Abstract
Thioredoxin (Trx) is an important redox regulator with cytosolic Trx1 and mitochondrial Trx2 isozymes. Trx has multi-physiological functions in cells and its bioavailability is negatively controlled through active site binding to a specific thioredoxin binding protein (TBP-2). This paper describes the delicate balance between TBP-2 and Trx, and the effect of overexpression of TBP-2 in the human lens epithelial cells. Cells overexpressing TBP-2 (TBP-2 OE) showed a 7-fold increase of TBP-2, and a nearly 40% suppression of Trx activity but no change in Trx expression. The TBP-2 OE cells grew slower and their population decreased to 30% by day 7. Cell cycle analysis showed that TBP-2 OE cells arrested at the G2-M stage, and that they displayed low expressions of the cell cycle elements P-cdc2 (Y15), cdc2, cdc25A and cdc25C. Furthermore, TBP-2 OE cells were more sensitive to oxidation. Under H2O2 (200 µM, 24 hrs) treatment, these cells lost 80% viability and became highly apoptotic. Brief oxidative stress (200 µM, 30 min) to TBP-2 OE cells disrupted the Trx anti-apoptotic function by dissociating the cytosolic and mitochondrial Trx-ASK binding complexes. The same H2O2-treated cells also showed activated ASK (P-ASK), Bax, lowered Bcl2, cytochrome c release, and elevated caspase 3/7 activities. We conclude from these studies that high cellular levels of TBP-2 can potentially suppress Trx bioavailability and increase oxidation sensitivity. Overexpression of TBP-2 also causes slow growth by mitotic arrest, and apoptosis by activating the ASK death pathway.
Keywords: Thioredoxin, Thioredoxin binding protein2 (TBP-2), apoptosis, oxidative stress, cell cycle
INTRODUCTION
Thioredoxin (Trx) is a 12-kDa ubiquitous protein present in all living cells. Trx has a wide range of physiological functions, including DNA synthesis, oxidation damage repair, and regulation of inflammation and apoptosis. Trx functions through its ability to control thiol/disulfide homeostasis using vicinal cysteine residues at its active site to dethiolate protein-protein disulfide bonds. Oxidized Trx in turn is reduced by thioredoxin reductase (TR) using donated electrons from NADPH to complete the catalytic cycle [1]. Two major isoforms of Trx have been found in mammalian cells, cytosolic Trx1 (Trx1) and mitochondrial Trx2 (Trx2).
Several proteins are known to bind with Trx. These include apoptosis activating kinase (ASK), and the NADPH oxidase subunit of p40phox, which is also called thioredoxin binding protein 1 (TBP-1). Recently another thioredoxin binding protein 2 (TBP-2) has been identified using a yeast-two hybrid screen. This protein is up-regulated in HL-60 leukemia cells treated with 1,25-dihydroxy vitamin D3 [2], and has been named vitamin D3 up-regulated protein 1 (VDUP1) or thioredoxin interacting protein (TXNIP) [3–5]. TBP-2 is a 46-kDa protein that is ubiquitously expressed primarily in the cytosol of many tissues; however, it is also present in the nucleus pancreatic beta cells [6]. Since TBP-2 only binds to reduced Trx and forms disulfide bonds with the cysteine residues at its catalytic center, its interaction with Trx suppresses Trx activity [3, 5]. Therefore, TBP-2 is considered to be a negative regulator of Trx that controls Trx bioavailability. In recent years, extensive studies have focused on examining the biological function of TBP-2. The inducible nature of TBP-2 under several stress conditions, including UV light, γ-rays, heat shock and high glucose [7, 8] suggests that TBP-2 may play a role in the cellular processes of cell differentiation, apoptosis, immune response, and energy metabolism [9–14]. Furthermore, it was found that TBP-2 over-expression renders the cells more vulnerable to oxidative stress [15], and slows cell proliferation with cell cycle arrest at G1 stage [16].
One of the functions of Trx is to prevent cell apoptosis by sequestering the intracellular death signaling ASK1 through its N-terminal end and inhibiting its kinase activity. However, binding between Trx1 and ASK1 is highly dependent on the redox status of Trx1 [17]. Oxidized Trx1 can dissociate from its complex allowing ASK1 to be released and activated. The activated ASK1 (P-ASK1) in turn activates downstream c-Jun N-terminal kinase (JNK) or p38 MAP kinase, or both, to initiate the apoptotic pathway [18]. ASK1 is activated by the production of ROS associated with stress from oxidation, tumor necrosis factor-α (TNF-α), and lipopolysaccharide (LPS) [19]. Some studies indicate that ROS-dependent activation of ASK1 is required for the oxidative stress-induced apoptosis in macrophages, mouse embryonic fibroblasts and other cell types [20, 21].
In mammalian tissues, the eye lens is most vulnerable to oxidative stress. The protein-rich lens depends on its ability to maintain the proteins in a reduced state by various anti-oxidants and oxidation defense enzymes to keep its transparency [22]. Trx1 is present in the lens and has been demonstrated to be an effective oxidation defense enzyme [23]. TBP-2 is also present in the lens, and under oxidative stress it has been shown to regulate the bioavailability of Trx [24]. Furthermore, it was shown that human lens epithelial B3 (HLE B3) cells with over-expressed TBP-2 displayed lower Trx activity, slower cell growth and increased susceptibility to H2O2-induced apoptosis [24]. However, little is known about the mechanism responsible for slow cell growth, and the accelerated H2O2-induced apoptosis in cells enriched with TBP-2. The intracellular localization and action of TBP-2 in lens epithelial cells remain largely unknown. In this study, we provided evidence that the slow growth of TBP-2 OE cells under non-stress conditions is likely linked to a malfunction of the cell cycle checkpoint signaling cdc2 and cdc25 molecules that leaves cells arrested at G2-M stage. Under oxidative stress, the major potential mechanism for activation of the cell death pathway in TBP-2 OE cells is the dissociation and activation of ASK1 from the cytosolic Trx1-ASK1 binding complex and the mitochondrial Trx2-ASK1 binding complex. Immunofluorescent studies in lens epithelial cells have verified that TBP-2 is primarily localized in the cytosol but translocates into mitochondria under oxidative stress.
MATERIALS AND METHODS
Cell culture and treatment
Human lens epithelial cells (HLE B3), immortalized by infecting with adenovirus 12-SV40, were grown and maintained in MEM medium (Gibco, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 50 µg/ml gentamycin (Gibco, Carlsbad, CA, USA) in humidified CO2 incubator. For growth analysis, one million of cells were plated into 60-mm dishes. Media were changed on day 1, 3, 5 and 7 and pictures of cells were taken using a microscope. On day 7, the cells were trypsinized and counted using hemacytometer. For the H2O2-induced apoptotic studies, HLE B3 cells were deprived serum gradually by overnight culture in MEM with 2% FBS followed by incubating in serum-free medium for 30 min before exposure to a bolus of 200 µM H2O2 for different times.
Over-expression and Knockdown of TBP-2 in HLE B3 cells
Sense cDNA for TBP-2 was introduced into the multi-cloning site of Geneticin (G418 sulfate)-resistant mammalian expression vector pcDNA3.1 (+) to construct plasmids. The plasmids were then transfected into HLE B3 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Cells transiently transfected with the cDNA were incubated with fresh culture medium for 24 hrs before use.
For TBP-2 knockdown (KD) process, HLE B3 cells were grown to 50–70% confluence in 24-well plates cultured in 20% serum under normal conditions. A 25 µl aliquot of serum-free medium containing 0.75 µl of siLentFect (Bio-Rad company) was mixed with 25 µl of serum-free medium containing 10 nM TBP-2 siRNA with a primer sequence of 5’-AAGAGCCAATTTAACAAACTA-3’ (SI03648827, Qiagen, Valencia, CA, USA) and incubated for 20 min at room temperate. This mixture was then added to each well of the cultured cells containing 250 µl of fresh culture medium allowing the transfection to continue for 72 hrs.
Isolation of cytosolic and mitochondrial fractions from HLE B3 cells
The mitochondrial fraction was isolated according to the method described by Rehncrona, et al [25]. Briefly, HLE B3 cells were trypsinized and centrifuged, and the cell pellets homogenized using 1 ml syringe in 1 ml buffer A (225 mM mannitol, 65 mM sucrose, 1 mM EGTA, 10 mM HEPES, pH 7.2), followed by centrifugation at 500 ×g for 10 min at 4°C to collect nuclei and membranes. The chilled supernatant was centrifuged twice at 500 ×g for 10 min, and the pooled supernatant was centrifuged again at 10,000 ×g for 10 min. The final supernatant was considered cytosolic fraction while the sediment was the mitochondrial fraction. The mitochondrial precipitants were solubilized in buffer B (225 mM mannitol, 65 mM sucrose 10 mM HEPES, pH 7.2), centrifuge again at 10,000 ×g for 10 min and the final sediment was re-suspended in 100 µl buffer B. All these steps were performed on ice.
Cytotoxicity assay
Cell viability was measured by a nonradioactive colorimetric cell viability kit (Promokine, Heidelberg, Germany) with tetrazolium salt WST-8 (2-(2-methoxy-4-nitrophenyl)-3- (4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt), which is reduced to water-soluble, orange formazan dye by dehydrogenases present in the metabolically active cells. The absorbance of the formazan dye is directly proportional to the number of living cells. HLE B3 cells were treated with 50, 100, 200, 300 µM of H2O2 for 24 hrs. After treatment, 10 µl of WST-8 solution was added to each well of the culture plate and incubated for 2 hrs in the incubator. The transmission was evaluated at 450 nm using a 96-well microplate reader (Bio-Rad, Richmond, CA, USA).
Flow cytometry analysis on dead cells by propidiumidodine staining
Vector and TBP2 OE HLE-B3 cells were cultured in 60 mm dishes. After gradual serum deprivation, the cells were exposed to serum-free medium with and without 200 µM H2O2 for 24 hrs followed by analysis for dead cells. To quantify dead cells, propidium iodide (PI), a nucleic acid binding dye, was used (Invitrogen, Carlsbad, CA, USA). The cells were trypsinized and washed in cold phosphate-buffered saline (PBS). Then the cells were stained with PI according to manufacturer’s protocol and analyzed using flow cytometry (FACScan flow cytometer, Becton Dickinson, San Jose, CA) by measuring the fluorescence emission at 575 nm using 488 nm excitation. The percentage of dead cells was calculated by CellQuestPro software (BD Biosciencs, San Jose, CA).
Inhibition of H2O2-induced apoptosis in HLE B3 cells by pan caspase 3 inhibitor
Human lens epithelial cells (HLE B3) were grown and maintained in MEM medium (Gibco, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 50 µg/ml gentamycin (Gibco, Carlsbad, CA, USA) in humidified CO2 incubator. For growth analysis, one million cells were plated into 60-mm dishes. For the H2O2-induced apoptotic studies, HLE-B3 cells were deprived serum gradually by overnight culture in MEM with 2% FBS followed by incubating in serum-free medium for 30 min. Then these cells were pre-incubated with 100 µM Z-VAD-FMK (R&D Systems, Minneapolis, MN, USA, Catalog number: FMK001) for 3 hrs before exposure to 200 µM of H2O2 for 24 hrs. After the treatment, HLE-B3 cells were harvested for apoptosis assay and stained with propidium iodide (Invitrogen, Carlsbad, CA, USA, Catalog number: P3566) followed by flow cytometric analysis described above.
Cell cycle analysis
Cells were fixed with 70% ethanol and incubated overnight at 4°C. Fixed cells were subsequently washed, treated with 5 µg/ml RNase A, and stained with 50 µg/ml propidium iodide. The analysis of DNA content was performed on list mode data using the Modfit 3.0 software (Verity Software House, Topsham ME).
Immunoprecipitation
Anti-ASK1 antibody was used for the immunoprecipitation of ASK1-Trx1 complex and ASK1-Trx2 complex. The cytosolic and mitochondrial fractions were incubated with anti-ASK1 agarose conjugate (#sc7931AC, Santa Cruz, Santa Cruz, CA, USA) overnight at 4°C. The immunoprecipitated complexes were collected by centrifugation at 3,000 rpm for 5 min at 4°C, washed three times with ice-cold washing buffer (1M Tris-HCL, 1.5 M NaCl, 50 mM EDTA), resuspended in electrophoresis SDS sample buffer, boiled for 5 min at 95°C, and analyzed on 10% SDS-PAGE. Immunoblotting was performed using the following primary antibodies: anti-ASK1 (1:250) (#sc7931, Santa Cruz, Santa Cruz, CA, USA), anti-Trx1 (1:1000, for cytosol fraction)(#ab86255, Abcam, Cambridge, MA, USA.), and anti-Trx2 (1:100, for mitochondria fraction) (#sc133200, Santa Cruz, Santa Cruz, CA, USA). The secondary antibodies used were as follows: anti-rabbit IgG (1:2000), anti-rabbit IgG (1:8000), and anti-mouse IgG (1:2500) (Santa Cruz, Santa Cruz, CA, USA).
Western blot analysis
Equal amounts of protein were subjected to SDS-PAGE on a 10% polyacrylamide gel, transferred to a polyvinylidenedifluoride (PVDF) membrane (GE Healthcare, Boulder, CO, USA), and blocked for 1 hr with blocking buffer containing 5% nonfat dry milk in Tris-buffered saline (TBS-T, 10 mM Tris-HCL [pH 7.5], 100 mM NaCl and 0.1% Tween 20). Primary antibody incubations were performed at the following dilutions: TBP-2, 1:1000 (#K0204-3, MBL, Des Plaines, IL, USA); P-ASK1, 1:250 (#3764, cell signaling, Danvers, MA, USA); ASK1, 1:250 (#sc7931, Santa Cruz, Santa Cruz, CA, USA); P-JNK, 1:500 (#9251, Cell Signaling, Danvers, MA, USA); JNK, 1:500 (#9258, Cell Signaling, Danvers, MA, USA); P-p38, 1:500 (#9211, Cell Signaling, Danvers, MA, USA); p38, 1:500 (#9212, Cell Signaling, Danvers, MA, USA); Bcl-2, 1:500 (# 2876, Cell Signaling, Danvers, MA, USA); Bax, 1:500 (#2772, Cell Signaling, Danvers, MA, USA); cytochrome c, 1:500 (# 4272, Cell Signaling, Danvers, MA, USA); caspase 3, 1:500 (#9262, Cell Signaling, Danvers, MA, USA); Trx1, 1:2000 (#ab86255, Abcam, Cambridge, MA, USA.); Trx2, 1:100 (#sc133200, Santa Cruz, Santa Cruz, CA, USA); GAPDH, 1:2000 (#sc32233, Santa Cruz, Santa Cruz, CA, USA); VDAC, 1:2000 (#4661, Cell Singaling, Danvers, MA, USA); and β-actin, 1:2000 (#A2228, Sigma, St. Louis, MO, USA). All incubations were done at 4°C overnight. Then, the membrane was washed three times with TBS-T for 30 min and followed by respective secondary antibody incubations with goat anti-rabbit IgG-horseradish peroxidase (for membrane probed with rabbit anti human P-ASK1, ASK1, VDAC, P-JNK, JNK, P-p38, p38, Bcl-2, Bax, cytochrome c, caspase 3, and Trx1 antibody) or goat anti-mouse IgG-horseradish peroxidase (for membranes probed with mouse anti human TBP-2, Trx2, GAPDH, and β-actin antibody) (Santa Cruz, Santa Cruz, CA, USA). Similar procedures were used for Pcdc2 (Y15), cdc2, cdc25A and cdc25C. Immunodetection was performed with the ECL Western Blotting Detection System (Thermo Scientific, Rockford, IL, USA). The immunoblot was analyzed with an imaging system (Versadoc 5000 MP Imaging System, Bio-Rad, Richmond, CA, USA). Densitometric analyses of immunoblots were carried out using an image processing and analysis program, Quantity One (Bio-Rad, Richmond, CA, USA)
Immunohistochemistry
HLE B3 cells were seeded on the cover slips overnight. After the media was removed from the dish, pre-warmed (37°C) staining solution containing MitoTrackerR probe (100 nM final concentration, MitoTrackerR probes, Invitrogen, Carlsbad, CA, USA) was added and incubated for 30 min under growth conditions. After staining, the cover-slip was rinsed with fresh pre-warmed PBS, fixed in 4% formaldehyde for 15 min at room temperature, followed by incubation for 10 min with PBS containing 0.25% Triton X-100. The cells were then washed in PBS three times for 5 min, and incubated with 1% BSA in PBST for 30 min to block unspecific binding of the antibodies, then in the 1:50 TBP-2 antibody (Santa Cruz, Santa Cruz, CA) in 1% BSA in PBST in a humidified chamber overnight at 4°C. After washing the cells three times in PBS (5 min each), incubate cells with donkey anti-goat Alexa Fluor 488 secondary antibody (Invitrogen, Carlsbad, CA, USA) at 1:10000 dilution for 1 hr at room temperature in dark. The cover-slip was rinsed three times in PBS, incubated with 300 nM DAPI (4',6-diamidino-2-phenylindole, dihydrochloride) solution for 1 min at room temperature, and then rinsed again in PBS three times. Microscopic study was performed on a Nikon C1 laser scanning confocal microscope.
Thioredoxin and caspase 3/7 activity assay
The activity of Trx was determined following a previously described method [24]. To compare the caspase 3/7 activity of vector-transfected and TBP-2-transfected HLE B3 cells, 100 cells were plated into 96-well plate. Caspase 3/7 activity assay was conducted using Caspase-Glo 3/7 Assay Kit from Promega according to manufacturer’s manual.
Protein determination
Protein concentrations were determined by BCA following manufacture’s protocol (PierceChemical Co., Rockford, IL) with bovine serum albumin as the standard.
Statistics
Each experiment was performed at least three times and statistical analyses were done with one-way ANOVA using the SPSS software. The number of experimental samples used in each group is presented in the figure legends. All data were expressed as mean ± S.D. and differences were considered significant at p<0.05.
RESULTS
Overexpression of TBP-2 reduced cell growth
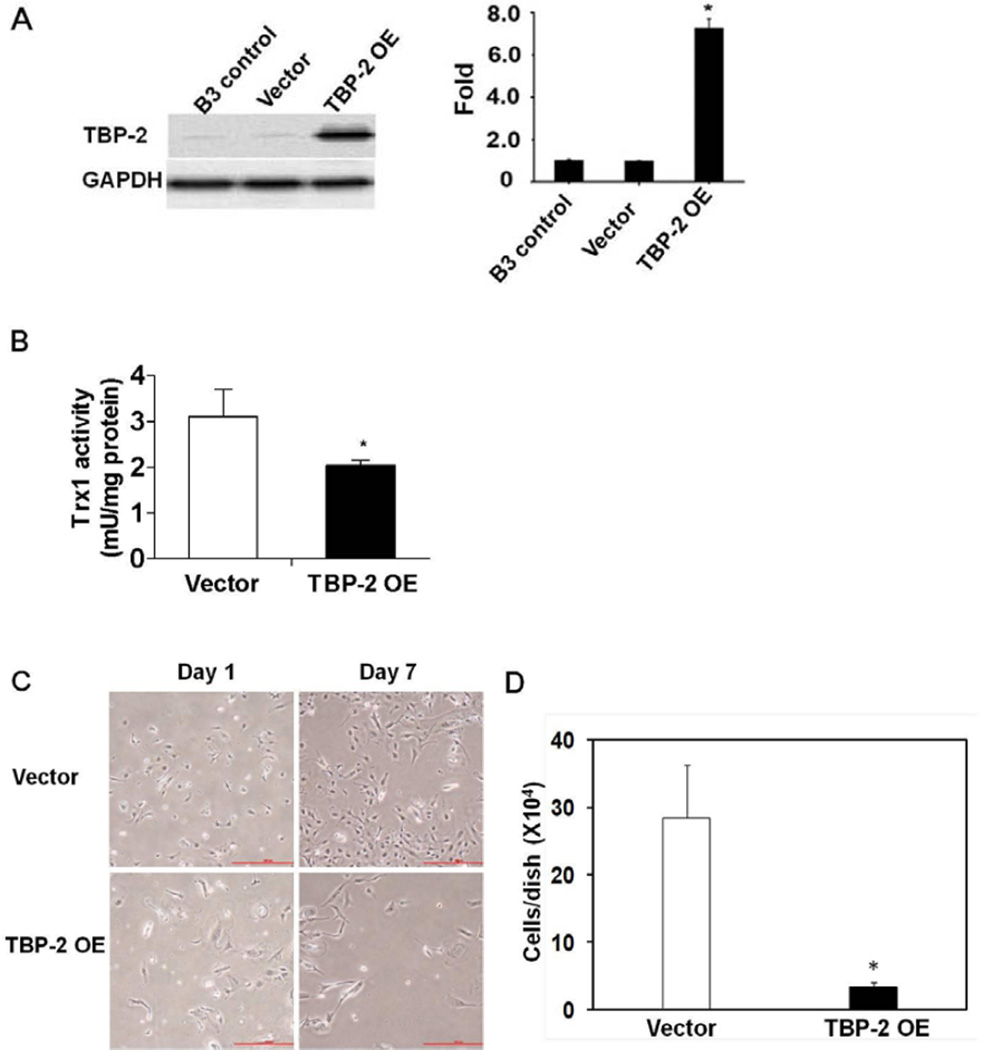
TBP-2 pcDNA was transfected into HLE B3 cells. The transient transfectant was selected by incubating the transfected cells with Geneticin and the overexpression of TBP-2 was confirmed by Western blot analysis. TBP-2 overexpression in HLE B3 cells showed a nearly 7-fold increase in TBP-2 protein (Fig. 1A) that suppressed Trx1 activity in the cell by almost 40% without affecting the expression level of Trx1 (Fig. 1B). Cells overexpressing TBP-2 showed a phenotype of slow growth. As shown in Figure 1C, where similar numbers of both TBP-2 and vector-transfected cells were seeded and cultured in the DMEM with 20% FBS, lower cell proliferation is observed in TBP-2 OE cells compared to the vector control cells. By the end of one week, the TBP-2 OE cell numbers were only about 20% of that of the vector control cells (Fig. 1D).
Figure 1. Over-expression of TBP-2 in human lens epithelial B3 (HLE B3) cells inhibited Trx activity and reduced cell growth.
HLE B3 cells were transfected with vector (pcDNA3.1 (+)) or TBP-2 plasmids for 24 hours, to obtain cells with a transient TBP-2 over-expression. The whole cell proteins isolated from HLE B3 cells (B3 control), vector transfected cells (Vector) and TBP-2 over-expressed cells (TBP-2 OE) were extracted and compared. (A). Western blot analysis of TBP-2 expression. GAPDH was used as loading control. The relative density analyses of TBP-2 protein bands were depicted in the right panel. (B). Assay of Trx activity in vector-transfected and TBP-2-transfected HLE B3 cells.(C). Pictures of both vector-transfected and TBP-2-transfected HLE B3 cells at day 1 and day 7. (D). The number of cells in each plate after growing for 7 days. All data are expressed as mean ± SD based on triplicate experiments. *p< 0.05 vector control vs TBP-2 OE.
Overexpression of TBP-2 altered cell signaling and activated caspases 3
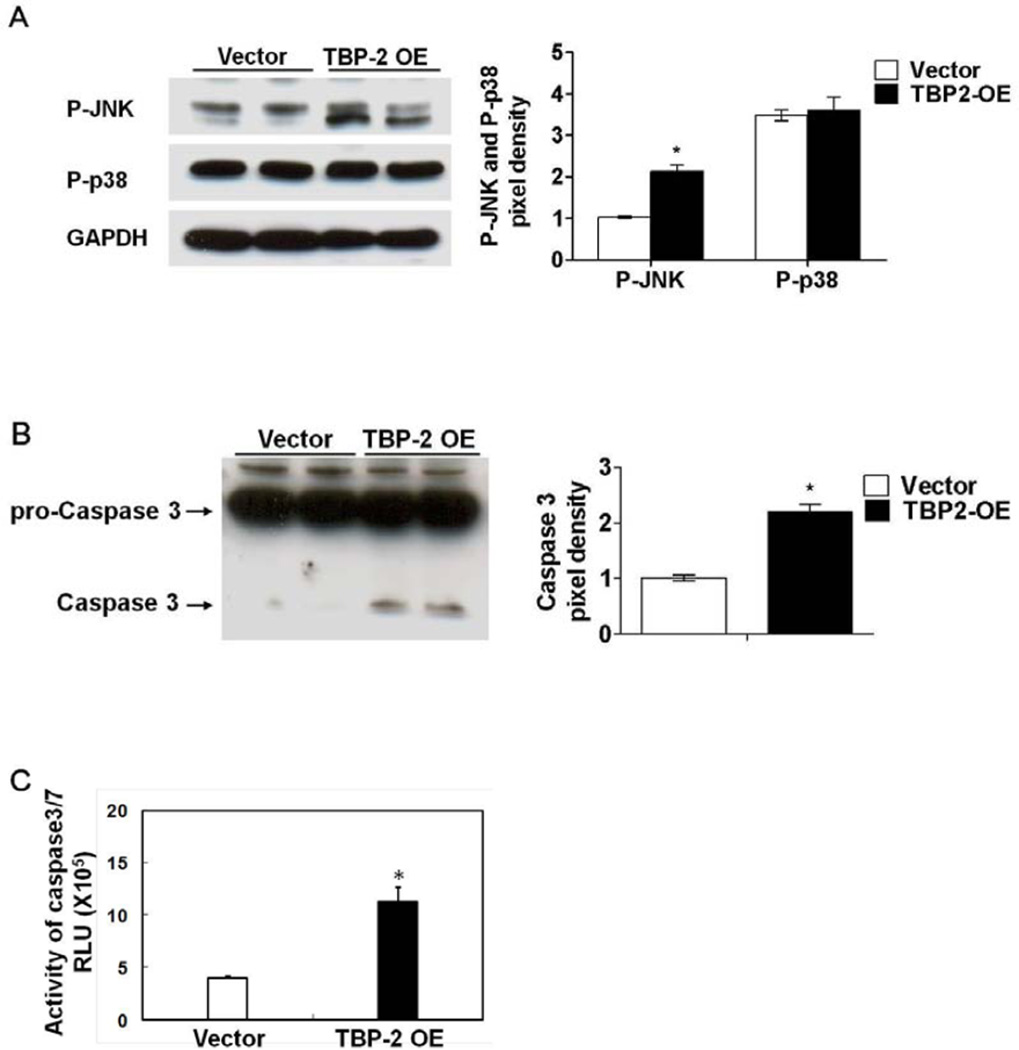
To investigate the possible mechanism of cell growth retardation in the TBP-2 overexpressed cells, we first examined the activations of the stress molecules JNK and p38 in the MAP Kinase signaling pathway. As shown in Figure 2A, compared to the vector control cells, TBP-2 enrichment activates JNK (P-JNK), but not the p38 signaling pathway. The potential effect of TBP-2 overexpression on cell apoptosis was analyzed by examining the status of caspases 3, a protease marker for apoptotic cell death. Western blot analysis showed that caspase 3 was barely detectable in the vector control cells but was very visible in the TBP-2 OE cells. This indicates that caspase 3 was cleaved from its precursor pro-caspase 3 to participate in apoptotic processes (Fig. 2B). The caspase 3/7 like activity was found to be increased nearly 3-fold over the vector control cells (Fig. 2C).
Figure 2. Over-expression of TBP-2 in HLE B3 cells altered cell signaling and initiated apoptosis.
(A). Western blot analysis of P-JNK and P-p38 in both vector-transfected and TBP-2-transfected HLE B3 cells. GAPDH was used as loading control. (B). Western blot analysis of procaspase 3 and caspase-3 in vector-transfected and TBP-2-transfected HLE B3 cells, using anti-procaspase 3 and anti-caspase-3 antibodies, respectively. (C) Activity assay of caspase-3/7 in vector-transfected and TBP-2-transfected HLE B3 cells. The pixel density of immunoblots was analyzed and compared with the vector control normalized to 1.0. The data are expressed as mean ± S.D. with n = 3. *P <0.05 vector control vs TBP-2 OE.
TBP-2 overexpression induced cell cycle arrest in G2-M
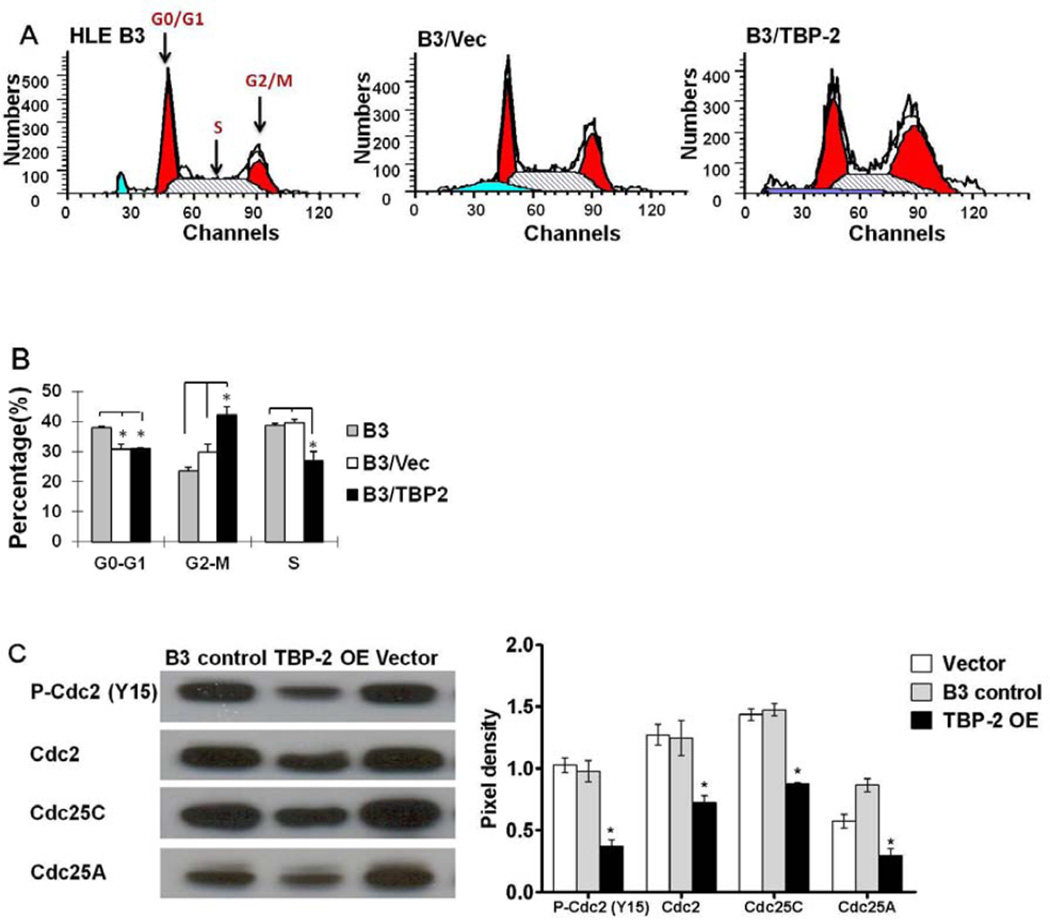
Since TBP-2 induces cell cycle arrest in other cell types [16], we examined the cell cycle distribution of TBP-2 OE cells by analyzing DNA levels in the cells using flow cytometry. A representative histogram of the cell cycle from TBP-2 OE cells compared to vector transfected and non-transfected HLE B3 cells is shown in Figure 3A. Compared to normal B3 cells, vector transfection did not significantly change the pattern of cell distribution other than a slightly shorter G0–G1M phase, and a little longer G2/M phase. However TBP-2 overexpression increased the number of cells in the G2-M phase, and decreased the S phase. As summarized in Figure 3B, the B3 control cells displayed a cell distribution in the cell cycle of 38% in G0–G1M, 22% in G2-M and 40% in S phase. The vector-transfected cells showed a distribution of 30% in G0–G1M, 29% in G2-M and 41% in S phase. While TBP-2 OE cells have the same number of cells in stage G0–G1 as the vector group, but the TBP-2 OE cells had14% more in the G2-M stage, and 13% less in the S phase. Therefore, TBP-2 overexpression resulted in more cells arresting in the G2-M phase of the cells cycle.
Figure 3. Over-expression of TBP-2 in HLE B3 cells altered cell cycle progression.
Cell cycle analysis of HLE B3 control cells (B3 control), vector-transfected HLE B3 cells (Vector) and TBP-2 transfected HLE B3 cells (TBP-2 OE) were carried out after 48 hrs growth by flow cytometry. (A). A representative cell cycle histogram for B3 control, Vector and TBP-2 OE cells, respectively. The G0/G1, S and G2/M phases are indicated on the B3 diagram. (B). Analysis of the % population of G0/G1, S and G2/M phases in B3 control, Vector and TBP-2 OE cells. The data are expressed as mean ± SD with n = 3. *P<0.05. (C). Western blot analysis of the cell cycle regulatory elements P-cdc2 (Y15), cdc2, and phosphatases of cdc25C and cdc25A in B3 control, TBP-2 OE and Vector cells. The pixel densities of the immunoblots were analyzed and compared with the control PCdc2 (Y15) normalized to 1.0. The data are expressed as mean ± SD with n = 3. *P<0.05.
It is known that cell cycle progression depends on several cyclin-dependent kinases (cdks, also called cell division control proteins, cdcs). Cdk1 (or cdc2) activation results from dephosphorylation of the inactive P-cdc2 (Y15) via phosphatases cdc25A/C. Activation of cdk1 (cdc2) is the checkpoint for G2-M stage to exit and complete the cycle [26, 27, 28]. Therefore, we examined the status of cdc2 and its antagonist cdc25 phosphatases A and C. As shown in Figure 3C, the TBP-2 OE cells showed much lower expression levels of Pcdc2 (Y15) and total cdc2 protein compared to that of untransfected HLE B3 cells and the vector control. The same cells also expressed lower levels of both phosphatase cdc25A and cdc25C. The results indicate that TBP-2 OE cells may have insufficient reduced Trx to regulate the cell cycle.
TBP-2 overexpressed cells are more sensitive to oxidative stress
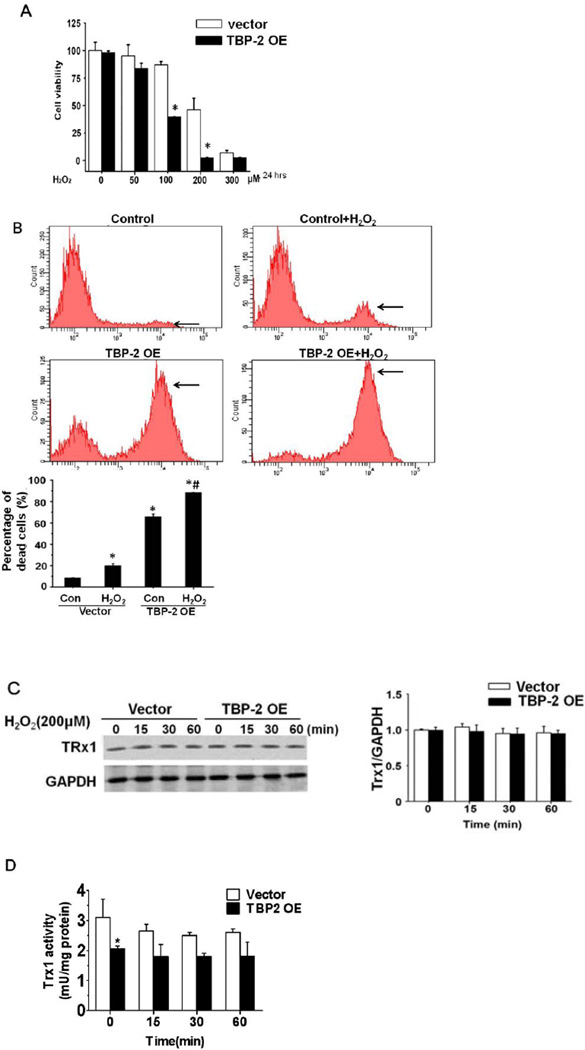
To test whether TBP-2 OE cells are more sensitive to oxidative stress, cell viability (WST-8 assay) in TBP-2 OE and vector control cells was examined after exposing each to a bolus amount of H2O2 (50–300 µM) for 24 hrs. Exposure to 50 µM H2O2 showed little effect on both control and TBP-2 OE cells (Fig. 4A); however, 100 µM H2O2 exposure resulted in a significant loss of cell viability with a 53% (P < 0.003) reduction in TBP-2 OE cells compared to 13% for the controls. Increasing H2O2 exposure to 200 µM resulted in a 97% loss of viability in TBP-2 OE cells compared to 46% in vector cells. Viability was also lost in both cell types exposed to 300 µM H2O2. These findings indicate that TBP-2 enriched cells are more sensitive to oxidative stress.
Figure 4. Over-expression of TBP-2 enhances H2O2-induced cytotoxicity in human lens epithelial B3 cells.
(A). Effects of TBP-2 over-expression on H2O2-induced cytotoxicity in HLE B3 cells. The HLE-B3 cells were transfected with vector (pcDNA3.1 (+)) or TBP-2 plasmids for 24 hrs. The cells were treated with 0, 50, 100, 200 and 300 µM of H2O2 for 24 hrs, and the cell viability was determined by WST-8 assay. The data are expressed as mean ± S.D. of three independent experiments. *p<0.05, vector vs TBP2 over-expression. (B). A representative PI-positive histogram from flow cytometry. Horizontal arrow indicates the location of PI-positive cells. The percent population of the PI-positive Vector control and TBP-2 OE cells with and without H2O2 treatment is summarized in the bar graph. The data were expressed as mean ± SD of three independent experiments. *p<0.05 compared to vector-transfected control cells. # p<0.05 compared to TBP-2 OE control cells.(C). Effects of TBP-2 over-expression on Trx expression in cells with and without oxidation (200 µM H2O2 for 0, 15, 30 and 60 min). GAPDH was used as a loading control, and the relative density of Trx was normalized against GAPDH. (D). Effect of TBP-2 over-expression on Trx activity in cells with and without oxidation (200 µM H2O2 for 0, 15,30 and 60 min). The data are expressed as mean ± SD of three independent experiments. * p<0.05 compared to vector transfected control cells.
Next we examined if TBP-2 enrichment accelerates oxidation-induced cell death. Both control cells and the TBP-2 OE cells were treated with 200 µM H2O2 for 24 hrs and then stained with propidium iodine (PI), followed by flow cytometry. A representative histogram on the distribution of the PI–positive cells is depicted in Figure 4B, in which the PI positive cells are minimal in the vector controls but they increase to 20% after H2O2 treatment. In contrast, PI positive cells were present in 65% of the TBP-2 population and elevated to 88% after exposure to H2O2. A quantitative measurement of the PI-positive population is summarized in the bar graph of Figure 4B.
Inhibition of H2O2-induced apoptosis in HLE B3 cells by pan caspase 3 inhibitor
To ensure that oxidative stress-induced apoptosis in human lens epithelial cells is mediated via caspase, the cells were treated with H2O2 and then compared with the cells treated with H2O2 in the presence of pan caspase inhibitor. As shown in Supplement Figure 1, the control cells in panel A of the flow cytometric histogram showed very minor apoptotic population (less than 1%), however, once the cells were treated with 200 µM of H2O2, the proportion of apoptotic cell was as high as 94% of the total population. However, when cells were pretreated with a pan caspase 3 inhibitor before oxidative stress, the population of apoptotic cells was only 23%. These results suggest that H2O2-induced HLE B3 cell injury is mediated primarily by caspase-associated apoptosis.
Effect of TBP-2 enrichment on the expression and activity of Trx1 in HLE B3 cells
Trx1 expression and catalytic activity in TBP-2 OE cells with and without oxidative stress exposure was compared to that in vector transfected control cells. As shown in Figures 4C–4D, the protein levels of Trx1 are not affected by TBP-2 overexpression, but the activity is suppressed down to 68% of the vector controls. Exposure to a bolus of H2O2 (200 µM) for 15, 30 or 60 min resulted in no change in the expression of Trx1 in both TBP-2 enriched and vector control cells. Short-term exposure to H2O2 only suppressed Trx1 activity 15% in the control cells but had minimal effect on Trx1 in TBP- 2 OE cells (Fig. 4D). It is likely that the bolus of 200 µM H2O2 was detoxified and dissipated after 15 min and the residual H2O2 was too weak to cause further inactivation of the oxidation-resistant Trx1. Whereas Trx activity in TBP-2 OE cells was already suppressed by conjugating with the excess TBP-2 to be inactivated further by H2O2 exposure (Fig. 4D).
TBP-2 overexpression accelerates H2O2-induced apoptosis in HLE B3 cells
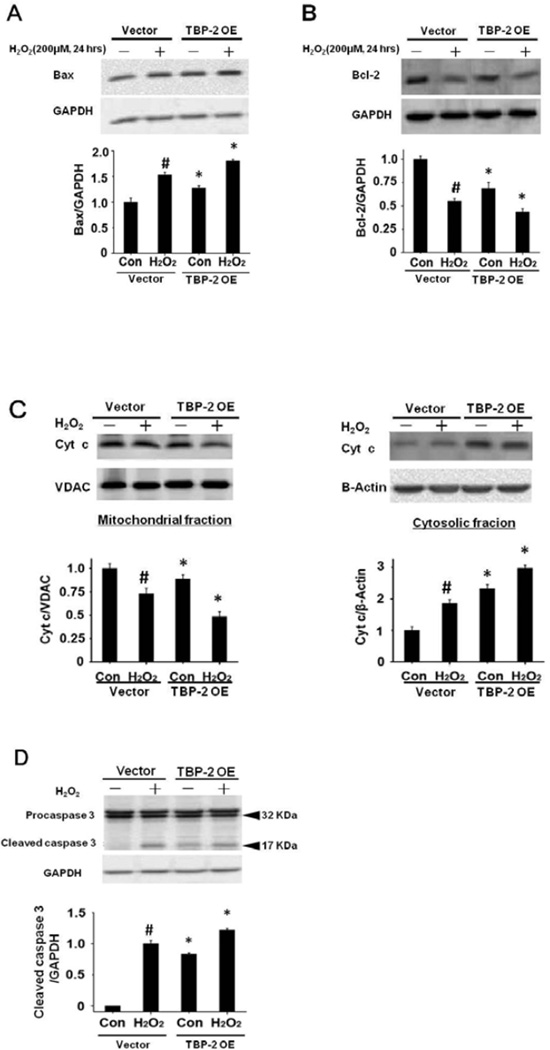
Since loss of cell viability and the presence of PI-positive cells may be associated with apoptosis, we examined the H2O2-treated control and TBP-2 OE cells (200 µM H2O2, 24 hrs) for the apoptotic signaling molecules in the mitochondria, including Bax, Bcl-2, caspase 3 and cytochrome c [29]. As shown in Figure 5A, unstressed cells showed higher Bax (pro-apoptotic factor) expression in TBP-2 OE than that of the control. After oxidative stress, Bax increased in both cells but the levels were much higher in the TBP-2 enriched cells. In contrast, the unstressed cells showed an extensive suppression of Bcl-2 expression (anti-apoptotic factor) when TBP-2 was enriched. Oxidative stress in both the control and TBP-2 OE cells showed a substantial loss in Bcl-2 (Fig. 5B). Similarly, TBP-2 OE induced cytochrome c loss in the mitochondria that leaked into the cytosol and oxidative stress amplified this migration (Fig. 5C). Lastly, TBP-2 OE also induced more caspase 3 release from its precursor pro-caspase 3, even in the absence of oxidative stress. After H2O2 treatment, caspase 3 appeared in the control cells but much more so in the TBP-2 OE cells (Fig. 5D). These results indicate that cells containing higher TBP-2 levels are more sensitive to oxidation-induced apoptosis.
Figure 5. TBP-2 over-expression accelerated H2O2-induced apoptosis.
Vector-transfected (pcDNA3.1 (+)) and TBP-2 over-expressed (TBP-2 OE) HLE B3 cells were treated with or without 200 µM H2O2 for 24 h. (A). TBP-2 over-expression up-regulated the Bax protein level in HLE B3 cells. Western blot analysis was done with anti-Bax, and the digital pixel density was normalized against GAPDH (loading control) and compared against untreated vector control standardized to 1.0. (B). TBP-2 over-expression down-regulated the Bcl-2 protein level in HLE B3 cells. Western blot analysis was done with anti-Bcl-2. The digital pixel density was normalized against GAPDH (loading control) and compared against untreated vector control standardized to 1.0. (C). TBP-2 overexpression suppressed cytochrome c protein level in the mitochondrial fraction and elevated cytochrome c protein level in the cytosolic fraction of H2O2-treated HLE B3 cells. VDAC and β-actin were detected in the mitochondrial and cytosolic fraction, respectively. The relative pixel density of cytochrome c over VDAC was analyzed for the mitochondrial fraction and cytochrome c over β-actin was analyzed for the cytosolic fraction. Cyt c: cytochrome c. (D). TBP-2 over-expression increased caspase 3 cleavage in HLE B3 cells. Whole cell lysates from both H2O2 treated and H2O2 untreated groups were immunoblotted with antibodies specific for procaspase 3 and caspase 3. GAPDH was probed as a control. Data shown are mean±S.D. of three independent experiments. *p<0.05 (comparison between vector-transfected cells and TBP-2 OE cells). # p<0.05 compared to vector-transfected control cells.
TBP-2 overexpression promotes oxidative stress-induced ASK1 release and activation
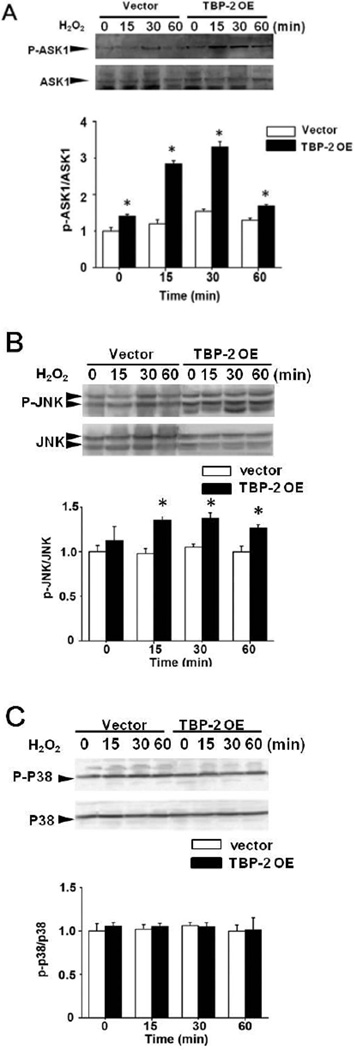
To understand why overexpression of TBP-2 causes cells to be more sensitive to oxidation and to become easily apoptotic, we examined whether TBP-2 OE cells promote the activation of ASK1 death pathway. TBP-2 OE and control cells exposed to 200 µM H2O2 for shorter time periods of 0–60 min were examined for ASK1, P-ASK1 (activated) and the downstream effectors JNK and p38. As shown in Figure 6A, P-ASK1 normalized to total ASK1 (P-ASK1/ASK1) showed a transient elevation in control cells starting at 15 min which peaked at 30 min and returned to the baseline level at 60 min. Under the same conditions, TBP-2 OE showed a similar transient activation pattern of P-ASK1/ASK1, which was much more exaggerated in each time point with the ratio of P-ASK1/ASK1 more than doubled during 15–30 min post-H2O2 treatment periods compared to the controls (Figure 6A). Total ASK1 was probed and was constant throughout the 60 min treatment.
Figure 6. TBP-2 regulates phosphorylation of ASK1 and JNK.
Vector-transfected and TBP-2 OE HLE B3 cells were treated with 200 µM H2O2 for 0, 15, 30 and 60 min and used for the following studies. (A). Time-dependent changes in ASK1 phosphorylation induced by H2O2. P-ASK1 protein level was detected by Western blot analysis, and the relative intensity of P-ASK1 was normalized to the total ASK1 and compared with the untreated vector control at 1.0. (B). Western blot analysis of the time-dependent effects on JNK phosphorylation (P-JNK) and the total JNK induced by H2O2. The relative intensity of P-JNK was normalized to JNK with untreated vector control standardized to 1.0. (C). p38 phosphorylation in Vector and TBP-2 OE cells after 200 µM H2O2 treatment. Western blot analysis was done with anti-P-p38 and anti-p38. The relative intensity of Pp38 was normalized to p38 and compared to the untreated control. All the data shown above are mean±S.D. of three independent experiments. *p<0.05 compared to vector-transfected cells by ANOVA.
H2O2-induced ASK1 activation also resulted in activation of JNK. As shown in Figure 6B, a transient JNK activation between 15–30 min was found in both control and TBP-2 OE cells; however, the effect on the latter was more pronounced. In contrast, the same experimental condition did not show any effects on p38 (Fig. 6C). This was in agreement with the results of unstressed TBP-2 OE cells in which only JNK but no p38 was activated (Fig. 2A).
Evidence that Trx2 is present in the mitochondria of HLE B3 cells and involved in Trx2/ASK1 binding
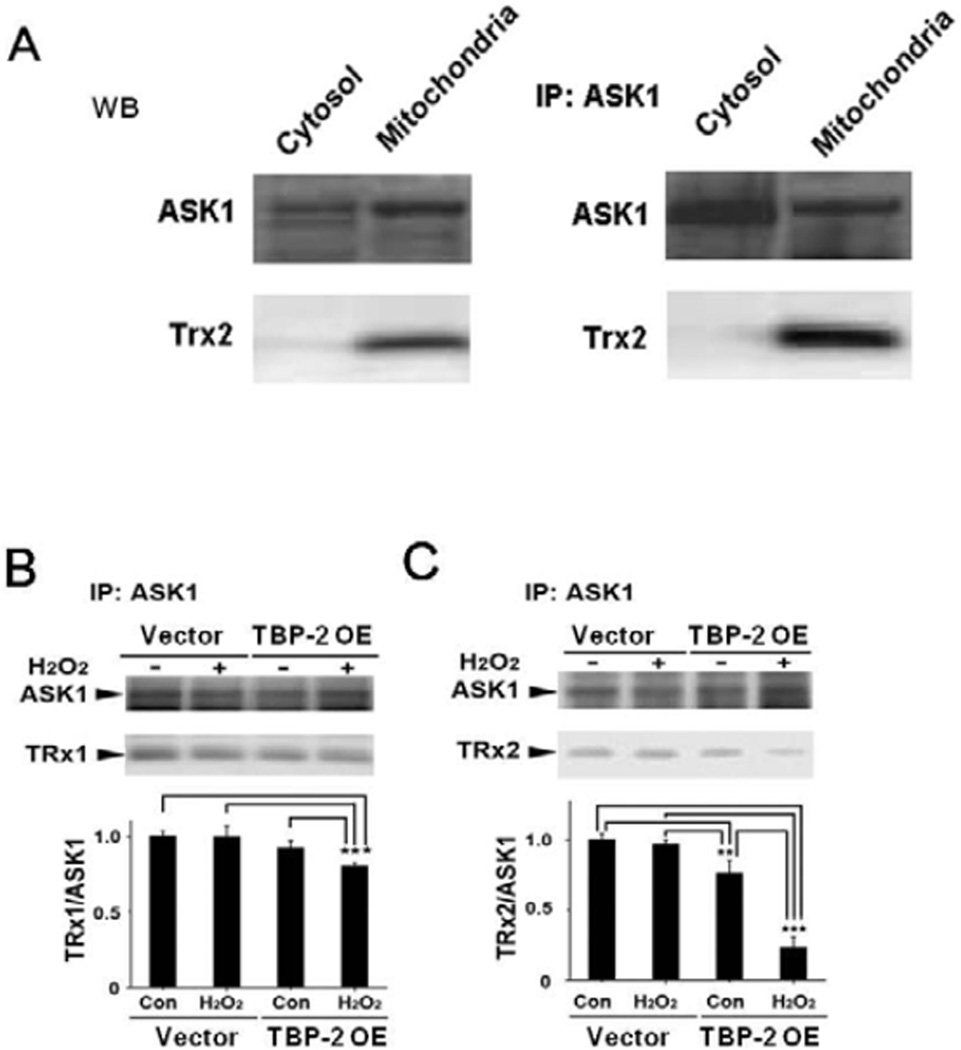
To prove that Trx2, a mitochondrial isozyme of Trx1, is present in the HLE B3 cells and is involved in Trx2/ASK1 binding. We used the untransfected HLE B3 cells and separated the cell lysate into cytosolic and mitochondrial fractions. Each fraction was probed for the presence of ASK1 and Trx2 with anti-ASK1 and anti-Trx2 antibodies, respectively. As shown in the left panels in Figure 7A, ASK1 is present in both the cytosolic and mitochondrial fractions while Trx2 is only seen in the mitochondria. The cell lysate was also used for immunoprecipitation with ASK1 antibody. The immunoprecipitants only showed the presence of Trx2 in the mitochondrial fraction (Fig. 7A, right panels). These results indicate that Trx2 is mitochondria specific and binds with ASK1.
Figure 7. Effect of H2O2 on TRx1/ASK1 and TRx2/ASK1 binding stability in vector control and TBP-2 OE cells.
(A). Normal HLE B3 cells (un-transfected) were lysed and separated into cytosolic and mitochondrial fractions. Western blot analysis of ASK1 and Trx2 was carried out in cytosolic and mitochondrial fractions (Left panel). ASK1 immunoprecipitants (IP) were analyzed for the presence of ASK1 and Trx2 (right panel). (B). Vector and TBP-2 OE HLE B3 cells were treated with and without H2O2 (200 µM, 30 min), followed by separation of mitochondrial and cytosolic fractions, and immunoprecipitated with anti-ASK1 antibodies. Western blot analysis was performed with anti-ASK1 and anti-Trx1 antibodies in cytosolic fraction, anti-ASK1 and anti-Trx2 antibodies in mitochondrial fraction. The relative intensity of Trx1 or Trx2 was normalized to ASK1 and compared against untreated vector cells standardized to 1.0. Data represent mean±S.D. of three independent experiments. *p<0.05 compared to vector-transfected cells by ANOVA.
TBP-2 overexpression dissociates ASK1/Trx1 and ASK1/Trx2 binding complexes
Since Trx1 is known to bind with ASK1 and to prevent ASK1 activation, we examined whether TBP-2 enrichment in cells can alter the ability of Trx1 to bind with ASK1 in the cytosol and Trx2 with ASK1 in the mitochondria. Both vector control and TBP-2 OE cells were incubated with 200 µM H2O2 for only 30 min, which is the optimal time for ASK1 activation shown in Figure 6A. The cells were lysed and separated into mitochondrial and cytosolic fractions followed by immunoprecipitation with anti-ASK1 antibody. Western blots were performed with anti-Trx1 antibody in cytosolic fraction, and anti-Trx2-antibody in mitochondrial fraction. With only 30 min treatment, H2O2 did not affect the cytosolic Trx1/ASK1 binding in the vector cells, but significantly decreased this ratio in the TBP-2 OE cells (Fig. 7B). In contrast, although H2O2 caused a minimal decrease of the Trx2/ASK1 binding complex in the mitochondrial fraction of the vector cells, it drastically suppressed the mitochondrial Trx2/ASK1 ratioin the TBP-2 OE cells. Interestingly, even without oxidative stress, Trx2/ASK1 binding was already lowered 20% by the presence of high cellular TBP-2 protein (Fig. 7C). This result suggests that Trx/ASK1 interaction occurs in both cytosolic and mitochondrial regions, and that this binding becomes unstable in cells enriched with TBP-2.
TBP-2 localization and migration in the HLE B3 cells
TBP-2 is localized predominantly in the cytosol of HLE B3 cells, as shown in the immunofluorescent image of cells in Figure 8A, where TBP-2 proteins (green dye, conjugated with Alexa Fluor 488 antibody) are heavily concentrated outside of the mitochondria (red dye with MitoTrackerR probe) and the nucleus (blue dye with DAPI). Immunoblotting of the isolated mitochondria and cytosol showed that TBP-2 was present in the cytosolic fraction of the control cells but decreased after cells were treated with 200 µM H2O2 for 24 hrs (Fig. 8B). However, the mitochondrial fraction of the same control cells displayed trace amounts of TBP-2, which increased after oxidative stress (Fig. 8C). TBP-2 in the TBP-2 OE cells showed a strong presence in both cytosolic and mitochondrial fractions; however, exposure to H2O2 stress decreased TBP-2 in the cytosol and increased in mitochondria. VDAC, a mitochondrial specific protein, was used to ensure the purity of the mitochondrial fraction used for this study (Fig. 8C). This suggests that TBP-2 is mainly present in the cytosol but can migrate into mitochondria upon oxidative stress.
Figure 8. Localization and H2O2-stimulated TBP-2 translocation in HLE-B3 cells.
(A). Immunofluorescent detection of endogenous TBP-2 in HLE B3 cells by confocal microscopy. TBP-2 was detected with JY2 antibody and Alexa Fluor 488 secondary antibody (green); mitochondria were visualized by MitoTrackerR probe (red); and nuclei were labeled by DAPI (blue). (B). Differential expression of TBP-2 in the cytosolic fractions of vector and TBP-2 OE cells with and without treatment with H2O2 (200 µM, 30 min). Beta-actin was probed to ensure equal protein loading and the relative intensity of TBP-2 was normalized against beta-actin with untreated control standardized to 1.0. (C). Differential expression of TBP-2 in mitochondrial fraction of vector and TBP-2 OE cells with and without treatment with H2O2 (200 µM, 30 min). The mitochondrial specific protein VDAC was probed to ensure equal protein loading and the relative intensity of TBP-2 was normalized against VDAC with untreated control standardized to 1.0. All data represent mean±S.D. of three independent experiments. *p<0.05 compared to vector-transfected cells by ANOVA.
Effect of TBP-2 knockdown on cell viability in HLE B3 cells
Next we examined the effect of TBP-2 knockdown (KD) on cell viability and Trx1 activity. As indicated in Figure 9A, TBP-2 siRNA successfully suppressed TBP-2 expression in the HLE B3 cells to 30% of the control while scramble siRNA (negative control) only showed an insignificant effect. GAPDH (or G3PD) was probed to ensure equal sample loading on the gel.
Figure 9. The effect of TBP-2 knockdown (KD) on cell viability and TRx1 activity in HLE B3 cells after H2O2 treatment.
(A). The HLE B3 cells were transfected with siLentFect and scramble siRNA or TBP-2 siRNA for 72 hrs, and cell lysates used for Western blot analysis with GAPDH as the loading control. The relative density analysis of TBP-2 protein band in control, scramble siRNA and TBP-2 siRNA is depicted in the right panel based on triplicate experiments. *p<0.05, vector vs TBP-2 down-expression. (B). Effects of TBP-2 down-expression on H2O2-induced cytotoxicity in HLE B3 cells. Cells with scramble siRNA or TBP-2 siRNA were treated with 0–500 µM H2O2 for 24 hrs, and the cell viability was determined by WST-8 assay. The data are expressed as mean±S.D. of three independent experiments. *p<0.05, vector vs TBP2 down-expression. (C). Trx1 activity increased in TBP-2 KD cells compared to scramble siRNA cells. There are no significant differences between two groups after H2O2 treatment (300 µM for 30 min).
As shown in Figure 9B, in comparison to the negative control, cells with suppressed TBP-2 expression show a marginally enhanced survival rate under oxidative stress conditions (24 hrs of H2O2, 50–500 µM). Furthermore, the TBP-2 KD cells showed a trend of higher Trx1 activity in comparison to that of the scramble siRNA control. This pattern of enhanced Trx1 activity in TBP-2 KD cells over the negative control cells persisted even when both cells were exposed to 300 µM H2O2 for 30 min (Fig. 9C).
DISCUSSION
Our results provide strong evidence that TBP-2 over-expression in cells upsets the redox balance needed for cell cycle progression and cell proliferation. We observed a slow growth in the TBP-2 OE cells with cell cycle arrest at G2-M phase in which the expression of key control elements cdc2 and cdc25 were markedly suppressed (Fig. 3C). In particular, as cdc25A/C is known to be a phosphatase that acts as a key checkpoint for cell cycle processing from the G2 phase into the M phase [28, 30]. Therefore suppressing cdc25A/C expression could affect the cell’s ability to dephophosrylate and convert the inactive target protein P-cdc2 (Y15)into the activate cdc2 form, preventing a large number of cells from committing to mitosis and be retained in the M stage. Interestingly, Pcdc2 (Y15) did not accumulate in the cells. Instead its presence was extensively depressed along with cdc2 and cdc25C and cdc25A. This may be due to the severely suppressed Pcdc2 (Y25) expression by overexpressed TBP-2 in cells (Fig. 3C, top panel).
Characterization of our transiently transfected TBP-2 OE cells showed a 7-fold increase in TBP-2 presence with no effect on Trx expression. However, the higher TBP-2 level in cells suppressed Trx activity down to only 60% of the control cells. Most likely, Trx has been oxidized through the competitive binding with TBP-2, resulting in a decreased bioavailability. Although we did not measure the redox status of the TBP-2 OE cells, we predict that the redox potential has been compromised to the extent that many growth regulatory molecules that are oxidant-sensitive have been damaged or have lost in their biological functions. For example, cdc25C is known to be extremely sensitive to oxidative stress because it can form intra protein disulfide bond that inactivates cdc25C to impede its normal function in regulating G2-M cell cycle progression [31]. The inactive disulfide cdc25C can only be reduced by Trx1 to restore its activity [32]. As cell cycle is controlled by the redox cycle [33], it is likely that enriched TBP-2 has depleted Trx bioavailability, making it unable to provide robust DNA synthesis during cell proliferation, and unable to maintain cdc25C in a reduced state, resulting in G2/M cell cycle arrest.
Previously it was reported [16] that TBP-2 (or UDVP1) was upregulated in tumor cells when the cells were stressed with growth inhibitor TGFβ or by simple TBP-2 transfection. The elevated TBP-2 in these cells induced cell cycle arrest at G0/G1 stage. Schultz et al [34] demonstrated in aortic smooth muscle cells that TBP-2 overexpression showed anti-proliferative effect by decreasing intrinsic Trx activity as well as preventing PDGF-induced DNA synthesis and Trx activation. Our results are in agreement with the concept that TBP-2 upregulation can directly affect Trx-regulated cell proliferation. However, our results differ in the site of the affected cell cycle phase. Unlike the reported effect at G0/G1, we observed cell arrest only in the G2-M stage and not the G0/G1 phase (Figs. 3A–3B). This difference may be cell type specific. Further studies are needed in this area for clarification. Apparently having cell cycle arrest at the G2-M stage can also trigger pro-apoptotic program. As shown in Figures 2B–2C, the apoptotic marker protein, caspase 3, is cleaved off of pro-caspase 3 and exhibits elevated caspase 3/7 like catalytic activity. It is not clear whether cell cycle arrest at G2/M phase can trigger the apoptotic event directly or indirectly. Further work is needed in this area.
The most striking effect of TBP-2 enrichment on cells is the increased sensitivity to oxidative stress. In the presence of a bolus of 100 µM H2O2 for 24 hrs, the non-transfected control HLE B3 cells or vector transfected cells remain viable, but the TBP-2 OE cells lose 50% of their survival capability (Fig. 4A). This high sensitivity to oxidation is even more obvious when these cells are exposed to 200 µM H2O2. Under this condition, the vector cells retained 50% viability while essentially all TBP-2 cells became non-viable (Fig. 4A). The latter conditions invoked extensive cell apoptosis as evident from the activation of the classical apoptotic pathway where elevated Bax expression, released mitochondrial cytochrome c to cytosol, cleavage of procaspase 3 into caspase 3, and loss of plasma membrane integrityall occurred in these cells (Fig. 4B, Fig. 5). We validated that HLE B3 cells could be induced into apoptosis by exposure to 200 µM H2O2 and that the apoptotic process involved caspase 3, as a pan inhibitor of caspase 3 substantially suppressed apoptotic process (see supplement figure 1).
Our evidence also indicates that the major cause for the accelerated cell apoptosis in TBP-2 OE cells under oxidative stress is the fact that extensive binding in these cells between TBP-2 and Trx disturbs the binding complex of Trx with pro-apoptotic factor ASK1. The changes included ASK1 release as well as ASK1-activation (P-ASK1) in the TBP-2 OE cells after oxidative stress. The weakened Trx-ASK1 binding allowed ASK1 to initiate apoptosis via the downstream signaling factor JNK (Fig. 6). However, it is important to note that TBP-2 OE not only disturbed the cytosolic Trx1-ASK1 binding, but also severely disrupted Trx2-ASK1 binding in the mitochondria (Fig. 7).
Since destabilizing Trx-ASK1 binding has been observed in other cell types when Trx is oxidized [35], our observation confirms this phenomenon in the lens epithelial cells. It has been reported that in the pancreatic cells TBP-2 resides in the nucleus and translocates into mitochondria under oxidative stress [6]. Interestingly, our data in Figure 8A did not show any evidence for TBP-2 being present in the nucleus because fluorescence-labeled TBP-2 was only observed in the cytosol. Under oxidative stress, however TBP-2 levels in the cytosolic fraction decreased and increased in the mitochondrial fraction. This indicates that TBP-2 potentially migrated from cytosol to the mitochondria where it interacted with Trx2 and dissociated the pre-existing Trx2-ASK1 binding complex. Thus, it is likely that TBP-2 may reside in different compartment, depending on the cell type. The translocation from cytosolic to the mitochondrial fraction may be unique to the lens epithelial cells. How this translocation can be accomplished is an intriguing question and worthy further investigation.
The relationship between Trx and TBP-2 was further clarified using cells that were genetically engineered to down-regulate TBP-2 expression. Using siRNA to decrease TBP-2 expression to 20% of the HLE-B3 control cells (Fig. 9A), the cells appeared healthier and became more resistant to oxidative stress. The Trx activity was also correspondingly elevated in the cells with lower presence of TBP-2, thus Trx was more bioavailable to cells to resist oxidative stress, and better regulate redox homeostasis. A similar observation has been reported in cancer cells where TBP-2 level was decreased while Trx activity was enhanced. Therefore, TBP-2 interaction with Trx is very delicate and important for maintaining the biological function and the well being of the cells.
In summary, we have shown that TBP-2 overexpression can damage the health and function of cells. It not only slows cell growth by cell cycle arrest at G2-M phase and inducing cell apoptosis. But, it also causes the cells to be more sensitive to oxidative stress that can lead to pro-apoptotic state. This latter case is shown as a result from disturbed anti-apoptotic function of Trx, in which the formation of a Trx-ASK1 binding complex is essential to keep the cells viable and resistant to cell death. Based on our data, we propose that TBP-2 over expression can cause two separate but parallel events. One is that TBP-2 leads to apoptosis by disturbing cell cycle progression out of the G2-M cycle. The other is that TBP-2 enrichment may cause cells to be more susceptible to H2O2-induced apoptosis. Such aberrations are mainly due to the displacement of Trx-ASK1 binding with Trx-TBP-2 binding, which renders an elevated population of oxidized, and non-effective Trx that fails to regulate the cell cycle, and a concomitant increase in free ASK1 pool to initiate the mitochondrial-mediated cell apoptosis. A diagram of such hypothesis is shown in Figure 10.
Figure 10. Potential mechanism of the effect of TBP-2 overexpression in human lens epithelial cells.
The diagram depicts the potential mechanism for TBP-2 overexpression-induced effect on human lens epithelial B3 cells (HLE B3). TBP-2 overexpression in HLE B3 cells can induce cell cycle arrest at G2/M stage, followed by JNK activation and apoptosis. In the presence of oxidative stress such as H2O2 (ROS), the excess TBP-2 disrupts Trx-ASK1 binding complex both in the cytosol and in the mitochondria, leading to the release and activation of ASK, followed by JNK activation and apoptosis. TBP-2 enriched cells have lower reduced Trx (SH) and higher Trx (S-S).
TBP-2: thioredoxin binding protein-2
ROS: reactive oxygen species
Trx1: thioredoxin 1
Trx2: thioresoxin 2
ASK1: apoptosis stimulating kinase 1
P-ASK1: phosphorylated ASK1 (activated)
P-JNK: phosphorylated JNK (activated)
Trx (S-S): oxidized thioredoxin
Supplementary Material
ACKNOWLEDGEMENTS
We deeply appreciate the assistance of XiaoliTian, PhD in preparation of the figures while Peter Kador, PhD for reading the manuscript. This research is support by a NIH grant RO1EY10595 (MFL). Flow cytometry support from COBRE (5P30 RR031151-02) to the Nebraska Center for Virology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented at the International Conference of the Lens, January 15–20, 2012, Kona, Hawaii, USA
REFERENCES
- 1.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from H-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219(1):26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274(31):21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 4.Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima YA. possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271(3):796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- 5.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164(12):6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 6.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe R, Nakamura H, Masutani H, Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacology and Therapeutics. 2010;127:261–270. doi: 10.1016/j.pharmthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146(5):2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 9.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflamma some activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 10.Nishinaka Y, Nishiyama A, Masutani H, Oka S, Ahsan KM, Nakayama Y, Ishii Y, Nakamura H, Maeda M, Yodoi J. Loss of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 in human T-cell leukemia virus type I-dependent T-cell transformation: implications for adult T-cell leukemia leukemo genesis. Cancer Res. 2004;64(4):1287–1292. doi: 10.1158/0008-5472.can-03-0908. [DOI] [PubMed] [Google Scholar]
- 11.Lee KN, Kang HS, Jeon JH, Kim EM, Yoon SR, Song H, Lyu CY, Piao ZH, Kim SU, Han YH, Song SS, Lee YH, Song KS, Kim YM, Yu DY, Choi I. VDUP1 is required for the development of natural killer cells. Immunity. 2005;22(2):195–208. doi: 10.1016/j.immuni.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Oka S, Yoshihara E, Bizen-Abe A, Liu W, Watanabe M, Yodoi J, Masutani H. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxi some proliferator-activated receptor function. Endocrinology. 2005;150(3):1225–1234. doi: 10.1210/en.2008-0646. [DOI] [PubMed] [Google Scholar]
- 13.Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, Yoshida T, Nakamura H, Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20(1):121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277(29):26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 15.Joguchi A, Otsuka I, Minagawa S, Suzuki T, Fujii M, Ayusawa D. Overexpression of VDUP1 mRNA sensitizes HeLa cells to paraquat. Biochem Biophys Res Commun. 2002;293(1):293–297. doi: 10.1016/S0006-291X(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN, Choi I. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxy vitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003;22:4035–4046. doi: 10.1038/sj.onc.1206610. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-Independent manner. Circ Res. 2004;94(11):1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 19.Katagiri K, Matsuzawa A, Ichijo H. Regulation of apoptosis signal-regulating kinase 1 in redox signaling. Methods Enzymol. 2010;474:277–288. doi: 10.1016/S0076-6879(10)74016-7. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283(12):7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 21.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yegorova S, Liu A, Lou MF. Human lens thioredoxin: molecular cloning and functional characterization. Invest Ophthalmol Vis Sci. 2003;44(8):3263–3271. doi: 10.1167/iovs.02-1322. [DOI] [PubMed] [Google Scholar]
- 23.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22(5):657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 24.Liyanage NP, Fernando MR, Lou MF. Regulation of the bioavailability of thioredoxin in the lens by a specific thioredoxin-binding protein (TBP-2) Exp Eye Res. 2007;85(2):270–279. doi: 10.1016/j.exer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehncrona S, Mela L, Siesjö BK. Recovery of brain mitochondrial function in the rat after complete and incomplete cerebral ischemia. Stroke. 1979;10(4):437–446. doi: 10.1161/01.str.10.4.437. [DOI] [PubMed] [Google Scholar]
- 26.Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17(6):471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- 28.Timofeev O, Cizmecioglu O, Settele F, Kempt T, Hoffmann I. Cdc25 phosphatase are required for timely assembly of cdk1-cyclinB at the G2/M transition. J. Biol. Chem. 2010;285:16978–16990. doi: 10.1074/jbc.M109.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J, Lukas J. Regulation of G(2)/M events by cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21(21):5911–5920. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savitsky PA, Finkel T. Redox regulation of Cdc25c. J. Biol. Chem. 2002;277(23):20535–42050. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 32.Sohn J, Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- 33.Burhans WC, Heintz NH. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Rad Biol Med. 2009;47:1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ. Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- 35.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27(23):8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.