils are dispensable for host defense against L. M (4, 5). Rather, Ly6Chi monocytes are essential for the control of bacterial infection (4, 5).
Ly6Chi Monocytes are mononuclear phagocytes that are generated in the bone marrow (BM) and circulate in the bloodstream (6). It has been shown that recruitment of Ly6Chi monocytes to the foci of infection is essential for the eradication of L.M. (7). Emigration of Ly6Chi monocytes from BM to the bloodstream is controlled by the interaction of chemokine receptor CCR2 and its ligands, MCP-1 and MCP-3, on monocytes (8, 9). Mice deficient in CCR2 or MCP-1, MCP-3 are susceptible to L.M. infection (8, 9). Interestingly, once monocytes are in the bloodstream, their migration to the liver is CCR2- and chemokine-independent, but is dependent on the interaction between CD11b on Ly6Chi monocytes and ICAM-1 on endothelial cells (10).
MPYS, also known as STING, MITA and TMEM173, is a four-transmembrane protein essential for L.M. induced type I IFN (IFN-I) production in vitro (11). MPYS mediates IFN-I production by sensing cytosolic DNA released from bacteria (12). Studies done in STING−/− mice (Tmem173<tm1Gnb>) have demonstrated that MPYS/STING is critical for host defense against DNA virus infections (12). However, increasing evidence suggests that MPYS/STING may also be important for host defense against non-viral pathogens such as Francisella tularensis, Brucella abortus, streptococcus pneumoniae, Chlamydia muridarum and plasmodium falciparum (13–17). The in vivo significance of MPYS/STING function in host defense against these non-viral infections has not been demonstrated. Using MPYS deficient mice (Tmem173<tm1Camb>), we show here that MPYS mediates host defense against L.M. infection in vivo by regulation of Ly6Chi monocyte recruitment.
Materials and Methods
Mice
Six to twelve week old mice were used for all experiments. IFNAR−/− (B6.129PF2/ifnab) have been described previously. Generation of MPYS deficient mice (Tmem173<tm1Camb>) has been described in detail previously (18). Briefly, exons 3–5 are flanked by loxp sites and a neo gene is inserted into intron 5, which is flanked by Frt elements. The presence of the neo gene disrupts expression of MPYS, causing null MPYS protein deficiency. Mice were housed and bred in the Biologic Resource Center (BRC) at National Jewish Health (Denver, CO). All in vivo experiments were performed in accordance with the regulations and with the approval of National Jewish Health and the Institutional Animal Care and Use Committee.
In vivo L. M Infections
Mice were infected (tail vein) with 4~7×103 cfu of log-phase L.M. (10403S). Spleens and livers were harvested at day 2, 3 and 4 post-infection. Bacterial CFUs in infected organs were determined by dilution plating, as previously described (18).
Cytokine and Chemokine ELISAs
Serum from infected mice was collected at indicated time points and concentrations of MCP-1 (eBioscience, 88–7391), MCP-3 (eBioscience, BMS6006INST), IL-12/p70 (eBioscience, 88–7921), TNF (eBioscience, 88–7324), IL-1β (eBioscience, 88–7013), IFNγ (BD Biosciences, 551866) and IFNβ (PBI InterferonSource, 42400) using commercial ELISAs.
Calcium mobilization ([Ca+2]i)
Intracellular calcium concentration was measured, as previously described (18). Briefly, BM cells were loaded with Indo-1AM (Molecular Probes, Eugene, OR) together with Ly6C-APC (BD Biosciences, clone AL-21) for 30 min at 37°C. Cells were then washed and suspended in IMDM supplemented with 2% FCS at 106 cells/ml. Cells were stimulated with MCP-1. Data were collected on LSR II and analyzed using Flow-Jo software (Tree Star, Inc., San Carlos, CA).
Flow Cytometric Assay of Immunofluorescence
Cells were stained with FITC- or PerCP-CD45 (BD Pharmingen, 30-F11), APC-F4/80 (AbD-Serotec, Cl:A3-1), PE-F4/80 (CALTAG, BM8), FITC- or APC- Ly6C (BD Pharmingen, AL-21), PE-Ly6G (BD Pharmingen, 1A8), APC-CY7-CD11b (BD Pharmingen, M1/70). FACS was performed using a FACScan flow cytometer (BD Biosciences) and analyzed by FlowJo (CellQuest).
Liver lymphocytes isolation
Mice were euthanized at the indicated time. Livers were perfused through the portal vein, and digested in collagenase IV (0.05%, Worthington, # 4188) and DNase I (0.002%, Roche, #12877200) buffer for 37°C for 40 min. Nonparenchymal cells were first isolated by centrifugation at low g force (20g), then further purified by 40%/80% Percoll gradient. Cells at the interface of the gradient were collected and subjected to further analysis.
Adoptive transfer
For competitive adoptive transfer experiments, BM cells were isolated and stained with Ly6C-APC (AL-21) on ice for 20 min. Ly6Chi monocytes (1×106) were sorted by the National Jewish Health Flow Cytometry Core Facility using the Moflo XDP Sorter. Cells were washed in PBS and differentially labeled with 0.5µM or 2.5µM CFSE in PBS at RT for 20 min. Cells were then washed in complete medium, suspended in PBS, mixed at a 1:1 ratio and injected (i.v) into recipient mice.
For the adoptive transfer of cells into L.M. infected animals, BM cells were isolated and stained with Ly6C-APC (AL-21) on ice for 20 min. Ly6Chi monocytes were sorted as previously described above. Sorted Ly6Chi cells (0.75×106) were suspended in 200µl PBS and injected (i.v) into recipient mice at 24hpi of L.M. infection.
Immunoblotting
Immunoblotting was done, as stated previously (19). Briefly, cells were lysed in RIPA buffer (50mM pH7.4 Tris-Cl, 1% NP40, 0.25% sodium deoxycholate, 150mm sodium chloride, 10mM iodoacetamide, 0.1 % SDS, 2 mM Na3VO4, 10 mM NaF, 0.4 mM EDTA, 1 mM PMSF, and 1 µg/ml each of aprotinin, α1-antitrypsin, and leupeptin). Whole cell lysates were loaded directly on a NuPAGE 10% Bis-Tris Gel (Invitrogen).
Intracellular MPYS Staining
Intracellular MPYS staining was done, as previously described (18). Briefly, cells were washed in FACS buffer (PBS with 2% FBS, 0.05% sodium azide and 0.2µg/ml 2.4g2 Fc-receptor blocking Ab) and then re-suspended in BD Cytofix/Cytoperm™ buffer (BD Bioscience, cat# 554722) for 20 min at RT. BD Perm/Wash buffer™ (BD Bioscience, cat# 554723) was added to the cell suspension. Cells were collected and washed with BD Perm/wash buffer™ again. Cells were suspended in BD Perm/wash buffer™ containing Alex-647 conjugated rabbit anti-MPYS Ab for 20min at RT. Cells were collected, washed with BD Perm/wash buffer™, and analyzed using a FACScan. Data were analyzed using Flow-Jo software (Tree Star, Inc., San Carlos, CA).
Generation of BMDM
Bone-marrow-derived macrophages were generated, as previously described (18). BM cells harvested from mouse femurs were cultured in DMEM (GIBCO, cat# 11965) containing 20% FBS (Biosource, cat# 200P-500HI), 10% M-CSF containing media, 2mM L-glutamine (GIBCO, cat# 25030), 1mM Sodium Pyruvate (GIBCO, cat# 11360), 100units/ml penicillin+100µg/ml streptomycin (GIBCO, cat# 15140) and 50µM 2-ME. The medium was exchanged after three days, and cells were stained for the macrophage marker F4/80 at day 7.
In vitro L. M Infection
Cells were seeded in 12-well plates at 1×106 cells/well. The following day the medium was exchanged with fresh antibiotic-free medium. GFP-expressing Listeria monocytogenes (Listeria-GFP) (strain 10403S) were grown to log phase and added to the cell cultures. After 30 min, gentamicin (GIBCO, cat# 15750-060, 50µg/ml) was added to the medium to kill all extracellular bacteria. At 1 hr post infection, the medium was exchanged. At indicated time points, supernatants were collected and analyzed for cytokines by ELISA. For c.f.u measurement, cells were harvested, washed in PBS, and then lysed in 0.02% NP40 buffer. Serial dilutions of lysate were plated onto TSB agar plates.
RT-PCR detection of MPYS transcript
Cells were sorted based on the indicated surface markers. Total RNA was isolated using the Qiagen RNeasy Plus mini kit (Qiagen) and reverse-transcribed using oligodeoxythymidylate primers and the IMPROM II FT system (Promega) as previously described (18). cDNAs were used as template for 29–32 cycles of PCR with Taq polymerase (Invitrogen). Primers used were:
GAPDH sense (GGGAAGCCCATCACCATCTT);
GAPDH antisense (ACATACTCAGCACCGGCCTC);
MPYS sense (CAGGAACACCGGTCTAGGAA);
MPYS antisense (GCCAAACATCCAACTGAGGT);
Statistical Analysis
All data are expressed as means ± SEM. Statistical significance was evaluated using Prism 4.0 software to perform a Student’s t test (unpaired, two tailed) for comparisons between mean values.
Online Supplemental Figures
The online supplemental materials include 4 supplemental figures.
Results
Increased bacterial burden in the liver of L.M. infected Tmem173<tm1Camb> (MPYS−/−) mice
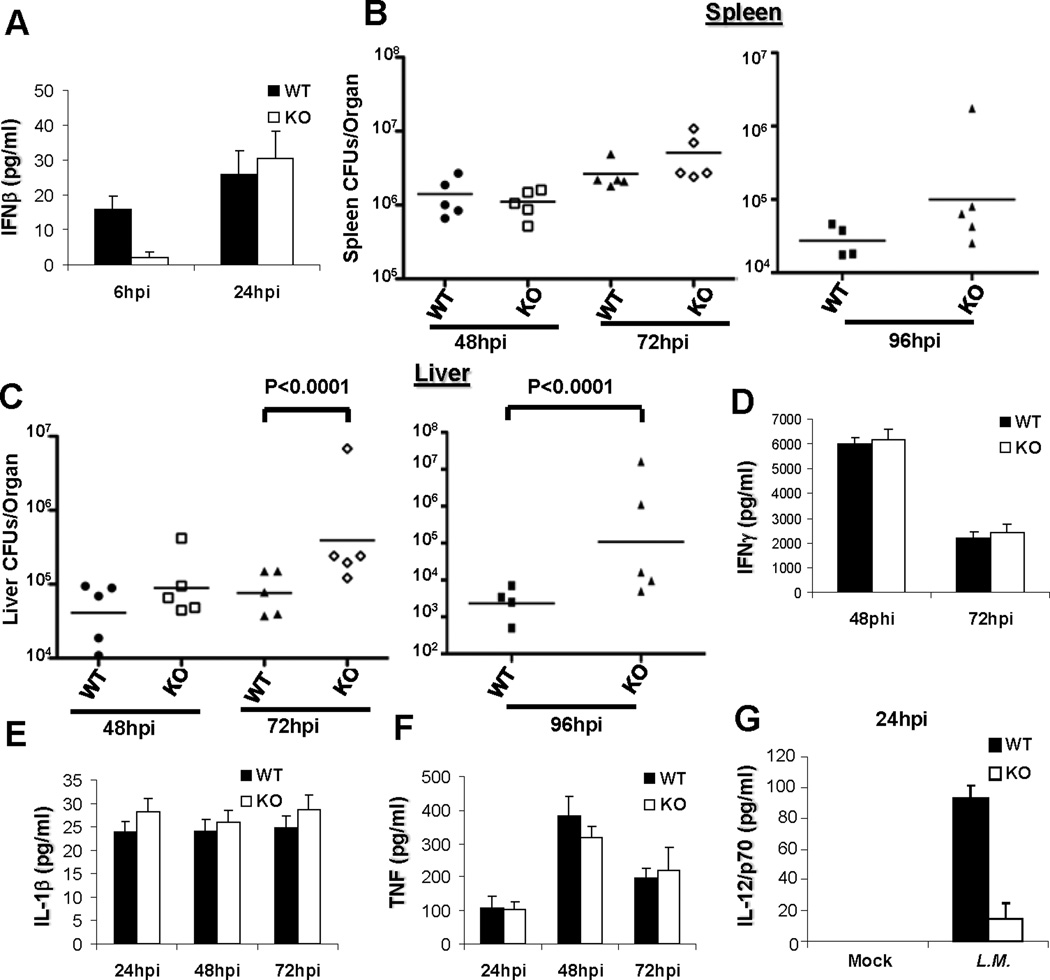
MPYS mediates L.M. induction of IFNβ in cultured Bone marrow-derived macrophages (BMDM) and Bone marrow-derived dendritic cells (BMDC) (Fig S1A) (12) via activation of transcriptional factor IRF3 (Fig S1B, S1C) (18). Previous studies have demonstrated that IFN-I is detrimental to the host defense against L.M. (20–22). We thus hypothesized, initially, that MPYS−/− mice will be resistant to L.M. infection, similar to the IFNRA−/− mice. To test this hypothesis, we infected the MPYS−/− mice (Tmem173<tm1Camb>) (18) with a sub-lethal dose of L.M. (i.v.). To our surprise, we found that MPYS is only partially required for L.M. induction of IFNβ production in vivo (Fig 1A). Furthermore, unlike the IFNRA−/− mice which have ~1,200 fold less bacterial load in the spleen 72 hrs post infection (hpi) (22), spleens from L.M. infected MPYS−/− mice have a similar bacterial burden to WT mice (Fig 1B).
Figure 1. MPYS−/− mice have an increased bacterial burden in the liver, but not the spleen.
MPYS−/− and their WT littermates were infected (i.v) with L.M. (7,000 cfu). Spleens (B) and livers (C) were harvested, homogenized, and dilution plated to determine bacterial burden at the indicated time after infection. Each data point indicates an individual mouse. Sera were collected at the same time and IFNβ (A), IFNγ (D), IL-1β (E), TNF (F) and IL-12/p70 (G) were measured by ELISA. Bars indicate the mean values. Experiments were repeated more than three times.
The spleen and liver are the major sites of infection during systemic L.M. infection, in vivo. We found that MPYS−/− mice have increased bacterial burden in the liver by 72hpi and 96hpi compared to WT mice (Fig 1C). This observation is in contrast to IFNRA−/− mice, which showed ~1,000-fold reduced bacterial burden in the liver compared to WT mice by 72hpi (22). We also examined cytokine production during L.M. infection. We found that MPYS−/− mice have normal serum IFNγ, IL-1β and TNF level for 3 days following infection (Fig 1D, 1E, 1F and S1D). Interestingly, L.M. infected MPYS−/− mice have decreased IL-12 production (Fig 1G), which is also different from the increased IL-12 production found in L.M infected IFNRA−/− mice (22). We concluded that MPYS mediates host defense against L.M through a mechanism distinct from its role in activating IFN-I signaling.
MPYS is highly expressed in CD45+ liver cells but not in CD45− hepatocytes
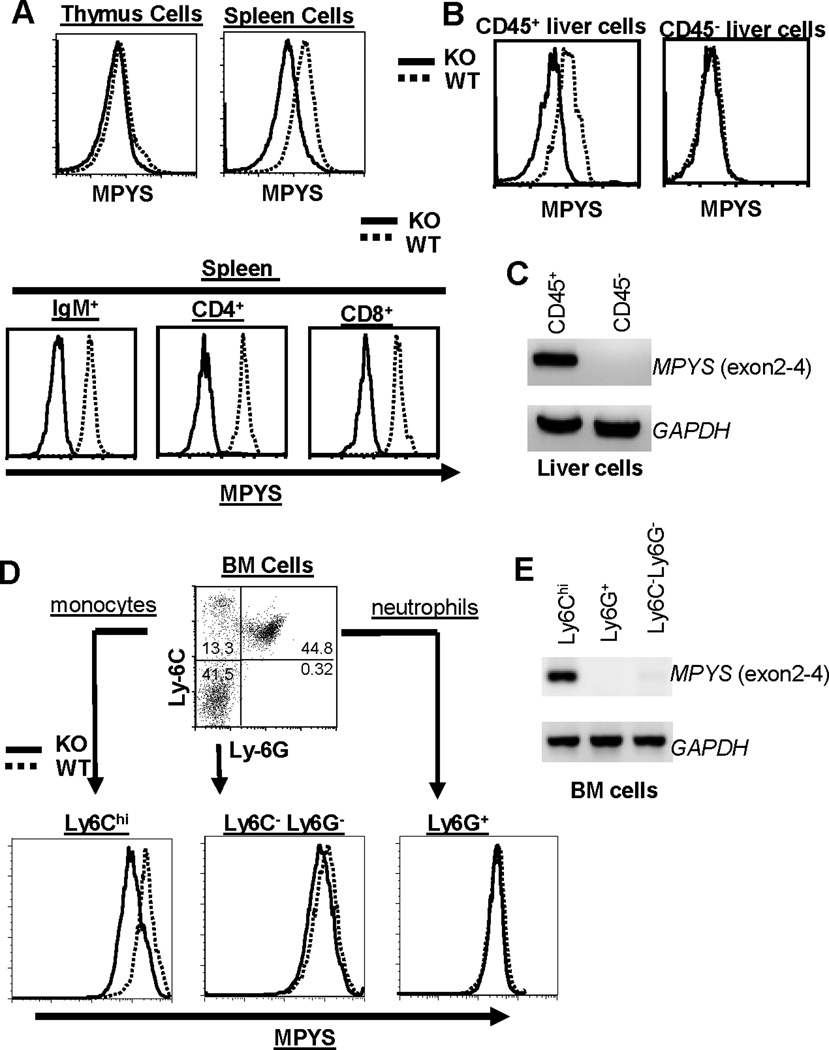
L.M. infected MPYS−/− mice have increased bacterial load in the liver but, not in the spleen relative to WT mice (Fig 1). To understand the seemingly different roles of MPYS in host defense against L.M. in the spleen and liver, we examined MPYS expression in spleen and liver cells by intracellular staining with an polyclonal antibody that recognizes the cytoplasmic tail of MPYS (18). Using the cells from MPYS−/− mice as negative controls, we found that MPYS is highly expressed in CD4+ T cells, CD8+ T cells, and IgM+ B cells in the spleen (Fig 2A). The deficiency of MPYS does not affect B cell and T cell development (12) (data not shown). Interestingly, MPYS is barely detectable in the thymus, where most cells are CD4+CD8+ T cells (Fig 2A). Surprisingly, we found that, in the liver, MPYS is only expressed in CD45+ cells while the majority of liver cells (CD45− hepatocytes) do not express MPYS (Fig 2B). RT-PCR analysis of MPYS level in CD45− hepatocytes confirms the lack of expression (Fig 2C). Thus, the increased liver bacterial burden in MPYS−/− mice is likely due to the deficiency of MPYS in CD45+ liver cells.
Figure 2. Selective Expression of MPYS in Various Cell Types.
A,B,D. Representative FACS plots of cells in the spleen, thymus, liver, and BM from naïve WT and MPYS−/− mice were stained with indicated Abs, as well as, MPYS by rabbit anti-mouse MPYS polyclonal Ab as described before (18). Experiments were done more than three times. C & E. RT-PCR to detect the MPYS transcript was done in c-DNA made from indicated cells from WT and MPYS−/− mice using primers for exon 2, 3, and 4 of the MPYS transcript. This experiment was repeated twice.
MPYS deficiency does not affect the ability of CD45+ liver cells to clear L.M
To examine the ability of residential CD45+ liver cells to clear L.M., we infected ex vivo CD45+ liver cells with GFP-expressing L.M. strain and tracked GFP-positive cells over time. We found that, unlike BMDM and BM neutrophils (Fig 3), CD45+ liver cells do not efficiently take up L.M. (Fig S2A). Furthermore, there is no difference in the number of L.M. positive CD45+ WT and MPYS−/− liver cells after overnight culture (Fig S2A). The MPYS−/− mice have similar populations of CD3+ T cells, B220+ B cells and NK1.1+ NK cells in the liver to WT mice (Fig S2B)
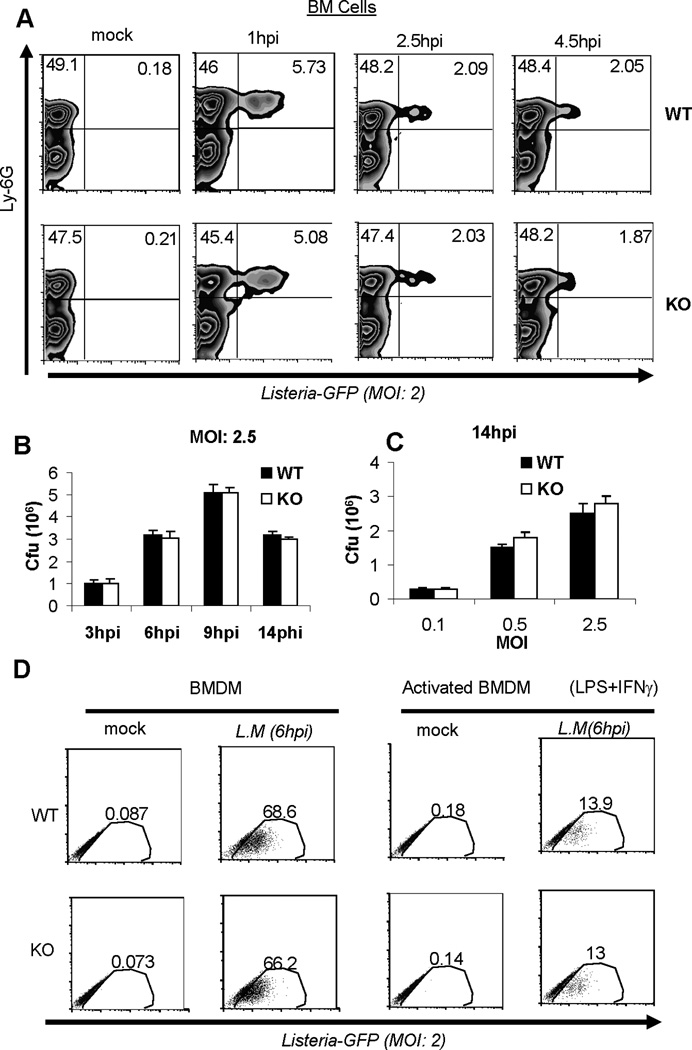
Figure 3. MPYS deficiency does not affect bacterial clearance by neutrophils and macrophages.
A. BM cells from WT and MPYS−/− mice were isolated and cultured together with Listeria-GFP (MOI: 2), as described in Materials and Methods. Cells were then stained for Ly6G expression and GFP positive cells were examined at the indicated time by Flow. Experiments were repeated twice. B–C. BMDM from WT and MPYS−/− mice were infected with L.M.. at indicated MOI. Cells were then lysed and c.f.u was determined, as described in Materials and Methods. Bars indicate the mean values. Experiments were repeated twice. D. WT and MPYS−/− BMDM cells or IFNγ (15U/ml) and LPS (100ng/ml)-treated BMDM (18hrs) were cultured with Listeria-GFP (MOI: 2) as described in Materials and Methods. GFP positive cells were determined by Flow at 6hpi. Experiments were done three times.
MPYS is expressed in bone marrow Ly6Chi monocytes but absent in Ly6G+ neutrophils
During systemic L.M. infection, there is an influx of monocytes from BM into the liver. These cells can represent ~50% of total CD45+ cells in the liver (23). Recent studies revealed that it is these Ly6Chi monocytes, but not Ly6G+ neutrophils, that mediate bacterial clearance in the liver (5). We found that MPYS is expressed in Ly6Chi monocytes, but not Ly6G+ neutrophils (Fig 2D). The expression of MPYS in Ly6C−Ly6G− BM cells is also low (Fig 2D). RT-PCR of MPYS in Ly6Chi and Ly6G+ cells confirmed these results (Fig 2E).
MPYS deficiency does not affect bacterial clearance by bone marrow cells
To examine their ability to clear L.M., we infected ex vivo BM cells with a GFP-expressing L.M. strain and tracked GFP-positive L.M. infected cells over the time. We found that L.M. was taken up mainly by Ly6G+ neutrophils (Fig 3A). Furthermore, there was no difference in bacterial clearance by Ly6G+ neutrophils cells from MPYS−/− and WT mice (Fig 3A). This is consistent with our finding that Ly6G+ neutrophils do not express MPYS (Fig 2).
MPYS deficiency does not affect bacterial clearance by BMDM
As shown in Fig 3A, Ly6Chi monocytes isolated from BM do not phagocytose bacteria well. However, Ly6Chi monocytes can differentiate into phagocytic macrophages in peripheral tissues. We examined bacterial clearance in BMDM. We did not find significant differences in phagocytosis of L.M. by BMDM from WT and MPYS−/− mice (Fig 3B and 3C). Macrophages can be further primed by IFNγ and LPS to generate activated macrophages that efficiently clear bacteria. Using the GFP-expressing L.M. strain, we found that activated BMDM from MPYS−/− mice have a similar ability to clear bacteria to WT mice (Fig 3D). We concluded that MPYS deficiency did not affect bacterial clearance by resting or activated BMDM.
Decreased L.M. infection-induced recruitment of Ly6Chi monocytes to liver in MPYS−/− mice
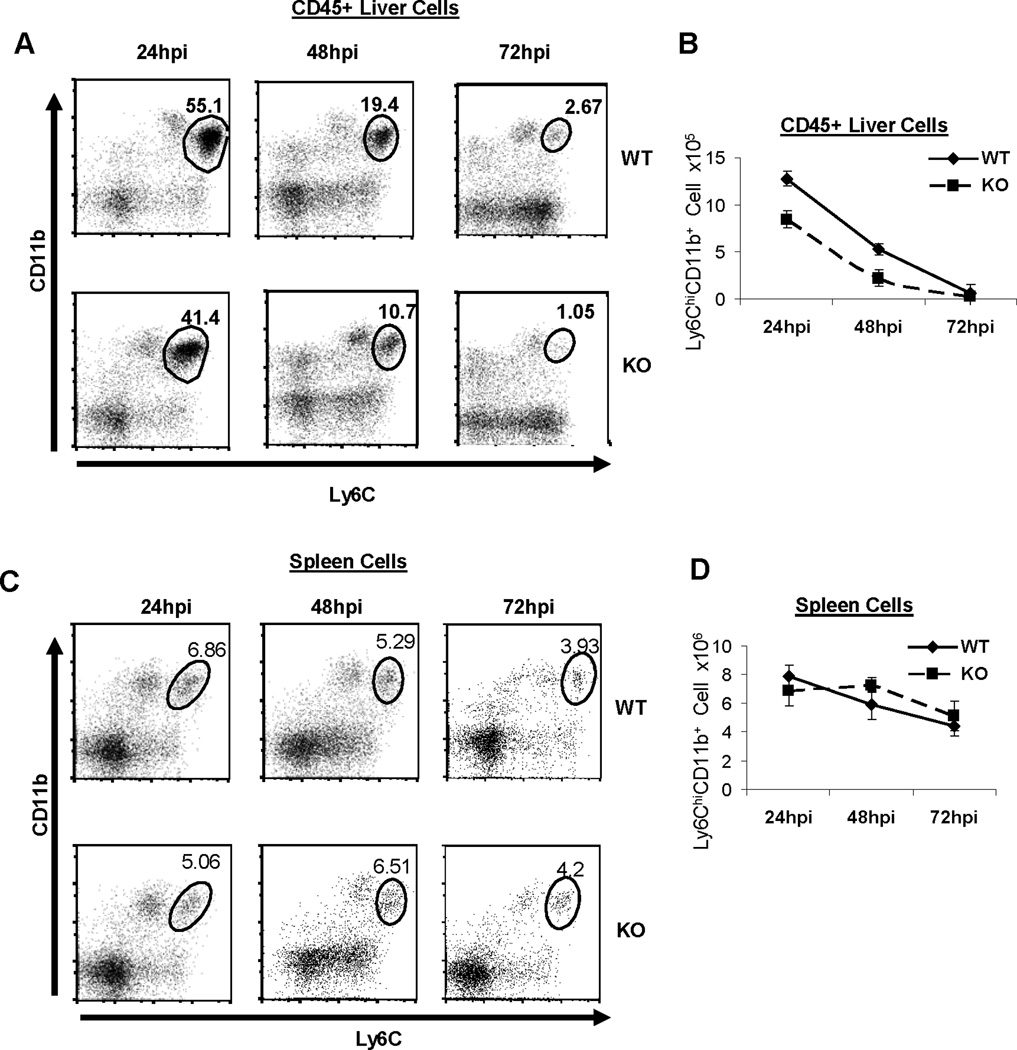
MPYS-deficiency does not affect macrophage clearance of L.M., which is consistent with the observation that there is no defect in bacterial clearance in spleens of infected MPYS−/− mice (Fig 1). However, we did see increased bacterial burden in livers of infected MPYS−/− mice (Fig 1). Unlike spleen, we found that MPYS is only expressed in CD45+ liver cells (Fig 2). Furthermore, during L.M. infection, ~50% of these CD45+ liver cells are Ly6Chi monocytes newly migrated from BM. We hypothesize that the increased bacterial load in the liver is due to the impaired recruitment of Ly6Chi monocytes. Indeed, we found that both the percentage, and total numbers, of Ly6Chi monocytes in the liver are decreased in L.M. infected MPYS−/− mice compared to WT mice over a three day period, post infection (Fig 4A, 4B).
Figure 4. MPYS−/− mice exhibit diminished Ly6Chi monocytes recruitment to liver but not spleen during systemic L.M. infection.
A & C. MPYS KO and their WT littermate controls were infected (i.v) with L.M. (~7,000cfu). Representative FACS plots of CD45+ leukocytes in livers (A) and the spleen (C) stained for Ly6C and CD11b expression are shown. B & D. The number of liver (B) or spleen (D) Ly6Chi monocytes from infected mice during the time period are shown. The data are presented as mean +/− SEM. A representative of 3 independent experiments is shown.
NK cells play an important role in the innate immunity phases to L.M. infection, producing IFNγ required for the maturation of Ly6Chi monocytes and generation of activated macrophage that effective clearance of bacteria (23). We found that the number of CD11b+ NK1.1+ activated NK cells in the liver, from infected MPYS−/− mice, is not significantly different from WT mice (Fig S3). This is consistent with the observation of normal IFNγ production in MPYS−/− mice (Fig 1D and S1D).
There is no difference in Ly6Chi monocyte infiltration in the spleens of infected WT and MPYS−/− mice (Fig 4C, 4D). This is consistent with the observation that MPYS−/− mice have comparable bacterial burden in spleen to WT mice (Fig 1).
Diminished MCP-1 and MCP-3 production in MPYS−/− mice
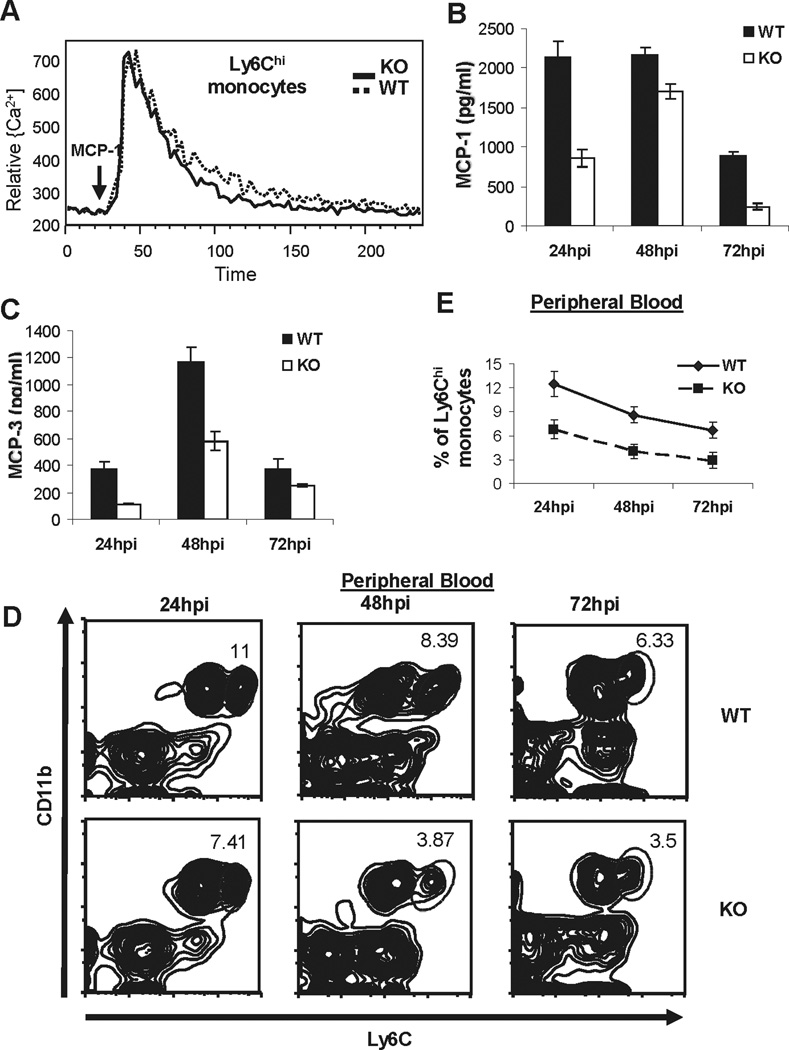
The recruitment of Ly6Chi monocytes to foci of infection is critical for the eradication of the bacteria (6). Previous studies have established that the chemokine receptor CCR2 is essential for monocyte emigration out of BM (9). However, we found that MPYS−/− Ly6Chi monocytes have normal CCR2 signaling (Fig 5A).
Figure 5. Decreased MCP-1 and MCP-3 production in L.M. infected MPYS−/− mice.
A. Bone marrow cells from MPYS KO and WT littermates were isolated, loaded with Indo-1 AM and stained with Ly6C-APC, then stimulated with recombinant mouse MCP-1 (10ng/ml, eBioscience). Cells were gated on Ly6Chi monocytes population. Experiments were repeated twice. B–C. MPYS−/− and their WT littermates were infected with L.M. and serum MCP-1, MCP-3 chemokines were measured as in Fig 1. Bars indicate the mean values. Experiments were repeated more than three times. D. Representative FACS plots of Ly6Chi CD11b+ monocytes in peripheral blood from infected mice are shown as in Fig 2. E. The percentages of Ly6Chi monocytes in blood from infected mice during the time period are shown. The data is presented as mean +/− SEM. A representative of 3 independent experiments is shown.
CCR2 is a receptor for the chemokines MCP-1 and MCP-3. Recent studies suggest that MPYS is required for MCP-1 production in Mouse Embryonic Fibroblast (MEF) cells (24). In view of this we determined whether MPYS deficiency affects in vivo MCP-1 and MCP-3 production during L.M. infection. We found that the production of both MCP-1 and MCP-3 are diminished in L.M. infected MPYS−/− mice (Fig 5B, 5C). In agreement with this finding, we found that MPYS−/− mice have decreased Ly6Chi monocytes in the bloodstream during systemic L.M. infections (Fig 5D, E).
Impaired Ly6Chi monocytes migration from bloodstream to livers in MPYS−/− mice
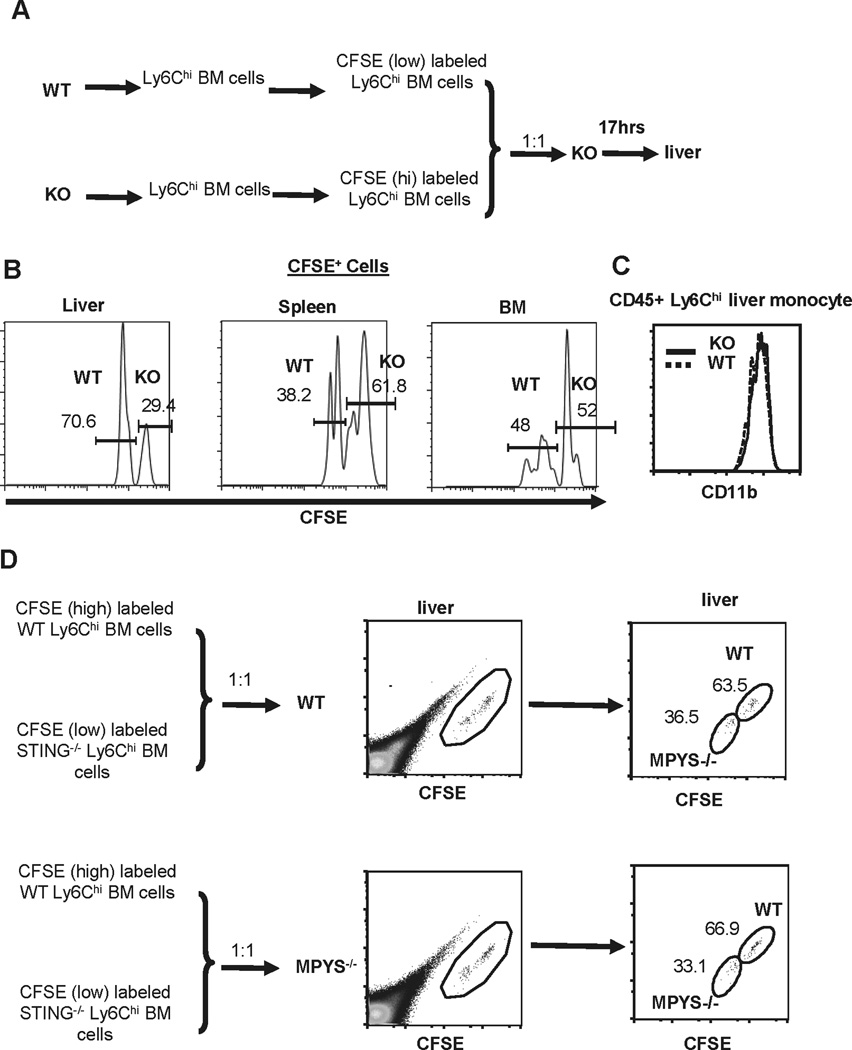
We then addressed why Ly6Chi monocyte infiltration into livers, but not spleens, is impaired in MPYS−/− mice. Migration of Ly6Chi monocytes from the bloodstream to the liver is CCR2-independent, but requires CD11b expression on Ly6Chi monocytes (10). However, migration of Ly6Chi monocytes from the bloodstream to the spleen depends on CX3CR1 expression on monocytes and CX3CL1 expression in the marginal zone on T cells (25). To begin to address this selectivity of infiltration, we performed the competitive adoptive transfer experiments. Sorted Ly6Chi monocytes from WT and MPYS−/− mice were differentially labeled with CFSE, then mixed at a 1:1 ratio and adoptively transferred into MPYS−/− mice (i.v.) (Fig 6A). After 17hrs, labeled positive cells in the liver, spleen, and BM of the recipient mice were quantified. We found that there were fewer MPYS−/− Ly6Chi monocytes than WT Ly6Chi monocytes in the liver (Fig 6B, left panel). Interestingly, there were more MPYS−/− Ly6Chi monocytes in the spleen than WT Ly6Chi monocytes (Fig 6B, middle panel). The more efficient migration of MPYS−/− Ly6Chi monocytes from the bloodstream to the spleen may explain why the numbers of Ly6Chi monocytes in MPYS−/− and WT spleens are similar. There was no significant difference in the recovery of WT and MPYS−/− Ly6Chi monocytes in BM (Fig 6B, right panel). The CD11b expression on WT and MPYS−/− monocytes Ly6Chi was also similar (Fig 6C).
Figure 6. MPYS−/− monocytes have impaired ability migrating from bloodstream to liver.
A. Ly6Chi BM cells from WT and MPYS−/− mice were sorted, labeled with low dose of CFSE (0.5µM, WT BM cells) or high dose of CFSE (2.5µM, MPYS−/− cells), then mixed at 1:1 ration and injected (i.v) into MPYS−/− mice. CFSE positive cells were analyzed after 17hrs. B. Representative FACS plots of CFSE+ monocytes in the liver, spleen, and BM of the recipient mice. C. Representative FACS plots of CD11b expression in Ly6Chi monocytes from WT and MPYS−/− livers. D. Ly6Chi BM cells from WT and MPYS−/− mice were sorted, labeled with low dose of CFSE (0.5µM, MPYS−/− cells) or high dose of CFSE (2.5µM, WT cells), mixed at 1:1 ratio and injected (i.v) into MPYS−/− or WT mice. CFSE positive cells were analyzed after 17hrs. Shown are representative FACS plots of CFSE+ monocytes in the liver of recipient mice. A representative of 3 independent experiments is shown.
The impaired MPYS−/− Ly6Chi monocytes migration from bloodstream to liver is cell intrinsic
To determine whether it is the lack of MPYS expression in Ly6Chi monocytes or other cell types that leads to the impaired MPYS−/− Ly6Chi monocyte migration, we adoptively transferred CFSE labeled WT and MPYS−/− Ly6Chi monocytes to WT or MPYS−/− mice and examined the ratio of CFSE labeled cells. We found that, in both cases, there were fewer MPYS−/− Ly6Chi monocytes than WT Ly6Chi cells in the liver (Fig 6D). We concluded that the homing defect is monocyte intrinsic, i.e. due to the lack of MPYS expression in Ly6Chi monocytes.
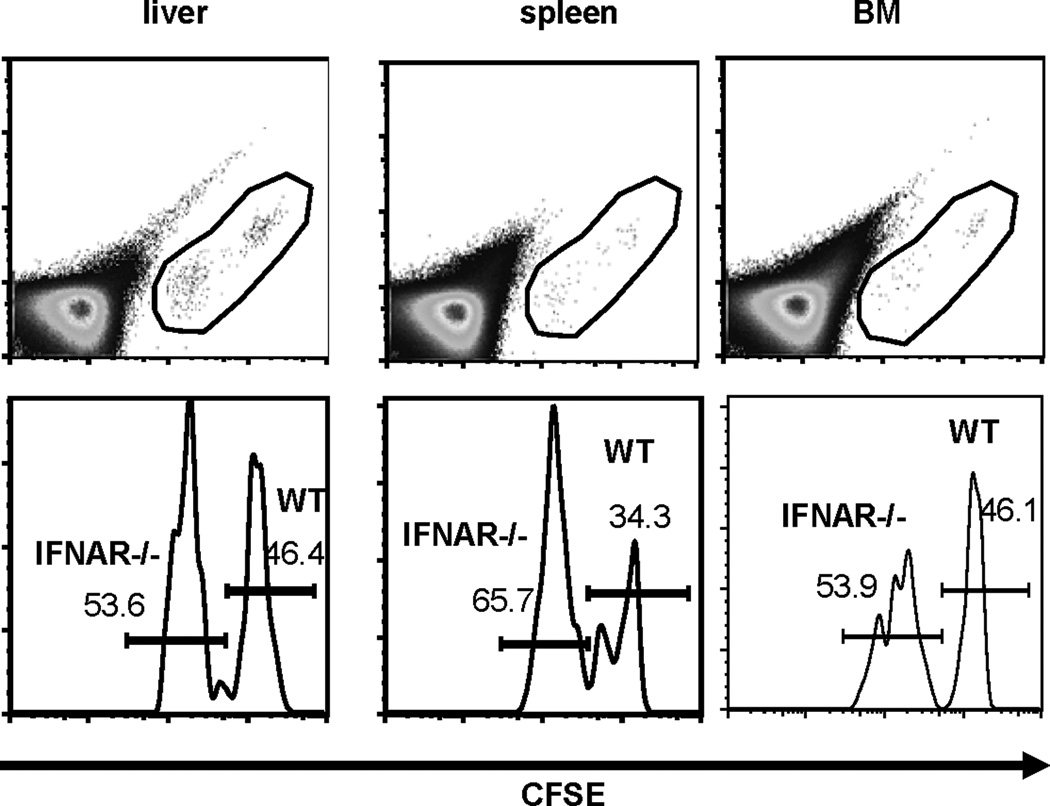
IFNRA−/− Ly6Chi monocytes migrate normally to liver
MPYS is an IFN-I stimulator. To determine if IFN-I signaling is required for Ly6Chi monocytes migration to liver, we performed a competitive adoptive transfer experiment in which WT and IFNAR−/− Ly6Chi monocytes were transferred to recipients and localization in liver compared. We found that, unlike MPYS−/− Ly6Chi monocytes, there was no significant difference in the ability of the two cell populations to home to the liver (Fig 7). Interestingly, like MPYS−/− cells, IFNAR−/− Ly6Chi monocytes migrate more efficiently to spleen than WT cells (Fig 7). There is no significant difference in the recovery of WT and IFNAR−/− Ly6Chi monocytes in BM (Fig 7). We concluded that defective localization of MPYS−/− Ly6Chi monocytes in liver is not due to the lack of IFN-I signaling.
Figure 7. IFNAR−/− monocytes migrates normally to liver.
Ly6Chi BM cells from WT and IFNAR−/− mice were sorted, labeled with low dose of CFSE (0.5µM, IFNAR−/− Ly6Chi cells) or high dose of CFSE (2.5µM, WT Ly6Chi cells), mixed at 1:1 ration, and injected (i.v) back into WT mice. CFSE positive cells were analyzed after 17hrs. Shown are representative FACS plots of total cells and CFSE+ liver, spleen, and BM of the recipient mice. Experiments were repeated twice.
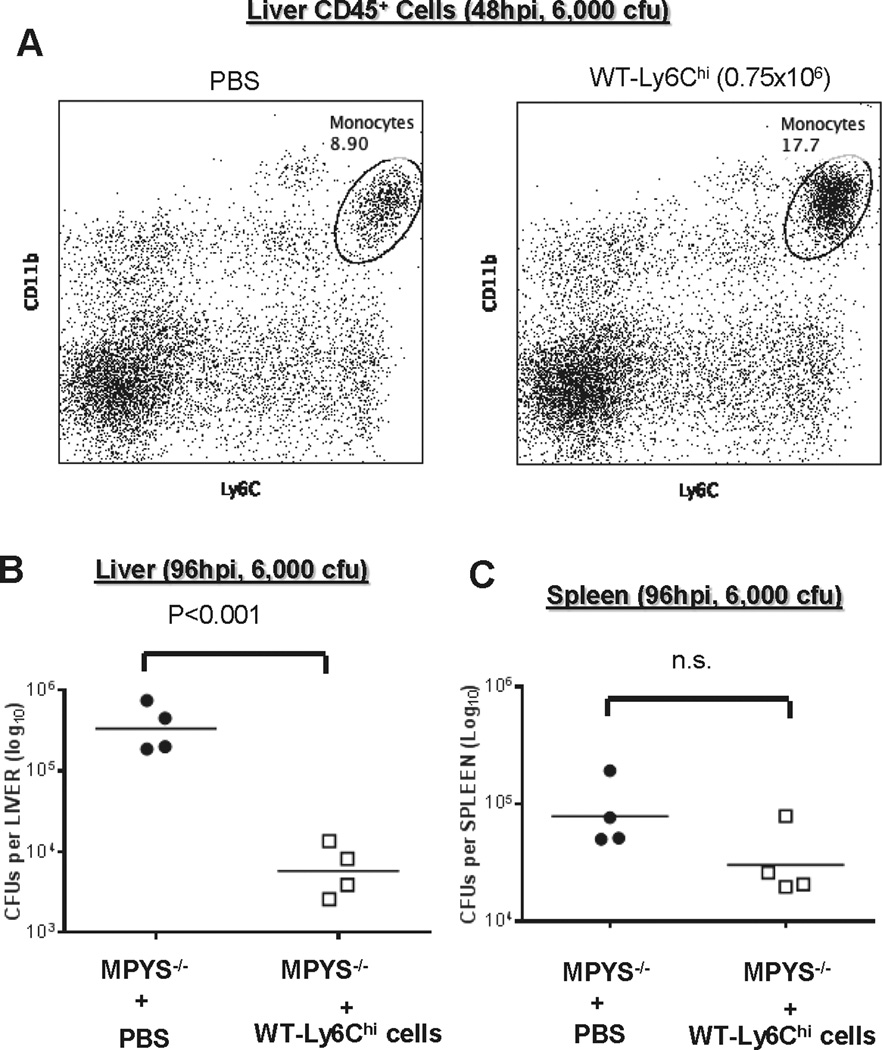
Adoptive transfer of WT Ly6Chi monocytes to MPYS−/− mice decreases liver bacterial burden
It was shown that the number of Ly6Chi monocytes recruited directly correlates with bacterial clearance efficiency in the liver (10). To test our hypothesis that the increased liver bacterial burden in MPYS−/− mice is due to the decreased recruitment of Ly6Chi monocytes, we adoptively transferred WT Ly6Chi monocyte into L.M.–infected MPYS−/− mice and assessed bacterial burden. We first showed that injecting (i.v.) WT Ly6Chi monocyte into L.M.-infected MPYS−/− mice indeed increase the numbers of liver Ly6Chi monocyte (Fig 8A). We next examined the liver bacterial burden in adoptively transferred MPYS−/− mice at 96hpi. Indeed, liver bacterial burden in these MPYS−/− mice is significantly decreased by the addition of WT Ly6Chi cells (Fig 8B). There is no significant change in the spleen (Fig 8C). We concluded that the increased bacterial burden in livers from MPYS−/− mice is due to the decreased liver localization of Ly6Chi cells.
Figure 8. Adoptive transfered of WT Ly6Chi monocytes decrease bacterial burden in livers of MPYS−/− mice.
A. Ly6Chi BM cells from WT mice were sorted and injected (i.v) into MPYS−/− mice at 24hpi of L.M. infection. Liver CD45+ cells were isolated and analyzed at 48hpi as described before. B–C. Livers (B) and spleens (C) from L.M. infected mice, receiving WT Ly6Chi BM or mock (PBS), were harvested at 96hpi, homogenized, and dilution plated to determine bacterial burden. Each data point indicates an individual mouse. Experiments were repeated twice.
Discussion
MPYS is critical for host defense against DNA virus infection due largely to its ability to mediate IFN-I production induced by the sensing of cytosolic viral DNA (12). In this report, we report a new in vivo mechanism by which MPYS mediates host defense against L.M. infection by controlling Ly6Chi monocytes recruitment in the liver. MPYS controls Ly6Chi monocytes migration by the regulation of chemokines production and Ly6Chi monocytes intrinsic homing to liver.
This novel function of MPYS described here is distinct from its ability to activate IFN-I signaling. This conclusion is supported by following observations: a) MPYS−/− mice have largely normal L.M. induced IFN-I production; b) While IFNRA−/− mice have over 1,000 folds of reduced bacterial load in liver during L.M. infection (22), MPYS−/− mice have increased bacterial loads in the liver relative to WT; c) While IFNRA−/− mice, which have elevated L.M. induced IL-12 production (22), MPYS−/− mice have decreased IL-12 production in vivo; d) Unlike monocytes from IFNRA−/− mice, MPYS−/− monocytes have intrinsic defect in homing to the liver. Thus, our results reveal a novel biologic function of MPYS by regulating Ly6Chi monocytes migration in vivo.
MPYS is essential for IFN-I production by L.M. in BMDM and BMDC. It is rather surprising that MPYS−/− mice have normal IFN-I production at 24hpi during L.M. infection in vivo, though IFN-I production at 6hpi was reduced. Several reports have shown that in vivo production of IFNβ is restricted to monocyte/macrophage during L.M. infection (26–28). Interestingly, naïve and activated macrophage adopt different mechanisms to produce IFNβ in response to L.M. infection (29). In naïve macrophages, L.M. activates host IFNβ production after escaping from phagosomes and entering cytosol (30). Cytosolic L.M. DNA or cyclic-di-AMP, secreted by L.M. then activates MPYS/STING-mediated IFNβ production (31, 32). The pore-forming protein listeriolysin O (LLO) deficient L.M. mutant, which fails to escape from phagosomes, fails to activate IFNβ in naïve macrophages (30). However, a LLO-deficient L.M. mutant induces similar IFNβ production to WT L.M. in IFN-γ treated BMDM (activated macrophage) (29). This response is partially dependent on nucleotide-binding oligomerization domain-containing protein 2 (NOD2)-mediated recognition of L.M. peptidoglycan in phagosome (29). Our result, which shows an early phase (6hpi), but not late phase (24hpi), deficiency of IFNβ production during L.M. is consistent with the presence of these two distinct IFNβ activation signaling pathway in vivo and suggests that MPYS may be only required for IFNβ production in response to L.M. in naïve macrophages but not activated macrophages.
A previous study by Sauer JD et.al found a major defect in IFN-I activity in the N-ethyl-N-nitrosourea (ENU)-induced goldenticket STING mutant mouse (STINGGt/Gt) during L.M. infection (33). The STINGGt/Gt mouse contains a T596A mutation in their MPYS/STING gene that results in an isoleucine to asparagine substitution (I199N) in the protein (33). It is noteworthy that, while BMDM from a STINGGt/Gt mouse has no detectable STING protein, the I199N STING mutant transfection in 293T cells leads to detectable STING protein expression (33). It is possible that additional mutations exist in the ENU-induced STINGGt/Gt mouse and confer the difference in L.M-induced IFN-I response in the STINGGt/Gt and our MPYS−/− mouse. Nevertheless, similar to the present study and distinct from the IFNRA−/− mice, the STINGGt/Gt mice have the same L.M. burden in the spleen as the WT mice (33).
Ly6Chi monocyte recruitment is critical for host defense against L.M. infection (6). CCR2−/−, as well as MCP-1−/−, MCP-3−/− mice, which are defective in Ly6Chi monocytes emigration from BM, are susceptible to L.M. infection (6). Accumulating evidence has demonstrated that both CCR2-dependent and the CCR2-independent mechanisms control Ly6Chi monocyte migration (6). We show, here, that following L.M. infection, MPYS−/− mice have diminished MCP-1 and MCP-3 production and decreased levels of Ly6Chi monocytes in bloodstream. Thus, MPYS participates in CCR2-dependent regulation of monocyte migration.
Many cell populations, including macrophages, fibroblasts and endothelial cells are capable of producing MCP-1 in vitro (34). We found that MPYS−/− BMDM and BMDC have decreased MCP-1 and MCP-3 production in response to L.M. infection (Fig S4). Using WT and Ccl2−/− (Ccl2 encodes MCP-1) bone marrow chimeric mice, Shi C et.al. demonstrated that, in vivo, the radiation-insensitive and presumably non-hematopoietic cells produce MCP-1 in response to L.M. infection (35). Specific ablation of the Ccl2 gene in mesenchymal stem cells (MSCs), CXCL12-abundant reticular (CARs) cells, or endothelial cells decrease the numbers of Ly6Chi monocytes in circulation during L.M. infection (35). However, unlike BMDM or BMDC, L.M. infection of these CD45− non-hematopoietic bone marrow cells, in vitro, do not induce MCP-1 production (Fig S4). This is likely due to the poor uptake of L.M. by these Ly6G− bone marrow cells (Fig 3A). The MPYS−/− mice (Tmem173<tm1Camb>) studied here can be used to generate conditional MPYS−/− mice (18). Thus, future studies, by specifically ablating MPYS expression in MSCs, CARs or endothelial cells, can determine whether MPYS expression in these cells is required for MCP-1 production during L.M. infection in vivo.
We observed nearly equivalent bacterial burden in the spleens of WT and MPYS−/− mice. A previous study found that, though CCR2−/− mice have increased bacterial burden in the liver 1 day after infection, the bacterial burden in the CCR2−/− spleen does not differ from WT mice until day 3 post infection (36). This is likely due to the fact that the spleen has more resident Ly6Chi monocytes than the liver. Thus, the decrease of Ly6Chi monocytes recruitment into the liver would have a greater impact on bacterial clearance. Also, we found that MPYS−/− Ly6Chi monocytes home more efficiently from the bloodstream to the spleen than WT cells, which could also account for the lack of effect of MPYS deficiency on the spleen.
MPYS also affects CCR2-independent monocyte migration. We further show that this migration defect is MPYS−/− monocytes intrinsic. It was demonstrated that reactive oxygen species (ROS) enhance the migration of monocytes across the blood-brain barrier (37). MPYS is a ROS sensor (38). We suggest that the impaired migration of MPYS−/− monocytes from the bloodstream to the liver may be a result of disrupted ROS signaling in these cells.
In summary, our findings reveal a novel in vivo mechanism by which MPYS regulates Ly6Chi monocyte migration and modulates host defense. Increasing evidence, mostly from in vitro studies, suggest that MPYS may play a role in host defense against many non-viral pathogens (13–17). Future studies will be focused on understanding the in vivo mechanism by which MPYS mediates host defense against these pathogens, whether by the production of IFN-I or regulation of monocytes migration, or both or some other as yet undefined mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Laurel Lenz for the GFP-expressing strain of L.M. and Dr. Ross Kedl for IFNAR−/− mice.
This work was supported by NIH 3R01AI062739-05S2 (J.C.C). J.C.C is Ida and Cecil Green Professor of Immunology. L.J is supported by a NIH Training Grant (5T32AI074491-03).
Abbreviations used
- L.M.
Listeria monocytogenes
- BMDM
bone-marrow derived macrophage
- BM
bone marrow
- hpi
hours post infection
- BMDC
bone-marrow derived dendritic cells
- IFN-I
type I IFN
- MSC
mesenchymal stem cells
- CARs
CXCL12-abundant reticular cells
- MEF
mouse embryonic fibroblast
- ENU
N-ethyl-N-nitrosourea
- LLO
listeriolysin O
- NOD2
Nucleotide-binding oligomerization domain-containing protein 2
Footnotes
The authors declare no competing financial interests.
Reference
- 1.Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM. Listeria--review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40:4–13. [PubMed] [Google Scholar]
- 2.Corr SC, O'Neill LA. Listeria monocytogenes infection in the face of innate immunity. Cell Microbiol. 2009;11:703–709. doi: 10.1111/j.1462-5822.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 3.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C, Hohl TM, Leiner I, Equinda MJ, Fan X, Pamer EG. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J Immunol. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serbina NV, Shi C, Pamer EG. Monocyte-Mediated Immune Defense Against Murine Listeria monocytogenes Infection. Adv Immunol. 2012;113:119–134. doi: 10.1016/B978-0-12-394590-7.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O'Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppe U, Hogner K, Doehn JM, Muller HC, Witzenrath M, Gutbier B, Bauer S, Pribyl T, Hammerschmidt S, Lohmeyer J, Suttorp N, Herold S, Opitz B. Streptococcus pneumoniae Stimulates a STING- and IFN Regulatory Factor 3-Dependent Type I IFN Production in Macrophages, which Regulates RANTES Production in Macrophages, Cocultured Alveolar Epithelial Cells, and Mouse Lungs. J Immunol. 2012;188:811–817. doi: 10.4049/jimmunol.1004143. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, Barber GN, Gazzinelli RT, Fitzgerald KA, Golenbock DT. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TL, Vasconcelos AC, Nogueira L, Bafica A, Silva AM, Oliveira SC. MyD88 and STING signaling pathways are required for IRF3-mediated IFN-beta induction in response to Brucella abortus infection. PLoS One. 6:e23135. doi: 10.1371/journal.pone.0023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prantner D, Darville T, Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol. 2010;184:2551–2560. doi: 10.4049/jimmunol.0903704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 2011;187:2595–2601. doi: 10.4049/jimmunol.1100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousens LP, Wing EJ. Innate defenses in the liver during Listeria infection. Immunol Rev. 2000;174:150–159. doi: 10.1034/j.1600-0528.2002.017407.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, Xia W, Gu J, Ishikawa H, Gutman D, Barber G, Qin Z, Jiang Z. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dresing P, Borkens S, Kocur M, Kropp S, Scheu S. A fluorescence reporter model defines"Tip-DCs" as the cellular source of interferon beta in murine listeriosis. PLoS One. 2010;5:e15567. doi: 10.1371/journal.pone.0015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockinger S, Kastner R, Kernbauer E, Pilz A, Westermayer S, Reutterer B, Soulat D, Stengl G, Vogl C, Frenz T, Waibler Z, Taniguchi T, Rulicke T, Kalinke U, Muller M, Decker T. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 2009;5:e1000355. doi: 10.1371/journal.ppat.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solodova E, Jablonska J, Weiss S, Lienenklaus S. Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage. PLoS One. 2011;6:e18543. doi: 10.1371/journal.pone.0018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2011;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 37.Van der Goes A, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. Faseb J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- 38.Jin L, Lenz LL, Cambier JC. Cellular reactive oxygen species inhibit MPYS induction of IFNbeta. PLoS One. 2010;5:e15142. doi: 10.1371/journal.pone.0015142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.