Abstract
Malaria remains one of the world’s most devastating diseases, causing over one million deaths every year. The most vulnerable stages of Plasmodium development in the vector mosquito occur in the midgut lumen, making the midgut a prime target for intervention. Mosquito transgenesis and paratransgenesis are two novel strategies that aim at rendering the vector incapable of sustaining Plasmodium development. Mosquito transgenesis involves direct genetic engineering of the mosquito itself for delivery of anti-Plasmodium effector molecules. Conversely, paratransgenesis involves the genetic modification of mosquito symbionts for expression of anti-pathogen effector molecules. Here we consider both genetic manipulation strategies for rendering mosquitoes refractory to Plasmodium infection, and discuss challenges for the translation of laboratory findings to field applications.
Keywords: malaria, transgenesis, paratransgenesis, mosquitoes, effector molecules
Malaria control: present and future
Malaria is a major cause of global morbidity and mortality. Close to half of the world’s population is at risk, about 300–500 million contract the disease annually and more than one million people die of malaria every year [1]. Clearly, the available means to fight the disease are insufficient. Unlike the two other major infectious diseases - AIDS and tuberculosis – Plasmodium, the causative agent of malaria, is absolutely dependent on completing a complex cycle in the vector Anopheles mosquito for transmission to occur [2]. Thus, eliminating the mosquito or interfering with its ability to support the parasite cycle will arrest malaria transmission. Current malaria control measures targeting the mosquito vector with insecticides have helped alleviate the malaria burden in many endemic areas [3]. However, the emergence and rapid spread of insecticide-resistant mosquitoes and of drug-resistant Plasmodium parasites combined with the lack of an effective vaccine severely undermine current control efforts [4]. Another rarely considered but equally important limitation is that insecticides leave intact the biological niche where mosquitoes reproduce. The mosquito populations that decline after insecticide use quickly rebound to pre-treatment levels when insecticide treatment stops or becomes ineffective.
Recently, the malERA consultative group stressed that malaria eradication cannot be achieved without introduction of novel control tools [5]. Transgenesis and paratransgenesis are two novel approaches for rendering mosquitoes refractory to Plasmodium infection. Here, we review recent advances on genetic approaches for interfering with the malaria parasite cycle in vector mosquitoes and consider the challenges in the translation of laboratory findings to field applications.
The malaria parasite cycle in the mosquito
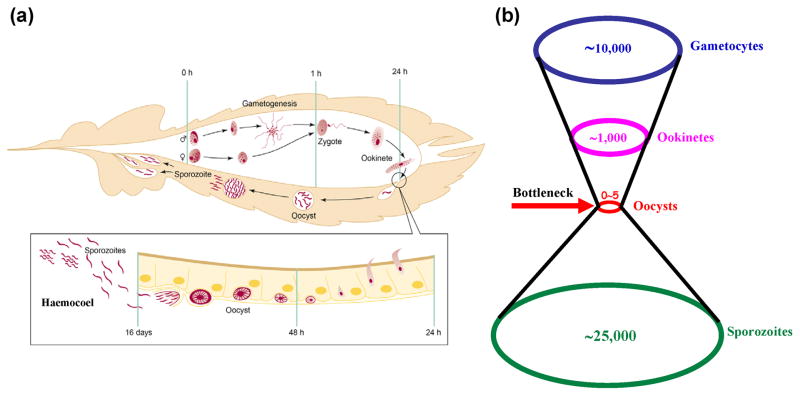
Plasmodium development in the mosquito is complex and involves the completion of multiple developmental steps in the midgut (gametogenesis, fertilization, followed by zygote, ookinete and oocyst formation) and the crossing of two mosquito epithelia (midgut and salivary gland) (Figure 1a). While thousands of gametocytes are ingested when a female mosquito feeds on an infected individual, only about 10% successfully develop into ookinetes and about five of these succeed in invading the midgut epithelium to form sessile oocysts [6]. This is followed by a dramatic amplification of parasite numbers, when each oocyst releases thousands of sporozoites into the hemocoel after which they invade the mosquito salivary glands. The parasite is transmitted to the next individual through the bite of an infected mosquito [7]. The severe bottleneck in the mosquito gut (Figure 1b) makes this compartment a prime target for interfering with the parasite cycle in its vector [8, 9].
Figure 1. The malaria parasite cycle in the mosquito vector.
(a) Life cycle of Plasmodium in the mosquito. The approximate developmental time at which each stage occurs in Plasmodium berghei (maintained at 20°C) is indicated. Transmission starts when the mosquito ingests an infected bloodmeal (0 h). Within minutes, gametocytes develop into gametes (the star-shaped figure illustrates exflagellation, which is the formation of male gametes) that fuse to form the zygote that differentiates into a motile ookinete. At 24 h, the ookinete invades the midgut epithelium and differentiates into an oocyst. About 2 weeks later, the oocyst ruptures, releasing thousands of sporozoites into the mosquito body cavity (hemocoel). Of all the tissues that sporozoites come in contact with, they can invade only the salivary gland. When the mosquito bites another vertebrate host, transmission is completed by release of sporozoites from the salivary glands (not shown). Reproduced with permission from Ref. [92]. (b) Plasmodium parasite numbers undergo a severe bottleneck during its development in the mosquito gut.
Genetic manipulation of mosquito vectorial competence
Since the mosquito is essential for parasite transmission, hindering the mosquito’s ability to support parasite development will reduce or eliminate malaria transmission. One option to interfere with parasite transmission is to genetically modify the mosquito for midgut expression of “effector genes” whose products inhibit parasite development. This proof of concept was tested for the first time by genetically engineering Anopheles stephensi for midgut expression of the SM1 peptide. This peptide binds to a putative ookinete receptor on the luminal surface of the midgut epithelium and strongly inhibits ookinete midgut invasion [10]. The genetically modified mosquitoes were substantially impaired in their ability to transmit the parasite [11]. Subsequent reports from different laboratories making use of a variety of effector molecules reached similar conclusions [12–17]. Collectively, these studies constituted proof-of-concept that it is possible to reduce Plasmodium transmission via genetic modification of the vector mosquito. One crucial challenge for translating these findings to the field is to devise effective means to drive anti-malaria effector genes into wild mosquito populations. It is not sufficient to simply release large numbers of transgenic mosquitoes. An effective drive mechanism must be devised to give mosquitoes carrying effector genes a competitive advantage. Of several potential approaches that have been proposed, including the use of transposable elements or Wolbachia, two – MEDEA (maternal-effect dominant embryonic arrest) and HEG (homing endonuclease gene) – are particularly promising. The MEDEA drive system has shown promisein experiments using the Drosophila model system [18]. The approach is based on linking the effector gene to a maternal gene that is required for embryonic development and is inactivated in the ovary, and a second gene that rescues the defect by embryonic expression of the same gene. The technologies for transferring this approach to mosquitoes (e.g., identification of mosquito maternal effect genes and of mosquito embryonic promoters for the rescue constructs) have not yet been developed. The Drosophila experiments indicated that a high initial introduction rate (~25%) was required which may constitute a limiting factor for field applications. More recently, another promising approach is being explored that makes use of HEG drive system [19–20]. This study showed that in cage experiments, the homing endonuclease gene I-SceI can rapidly spread among the transgenic An. gambiae progeny. An additional consideration for any gene drive approach is that there are on the order of 430 anopheline species, about 30–40 of which are natural vectors for human malaria [21] and very few of them have been shown to be amenable to genetic manipulation [22]. Moreover, anopheline vectors frequently exist as reproductively isolated populations (cryptic species) [23], thus preventing gene flow from one population to another. In addition, fitness load imposed by refractory gene(s), insertional mutagenesis and positional effects will have to be considered [15, 24, 25]. Once these issues are resolved, transgenesis could provide a powerful tool to combat malaria.
Anti-malarial effector genes
The identification of efficient anti-Plasmodium effector genes is an essential prerequisite for the generation of a refractory mosquito. Ideally, the effector molecules should interfere with parasite transmission without imposing a fitness cost to the mosquito. Based on their mode of action, the existing anti-Plasmodium effector molecules can be grouped into four classes (Table 2).
Table 2.
Anti-Plasmodium effector molecules
| Effector | Properties | Target parasite | Parasite stage(s) | Function or mechanism | Refs |
|---|---|---|---|---|---|
| Parasite killing | |||||
| Scorpine | Scorpion Pandinus imperator venom peptide |
P. falciparum P. berghei |
Gametocyte to Ookinete | Cecropin and defensin-like lytic peptide. | [29] |
| Shiva1 | Cecropin-like synthetic peptide |
P. berghei P. falciparum |
Gametocyte to Ookinete | Lyses parasites | [93] |
| Shiva-3 | Cecropin-like synthetic peptide |
P. falciparum P. berghei |
Gametocyte to ookinete | Lyses parasites | [33] |
| Cec A | An. gambiae cecropin A | P. berghei | Ookinete | Lyse parasites. | [28] |
| Cec B | An. gambiae cecropin B | P. falciparum | Oocyst | Lyses parasites | [32] |
| DEF1 A | An. gambiae defensin A |
P. falciparum P. berghei |
Ookinete | Lyses parasites | [26] |
| Gambicin | An. gambiae antimicrobial peptide |
P. falciparum P. berghei |
Ookinete | Lyses parasites | [27] |
| Angiotensin II | P. gallinaceum | Sporozoite | Lyses parasites | [31] | |
| Magainins | Peptides from the African clawed frog Xenopus laevis skin |
P. falciparum P. knowlesi P. cynomolgi |
All mosquito stages | Lyses parasites | [32] |
| Gomesin | A antimicrobial peptide from Spider |
P. falciparum P. berghei |
All mosquito stages | Lyses parasites | [34] |
| CEL-III | Hemolytic C-Type Lectin |
P. falciparum P. berghei |
Ookinete, oocyst | Lyses parasites | [13] |
| TP10 | Wasp venom peptide | P. falciparum | Gametocyte to ookinete | Lyses the parasites. | [94] |
| AdDLP | Anaeromyxobacter dehalogenans defensin-like peptide | P. berghei | Ookinete | Lyses the parasites | [95] |
| Meucin-25 | Scorpion Mesobuthus eupeus venom gland |
P.. berghei P. falciparum |
Gametocyte | Anti-microbial linear cationic peptide | [96] |
| Drosomycin | An inducible antifungal peptide initially isolated from the Drosophila melanogaster haemolymph | P. berghei | Ookinete | Lyses the parasites | [97] |
| Interaction with parasites | |||||
| EPIP | Enolase-Plasminogen Interaction Peptide |
P. falciparum P. berghei |
Ookinete | Inhibits mosquito midgut invasion by preventing plasminogen binding to the ookinete surface | [35] |
| Pro:EPIP | A fusion peptide composed of a chitinase propeptide (Pro) and EPIP |
P. falciparum P. berghei |
Ookinete | Blocks ookinete traversal of the mosquito peritrophic matrix and prevents plasminogen binding to the ookinete surface | [30] |
| Pbs21scFv-Shiva1 | Single-chain immunotoxin |
P. falciparum P. berghei |
Gametocyte to Oocyst | A single-chain monoclonal antibody (scFv) targeting the major ookinete surface protein pbs21 and linked to the lytic peptide Shiva1. | [37] |
| scFv 4B7 | A single-chain antibody | P. falciparum | Ookinete | Binds to P. falciparum ookinete surface protein Pfs25 | [16, 36] |
| scFv 2A10 | Single-chain antibody | P. falciparum | Ookinete | Targets the P. falciparum circumsporozoite protein (CSP) | [16, 36] |
| PfNPNA-1 | Single-chain antibody | P. falciparum | Sporozoite | Recognizes the repeat region (Asn-Pro-Asn-Ala) of the P. falciparum surface circumsporozoite protein | [50] |
| scFv 1C3 | Single-chain antibody | P. falciparum | Ookinete | Binds a P. falciparum secreted enzyme chitinase 1 | [16] |
| Interaction with mosquito midgut or salivary gland epithelia | |||||
| SM1 | Salivary gland and midgut peptide 1 |
P. berghei P. falciparum |
Ookinete, sporozoite | Blocks ookinete invasion of the midgut epithelium or sporozoite invasion of the salivary gland epithelium. | [10] |
| mPLA2 | Bee venom phospholipase |
P. falciparum P. berghei |
Ookinete | Inhibits ookinete midgut invasion, probably by modifying the properties of the midgut epithelial membrane | [12] |
| Pro | A chitinase propeptide |
P. falciparum P. berghei |
Ookinete | Inhibits the enzyme and blocks ookinete traversal of the mosquito peritrophic matrix | [39] |
| Pchtscfv | Single-chain antibody | P. falciparum | Ookinete | Inhibits the P. falciparum chitinase and blocks ookinete traversal of the mosquito peritrophic matrix | [98] |
| Manipulation of mosquito immune system | |||||
| Akt | A protein kinase |
P. falciparum P. berghei |
Ookinete | Akt boosts mosquito innate insulin immunity via signaling | [15] |
| Rel2 | Anopheles IMD pathway transcription factor |
P. falciparum P. berghei |
Ookinete, sporozoite | Rel2 overexpression enhances mosquito IMD pathway | [14] |
Parasite killing. This class includes peptides from the insect’s innate immune system such as defensins [26], gambicin [27] and cecropins [28], and peptides from other sources that lyse parasites but do not affect the host insect, such as scorpine, a scorpion anti-malaria lytic peptide that has hybrid properties of the lytic peptides cecropin and defensin [29, 30], Hemolytic C-Type Lectin CEL-III [13], angiotensin II [31], magainins [32], synthetic anti-parasitic lytic peptides Shiva-1 and Shiva-3 [33] and gomesin [34].
Interaction with parasites: EPIP, a Plasmodium Enolase-Plasminogen Interaction Peptide, is a peptide that inhibits mosquito midgut invasion by preventing plasminogen binding to the ookinete surface [35]. Other agents are single-chain monoclonal antibodies (scFvs) that bind to ookinete or sporozoite surface or secreted proteins. For instance, scFv 4B7 binds to P. falciparum ookinete surface protein Pfs25, 2A10 targets the P. falciparum circumsporozoite protein (CSP) [16, 36], anti-Pbs21 single chain antibody targets the P. berghei major ookinete surface protein Pbs21 [37], and scFv 1C3 binds a P. falciparum secreted enzyme chitinase 1 [16].
Interaction with mosquito midgut or salivary gland epithelia: Examples of this class are SM1 – a 12-amino acid Salivary gland and Midgut peptide 1, which binds to putative receptors on the luminal surface of the mosquito midgut and basal surface of the salivary gland epithelia, blocking ookinete and sporozoite invasion [10]; mPLA2 is a mutant phospholipase A2 that inhibits ookinete invasion, possibly by modifying the properties of the midgut epithelial membrane [12, 30, 38]; and a chitinase propeptide that inhibits this enzyme and in this way hinders ookinete traversal of the mosquito peritrophic matrix (PM) [39]. The PM is a chitin-based extracellular structure that surrounds the entire blood meal and must be crossed by the ookinete for reaching the mosquito midgut [40].
Manipulation of mosquito immune system. Several laboratories have shown that alteration of mosquito immune-related genes can lead to decreased mosquito vectorial competence. Blood meal-induced expression of Akt, a key signaling component in the insulin signaling pathway renders the mosquito refractory to Plasmodium infection [15]. Overexpression of IMD pathway-mediated transcription factor Rel2 renders the mosquito resistant to Plasmodium infection [14]. Manipulation of mosquito immune pathway using RNA interference or ‘smart sprays’ enhances mosquito anti-microbial response [41, 42].
We note that the identification of efficient effector molecules is as valuable to transgenesis as to paratransgenesis. A potential issue regarding anti-malaria effector molecules is that in the long run, parasites may develop resistance to their action in similar ways that they develop resistance to drugs that kill them in the human blood. For this reason, it will be important to combine multiple anti-Plasmodium effector proteins with different modes of action. In addition, the use of multiple effector genes will also maximize the effectiveness of interference with parasite development (additive or synergistic effects).
Genetic manipulation of mosquito pathogenic fungi and viruses
Insect fungal pathogens, Metarhizium robertsii and Beauveria bassiana are natural killer of insects including mosquitoes [43]. Several studies highlight the promising use of insect fungal pathogens for controlling adult malaria mosquitoes and reducing malaria transmission rates [44–49]. Recently, M. robertsii was genetically engineered to deliver anti-Plasmodium peptides or proteins into the mosquito hemocoel for killing sporozoites or preventing sporozoite invasion of mosquito salivary glands. The transgenic fungi significantly reduced sporozoite density in salivary glands [50], indicating that genetic modification of pathogenic fungi provides an enhanced tool to reduce malaria transmission.
Linear single-stranded DNA densoviruses were also found to infect several important vector mosquitoes (Aedes aegypti [51], An. gambiae [52], and Culex pipiens [53]), and to be able to be vertically transmitted [52, 54]. The densovirus AgDNV was found to efficiently infect An. gambiae larvae and to spread to the adult midgut, fat body and ovaries [52]. This virus was also found to be vertically transmitted to subsequent mosquito generations [52]. These properties suggest that densoviruses could be used to produce effector molecules in host mosquitoes [54]. However, the limited length of foreign DNA that these viruses can carry may become a limiting factor.
Mosquito symbionts and other associated organisms
Microbial associations with insects are ubiquitous and play an important role in shaping many aspects of insect digestive physiology, ecological adaptation and evolution. The gut microbiota is thought to be beneficial to the mosquito by providing nutritional supplements, tolerance to environmental perturbations and manipulation of host immune homeostasis [55]. Recently, the association between symbionts and their hosts has attracted increased attention from the perspective of their engineering to combat pathogens [56–60]. Many bacterial species have been identified from the midgut of field-collected anophelines, mostly Gram-negative proteobacteria and enterobacteria [61, 62]. The bacterial population structure in laboratory-reared adult mosquitoes was found to be similar to that of wild mosquitoes, suggesting that anopheline mosquitoes harbor their microbiome in a selective way [62]. A non-pathogenic bacterium, Pantoea agglomerans, was reported to be a dominant symbiotic bacterium in different mosquito species in Kenya and Mali [61], and also found in laboratory-reared An. stephensi, An. gambiae and An. albimanus mosquitoes [60, 63]. This bacterium is normally found on plant surfaces and blossoms [64–66], suggesting that flower nectar is a possible source of the mosquito microbiota in the field. Also, this property could potentially facilitate re-introduction of genetically-modified P. agglomerans into field mosquito populations.
The bacterial population in the mosquito gut increases by hundreds to thousands of times within 24 hours after a bloodmeal [30, 63]. The rapid proliferation of gut microbiota may stimulate mosquito immune responses that limit the infection by malaria parasites [67, 68]. Reduction of the gut microbiota with antibiotics renders the mosquito more susceptible to Plasmodium infection. Conversely, co-infection of bacteria with Plasmodium gametocytes reduces the oocyst load in the mosquito midgut [67]. Recently, an Enterobacter bacterium strain Esp_Z, isolated from wild An. arabiensis mosquitoes in Zambia was found to significantly inhibit P. falciparum infection after co -feeding An. gambiae with bacteria and infectious blood. Reactive oxygen species (ROS) produced by this bacterium seems to mediate parasite killing in the midgut lumen prior to mosquito midgut invasion [69]. A recent study revealed a positive correlation between the abundance of commensal Enterobacteriaceae and Plasmodium infection in An. gambiae mosquito midgut, and suggested that Enterobacteriaceae might play a positive protective role in the natural infection of P. falciparum [70].
The acetic acid bacterium Asaia sp. was identified as a stable symbiont in laboratory An. stephensi colonies and in wild An. gambiae populations. Asaia sp. was also observed in several mosquito organs, including salivary glands and ovaries [58]. Importantly, Asaia sp. appears to be vertically transmitted from female to larval progeny, venereally transmitted from male to female during mating, and transstadially transmitted from larva to adult [58, 71, 72]. These features favor dissemination and should be helpful when considering potential introduction of genetically-modified bacteria into mosquito populations in the field.
The intracellular endosymbiotic bacterium Wolbachia can infect a large number of insects and other arthropod species, and may play key roles in modulating pathogen infection and transmission in several insect species [73, 74]. Recent studies showed that Wolbachia infection reduces pathogen levels in multiple mosquito species [75–77]. Somatic Wolbachia infections of Anopheles can also significantly inhibit Plasmodium oocyst formation through activation of the mosquito immune system [78]. However, Wolbachia-infected anopheline mosquitoes have not been found in nature and stable mosquito infections have not yet been reported [79].
Several yeasts such as Candida, Pichia, Wickerhamomyces anomalus were found in the guts of Anopheles mosquitoes [80–82]. Moreover, W. anomalus was also identified in the reproductive organs of malaria vectors An. gambiae and An. stephensi [81, 82], raising the possibility of use of symbiotic yeasts for delivery of anti-malaria effector proteins to anopheline vectors.
Paratransgenesis
Paratransgenesis refers to the genetic engineering of a microorganism associated with its insect host, as opposed to genetic modification of the insect itself. At the heart of the paratransgenesis strategy for malaria control is the fact that the mosquito microbiota and Plasmodium share the same compartment – the midgut – where the most vulnerable stage of the parasite development occurs (Figure 1b). These considerations suggest an alternate approach to interfere with malaria transmission by genetically engineering midgut symbiotic microorganisms to deliver anti-Plasmodium effector molecules [83]. Paratransgenesis has a number of attractive features [84]. i) As for most higher organisms, the mosquito carries a significant microbiota in its midgut; ii)A severe bottleneck of Plasmodium development occurs in the mosquito midgut lumen, making this compartment a prime target for intervention [8, 9]; iii) The developing parasite and the microbiota share the same midgut compartment, directly exposing parasites to molecules produced by engineered symbiotic bacteria; iv) the midgut bacterial population increases dramatically, by 100-to 1000 -fold, after ingestion of a blood meal [7], correspondingly increasing the output of effector molecules produced by recombinant bacteria (Figure 2 a–b). The basic requirements for a paratransgenesis approach are listed in Table 1.
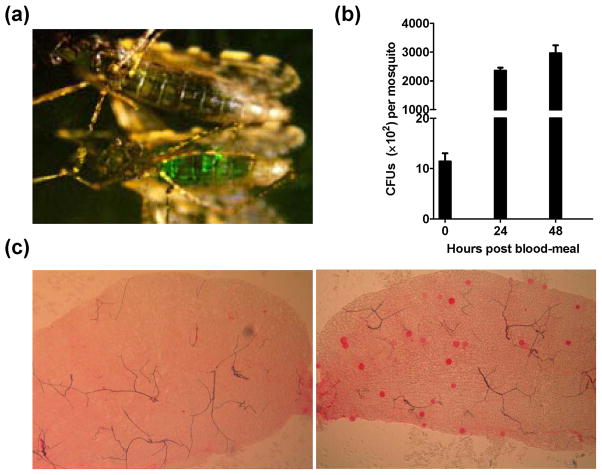
Figure 2. Engineered Pantoea agglomerans efficiently inhibits Plasmodium falciparum development in mosquitoes.
(a) Visualization of GFP -tagged P. agglomeransin the mosquito midgut 24 h after a blood meal. GFP-expressing P. agglomerans were administered to 2 day-old Anopheles gambiae via a sugar meal. The upper mosquito fed on wild-type bacteria, and the lower mosquito fed GFP-tagged bacteria. (b) P. agglomerans rapidly proliferate in the midgut after a blood meal. The number of fluorescent bacteria colony-forming units (CFUs) was determined at each of the indicated times by plating serially diluted midgut homogenates on LB/kanamycin agar plates. (c) Inhibition of P. falciparum development in An. gambiae by recombinant P. agglomerans engineered to secrete scorpine, a potent antiplasmodial peptide. Wild type P. agglomerans was fed to one group of An. gambiae mosquitoes via a sugar meal while P. agglomerans engineered to secrete scorpine was fed to the other group of mosquitoes. After 32 h both groups of mosquitoes were fed on the same P. falciparum-infected blood meal. Midguts were dissected 8 d post infection and oocyst number per midgut was determined after staining with 0.1% (wt/vol) mercurochrome. Left panel: a midgut from a mosquito carrying scorpine-secreting P. agglomerans; Right panel: a midgut from a control mosquito carrying an equal number of wild type P. agglomerans. Note the strong reduction in oocyst numbers in the midgut from the mosquito fed scorpine-secreting bacteria.
Table 1.
The basic requirements for paratransgenesis
| i | Stable symbiotic relationship between microorganism and vector |
| ii | Symbiotic microorganism can be cultured in vitro and genetically manipulated |
| iii | Effector gene product should not impair symbiont and vector fitness |
| iv | Effector gene product should be secreted to assure interaction with the target pathogen |
| v | An efficient means of introducing the engineered symbiont into field must be devised |
Paratransgenesis has already been proposed as a feasible means to control other insect borne diseases. The parasitic protozoan Trypanosoma cruzi, the causative agent of Chagas disease, is transmitted by the triatomid bug Rhodnius prolixus [56]. The Rhodnius obligate gram-positive bacterium Rhodococcus rhodnii was genetically engineered to produce the antimicrobial peptide cecropin A, and fed to naïve R. prolixus nymphs. Durvasula and collaborators [56] showed that expression of the anti -parasite peptide by the genetically modified symbionts significantly reduces T. cruzi’s ability to survive in the bug.
Fighting malaria with paratransgenesis
Early reports on the use of paratransgenesis to fight malaria were based on the recombinant laboratory bacterium E. coli expressing a single-chain immunotoxin [85]. In another study, a dimer of the SM1 peptide or a modified phospholipase A2 [60] was used, resulting in a significant decrease of P. berghei oocyst numbersin An. stephensi. However, inhibition of parasite development was modest for two main reasons: i) the E. coli bacterium used for these studies were attenuated laboratory strains that survived poorly in the mosquito midgut, and ii) the recombinant anti-Plasmodium effector proteins either formed insoluble inclusion bodies [37] or were attached to the bacteria surface [60]. In either case, the effector molecules could not diffuse to their intended parasite or mosquito midgut targets.
Some of these shortcomings were addressed in recent studies with P. agglomerans, a bacterium commonly found in field vector mosquitoes in Africa [61] as well as in laboratory-reared An. stephensi, An. gambiae and An. albimanus [60, 63]. P. agglomerans easily grows in culture and can be engineered to secrete anti-Plasmodium proteins using the HlyA system [30, 86]. The engineered bacteria were tested for their ability to thwart Plasmodium development in the mosquito as follows. Recombinant bacteria were fed to mosquitoes via cotton balls soaked with a bacteria suspension in sugar solution. One day later, these mosquitoes were fed on a Plasmodium-infected blood meal. Control mosquitoes were fed bacteria transformed with the HlyA parental plasmid not fused to an effector protein. One or two weeks after the infectious blood meal, success of parasite development was measured by counting the number of oocysts per gut (only ookinetes that successfully cross the mosquito midgut epithelium can form oocysts). These experiments showed that recombinant bacteria secreting anti-malaria effector proteins strongly inhibit Plasmodium development in mosquitoes, as compared with mosquitoes fed control bacteria (Figure 2c). Inhibition varied from 85% for mPLA2 to 98% for scorpine and (EPIP)4 without any detectable fitness cost to the transgenic bacteria [87] and or to the host mosquitoes [30]. Considering that a mosquito that produces one oocyst is as infective to a human host as a mosquito producing a large number of oocysts, a more important measure of transmission blocking potential is to compare the number of infected mosquitoes carrying one or more oocysts (infected mosquitoes) with the number of non-infected mosquitoes. In the experiments described above, the percentage of infected mosquitoes dropped from 90% in controls to 14~18% in mosquitoes carrying scorpine- or (EPIP)4-expressing bacteria [30]. This strong reduction in the proportion of infected mosquitoes should translate into important reduction of transmission in the field. Moreover, the use of multiple effector molecules, each acting by a different mechanism, should greatly reduce the probability of selecting resistant parasites. The inhibition of parasite development was equivalent when using an African mosquito (An. gambiae) and an Asian mosquito (An. stephensi). Also, inhibition of P. berghei (a rodent parasite) and P. falciparum (a human parasite) was equivalent, suggesting that this approach may also work for other human parasites, such as P. vivax. Thus, the paratransgenesis strategy may well turn out to be “universal”, being effective for multiple mosquito and parasite species. These promising laboratory results will next need to be translated to field applications. A major outstanding issue is how to efficiently introduce the engineered bacteria into wild mosquito populations. While some approaches have been proposed (Box 1), none have as yet been experimentally verified.
Box 1. Genetic manipulation of mosquito vectorial competence via transgenesis and paratransgenesis.
Mosquito transgenesis has the advantage of having no off-target effects as transgene expression is restricted to the engineered mosquito. The anti-Plasmodium effector genes can be engineered to express in specific tissues (midgut, fat body and salivary glands), only in females and in a blood-induced manner. While it has been shown that a mostly refractory mosquito can be produced in the laboratory, challenges remain for translating these findings to field applications. A method to drive effector genes into mosquitoes in the field still needs to be devised. The MEDEA [18] and homing nuclease (HEG) [19] approaches are among the most promising ones. Additional issues that need to be considered are the multiplicity of anopheline vector species (each needs to be separately engineered), the reproductive barriers within a given species (cryptic species), mass production and sex selection of transgenic mosquitoes (females cannot be mass-released in the field), the large size of the constructs expressing multiple effector genes and the possible loss of transgene expression over time [99].
Paratransgenesis refers to an alternative approach for delivery of effector molecules via the genetic modification of mosquito symbionts. Advantages of paratransgenesis are the simplicity of genetic modification of bacteria, the ease of growing the genetically modified bacteria in large scale, the fact that it by passes genetic barriers of reproductively isolated mosquito populations and effectiveness does not appear to be in fluenced by mosquito species. However, many challenges lay ahead. A major challenge is to devise effective means to introduce engineered bacteria into field mosquito populations. This may be accomplished by placing around villages, bating stations (cotton balls soaked with sugar and bacteria placed in clay jar refuges) [100] using engineered symbiotic bacteria that are vertically and horizontally transmitted among mosquito populations [58]. However, no experimental evidence for the effectiveness of such approach is presently available. Moreover, for future use in the field, the effector genes need to be integrated into the bacterial genome to avoid gene loss and also to minimize the risk of horizontal transgene transfer.
For both the transgenic and the paratrasngenic approaches, a major challenge for ultimate implementation will be to obtain the required approval from regulatory agencies and from the local population.
Perspectives
Current insecticide-based vector control strategies such as insecticide-impregnated bed nets, as well as other population-suppression strategies (e.g., Sterile-Insect Technique or SIT [88, 89], RIDL (Release of Insects carrying a Dominant Lethal) [90] have the disadvantage that they create an “empty ecological niche”. This is because the use of these approaches leaves the environment where mosquitoes thrive unchanged and consequently mosquito populations revert to original density as soon as treatments end or when mosquitoes become resistant to the insecticide. In other words, any population-suppression strategy needs to be implemented forever.
Transgenesis and paratransgenesis are two novel promising means for interfering with Plasmodium development or infection of the vector mosquito through delivery of anti-Plasmodium effector molecules within the mosquito. Both are “population replacement” strategies that once implemented, should require much less follow-up effort than population-suppression strategies. The main properties of transgenesis and paratransgenesis are shown in Box 1. While many technical aspects have been successfully addressed, several major issues need to be resolved before transgenesis and paratransgenesis can be implemented in the field. One key issue for both approaches is to devise means to effectively drive transgene or the engineered bacteria into mosquito populations in the field. Other major topics that need to be addressed are the resolution of regulatory, ethical and the public acceptance issues relating to the release of genetically modified (GM)organisms in nature. While the GM subject is controversial, its resolution will ultimately rely on weighing risks against benefits. As these issues are considered, the benefit of saving lives should provide strong argument in its favor.
Transgenesis or paratransgenesis is not a cure-all solution for malaria control. Rather, both are envisioned as a complement to existing and future control measures. In this regard, transgenesis and paratransgenesis are compatible with each other (possibly additive) and with insecticides and population suppression approaches. Moreover, the diversity of effector proteins [91] make both approaches not unique to malaria but might also be extended to the control of other major mosquito-borne diseases, such as dengue and yellow fever.
Highlights.
Mosquito gut is a prime target for interfering with Plasmodium cycle in its vector.
Genetic approaches and challenges to block malaria transmission are considered.
Transgenic mosquitoes are engineered to produce anti-Plasmodium molecules.
Paratransgenesis uses engineered symbionts to deliver anti-Plasmodium molecules.
Both approaches hold promise but field implementation issues remain to be resolved.
Acknowledgments
Work in our laboratory was supported by a grant from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A, et al. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood BM, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trape JF, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 5.The malERA Consultative Group on Basic Science and Enabling Technologies. A research agenda for malaria eradication: basic science and enabling technologies. Plos Med. 2011;8:e1000399. doi: 10.1371/journal.pmed.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor LH. Infection rates in, and the number of Plasmodium falciparum genotypes carried by Anopheles mosquitoes in Tanzania. Ann Trop Med Parasitol. 1999;93:659–662. doi: 10.1080/00034989958168. [DOI] [PubMed] [Google Scholar]
- 7.Whitten MMA, et al. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28:121–130. doi: 10.1111/j.1365-3024.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- 8.Abraham EG, Jacobs-Lorena M. Mosquito midgut barriers to malaria parasite development. Insect Biochem Molec. 2004;34:667–671. doi: 10.1016/j.ibmb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Drexler AL, et al. Plasmodium development in the mosquito: biology bottlenecks and opportunities for mathematical modeling. Trends Parasitol. 2008;24:333–336. doi: 10.1016/j.pt.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh AK, et al. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. P Natl Acad Sci USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito J, et al. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 12.Moreira LA, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, et al. Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog. 2007;3:e192. doi: 10.1371/journal.ppat.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y, et al. Engineered anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7:e1002458. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corby-Harris V, et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs AT, et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. Plos Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham EG, et al. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol Biol. 2005;14:271–279. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 19.Windbichler N, et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212–215. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deredec A, et al. Requirements for effective malaria control with homing endonuclease genes. Proc Natl Acad, Sci, USA. 2011;108:E874–880. doi: 10.1073/pnas.1110717108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National center for infection disease (NCID) Mosquitoes. Division of parasitic disease; Atlanta: 2004. p. 6. [Google Scholar]
- 22.Coutinho-Abreu IV, et al. Transgenesis and paratransgenesis to control insect-borne diseases: current status and future challenges. Parasitol Int. 2010;59:1–8. doi: 10.1016/j.parint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell JR, et al. Population structure, speciation, and introgression in the Anopheles gambiae complex. Parassitologia. 1999;41:101–113. [PubMed] [Google Scholar]
- 24.Catteruccia F, et al. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- 25.Li C, et al. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J Hered. 2008;99:275–282. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokoza V, et al. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad, Sci, USA. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vizioli J, et al. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad, Sci, USA. 2001;98:12630–12635. doi: 10.1073/pnas.221466798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim W, et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J Med Entomol. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- 29.Conde R, et al. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000;471:165–168. doi: 10.1016/s0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, et al. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad, Sci, USA. 2012;109:12734–12739. doi: 10.1073/pnas.1204158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maciel C, et al. Anti-plasmodium activity of angiotensin II and related synthetic peptides. PLoS One. 2008;3:e3296. doi: 10.1371/journal.pone.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwadz RW, et al. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect Immun. 1989;57:2628–2633. doi: 10.1128/iai.57.9.2628-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Possani LD, et al. From noxiustoxin to Shiva-3, a peptide toxic to the sporogonic development of Plasmodium berghei. Toxicon. 1998;36:1683–1692. doi: 10.1016/s0041-0101(98)00161-5. [DOI] [PubMed] [Google Scholar]
- 34.Moreira CK, et al. Effect of the antimicrobial peptide gomesin against different life stages of Plasmodium spp. Exp Parasitol. 2007;116:346–353. doi: 10.1016/j.exppara.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh AK, et al. Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc Natl Acad, Sci, USA. 2011;108:17153–17158. doi: 10.1073/pnas.1103657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lara Capurro M, et al. Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am J Trop Med Hyg. 2000;62:427–433. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida S, et al. Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes. Mol Biochem Parasitol. 2001;113:89–96. doi: 10.1016/s0166-6851(00)00387-x. [DOI] [PubMed] [Google Scholar]
- 38.Zieler H, et al. A snake venom phospholipase A(2) blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J Exp Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]
- 39.Bhatnagar RK, et al. Synthetic propeptide inhibits mosquito midgut chitinase and blocks sporogonic development of malaria parasite. Biochem Biophys Res Commun. 2003;304:783–787. doi: 10.1016/s0006-291x(03)00682-x. [DOI] [PubMed] [Google Scholar]
- 40.Shao L, et al. The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- 41.Christophides GK, et al. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown AE, et al. Stable and heritable gene silencing in the malaria vector Anopheles stephensi. Nucleic Acids Res. 2003;31:e85. doi: 10.1093/nar/gng085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, et al. Local adaptation of an introduced transgenic insect fungal pathogen due to new beneficial mutations. Proc Natl Acad, Sci, USA. 2011;108:20449–20454. doi: 10.1073/pnas.1113824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farenhorst M, et al. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc Natl Acad, Sci, USA. 2009;106:17443–17447. doi: 10.1073/pnas.0908530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mnyone LL, et al. First report of Metarhizium anisopliae IP 46 pathogenicity in adult Anopheles gambiae ss and An arabiensis (Diptera; Culicidae) Parasit Vectors. 2009;2:59. doi: 10.1186/1756-3305-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholte EJ, et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- 47.Blanford S, et al. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- 48.Hancock PA, et al. An age-structured model to evaluate the potential of novel malaria-control interventions: a case study of fungal biopesticide sprays. Proc Biol Sci. 2009;276:71–80. doi: 10.1098/rspb.2008.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas MB, Read AF. Can fungal biopesticides control malaria? Nat Rev Microbiol. 2007;5:377–383. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- 50.Fang WG, et al. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331:1074–1077. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward TW, et al. Aedes aegypti transducing densovirus pathogenesis and expression in Aedes aegypti and Anopheles gambiae larvae. Insect Mol Biol. 2001;10:397–405. doi: 10.1046/j.0962-1075.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 52.Ren X, et al. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008;4:e1000135. doi: 10.1371/journal.ppat.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma M, et al. Discovery of DNA viruses in wild-caught mosquitoes using small RNA high throughput sequencing. PLoS One. 2011;6:e24758. doi: 10.1371/journal.pone.0024758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson J, et al. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res. 2006;68:361–392. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- 55.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durvasula RV, et al. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad, Sci, USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang TL, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad, Sci, USA. 2003;100:11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Favia G, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad, Sci, USA. 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao S, et al. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad, Sci, USA. 2005;102:11993–11998. doi: 10.1073/pnas.0504881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riehle MA, et al. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol. 2007;37:595–603. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Straif SC, et al. Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J Med Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, et al. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pumpuni CB, et al. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- 64.Andrews JH, Harris RF. The ecology and biogeography of microorganisms on plant surfaces. Annu Rev Phytopathol. 2000;38:145–180. doi: 10.1146/annurev.phyto.38.1.145. [DOI] [PubMed] [Google Scholar]
- 65.Pusey PL. Biological control agents for fire blight of apple compared under conditions limiting natural dispersal. Plant Dis. 2002;86:639–644. doi: 10.1094/PDIS.2002.86.6.639. [DOI] [PubMed] [Google Scholar]
- 66.Pusey PL. Crab apple blossoms as a model for research on biological control of fire blight. Phytopathology. 1997;87:1096–1102. doi: 10.1094/PHYTO.1997.87.11.1096. [DOI] [PubMed] [Google Scholar]
- 67.Dong Y, et al. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meister S, et al. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad, Sci, USA. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cirimotich CM, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boissiere A, et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damiani C, et al. Paternal transmission of symbiotic bacteria in malaria vectors. Curr Biol. 2008;18:R1087–1088. doi: 10.1016/j.cub.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 72.Favia G, et al. Bacteria of the genus Asaia: a potential paratransgenic weapon against malaria. Adv Exp Med Biol. 2008;627:49–59. doi: 10.1007/978-0-387-78225-6_4. [DOI] [PubMed] [Google Scholar]
- 73.Werren JH, et al. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 74.Iturbe-Ormaetxe I, et al. Wolbachia and the biological control of mosquito-borne disease. Embo Rep. 2011;12:508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 76.Kambris Z, et al. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bian G, et al. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes GL, et al. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker T, Moreira LA. Can Wolbachia be used to control malaria? Mem I Oswaldo Cruz. 2011;106:212–217. doi: 10.1590/s0074-02762011000900026. [DOI] [PubMed] [Google Scholar]
- 80.Ignatova EA, et al. The yeast flora of blood-sucking mosquitoes. Mikrobiol Z. 1996;58:12–15. [PubMed] [Google Scholar]
- 81.Ricci I, et al. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Environ Microbiol. 2011;13:911–921. doi: 10.1111/j.1462-2920.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- 82.Ricci I, et al. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): perspectives on vector-borne diseases symbiotic control. Antonie Van Leeuwenhoek. 2011;99:43–50. doi: 10.1007/s10482-010-9532-3. [DOI] [PubMed] [Google Scholar]
- 83.Hurwitz I, et al. Paratransgenic control of vector borne diseases. Int J Biol Sci. 2011;7:1334–1344. doi: 10.7150/ijbs.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riehle MA, Jacobs-Lorena M. Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Molec. 2005;35:699–707. doi: 10.1016/j.ibmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida S, et al. A single-chain antibody fragment specific for the Plasmodium berghei ookinete protein Pbs21 confers transmission blockade in the mosquito midgut. Mol Biochem Parasitol. 1999;104:195–204. doi: 10.1016/s0166-6851(99)00158-9. [DOI] [PubMed] [Google Scholar]
- 86.Tzschaschel BD, et al. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat Biotechnol. 1996;14:765–769. doi: 10.1038/nbt0696-765. [DOI] [PubMed] [Google Scholar]
- 87.Bisi DC, Lampe DJ. Secretion of anti-Plasmodium effector proteins from a natural Pantoea agglomerans isolate by using PelB and HlyA secretion signals. Appl Environ Microb. 2011;77:4669–4675. doi: 10.1128/AEM.00514-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 89.Nolan T, et al. Developing transgenic Anopheles mosquitoes for the sterile insect technique. Genetica. 2011;139:33–39. doi: 10.1007/s10709-010-9482-8. [DOI] [PubMed] [Google Scholar]
- 90.Alphey L, et al. Insect population suppression using engineered insects. Adv Exp Med Biol. 2008;627:93–103. doi: 10.1007/978-0-387-78225-6_8. [DOI] [PubMed] [Google Scholar]
- 91.Carter V, Hurd H. Choosing anti-Plasmodium molecules for genetically modifying mosquitoes: focus on peptides. Trends Parasitol. 2010;26:582–590. doi: 10.1016/j.pt.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Ghosh A, et al. Molecular strategies to study Plasmodium-mosquito interactions. Trends Parasitol. 2003;19:94–101. doi: 10.1016/s1471-4922(02)00052-1. [DOI] [PubMed] [Google Scholar]
- 93.Jaynes JM, et al. In vitro cytocidal effect of novel lytic peptides on Plasmodium falciparum and Trypanosoma cruzi. FASEB J. 1988;2:2878–2883. doi: 10.1096/fasebj.2.13.3049204. [DOI] [PubMed] [Google Scholar]
- 94.Arrighi RB, et al. Cell-penetrating peptide TP10 shows broad-spectrum activity against both Plasmodium falciparum and Trypanosoma brucei brucei. Antimicrob Agents Chemother. 2008;52:3414–3417. doi: 10.1128/AAC.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao B, et al. AdDLP, a bacterial defensin-like peptide, exhibits anti-Plasmodium activity. Biochem Biophys Res Commun. 2009;387:393–398. doi: 10.1016/j.bbrc.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 96.Gao B, et al. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie. 2010;92:350–359. doi: 10.1016/j.biochi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Tian C, et al. Gene expression, antiparasitic activity, and functional evolution of the drosomycin family. Mol Immunol. 2008;45:3909–3916. doi: 10.1016/j.molimm.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 98.Li F, et al. An anti-Chitinase malaria transmission-blocking single-chain antibody as an effector molecule for creating a Plasmodium falciparum-refractory mosquito. J Infect Dis. 2005;192:878–887. doi: 10.1086/432552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Franz AW, et al. Stability and loss of a virus resistance phenotype over time in transgenic mosquitoes harbouring an antiviral effector gene. Insect Mol Biol. 2009;18:661–672. doi: 10.1111/j.1365-2583.2009.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller GC, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]