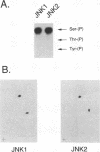
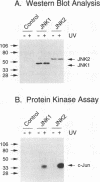
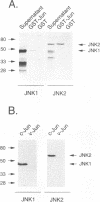
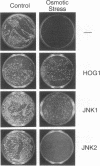
Abstract
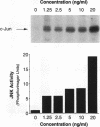
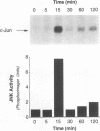
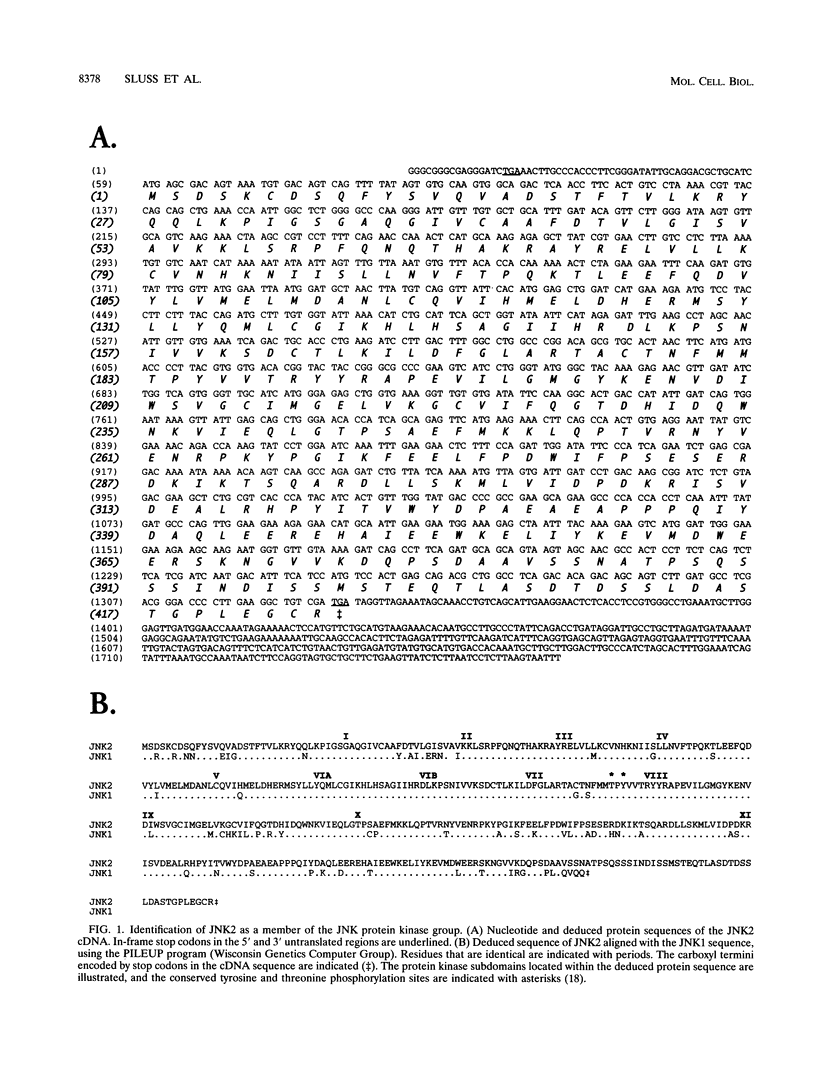
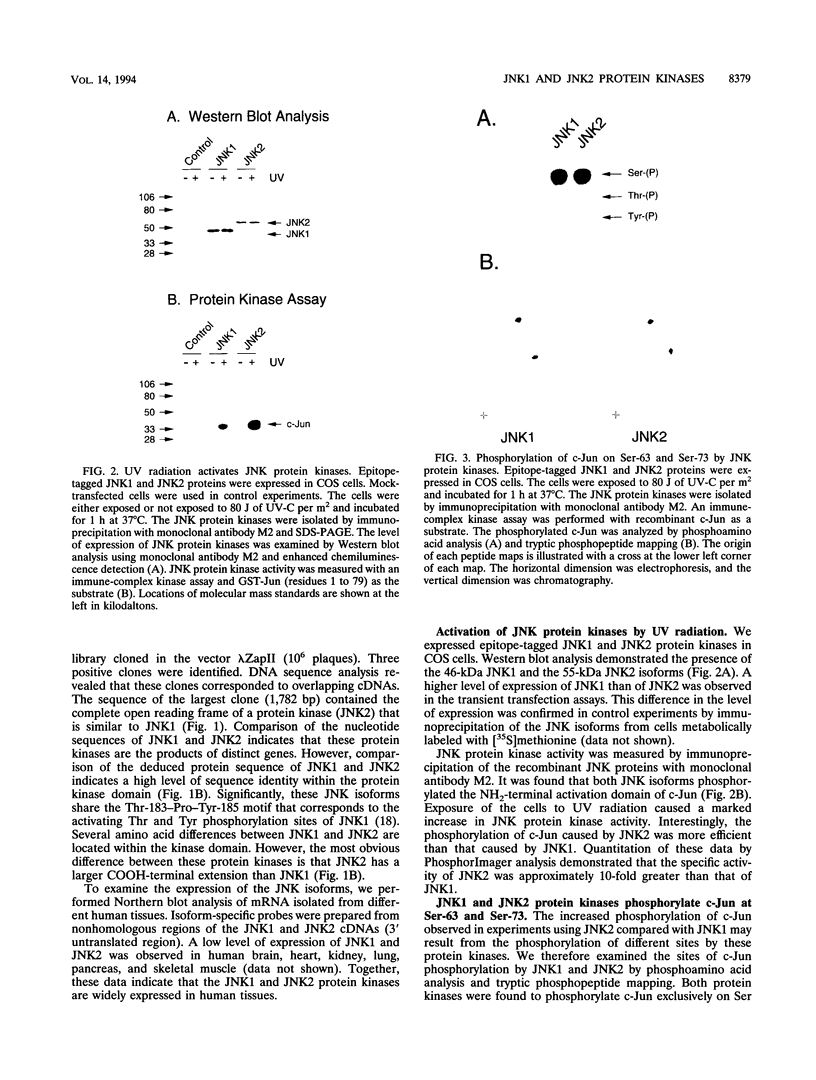
JNK protein kinases are distantly related to mitogen-activated protein kinases (ERKs) and are activated by dual phosphorylation on Tyr and Thr. The JNK protein kinase group includes the 46-kDa isoform JNK1. Here we describe the molecular cloning of a second member of the JNK group, the 55-kDa protein kinase JNK2. The activities of both JNK isoforms are markedly increased by exposure of cells to UV radiation. Furthermore, JNK protein kinase activation is observed in cells treated with tumor necrosis factor. Although both JNK isoforms phosphorylate the NH2-terminal activation domain of the transcription factor c-Jun, the activity of JNK2 was approximately 10-fold greater than that of JNK1. This difference in c-Jun phosphorylation correlates with increased binding of c-Jun to JNK2 compared with JNK1. The distinct in vitro biochemical properties of these JNK isoforms suggest that they may have different functions in vivo. Evidence in favor of this hypothesis was obtained from the observation that JNK1, but not JNK2, complements a defect in the expression of the mitogen-activated protein kinase HOG1 in the yeast Saccharomyces cerevisiae. Together, these data indicate a role for the JNK group of protein kinases in the signal transduction pathway initiated by proinflammatory cytokines and UV radiation.
Full text
PDF

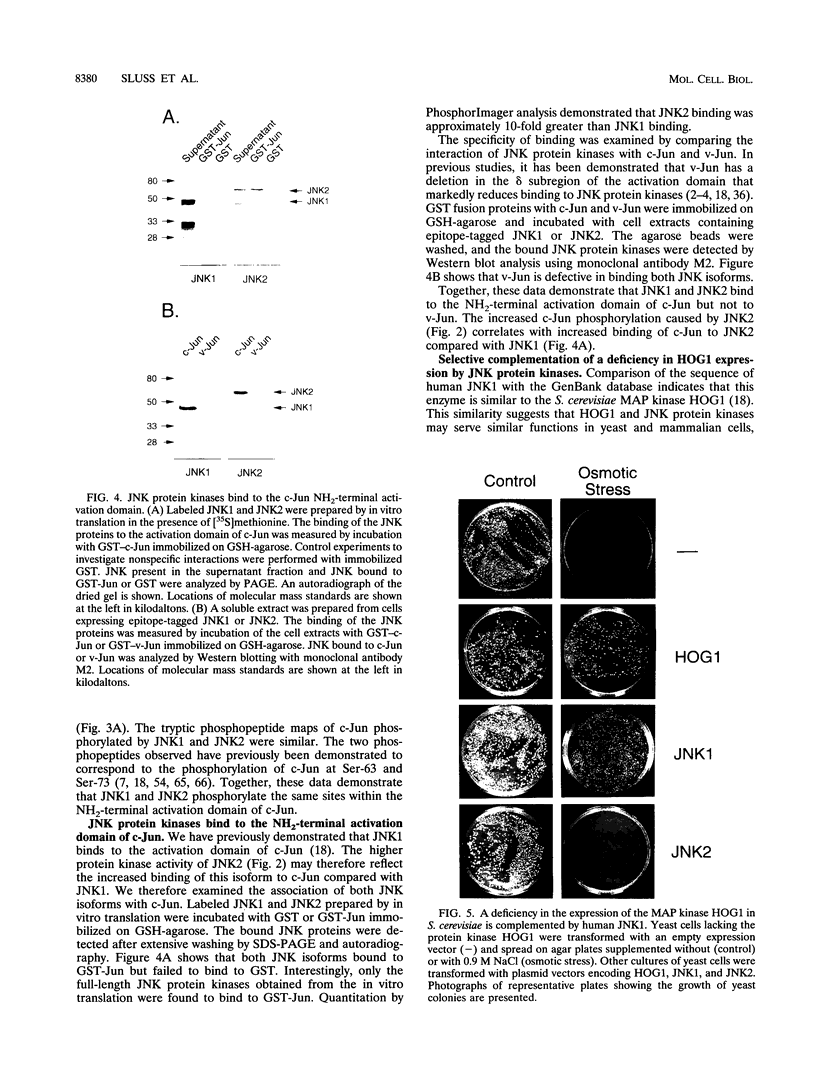
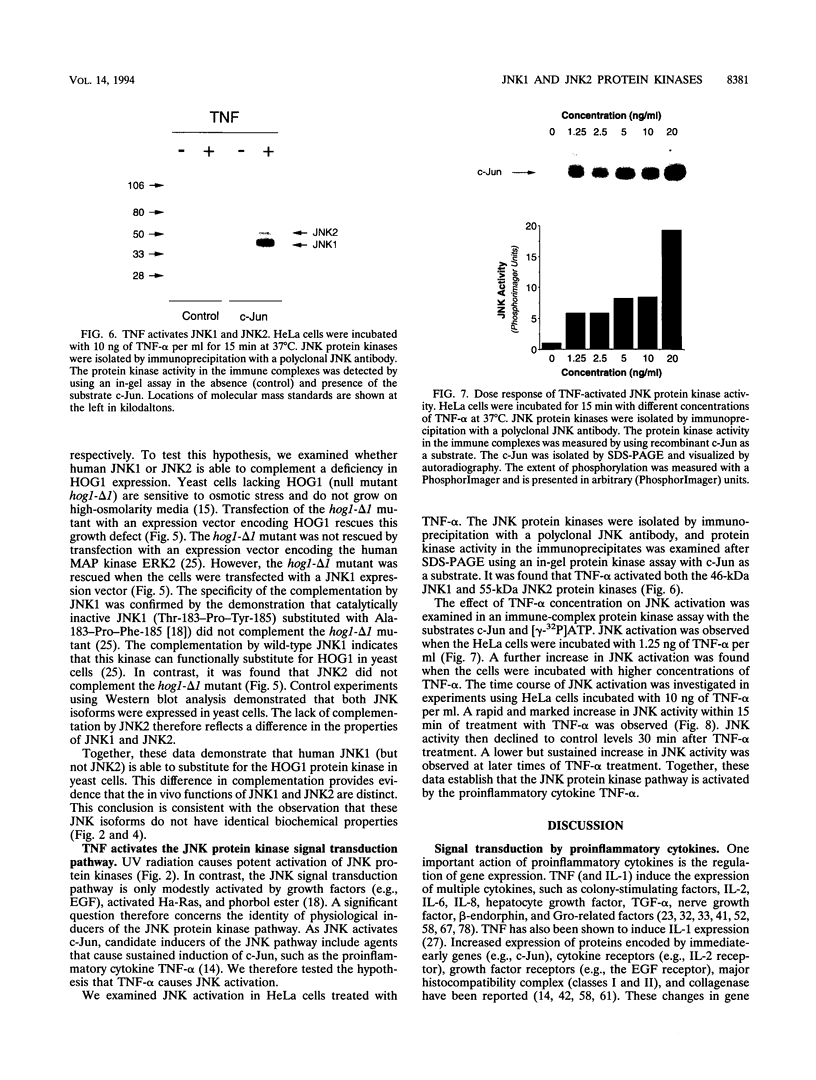
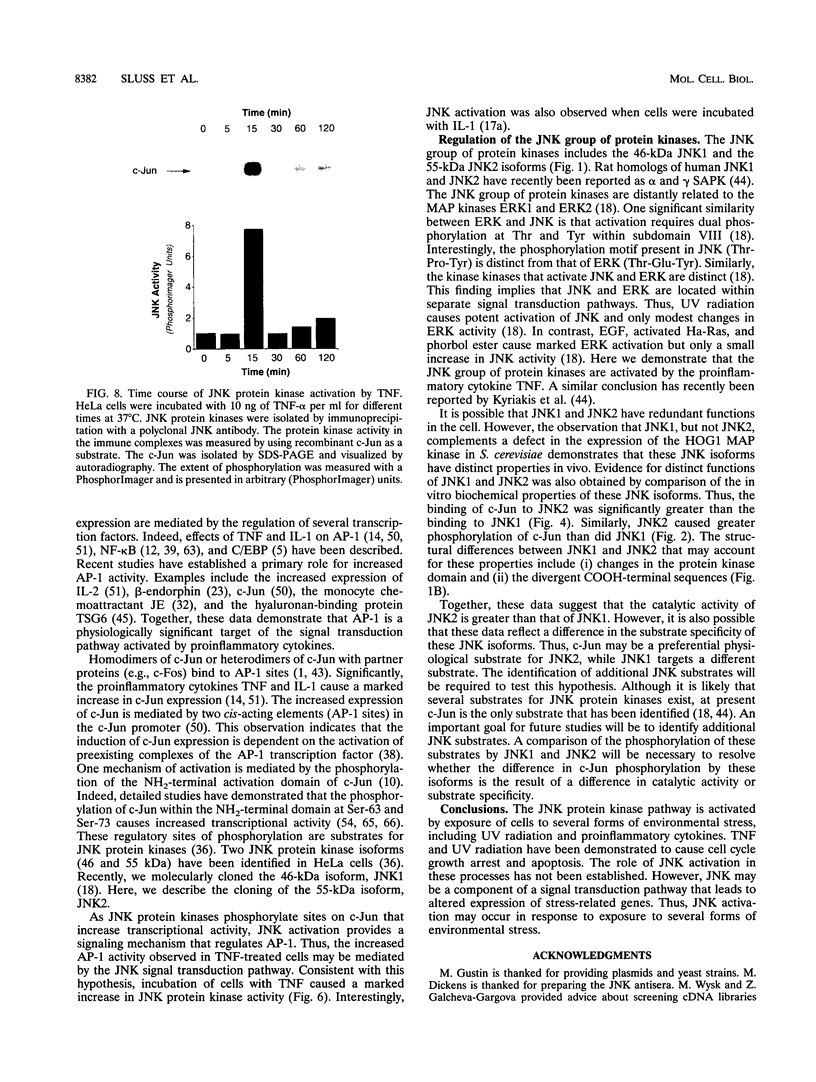


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Curran T. Encounters with Fos and Jun on the road to AP-1. Semin Cancer Biol. 1990 Feb;1(1):19–26. [PubMed] [Google Scholar]
- Adler V., Franklin C. C., Kraft A. S. Phorbol esters stimulate the phosphorylation of c-Jun but not v-Jun: regulation by the N-terminal delta domain. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5341–5345. doi: 10.1073/pnas.89.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V., Polotskaya A., Wagner F., Kraft A. S. Affinity-purified c-Jun amino-terminal protein kinase requires serine/threonine phosphorylation for activity. J Biol Chem. 1992 Aug 25;267(24):17001–17005. [PubMed] [Google Scholar]
- Adler V., Unlap T., Kraft A. S. A peptide encoding the c-Jun delta domain inhibits the activity of a c-jun amino-terminal protein kinase. J Biol Chem. 1994 Apr 15;269(15):11186–11191. [PubMed] [Google Scholar]
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Baker S. J., Kerppola T. K., Luk D., Vandenberg M. T., Marshak D. R., Curran T., Abate C. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Mol Cell Biol. 1992 Oct;12(10):4694–4705. doi: 10.1128/mcb.12.10.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner D. W., D'Arcy A., Janes W., Gentz R., Schoenfeld H. J., Broger C., Loetscher H., Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993 May 7;73(3):431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. IL-1 and TNF transmodulate epidermal growth factor receptors by a protein kinase C-independent mechanism. J Immunol. 1989 Jan 1;142(1):126–133. [PubMed] [Google Scholar]
- Bomsztyk K., Toivola B., Emery D. W., Rooney J. W., Dower S. K., Rachie N. A., Sibley C. H. Role of cAMP in interleukin-1-induced kappa light chain gene expression in murine B cell line. J Biol Chem. 1990 Jun 5;265(16):9413–9417. [PubMed] [Google Scholar]
- Brakebusch C., Nophar Y., Kemper O., Engelmann H., Wallach D. Cytoplasmic truncation of the p55 tumour necrosis factor (TNF) receptor abolishes signalling, but not induced shedding of the receptor. EMBO J. 1992 Mar;11(3):943–950. doi: 10.1002/j.1460-2075.1992.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamio L., Clayton L. K., Awad K. M., Reinherz E. L. Negative selection of thymocytes. A novel polymerase chain reaction-based molecular analysis detects requirements for macromolecular synthesis. J Immunol. 1992 Dec 1;149(11):3550–3553. [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler K. A., Mathias S., Kolesnick R. N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992 Mar 27;255(5052):1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Holtmann H., Brakebusch C., Avni Y. S., Sarov I., Nophar Y., Hadas E., Leitner O., Wallach D. Antibodies to a soluble form of a tumor necrosis factor (TNF) receptor have TNF-like activity. J Biol Chem. 1990 Aug 25;265(24):14497–14504. [PubMed] [Google Scholar]
- Espevik T., Brockhaus M., Loetscher H., Nonstad U., Shalaby R. Characterization of binding and biological effects of monoclonal antibodies against a human tumor necrosis factor receptor. J Exp Med. 1990 Feb 1;171(2):415–426. doi: 10.1084/jem.171.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Făgărăsan M. O., Aiello F., Muegge K., Durum S., Axelrod J. Interleukin 1 induces beta-endorphin secretion via Fos and Jun in AtT-20 pituitary cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7871–7874. doi: 10.1073/pnas.87.20.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Aggarwal B. B., Gray P. W., Leung D. W., Nedwin G. E., Palladino M. A., Patton J. S., Pennica D., Shepard H. M., Sugarman B. J. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- Gorospe M., Kumar S., Baglioni C. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993 Mar 25;268(9):6214–6220. [PubMed] [Google Scholar]
- Guesdon F., Freshney N., Waller R. J., Rawlinson L., Saklatvala J. Interleukin 1 and tumor necrosis factor stimulate two novel protein kinases that phosphorylate the heat shock protein hsp27 and beta-casein. J Biol Chem. 1993 Feb 25;268(6):4236–4243. [PubMed] [Google Scholar]
- Guy G. R., Cairns J., Ng S. B., Tan Y. H. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993 Jan 25;268(3):2141–2148. [PubMed] [Google Scholar]
- Guy G. R., Cao X., Chua S. P., Tan Y. H. Okadaic acid mimics multiple changes in early protein phosphorylation and gene expression induced by tumor necrosis factor or interleukin-1. J Biol Chem. 1992 Jan 25;267(3):1846–1852. [PubMed] [Google Scholar]
- Guy G. R., Chua S. P., Wong N. S., Ng S. B., Tan Y. H. Interleukin 1 and tumor necrosis factor activate common multiple protein kinases in human fibroblasts. J Biol Chem. 1991 Aug 5;266(22):14343–14352. [PubMed] [Google Scholar]
- Hanazawa S., Takeshita A., Amano S., Semba T., Nirazuka T., Katoh H., Kitano S. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 May 5;268(13):9526–9532. [PubMed] [Google Scholar]
- Hattori A., Tanaka E., Murase K., Ishida N., Chatani Y., Tsujimoto M., Hayashi K., Kohno M. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. J Biol Chem. 1993 Feb 5;268(4):2577–2582. [PubMed] [Google Scholar]
- Heller R. A., Song K., Fan N., Chang D. J. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992 Jul 10;70(1):47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993 Nov;7(11):2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Uehara Y., Graves L., Rachie N., Bomsztyk K. Herbimycin A blocks IL-1-induced NF-kappa B DNA-binding activity in lymphoid cell lines. FEBS Lett. 1992 Feb 24;298(2-3):240–244. doi: 10.1016/0014-5793(92)80067-q. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S. S., Rangnekar V. V., Sells S. F., Rangnekar V. M. Interleukin-1-inducible expression of gro-beta via NF-kappa B activation is dependent upon tyrosine kinase signaling. J Biol Chem. 1993 Aug 25;268(24):18018–18029. [PubMed] [Google Scholar]
- Kalthoff H., Roeder C., Brockhaus M., Thiele H. G., Schmiegel W. Tumor necrosis factor (TNF) up-regulates the expression of p75 but not p55 TNF receptors, and both receptors mediate, independently of each other, up-regulation of transforming growth factor alpha and epidermal growth factor receptor mRNA. J Biol Chem. 1993 Feb 5;268(4):2762–2766. [PubMed] [Google Scholar]
- Karin M. Signal transduction from cell surface to nucleus in development and disease. FASEB J. 1992 May;6(8):2581–2590. doi: 10.1096/fasebj.6.8.1317309. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Klampfer L., Shows T. B., Vilcek J. Transcriptional regulation of TSG6, a tumor necrosis factor- and interleukin-1-inducible primary response gene coding for a secreted hyaluronan-binding protein. J Biol Chem. 1993 Mar 25;268(9):6154–6160. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Stueber D., Banner D., Mackay F., Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993 Dec 15;268(35):26350–26357. [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S., Younes A., Kan C. C., Orlow I., Joseph C., Kolesnick R. N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993 Jan 22;259(5094):519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- Muegge K., Vila M., Gusella G. L., Musso T., Herrlich P., Stein B., Durum S. K. Interleukin 1 induction of the c-jun promoter. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7054–7058. doi: 10.1073/pnas.90.15.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Ostrowski J., Meier K. E., Stanton T. H., Smith L. L., Bomsztyk K. Interferon-gamma and interleukin-1 alpha induce transient translocation of protein kinase C activity to membranes in a B lymphoid cell line. Evidence for a protein kinase C-independent pathway in lymphokine-induced cytoplasmic alkalinization. J Biol Chem. 1988 Sep 25;263(27):13786–13790. [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Qwarnström E. E., MacFarlane S. A., Page R. C., Dower S. K. Interleukin 1 beta induces rapid phosphorylation and redistribution of talin: a possible mechanism for modulation of fibroblast focal adhesion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1232–1236. doi: 10.1073/pnas.88.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachie N. A., Seger R., Valentine M. A., Ostrowski J., Bomsztyk K. Identification of an inducible 85-kDa nuclear protein kinase. J Biol Chem. 1993 Oct 15;268(29):22143–22149. [PubMed] [Google Scholar]
- Raines M. A., Kolesnick R. N., Golde D. W. Sphingomyelinase and ceramide activate mitogen-activated protein kinase in myeloid HL-60 cells. J Biol Chem. 1993 Jul 15;268(20):14572–14575. [PubMed] [Google Scholar]
- Rangnekar V. V., Waheed S., Rangnekar V. M. Interleukin-1-inducible tumor growth arrest is characterized by activation of cell type-specific "early" gene expression programs. J Biol Chem. 1992 Mar 25;267(9):6240–6248. [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science. 1967 Sep 1;157(3792):1060–1062. doi: 10.1126/science.157.3792.1060. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988 Nov;13(11):443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Büscher M., Angel P., Rahmsdorf H. J., Ponta H., Hattori K., Chiu R., Karin M., Herrlich P. The Fos and Jun/AP-1 proteins are involved in the downregulation of Fos transcription. Oncogene. 1989 May;4(5):629–636. [PubMed] [Google Scholar]
- Seth A., Gonzalez F. A., Gupta S., Raden D. L., Davis R. J. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992 Dec 5;267(34):24796–24804. [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Arakaki N., Tsubouchi H., Takada H., Daikuhara Y. Enhancement of human hepatocyte growth factor production by interleukin-1 alpha and -1 beta and tumor necrosis factor-alpha by fibroblasts in culture. J Biol Chem. 1993 Apr 15;268(11):8140–8145. [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Tumor necrosis factor receptor signaling. A dominant negative mutation suppresses the activation of the 55-kDa tumor necrosis factor receptor. J Biol Chem. 1992 Mar 5;267(7):4304–4307. [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Two TNF receptors. Immunol Today. 1992 May;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Pennica D., Goeddel D. V. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993 Sep 5;268(25):18542–18548. [PubMed] [Google Scholar]
- Tartaglia L. A., Rothe M., Hu Y. F., Goeddel D. V. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993 Apr 23;73(2):213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietor I., Schwenger P., Li W., Schlessinger J., Vilcek J. Tumor necrosis factor-induced activation and increased tyrosine phosphorylation of mitogen-activated protein (MAP) kinase in human fibroblasts. J Biol Chem. 1993 Sep 5;268(25):18994–18999. [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Kampen E., Himmler A., Machleidt T., Krönke M. Human 55-kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Biol Chem. 1992 Sep 5;267(25):17997–18001. [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990 Jul;10(7):3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Yip Y. K., Vilcek J. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6802–6805. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]