Abstract
Surgically menopausal women incur a 2–5 fold increased risk for dementia and mortality from neurological diseases, but the mechanisms underlying these increased risks remain unclear. Previously, we demonstrated that after global cerebral ischemia (GCI), 17β-estradiol (E2 or estrogen) suppresses hippocampal elevation of the Wnt antagonist Dickkopf-1 (Dkk1), a neurodegenerative factor. We, thus, hypothesized that prolonged loss of E2 may lead to dysregulation of neural Dkk1 and Wnt/β-Catenin signaling, which could contribute to an increased risk of neurodegeneration. To test this hypothesis, we examined the effect of shortterm (1 week - STED) and long-term E2 deprivation (10 weeks - LTED) via ovariectomy upon basal and E2-regulated Dkk1 levels and Wnt/β-Catenin signaling in the hippocampal CA1 region following GCI. In STED rats, E2 exerted robust neuroprotection against GCI, suppressed postischemic elevation of Dkk1, and enhanced pro-survival Wnt/β-Catenin signaling, effects that were lost in LTED rats. Intriguingly, LTED rats displayed modest basal changes in Dkk1 and survivin expression. Further work showed that c-Jun N-Terminal Kinase (JNK) mediated GCI-induced changes in Dkk1 and survivin, and JNK inhibition afforded neuroprotection in LTED rats. Finally, we extended our findings to natural aging, as 24-month-old, reproductively senescent female rats also displayed a modest increase in basal Dkk1 in the CA1, which consistently co-localized with the apoptotic marker TUNEL after GCI and coincided with a loss of E2 neuroprotection. As a whole, this study supports the “critical period hypothesis” and further suggests that perimenopausal estradiol replacement may prevent neurodegenerative changes in the hippocampus by maintaining favorable Wnt/β-Catenin signaling.
Keywords: Estrogen, Dkk1, Hippocampus, Long-Term Estrogen Deprivation, Menopause, Neuroprotection
INTRODUCTION
17β-estradiol (E2) is an ovarian steroid hormone that serves as a neuroprotective agent in rodent models of focal and global cerebral ischemia [1–4]. Clinically, it is also implicated in neuroprotection from a variety of neurodegenerative conditions, such as stroke and Alzheimer’s disease [1, 5–9]. In support of E2’s neuroprotective effects, premenopausal women are relatively protected from stroke, compared to men [10–12]. However, after a period of long-term E2 deprivation (LTED), such as menopause, women’s incidence of stroke increases significantly, and women begin to have worse clinical outcomes following stroke than men [10, 13]. Along these lines, observational studies have revealed a 5-fold increased risk of mortality from neurological disorders in women who enter menopause prematurely due to bilateral oophorectomy [14–17]. Furthermore, observational studies also attribute mid-life E2 exposure to a 29–44% reduced risk of dementia in postmenopausal women [1]. Unfortunately, the Women’s Health Initiative (WHI), a large randomized controlled trial that subjected postmenopausal women to oral hormone replacement therapy (conjugated equine estrogens ± medroxyprogesterone acetate), was stopped prematurely due to an unexpected increase of ischemic stroke and dementia in the treatment arm [18, 19]. One important caveat of the study was that the average age of the WHI patients was 63.3 years, which is more than a decade after the onset of natural menopause in developed countries [20]. This led to conception of the “critical period hypothesis,” which suggests that a window of opportunity exists for E2 to provide neurological benefit after menopause and that significantly delayed E2 replacement may be ineffective or even detrimental to the brain [21–24].
Intriguingly, recent work has implicated a role for the neurodegenerative factor Dickkopf-1 (Dkk1) in a variety of neurodegenerative conditions, including stroke, temporal lobe epilepsy, and Alzheimer’s disease [25–30]. Dkk1 is an antagonist of canonical Wnt/β -Catenin signaling [31], and Dkk1 has been shown to mediate neuronal death in the hippocampus, as Dkk1 anti-sense oligonucleotides were able to prevent NMDA excitotoxicity in vitro and cerebral ischemia-induced cell death in vivo [32]. Along these lines, doubleridge mice, which have substantially reduced expression of Dkk1 [33, 34], display smaller ischemic infarcts after middle cerebral artery occlusion (MCAO) [28]. Furthermore, transgenic mouse models with mutations characteristic of frontotemporal dementia or early-onset Alzheimer’s disease were found to overexpress Dkk1 in brain regions affected by the respective neurodegenerative condition [29].
The hippocampus is an important brain region involved in learning and memory, and the hippocampal CA1 region is well known to be highly vulnerable to damage from stressors, such as global cerebral ischemia [35, 36]. Along these lines, our group and others have utilized a rodent model of global cerebral ischemia (GCI or 4-vessel occlusion), which involves occlusion of the common carotid and vertebral arteries, to selectively damage the CA1 region [4, 37–41]. As such, the GCI model has proven invaluable in identifying the neurodegenerative processes that can occur in the hippocampus and in helping to elucidate the mechanisms underlying E2 neuroprotection. In fact, we previously showed that E2 mediates robust neuroprotection of the hippocampal CA1 region during GCI, in part, by suppressing cerebral ischemia-induced elevation of Dkk1 and simultaneously activating pro-survival Wnt/β-Catenin signaling in pyramidal neurons [30]. Wnt is a secreted glycoprotein that serves as a ligand for the transmembrane Frizzled (Fzd) receptor and the low density lipoprotein-related protein 5/6 (LRP5/6) co-receptor, and Wnt promotes de-phosphorylation and nuclear retention of the transcriptional co-activator β-catenin [31]. Interestingly, β-catenin has been shown to interact with T-Cell Factor/Lymphoid Enhancing Factor (TCF/LEF) family of transcription factors inside the nucleus [31], leading to enhanced expression of key pro-survival factors, such as survivin, a protein which inhibits activation of pro-apoptotic caspases [42]. In light of this knowledge and our previous finding that E2 neuroprotection is lost in female rats subjected to LTED via ovariectomy or natural aging [38, 43], we hypothesized that E2’s failure to exert neuroprotection in LTED animals following GCI could be due to loss of its ability to suppress Dkk1 and facilitate pro-survival Wnt/ -catenin signaling in the hippocampus. Herein, for the first time, we demonstrate that LTED via surgical menopause (bilateral ovariectomy) or age-related reproductive senescence leads to elevation of basal Dkk1 expression and dysregulation of prosurvival Wnt/β-catenin signaling in the CA1 hippocampal region of female rats. We also demonstrate a role for JNK signaling in Dkk1 elevation after GCI and show that a significant delay in E2 replacement therapy after LTED leads to a loss of E2’s ability to prevent the ischemia-induced elevation of neurodegenerative Dkk1 in CA1 hippocampal neurons.
EXPERIMENTAL
Animals and Global Cerebral Ischemia
All procedures were approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee (AUP# 09-03-174) and were conducted in accordance with the National Institutes of Health guidelines for animal research. 3-month-old female Sprague-Dawley rats were bilaterally ovariectomized under isoflurane anesthesia one week or 11 weeks before induction of global cerebral ischemia. At the time of ovariectomy (STED) or 10 weeks later (LTED), placebo (20% β-Cyclodextrin) or 17β-estradiol osmotic mini-pumps (0.0167 mg E2 in 20% β-Cyclodextrin, 0.5 µL/hr, 14-day release; Alzet, Cupertino, CA) were implanted subcutaneously between the scapulae to mimic physiological E2 levels during Diestrus I (10–15 pg/mL) [30]. All animals (except sham control) underwent global cerebral ischemia (GCI) via 4-vessel occlusion as described previously [44–46]. The day before GCI, animals were anesthetized using chloral hydrate (350 mg/kg, ip), and both vertebral arteries (VA) were permanently occluded at the level of the alar foramina via electrocauterization. Immediately after bilateral VA occlusion, both common carotid arteries (CCA) were carefully isolated and loosely ligated with suture thread without interrupting blood flow. After a 24-hr recovery period, animals were re-anesthetized with chloral hydrate (300 mg/kg, ip), and the bilateral CCA were exposed and occluded with hemostatic clips to induce 10 minutes of complete forebrain ischemia. Animals which lost their righting reflex within 30 seconds and whose pupils were dilated and unresponsive to light during cerebral ischemia were selected for the experiments. After 10 minutes, the clips were removed, and reperfusion was confirmed before the wound was sutured. Rectal temperature was maintained at 36.5 to 37.5°C throughout the experiment with a thermal blanket. For reproductively senescent rat studies, either 3-month-old (Young) or 24-month-old (Aged) female Fisher 344 rats were used. All Fisher 344 females were ovariectomized one week before ischemia and treated with osmotic mini-pumps immediately following ovariectomy. Sham animals underwent identical surgical procedures except that the CCA were simply exposed but not occluded.
Histochemical Analysis of Neuroprotection
For brain harvesting, animals were deeply anesthetized with chloral hydrate and transcardially perfused with 0.9% saline containing 10 U/ml of heparin either 1, 5, or 7 days post ischemia-reperfusion, followed by fixation with cold 4% paraformaldehyde in 0.1M phosphate buffer (PB). Brains were post-fixed in the same fixative overnight at 4°C and cryoprotected with 30% sucrose in 0.1 M PB, pH 7.4 for 24–36 hr. Coronal sections (20 µm) were collected throughout the entire dorsal hippocampus (~2.5–4.5 mm posterior from bregma, ~100 sections per brain) for each animal. For analysis of neuroprotection, every fifth section was collected and stained. Briefly, sections were washed for 10 min in PBS followed by 0.1 % PBS-Triton-X100 for additional 10 min. After incubation with blocking solutions containing 10% donkey serum for 1h at room temperature in PBS containing 0.1% Triton X-100, sections were exposed overnight at 4°C to mouse anti-NeuN monoclonal antibody (1:500, Chemicon, MA). Sections were washed for 4 × 10 min, followed by incubation with Alexa Fluor 488 donkey anti-mouse antibody (1:500; Invitrogen, CA) for 1 h at room temperature. Staining solution was removed, and sections were washed with PBS-Triton-X100, followed by PBS and water. Then, sections were mounted using a water-based mounting medium containing anti-fading agents, and images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss, Germany) as described previously [47]. The number of NeuN-positive CA1 neurons per 1mm length of the medial CA1 pyramidal cell layer was counted bilaterally in five sections per animal. Cell counts from the right and left hippocampus on each of the five sections were averaged to provide the mean value. A Mean ± SE was calculated from the data in each group and statistical analysis performed as described below.
DAB staining
For diaminobenzidine staining, sections were incubated with 10% normal horse serum in PBS containing 0.1% Triton-X100 and 0.3% H2O2 for 1h at room temperature to block nonspecific surfaces. Sections were then incubated with primary antibody: polyclonal rabbit anti-Dkk1 (1:50, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal rabbit anti-survivin (1:100, Cell Signaling Technology, Inc., Danvers, MA), or polyclonal rabbit anti-p-β-Catenin (1:100, Cell Signaling Technology, Inc., Danvers, MA), overnight at 4oC in PBS containing 0.1% Triton-X100. Afterward, sections were washed with the same buffer, followed by incubation with secondary biotinylated horse anti-rabbit antibodies (1:500, Vector Laboratories, Inc., CA) in PBS containing 0.1% Triton X-100 for 1 h at room temperature. Sections were then washed and incubated with ABC reagent for 20 minutes at room temperature. Finally, sections were rinsed and incubated with DAB reagent for 2–10 min., according to the manufacturer’s instructions (Vector Laboratories, Inc.). Following DAB incubation, sections were washed briefly with distilled water, dehydrated in increasing concentrations of ethanol, cleared in xylene, and mounted using a xylene-based mounting medium. Images were captured on an Axiophot-2 visible/fluorescence microscope using an AxioVision4Ac software system (Carl Zeiss, Germany). Integrated density of immunostaining was then analyzed using ImageJ analysis software (Version 1.45s; Wayne Rasband, NIH, USA), and a Mean ± SE was calculated for each treatment group, which consisted of 4–7 animals each and 3–5 sections per animal. Statistical analysis was performed as described below, and all results were expressed as mean integrated density in arbitrary units + SE.
Double Immunofluorescence Staining with TUNEL
Coronal sections were incubated with 10% normal donkey serum for 1h at room temperature in PBS containing 0.1% Triton X-100, followed by incubation with the primary antibody anti-Dkk1 (1:50, Santa Cruz Biotechnology) overnight at 4oC in the same buffer. After primary antibody incubation, sections were washed for 3 × 10 min at room temperature, followed by incubation with Alexa-Fluor488 donkey anti-rabbit (1:500; Invitrogen Corporation, Carlsbad, CA), and TUNEL (In Situ Cell Death Detection Kit, TMR Red, Roche Diagnostics, Manheim, Germany) for 1 h at 37°C. Sections were then washed with PBS containing 0.1% Triton X-100 for 3 × 10 min, followed by 2 × 5 min with PBS and briefly with water, and then mounted with water-based mounting medium containing anti-fading agents (Biomeda, Fischer Scientific, Pittsburgh, PA). Simultaneous examination of negative controls confirmed the absence of nonspecific immunofluorescent staining, cross-immunostaining, or fluorescence bleed-through. The number of Dkk1- and TUNEL- double positive neurons per 250 µm length of the medial CA1 pyramidal cell layer was counted in five sections per animal, and a Mean ± SE was calculated from the data in each group (n = 5–7). Statistical analysis was performed as described below.
Confocal Microscopy and Image Analysis
All images were captured on an LSM510 Meta confocal laser microscope (Carl Zeiss, Germany) using a 40X oil immersion Neofluor objective (NA, 1.3) with the image size set at 1024 × 1024 pixels. The following excitation lasers/emission filters settings were used for various chromophores: argon/2 laser was used for Alexa Fluor488, with excitation maximum at 490 nm and emission in the range of 505–530 nm, HeNe1 laser was used for TMR with excitation maximum at 543 nm and emission in the range of 568–615 nm, and HeNe2 laser was used for Alexa Fluor647 with excitation maximum at 633 nm and emission in the range of 650–800 nm. The captured images were viewed and analyzed using LSM510 Meta imaging software.
Brain Homogenization
For brain tissue preparation, rats were sacrificed under anesthesia 24 hr. post ischemia-reperfusion. Once the whole brains were removed, the hippocampal CA1 regions were microdissected from both sides of the hippocampal fissure, immediately frozen in dry ice, and stored at −80°C until use. Tissues were homogenized with a Teflon-glass homogenizer in ice cold homogenization medium consisting of 50 mM HEPES (pH 7.4), 150 mM NaCl, 12 mM β-glycerophosphate, 3 mM dithiothreitol (DTT), 2 mM sodium orthovanadate (Na3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100, and inhibitors of proteases and enzymes (0.5mM PMSF, 10 µg/ml each of aprotinin, leupeptin, and pepstatin A). The homogenates were centrifuged at 15,000 ×g for 30 min at 4°C, and supernatants were collected and stored at −80°C until use. The protein concentrations were determined by a Lowry protein assay kit using bovine serum albumin as a standard.
Western Blot Analysis
For Western blotting analysis, total CA1 hippocampal lysates were mixed with Laemmli loading buffer and boiled for 5 min. An aliquot of 20–50 µg of protein was separated via 4–20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Millipore), blocked for 3 h, and incubated with 1° antibody against Dkk1 (1:200, Santa Cruz Biotechnology) or Survivin (1:1000, Cell Signaling Technology) at 4 °C overnight. -Tubulin (1:200, Santa Cruz Biotechnology) was used as a loading control. The membrane was then washed with Tween20- PBS to remove unbound antibody and incubated with 2° Alexa Fluor 680 goat anti-rabbit/mouse IgG for 1 h at room temperature. Bound proteins were visualized using the Odyssey Imaging System (LI-COR Bioscience, Lincoln, NB) and semi-quantitative analysis of the bands was performed using ImageJ analysis software (Version 1.45s; Wayne Rasband, NIH, USA). To quantitate hippocampal protein abundance, band densities of the indicated total proteins were analyzed and expressed as a ratio relative to -tubulin signals, and a Mean ± SE was calculated from each group for graphical presentation and statistical comparison.
Administration of drugs
The JNK inhibitor SP600125 was dissolved in PPCES vehicle (30% PEG–400/20% polypropylene glycol/15% cremophor EL/5% ethanol/30% saline) as described previously [48], and delivered to LTED females by tail vein injection 15 minutes before ischemia at a dose of 10 mg/kg. Controls consisted of LTED females that received a tail vein injection of PPCES vehicle alone 15 minutes prior to GCI (Vehicle). LTED animals that received placebo mini-pumps only (Placebo) were also included as an additional control.
Statistical Analysis
Statistical analysis was performed using one-way or two-way analysis of variance (ANOVA), as appropriate, followed by a Tukey-Kramer multiple comparison procedure. Statistical significance was accepted at the 95% confidence level (P < 0.05). Data are expressed as Mean + Standard Error (SE).
RESULTS
Long-Term Estrogen Deprivation Leads to Elevation of Basal Dkk1 and Loss of E2 Regulation in the Hippocampal CA1 Region
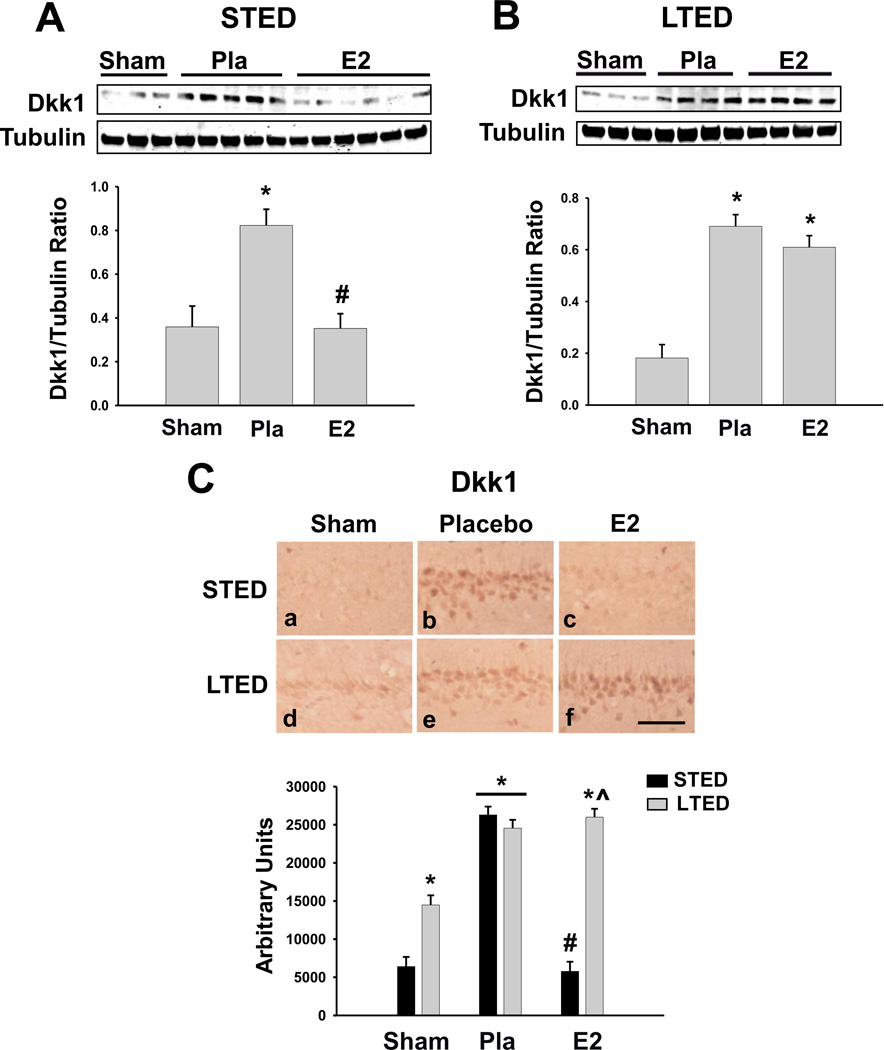
Since we previously showed that pre-treatment with physiological levels of E2 is able to prevent the ischemia-induced elevation of Dkk1 [30] and that E2 neuroprotection is lost in LTED animals [38], we sought to determine whether E2’s ability to suppress the neurodegenerative Wnt antagonist Dkk1was also lost following surgical menopause and to determine whether chronic loss of ovarian E2 affects basal Dkk1 expression in the hippocampus. Dkk1 levels were examined in the hippocampal CA1 region at 24h after GCI, a time-point that we previously found to demonstrate a robust elevation of Dkk1 after ischemia-reperfusion [30]. As shown in Figure 1A and 1C, western blot analysis and immunohistochemical analysis both revealed that Dkk1 was significantly elevated in the hippocampal CA1 of short-term (STED: short-term E2-deprived) ovariectomized rats following GCI as compared to non-ischemic sham controls, and immediate, low-dose E2 replacement was able to strongly attenuate the post-ischemic Dkk1 elevation (Fig. 1A and Fig. 1C: a–c, STED), a finding that is in agreement with our previous study [30]. In contrast, if E2 treatment was delayed 10 weeks following ovariectomy, and long-term E2 deprivation (LTED) was allowed to occur, the same low-dose E2 treatment was unable to attenuate Dkk1 elevation in the CA1 region following GCI (Fig. 1B and Fig. 1C: d–f). Intriguingly, we also noted that basal Dkk1 expression was modestly, but significantly increased in the hippocampal CA1 region of LTED rats as compared to STED rats (Fig. 1C: d).
Figure 1. Long-Term Estrogen Deprivation Leads to Loss of Estradiol Suppression of Neurodegenerative Dkk1 in CA1 Hippocampal Neurons.
(A) Western blotting shows Dkk1 expression 24 hours post global cerebral ischemia in total CA1 hippocampal lysates of animals treated with placebo (Pla) or low-dose estradiol (E2) immediately following ovariectomy (STED). Quantifications of band densities (Means + SE, n = 3–6 animals per group) are expressed as densitometric ratios of Dkk1 to -Tubulin. (B) Western blotting shows Dkk1 expression 24 hours post global cerebral ischemia in total CA1 hippocampal lysates of long-term E2-deprived (LTED) female rats treated with placebo (Pla) or low-dose estrogen (E2) 10 weeks following bilateral ovariectomy. Quantifications of band densities (Means + SE, n = 3–4 animals per group) are expressed as densitometric ratios of Dkk1 to -Tubulin. (C) Representative DAB staining (top) demonstrates Dkk1 expression in the CA1 region 24 hours post global cerebral ischemia. Quantitative summary (Bottom, Means + SE, n = 4–6 animals per group) indicates the raw integrated density of Dkk1 immunostaining in the medial CA1. * = p < 0.05 compared to Sham; # = p < 0.05 compared to Placebo; ^ = p < 0.05 compared to STED E2. Magnification = 20X; Scale bar = 50µm.
Long-Term Estrogen Deprivation Leads to Diminished Basal Wnt/β-Catenin Signaling and Loss of E2 Regulation in the CA1 Hippocampal Region
Since basal and post-ischemic E2 regulation of the Wnt antagonist Dkk1 was disrupted in long-term ovariectomized animals, we hypothesized that Wnt/β-catenin signaling in the hippocampal CA1 region of LTED animals would undergo equivalent changes. To test this hypothesis, we first examined the phosphorylation state of β-catenin, as active Wnt signaling promotes de-phosphorylation and nuclear retention of β-catenin, and phosphorylation of β-catenin at residues Ser33, Ser37, and Thr41 by GSK-3β is known to target it for degradation [30]. As shown in Figure 2, immunostaining of hippocampal CA1 sections revealed that low-dose E2 strongly suppressed GCI-induced elevation of phospho-β-catenin levels in STED rats (Fig. 2A:a–c, 2B) a finding that is in agreement with our previous study [30], but E2’s ability to prevent elevation of phospho-β-catenin levels after GCI was lost in LTED rats (Fig. 2A: d–f, 2B). Furthermore, basal phospho-β-catenin levels were significantly elevated in LTED as compared to STED surgically menopausal rats, further suggesting a role for ovarian E2 in control of basal Wnt/ β-catenin signaling in the hippocampal CA1 region (Fig. 2A:a,d, 2B).
Figure 2. Long-Term Estrogen Deprivation Promotes Phosphorylation of β-Catenin.
(A) Representative DAB staining demonstrates expression of β-catenin phosphorylated at Ser33, Ser37, and Thr41 24 hours post global cerebral ischemia. (B) Quantification (Means + SE, n = 4–6 animals per group) shows the raw integrated density of phospho-β-catenin immunostaining in the medial CA1. * = p < 0.05 compared to STED Sham; # = p < 0.05 compared to Placebo; ^ = p < 0.05 compared to STED E2. Magnification = 20X; Scale bar = 50µm.
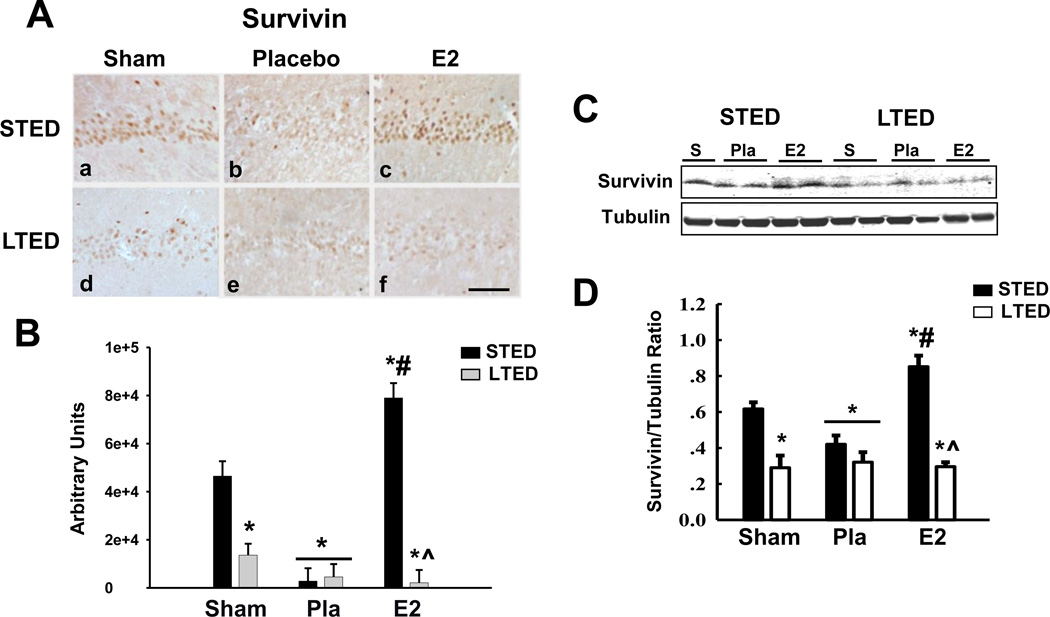
Since survivin is a key pro-survival protein that is a transcriptional target of β-catenin, and since we previously published that low-dose E2 is able to induce expression of survivin in the event of GCI [30], we next decided to examine survivin expression following GCI in LTED rats treated with delayed E2 therapy. As shown in Fig. 3, while immunohistochemistry and Western blot analysis revealed that low-dose E2 treatment strongly induced survivin levels in the CA1 region 24h after GCI in STED rats (Fig. 3A:c, B–D) a finding that confirms the results of our previous study [30], E2’s ability to enhance survivin expression was lost in LTED rats (Fig. 3A:f, B–D). Furthermore, basal survivin levels were significantly reduced in the hippocampal CA1 region of LTED rats as compared to STED rats (Fig. 3A:d, B–D), a finding that corroborates the elevation of basal Dkk1 and basal phospho-β-catenin levels in long-term ovariectomized rats (Fig. 1 and Fig. 2). Importantly, cell counts of Dkk1-, phospho-β-Catenin-, and Survivin-immunopositive neurons in the hippocampal CA1 showed similar patterns to those observed using intensity analysis (data not shown).
Figure 3. Delayed Estrogen Replacement Following Ovariectomy Fails to Induce Survivin Expression in CA1 Hippocampal Neurons.
(A) Representative DAB staining demonstrates survivin expression in the hippocampal CA1 region 24 hours post ischemia-reperfusion. (B) Quantitative summary of data (Means + SE, n = 4–6 animals per group) displays the raw integrated density of survivin immunostaining in the medial CA1. (C) Western blotting of survivin present in total CA1 hippocampal lysates corroborates DAB staining and is compared to α-Tubulin. (D) Quantification of Western blotting via densitometric band analysis is expressed as ratio of Survivin to -Tubulin (Means + SE, n = 4–6 animals per group). * = p < 0.05 compared to STED Sham; # = p < 0.05 compared to Placebo; ^ = p < 0.05 compared to STED E2; Magnification = 10X; Scale bar = 50µm
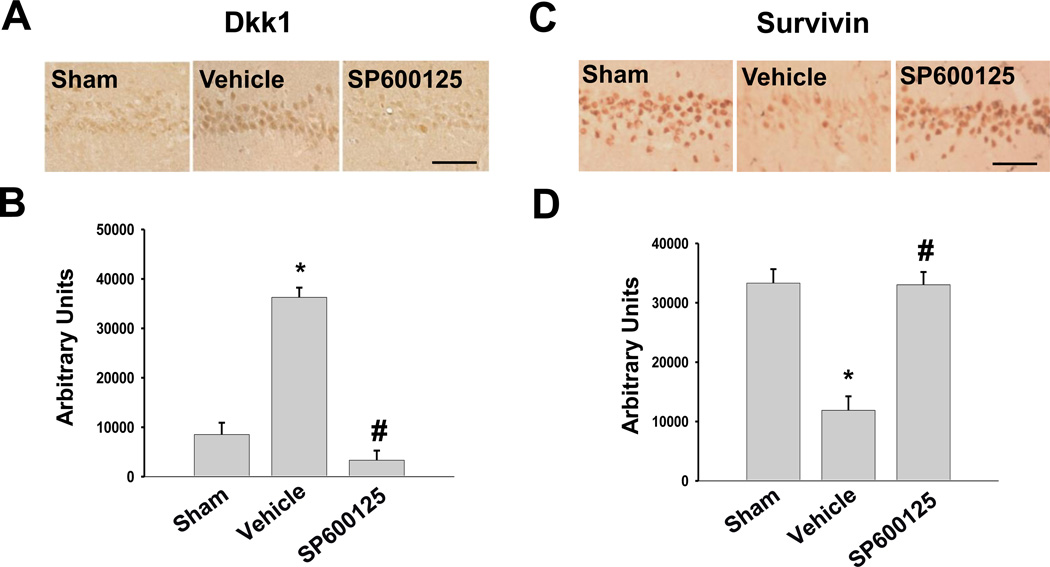
C-Jun N-Terminal Kinase Plays a Critical Role in Ischemic Modulation of Dkk1, Survivin, and Hippocampal Neuronal Cell Death in Long-Term Estrogen-Deprived Rats
C-Jun N-terminal kinase (JNK) has been previously implicated by our laboratory as an upstream regulator of Dkk1 due to its ability to activate the transcription factor c-Jun by phosphorylation [30]. We, thus, sought to determine whether JNK was a critical mediator of the profound Dkk1 elevation and survivin depression observed in LTED rats after GCI. To address this issue, we employed a specific JNK inhibitor, SP600125, which was administered to LTED females via tail vein injection 15 minutes prior to GCI. Figure 4 shows that GCI led to a robust induction of neurodegenerative Dkk1 in CA1 hippocampal neurons of LTED female rats 24 hours following ischemia-reperfusion (Fig. 4A–B: Vehicle), and administration of the JNK inhibitor SP600125 dramatically attenuated ischemia-induced Dkk1 elevation in LTED animals (Fig. 4A–B: SP600125). We also examined the effect of JNK inhibition on survivin expression in ischemic LTED rats. Along these lines, immunostaining revealed that GCI led to a profound reduction of survivin expression in LTED animals (Fig. 4C–D: Vehicle) and that this effect was prevented by the inhibition of JNK (Fig. 4C–D: SP600125). These findings suggest that JNK activation is critical for the ischemia-induced elevation of neurodegenerative Dkk1 and the ischemia-induced loss of the anti-apoptotic factor survivin in LTED female rats.
Figure 4. JNK is required for Ischemia-Induced Elevation of Dkk1 and Loss of Survivin in Long-Term Estrogen-Deprived Female Rats.
Representative DAB staining demonstrates Dkk1 (A) or Survivin (C) expression in CA1 hippocampal neurons of LTED female rats 24 hours following ischemia-reperfusion. The JNK inhibitor SP600125 was administered 15 minutes prior to ischemia via tail vein injection (10mg/kg in PPCES Vehicle). Controls consisted of LTED females that received a tail vein injection of PPCES alone 15 minutes prior to GCI (Vehicle). Quantification (Means + SE, n = 4–6 animals per group) displays raw integrated density of Dkk1 (B) or Survivin (D) immunostaining in the medial CA1. * = p < 0.05 compared to Sham; # = p < 0.05 compared to Vehicle (PPCES alone). Magnification = 20x; Scale bar = 50µm.
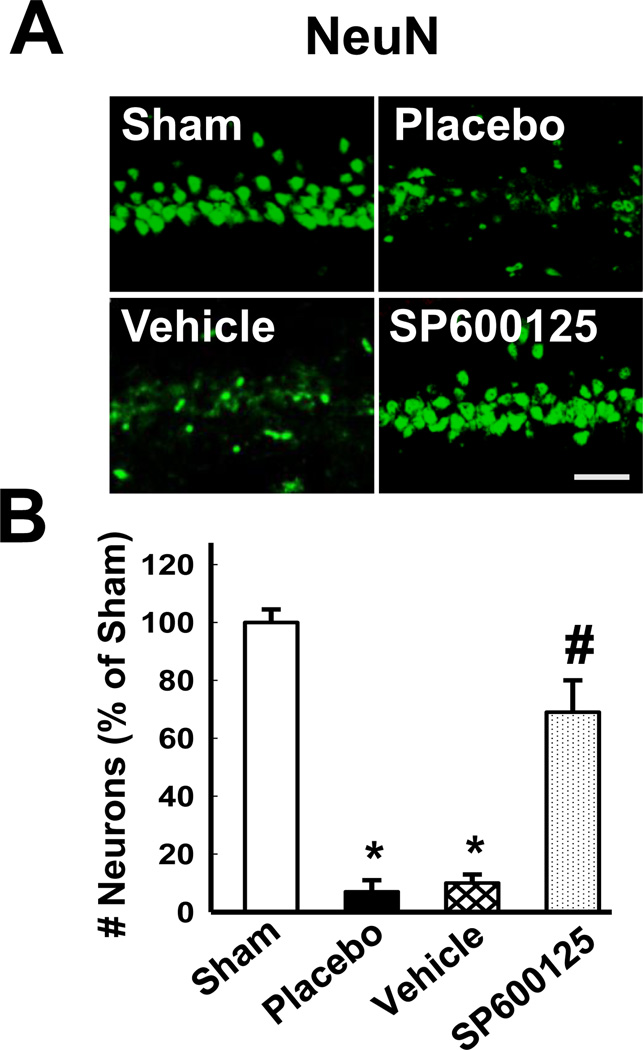
Furthermore, since inhibition of JNK prevented post-ischemic Dkk1 elevation (Fig. 4A–B) and maintained survivin expression (Fig. 4C–D) in the CA1 region of LTED females, we next sought to determine whether inhibition of JNK was sufficient to protect the hippocampal CA1 region from GCI-induced cell death after chronic loss of ovarian E2. As shown in Figure 5, administration of the JNK inhibitor SP600125 promoted robust neuroprotection in long-term ovariectomized females. Neuronal survival was visualized in the hippocampal CA1 via staining with the neuronal nuclear marker NeuN at 7 days post ischemia-reperfusion (Fig. 5A). The results revealed that non-ischemic LTED animals displayed a high number of NeuN-positive cells, indicating a dense population of healthy neurons in the hippocampal CA1 region. In contrast, placebo- and vehicle-treated LTED animals displayed significantly fewer NeuN-positive neurons, indicative of profound neuronal loss following GCI (Fig. 5A–B). Of significant interest, NeuN staining of healthy neurons was extensive in the hippocampal CA1 region of ischemic LTED animals treated with SP600125 (Fig. 5A) and, in fact, comparable to non-ischemic sham levels (Fig. 5B), suggesting that JNK activation also plays a critical role in mediating ischemic neuronal cell death in the CA1 region of LTED female rats.
Figure 5. The JNK Inhibitor SP600125 is Neuroprotective in the Hippocampal CA1 Region of Long-Term Estrogen-Deprived Female Rats Following Global Cerebral Ischemia.
(A) The JNK inhibitor SP600125 was administered 15 minutes prior to ischemia via tail vein injection (10mg/kg in PPCES Vehicle). Controls consisted of LTED females that received a tail vein injection of PPCES alone (Vehicle) and LTED Placebo females that did not receive a tail vein injection (Placebo). Typical photomicrographs of hippocampal CA1 region depicting NeuN staining 7 days following ischemia-reperfusion in LTED females are shown. (B) Quantitative summary of data (Means + SE, n = 6 animals per group) shows the number of surviving neurons per 250 µm of medial CA1 expressed as % of Sham. NeuN-positive pyramidal cells showing intact, round nuclei were counted as surviving cells. Magnification = 40X; Scale bar = 50 µm. * p < 0.01 vs. sham; # p < 0.05 vs. Vehicle (PPCES alone) and Placebo.
Natural Aging Leads to Basal Elevation of Dkk1 in the Hippocampal CA1 Region and Loss of E2 Regulation Following GCI
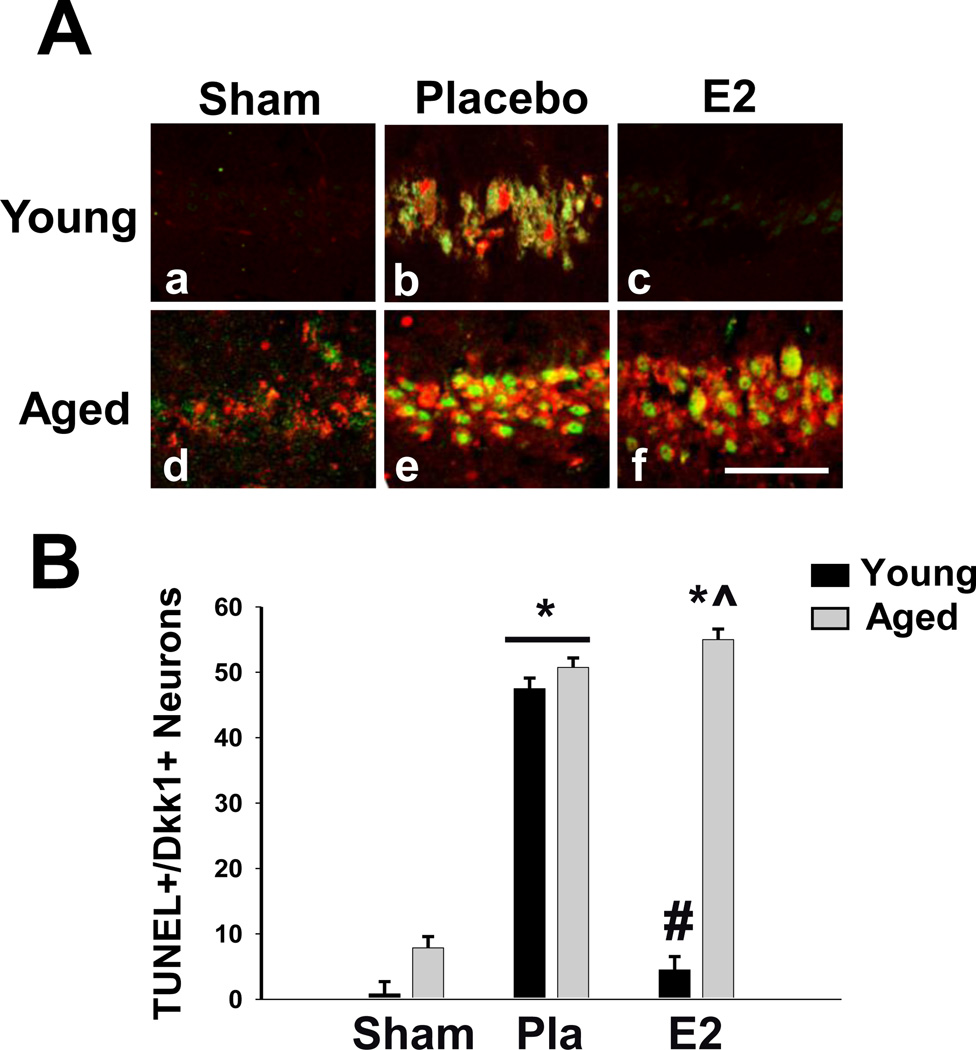
In a previous study, we reported that E2 is neuroprotective in young and middle-aged rats (3 and 11 months old, respectively) but loses this effect in aged female rats (24 months old) [43]. We, thus, sought to determine whether natural aging, or reproductive senescence, also leads to dysregulation of Dkk1 in the CA1 hippocampal region, and whether E2’s ability to attenuate ischemic induction of Dkk1 is also lost in aged, reproductively senescent female rats. Additionally, since we and others have reported that aged rats are more susceptible to damage from GCI [43, 49–52], we sought to determine whether induction of the neurodegenerative factor Dkk1 was associated with neuronal cell death by examining co-localization of Dkk1 with TUNEL, a marker of DNA damage and apoptosis [53– 55]. To address these questions, we subjected young (3-month-old) and aged (24-month-old) Fisher 344 rats to GCI one week following bilateral ovariectomy and examined immunostaining of Dkk1 and TUNEL in hippocampal CA1 sections. As shown in Figure 6, young, non-ischemic sham rats showed almost no Dkk1 or TUNEL staining in the CA1 region, indicating little Dkk1 expression and very little DNA damage in the basal, non-ischemic state (Fig. 6A:a, 6B). Examination of aged, non-ischemic rats revealed modest Dkk1 and TUNEL staining in the CA1 region, indicative of elevated basal Dkk1 expression and hippocampal DNA damage following aging alone (Fig. 6A:d, 6B). However, while both Dkk1 and TUNEL appeared to be mildly elevated in the CA1 hippocampal region of non-ischemic aged rats, Dkk1 and TUNEL staining showed little co-localization in these animals. In contrast, GCI induced a robust increase in both Dkk1 and TUNEL staining in the hippocampal CA1 region, and Dkk1 was highly co-localized with TUNEL-positive cells in the CA1 region of both young and aged rats (Fig. 6A:b,e, 6B). Furthermore, while low-dose E2 replacement immediately after ovariectomy was able to prevent CA1 hippocampal induction of both Dkk1 and the apoptotic marker TUNEL following 10 minutes of GCI in young rats (Fig. 6A:c), low-dose E2 replacement immediately after ovariectomy was unable to prevent CA1 hippocampal induction of Dkk1 and TUNEL following GCI in aged rats (Fig. 6A:f).
Figure 6. Neurodegenerative Dkk1 Co-Localizes with the Apoptotic Marker TUNEL in CA1 Hippocampal Neurons After Global Cerebral Ischemia in Aged, Reproductively Senescent Female Rats.
(A) Representative photomicrographs depicting Dkk1 (green) and TUNEL (red) expression in the hippocampal CA1 7 days (Young) and 3 days (Aged) following global cerebral ischemia. (B) Quantitative summary of data (Means + SE, n = 5–7 animals per group) indicates the number of neurons expressing both Dkk1 and TUNEL per 250µm medial CA1. * = p < 0.05 compared to Young Sham; # = p < 0.05 compared to Placebo; ^ = p < 0.05 compared to both Young E2. Magnification = 40X; Scale bar = 50µm.
DISCUSSION
The current study yielded two major findings - 1) LTED (either naturally via aging or surgically via bilateral ovariectomy) leads to dysregulation of basal Dkk1-Wnt/β-catenin signaling in the hippocampal CA1 region of female rats, and 2) E2 loses its ability to attenuate ischemia-induced Dkk1 elevation following LTED. With respect to the first finding, up-regulation of basal Dkk1 and a corresponding down-regulation of basal survivin in the hippocampal CA1 region of LTED rats could suggest that there is increased neuronal cell death occurring after LTED in the non-ischemic state. However, immunostaining for the neuronal marker NeuN in the hippocampal CA1 region failed to show any evidence of increased neuronal cell death in non-ischemic LTED animals as compared to non-ischemic STED animals (Supplemental Fig. 1). Furthermore, the modest Dkk1 elevation in the CA1 region of nonischemic aged animals did not show significant co-localization with the DNA damage/apoptotic marker TUNEL. In contrast, the robust induction of Dkk1 observed following GCI in aged rats was highly co-localized with TUNEL. Taken as a whole, these findings suggest that the basal elevation of Dkk1 in LTED and aged non-ischemic rats is not sufficient to induce neuronal cell death.
However, it is possible that basal changes in the hippocampal expression of Dkk1 and survivin in LTED and aged non-ischemic rats may cause these animals to be more sensitive to ischemic insults. In support of this contention, we [38, 43] and others [39, 49, 56, 57] have reported that the LTED and aged rat hippocampus is more susceptible to damage from global cerebral ischemia. Obviously, in regard to the current study, lower survivin levels could make the hippocampal neurons more susceptible to ischemic stress because survivin is known to prevent cell death by inhibiting the cleavage and, thus, activation of pro-apoptotic caspases [42, 58–60]. Along these lines, the decrease in survivin observed in the current study is likely due to the fact that Dkk1, as a Wnt/β-catenin pathway antagonist, prevents expression of critical β- catenin pro-survival genes, such as survivin. It is interesting to note that there is also a report of basal changes in activation of cAMP response element binding protein (CREB), Akt, and STAT3 in the hippocampal CA1 of LTED female rats [39], which may also play a role in the observed increased sensitivity of the LTED hippocampus to damage from GCI.
Intriguingly, Dkk1 is thought to be under transcriptional control of the stress sensor p53 [32, 61], and we have reported that p53 appears to be stabilized via acetylation in non-ischemic LTED animals [62]. Therefore, it is possible that the modest increase we observed in basal Dkk1 levels following LTED could be due to enhanced p53-mediated transcription of Dkk1 in the absence of ischemic stress. Once ischemia occurs, however, Dkk1 is then dramatically elevated above the threshold required to induce cell death. We propose that this event occurs due to robust activation of the pro-death JNK/c-Jun signaling cascade, which ultimately leads to neuronal apoptosis in the hippocampal CA1 region following GCI. Along these lines, Dkk1 is also thought to be under transcriptional control of c-jun [32, 63], and we hypothesized that JNK/c-jun activation might be responsible for the dramatic, post-ischemic elevation of Dkk1. In support of these contentions, we demonstrated that inhibition of JNK strongly attenuates GCI-induced Dkk1 elevation, survivin depression, and neuronal apoptosis in the CA1 region of LTED rats. Thus, in the current study, we provide evidence that JNK/c-jun activation plays a critical role in the regulation of Dkk1 and survivin and the resultant hippocampal neuronal cell death in LTED animals after GCI.
The second major finding of the current study was that the ability of E2 to modulate Dkk1/Wnt-β-catenin signaling is lost in LTED rats. As such, the loss of E2’s ability to regulate Dkk1 and Wnt-β-catenin signaling after prolonged hypoestrogenicity may help explain previous reports that LTED is associated with a loss of E2 neuroprotection in the hippocampal CA1 region following GCI [38, 43]. Since Dkk1 has been found to be elevated in human neurodegenerative disorders, such as stroke and Alzheimer’s disease, the current findings, if applicable to humans, could also help explain the doubled lifetime risk of dementia and 5-fold increase in mortality from neurological disorders in women who enter menopause prematurely due to bilateral oophorectomy [15–17, 64]. Furthermore, E2’s inability to suppress ischemiainduced Dkk1 following LTED supports the critical period hypothesis of E2 replacement [21, 24], which holds that E2 replacement must be initiated at peri-menopause to be beneficial. Along these lines, this observation could also help shed light on the unexpected negative results of the WHI study, which suggested that oral hormone replacement therapy led to an increased risk of ischemic stroke and dementia in postmenopausal women aged 65 and older [18, 19], and provide critical support for the implementation of perimenopausal, transdermal E2 replacement.
In conclusion, LTED, either through models of surgical menopause or age-related reproductive senescence, appears to have detrimental effects on the CA1 region of the hippocampus. The ability of low-dose E2 to protect the CA1 region from global cerebral ischemia is lost following prolonged E2 deprivation, and CA1 hippocampal neurons also appear to undergo unfavorable changes during chronic loss of ovarian E2. These changes include: 1) upregulation of neurodegenerative Dkk1, 2) phosphorylation of β-catenin, and 3) down-regulation of survivin, all of which may predispose hippocampal neurons to apoptosis following a stressor, such as cerebral ischemia. Importantly, the current study showed that early, but not delayed, E2 replacement maintained pro-survival Wnt/β-catenin signaling in the hippocampus after LTED, a finding that is consistent with both the critical period hypothesis and healthy cell bias of E2 replacement [15, 16, 21, 23, 24, 65, 66]. Finally, since the Wnt antagonist Dkk1 has been implicated in the pathophysiology of several neurodegenerative diseases [25–29, 67, 68], pharmacological inhibition of Dkk1 may yield a new avenue for the treatment of neurodegenerative disease, which should be further explored.
Supplementary Material
HIGHLIGHTS.
Hippocampal Dkk1 is elevated after long-term ovariectomy and natural aging.
Hippocampal Wnt signaling is impaired following chronic loss of ovarian estradiol.
Delayed estradiol replacement can no longer suppress Dkk1 or induce Wnt signaling.
C-Jun-N-Terminal Kinase is a critical mediator of post-ischemic Dkk1 elevation.
These findings support the critical period hypothesis of estrogen replacement.
ACKNOWLEDGEMENTS
The research presented in this article was conducted in partial fulfillment of the MD/PhD dual degree requirements set forth for ELS and was supported by a pre-doctoral fellowship to ELS from the American Heart Association (12PRE11530009), a Scientist Development Grant to QZ from the American Heart Association (10SDG2560092), and a grant to DWB from the NINDS, National Institutes of Health (NS050730).
Abbreviations
- Dkk1
Dickkopf-1
- E2 or Estrogen
17β-Estradiol
- GCI
Global Cerebral Ischemia
- Fzd
Frizzled
- GSK3β
Glycogen Synthase Kinase 3β
- HRT
Hormone Replacement Therapy
- JNK
c-Jun N-terminal Kinase
- LRP5/6
Low Density Lipoprotein-Related Protein 5/6
- LTED
Long-Term Estrogen Deprivation or Long-Term Estrogen-Deprived
- MCAO
Middle Cerebral Artery Occlusion
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP-X Nick-End Ligase
- Wnt
Wingless
- WHI
Women’s Health Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 3.Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96:119–130. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpkins JW, Perez E, Wang X, Yang S, Wen Y, Singh M. The Potential for Estrogens in Preventing Alzheimer's Disease. Ther Adv Neurol Disord. 2009;2:31–49. doi: 10.1177/1756285608100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 7.Simpkins JW, Yi KD, Yang SH. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol. 2009;30:93–105. doi: 10.1016/j.yfrne.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 11.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. Ilar J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 13.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 14.Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, et al. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33:32–40. doi: 10.1159/000211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. Jama. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 19.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 20.Harman SM, Brinton EA, Clarkson T, Heward CB, Hecht HS, Karas RH, et al. Is the WHI relevant to HRT started in the perimenopause? Endocrine. 2004;24:195–202. doi: 10.1385/ENDO:24:3:195. [DOI] [PubMed] [Google Scholar]
- 21.Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 23.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 25.Busceti CL, Biagioni F, Aronica E, Riozzi B, Storto M, Battaglia G, et al. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 26.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 28.Mastroiacovo F, Busceti CL, Biagioni F, Moyanova SG, Meisler MH, Battaglia G, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 29.Rosi MC, Luccarini I, Grossi C, Fiorentini A, Spillantini MG, Prisco A, et al. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. J Neurochem. 2010;112:1539–1551. doi: 10.1111/j.1471-4159.2009.06566.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer's disease: up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, et al. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- 36.Mattson MP, Guthrie PB, Kater SB. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333–351. [PubMed] [Google Scholar]
- 37.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 38.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Butte-Smith M, Zukin RS, Etgen AM. Effects of global ischemia and estradiol pretreatment on phosphorylation of Akt, CREB and STAT3 in hippocampal CA1 of young and middle-aged female rats. Brain Res. 2012;1471:118–128. doi: 10.1016/j.brainres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part two: modeling cerebral ischemia. Open Neurol J. 2010;4:34–38. doi: 10.2174/1874205X01004020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and-independent mechanisms. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Fukuda S, Pelus LM. Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146–8153. doi: 10.1038/sj.onc.1207992. [DOI] [PubMed] [Google Scholar]
- 43.Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, et al. From the Cover: C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-{alpha} and the critical period hypothesis of estrogen neuroprotection. PNAS. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang QG, Tian H, Li HC, Zhang GY. Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett. 2006;408:159–164. doi: 10.1016/j.neulet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 46.Pulsinelli WA, Buchan AM. The four-vessel occlusion rat model: method for complete occlusion of vertebral arteries and control of collateral circulation. Stroke. 1988;19:913–914. doi: 10.1161/01.str.19.7.913. [DOI] [PubMed] [Google Scholar]
- 47.Wakade C, Khan MM, De Sevilla LM, Zhang QG, Mahesh VB, Brann DW. Tamoxifen neuroprotection in cerebral ischemia involves attenuation of kinase activation and superoxide production and potentiation of mitochondrial superoxide dismutase. Endocrinology. 2008;149:367–379. doi: 10.1210/en.2007-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu K, Sun X, Puchowicz MA, LaManna JC. Increased sensitivity to transient global ischemia in aging rat brain. Adv Exp Med Biol. 2007;599:199–206. doi: 10.1007/978-0-387-71764-7_26. [DOI] [PubMed] [Google Scholar]
- 50.Xu K, Puchowicz MA, Sun X, LaManna JC. Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res. 2010;1328:181–189. doi: 10.1016/j.brainres.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shetty PK, Galeffi F, Turner DA. Age-Induced Alterations in Hippocampal Function and Metabolism. Aging Dis. 2011;2:196–218. [PMC free article] [PubMed] [Google Scholar]
- 52.Di Napoli M, Shah IM. Neuroinflammation and cerebrovascular disease in old age: a translational medicine perspective. J Aging Res. 2011;2011 doi: 10.4061/2011/857484. 857484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thal SE, Zhu C, Thal SC, Blomgren K, Plesnila N. Role of apoptosis inducing factor (AIF) for hippocampal neuronal cell death following global cerebral ischemia in mice. Neurosci Lett. 2011;499:1–3. doi: 10.1016/j.neulet.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Suman S, Maniar M, Fornace AJ, Jr, Datta K. Administration of ON 01210.Na after exposure to ionizing radiation protects bone marrow cells by attenuating DNA damage response. Radiat Oncol. 2012;7:6. doi: 10.1186/1748-717X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li GY, Fan B, Ma TH. Visible light may directly induce nuclear DNA damage triggering the death pathway in RGC-5 cells. Mol Vis. 2011;17:3279–3289. [PMC free article] [PubMed] [Google Scholar]
- 56.Xu K, Puchowicz MA, Sun X, LaManna JC. Mitochondrial dysfunction in aging rat brain following transient global ischemia. Adv Exp Med Biol. 2008;614:379–386. doi: 10.1007/978-0-387-74911-2_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inagaki T, Kaneko N, Zukin RS, Castillo PE, Etgen AM. Estradiol attenuates ischemia-induced death of hippocampal neurons and enhances synaptic transmission in aged, long-term hormone-deprived female rats. PLoS One. 2012;7:e38018. doi: 10.1371/journal.pone.0038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JX, Zheng S. Caspase-3 and survivin expression in pediatric neuroblastoma and their roles in apoptosis. Chin Med J (Engl) 2004;117:1821–1824. [PubMed] [Google Scholar]
- 59.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 60.Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984–1988. doi: 10.3748/wjg.v10.i13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19:1843–1848. doi: 10.1038/sj.onc.1203503. [DOI] [PubMed] [Google Scholar]
- 62.Raz L, Zhang QG, Han D, Dong Y, De Sevilla L, Brann DW. Acetylation of the pro-apoptotic factor, p53 in the hippocampus following cerebral ischemia and modulation by estrogen. PLoS One. 2011;6:e27039. doi: 10.1371/journal.pone.0027039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. Embo J. 2002;21:966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maki PM. Potential importance of early initiation of hormone therapy for cognitive benefit. Menopause. 2006;13:6–7. doi: 10.1097/01.gme.0000194822.76774.30. [DOI] [PubMed] [Google Scholar]
- 66.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caraci F, Busceti C, Biagioni F, Aronica E, Mastroiacovo F, Cappuccio I, et al. The Wnt antagonist, Dickkopf-1, as a target for the treatment of neurodegenerative disorders. Neurochem Res. 2008;33:2401–2406. doi: 10.1007/s11064-008-9710-0. [DOI] [PubMed] [Google Scholar]
- 68.Dun Y, Li G, Yang Y, Xiong Z, Feng M, Wang M, et al. Inhibition of the canonical Wnt pathway by Dickkopf-1 contributes to the neurodegeneration in 6-OHDA-lesioned rats. Neurosci Lett. 2012;525:83–88. doi: 10.1016/j.neulet.2012.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.