Abstract
Nicotinic acetylcholine receptors are calcium-permeable and the initial targets for nicotine. Studies suggest that calcium-dependent mechanisms mediate some behavioral responses to nicotine; however, the post-receptor calcium-dependent mechanisms associated with chronic nicotine and nicotine withdrawal remain unclear. The proteins calcium/calmodulin-dependent protein kinase II (CaMKII) and synapsin I are essential for neurotransmitter release and were shown to be involved in drug dependence. In the current study, using pharmacological techniques, we sought to (a) complement previously published behavioral findings from our lab indicating a role for calcium-dependent signaling in nicotine dependence and (b) expand on previously published acute biochemical and pharmacological findings indicating the relevance of calcium-dependent mechanisms in acute nicotine responses by evaluating the function of CaMKII and synapsin I after chronic nicotine and withdrawal in the nucleus accumbens, a brain region implicated in drug dependence. Male mice were chronically infused with nicotine for 14 days, and treated with the β2 -selective antagonist dihydro-β-erythroidine (DHβE), or the α7 antagonist, methyllycaconitine citrate (MLA) 20 minutes prior to dissection of the nucleus accumbens. Results show that phosphorylated and total CaMKII and synapsin I protein levels were significantly increased in the nucleus accumbens after chronic nicotine infusion, and reduced after treatment with DHβE, but not MLA. A spontaneous nicotine withdrawal assessment also revealed significant reductions in phosphorylated CaMKII and synapsin I levels 24 h after cessation of nicotine treatment. Our findings suggest that post-receptor calcium-dependent mechanisms associated with nicotine withdrawal are mediated through β2-containing nicotinic receptors.
Keywords: CaMKII, calcium signaling, nicotine withdrawal, nucleus accumbens, nicotine dependence, synapsin I, nicotinic receptors
1. Introduction
Nicotine-associated behaviors are mediated through nicotinic acetylcholine receptors, which are calcium-permeable, and the initial targets for nicotine. Upon nicotine binding, a direct influx of calcium through nicotinic receptors leads to an indirect calcium influx through voltage-gated calcium channels and intracellular calcium stores (Rathouz and Berg, 1994; Dajas-Bailador et al., 2002). The subsequent rise in intracellular calcium induces activation of various downstream second-messengers, including calcium/calmodulin-dependent protein kinase II (CaMKII), one of the most abundant proteins in neurons (Deisseroth et al., 1998), and a protein involved in several essential processes, including induction of long term potentiation (Lisman et al., 2002) and neurotransmitter release (Schulman and Hanson, 1993). CaMKII, in turn, activates various substrates, including synapsin I, a presynaptic vesicle-associated protein essential for neurotransmitter release and phosphorylated by CaMKII at Ser-566 and Ser-603 (De Camilli et al., 1990; Hilfiker et al., 1999).
Activation of these post-receptor calcium-dependent signaling cascades is involved in nicotine-mediated responses. Acute nicotine-induced increases in CaMKII activity are mediated through β2-containing nicotinic receptors in brain regions implicated in drug dependence, including the ventral tegmental area, nucleus accumbens, and amygdala (Jackson et al., 2009d). An acute systemic injection of nicotine also elevates CaMKII in the spinal cord (Damaj, 2000; Damaj, 2007). Further, L-type calcium channel blockers and CaMKII inhibitors block development and expression of nicotine-induced antinociception at the spinal level (Damaj, 2005). Calcium-dependent mechanisms are also involved in physical and affective nicotine withdrawal behaviors (Biala and Weglinska, 2005; Jackson et al., 2009a). In addition to CaMKII, synapsin I activity is also increased in the nucleus accumbens after acute nicotine (Jackson et al., 2009d). Synapsin I mRNA is also increased in the locus coeruleus, amygdala, spinal cord, and pontine central gray area after chronic morphine treatment in rats (Matus-Leibovitch et al., 1995). Further, increased synapsin I phosphorylation and subsequent dopamine release were noted after amphetamine sensitization in rats, and after chronic amphetamine treatment in rat striatal synaptosomes (Iwata et al., 1996; Iwata et al., 1997a; Iwata et al., 1997b).
While these findings show that calcium-dependent mechanisms are relevant to drug-dependence behaviors, the changes occurring in post-receptor calcium-dependent signaling after chronic nicotine and withdrawal remain unclear. Thus, in the current study, we sought to complement previous behavioral findings from our lab indicating a role for calcium-dependent signaling in nicotine dependence and withdrawal (Damaj, 2005; Jackson et al., 2009a) and expand on acute nicotine studies suggesting the importance of calcium-dependent mechanisms in mediating acute nicotine responses (Damaj, 2000; Damaj, 2007; Jackson et al., 2009d) by examining CaMKII and synapsin I function in the nucleus accumbens after chronic nicotine exposure and withdrawal. Mice were chronically treated with nicotine for 14 days, and withdrawal was precipitated using the β2 nicotinic receptor -selective antagonist, dihydro-β-erythroidine (DHβE), and the α7 nicotinic receptor antagonist, methyllycaconitine citrate (MLA). A spontaneous withdrawal assessment was also conducted to complement our precipitated approach.
2. Materials and Methods
2.1. Animals
Male adult C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Animals were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with food and water available ad libitum. The rooms were on a 12 h light/dark cycle (lights on at 7:00 A.M.). Mice were about 8–10 weeks of age and weighed approximately 25–30 g at the start of the experiment. All experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Drugs
(−)-Nicotine hydrogen tartrate salt was purchased from Sigma Chemical Company (Milwaukee, WI). DHβE and MLA were purchased from RBI (Natick, MA, USA) and were dissolved in saline. Drugs were injected s.c. at a volume of 10 ml/kg body weight. The pH of the nicotine solution was checked and neutralized if necessary. All doses are expressed as the free base of the drug.
2.3. Chronic nicotine administration
Mice were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and implanted with Alzet osmotic minipumps [model 2002 (14 days); Durect Corporation, Cupertino, CA, USA] filled with (−)-nicotine or saline solution. The minipumps infused nicotine (36 mg/kg/day, s.c.) or saline for 14 days. The concentration of nicotine was adjusted according to animal weight and minipump flow rate.
2.4. Chronic nicotine and withdrawal studies
On the morning of day 15, male C57Bl/6 mice (n= 32) were injected with vehicle or antagonist [DHβE (2 mg/kg, s.c.) or MLA (10 mg/kg, s.c.)]. Mice were sacrificed by cervical dislocation 20 minutes after antagonist injection. To assess protein levels after spontaneous withdrawal, in a separate group of male C57Bl\6 mice (n= 8), minipumps were removed the afternoon of day 14. In brief, mice were anesthetized using isofurane anesthesia. A small 5mm incision was made on the skin surrounding the mini pump. The pump was removed through the incision, and the incision sutured with nylon monofilament. Mice were awake and active approximately 5 min after removal from isoflurane, and allowed to recover from surgery overnight. Mice were sacrificed the morning of day 15, approximately 24 hours after cessation of nicotine treatment. For all studies, the brains were rapidly removed and sliced into 1 mm thick sections using a mouse brain matrix (Braintree Scientific Co., Braintree, MA, USA) on ice. The nucleus accumbens, consisting of both the shell and core divisions, was identified using a stereotaxic atlas (Paxinos and Franklin, 2001), dissected from the appropriate section (approximate coordinates Nucleus accumbens: Bregma 1.10 mm), and placed immediately in cold extraction buffer.
2.5. Western blot assays
Nucleus accumbens brain sections were homogenized in extraction buffer containing 50 mM Tris, 1 % SDS, 1 mM PMSF, 1 mM EDTA, 5 mM EGTA, 1 mM Na+ orthovanadate, 20 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 μM okadaic acid. Protein concentrations were determined using the Bradford assay. 30 μg of protein were incubated with 6X blue gel loading dye (New England Biolabs, Ipswich, MA, USA), and heated for 5 minutes at 95°C. Samples were then separated by SDS-polyacrylamide gel electrophoresis on a 10% TRIS-HCl gel and subjected to immunoblotting. Non-specific protein was blocked in 5% milk solution in TBS-T for 1 hour at room temperature. Primary antibodies for α-CaMKII (1:1000; Sigma, St. Louis, MO, USA), α-pCaMKII (1:10000; Fisher Scientific, USA), synapsin I (1:2000; Chemicon International, Inc, Billerica, MA, USA), or pSynapsin I Ser603 (an antibody specific for the site phosphorylated by CaMKII) (1:2000; Sigma, St. Louis, MO, USA) and α-tubulin antibody (1:5000; Upstate, Temecula, CA, USA) were incubated overnight at 4°C. Secondary antibodies (1:5000; LiCor Biosciences, Inc., Lincoln, NE, USA) were incubated for 1 hour at room temperature the next day. Bound antibody was detected using the LiCor Odyssey Infrared Imaging System (LiCor Biosciences, Inc., Lincoln, NE, USA). α-CaMKII bands were detected at 50 kDa, α-pCaMK II bands were detected at 52 kDa, Synapsin I bands were detected at 80 kDa, pSynapsin I Ser 603 bands were detected at 78 kDa, and α-tubulin bands were detected at 55 kDa. Blots were analyzed by taking the ratio of protein:αtubulin. Results from two independent blots were combined, normalized, and represented as a percentage of saline baseline. The ratio of phosphorylated to total protein was also calculated using the formula [(phospho/αtubulin)/(total/α-tubulin)].
2.6. Statistical analysis
For all data, statistical analyses were performed using StatView®. Data from precipitated western blot studies were analyzed using one-way analysis of variance with treatment as the between subject factor. Significant results were further assessed using a Neuman-Keuls post hoc test. For spontaneous nicotine withdrawal studies, data were analyzed using a student’s unpaired t-test. P-values less than 0.05 were considered significant.
3. Results
3.1. DHβE, but not MLA, decreases total and phosphorylated CaMKII and synapsin I protein levels in the nucleus accumbens
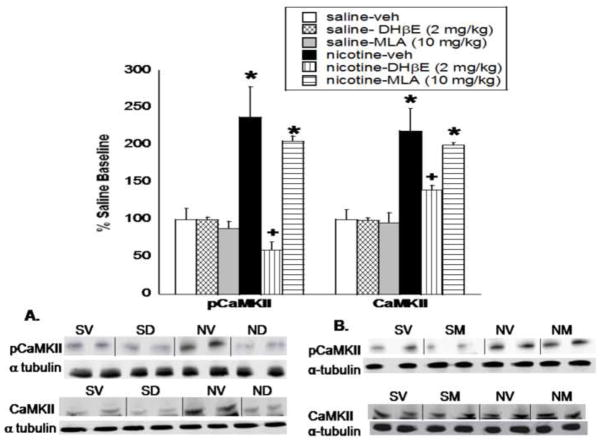
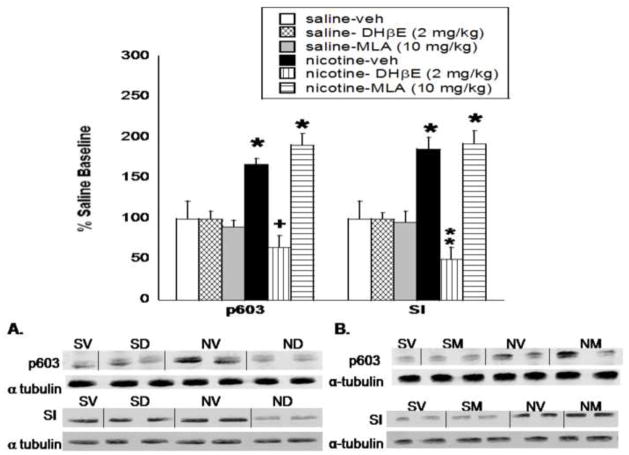
Chronic nicotine infused for 14 days induced a significant increase in pCaMKII (F(5,27) = 12.73, P < 0.0001) and CaMKII (F(5,27) = 10.985, P =0.0002) protein level in the nucleus accumbens as shown in Fig. 1, as well as in pSynapsin I Ser603 (p603; (F(5,27) = 7.44, P = 0.005) and synapsin I (F(5,27) = 8.89, P = 0.001) protein level (Fig. 2). Post-hoc analysis indicate that treatment with the β2 nicotinic receptor selective antagonist DHβE (2 mg/kg, s.c.) significantly reduced the chronic nicotine-induced increase in pCaMKII, CaMKII, p603, and synapsin I protein expression in the nucleus accumbens. Conversely, the α7 nicotinic receptor antagonist, MLA (10 mg/kg, s.c.), failed to reduce the nicotine-induced increase, as this effect was still observed in the nucleus accumbens for all proteins after MLA treatment. Representative blots are shown in Fig. 1A and B for pCaMKII and CaMKII respectively and Fig. 2A and B for p603 and synapsin I respectively. DHβE and MLA alone had no significant effect on protein expression at the doses tested. The ratios of phosphorylated to total protein in the chronic nicotine, nicotine-DHβE, and nicotine-MLA treated groups, shown in Table 1, show no significant change in the pCaMKII:CaMKII or p603:synapsin I ratios when expressed as a percentage of saline-control.
Fig. 1. DHβE, but not MLA, precipitates a reduction in CaMKII activity and protein level in the nucleus accumbens.
Mice were chronically infused with nicotine or saline for 14 days, and treated with vehicle, DHβE (2 mg/kg, s.c.) or MLA (10 mg/kg, s.c.) on the morning of day 15. Western blot analysis shows that CaMKII activity (pCaMKII) and total protein level (CaMKII) were significantly reduced in the nucleus accumbens after treatment with A. DHβE, but not B. MLA. Results from two independent blots were combined, normalized, and represented as a percentage of saline baseline. The x-axis represents the protein analyzed. the y-axis represents the percent expression compared to saline baseline. Each point represents the mean ± S.E.M of 4–8 mice per group. * denotes P < 0.05 vs. saline and nicotine-antagonist groups. + denotes P < 0.05 vs. chronic nicotine groups. veh, vehicle; SV, saline-vehicle; SD, saline-DHβE; SM, saline-MLA; NV, nicotine-vehicle; ND- nicotine-DHβE; NM-nicotine-MLA
Fig. 2. DHβE, but not MLA, precipitates a reduction in synapsin I activity and protein level in the nucleus accumbens.
Mice were chronically infused with nicotine or saline for 14 days, and treated with vehicle, DHβE (2 mg/kg, s.c.) or MLA (10 mg/kg, s.c.) on the morning of day 15. Western blot analysis shows that synapsin I activity (p603) and total protein level (synapsin I; SI) were significantly reduced in the nucleus accumbens after treatment with A. DHβE, but not B. MLA. Results from two independent blots were combined, normalized, and represented as a percentage of saline baseline. The x-axis represents the protein analyzed. the y-axis represents the percent expression compared to saline baseline. Each point represents the mean ± S.E.M of 4–8 mice per group. * denotes P < 0.05 vs. saline and nicotine-antagonist groups. + denotes P < 0.05 vs. chronic nicotine groups. ** denotes P < 0.05 vs. SV, SD, and chronic nicotine groups. veh, vehicle; SV, saline-vehicle; SD, saline-DHβE; SM, saline-MLA; NV, nicotine-vehicle; ND- nicotine-DHβE; NM-nicotine-MLA
Table 1.
Ratio of phosphorylated to total protein levels after nicotine-DHβE treatment and 24 hr after spontaneous nicotine withdrawal. Ratios are expressed as a percentage of saline control.
| Groups | pCaMKII:CaMKII | p603:synapsin I |
|---|---|---|
| Veh-veh precipitated | 99.96 ± 2.57 | 100 ± 12.24 |
| Chronic nicotine | 128.74 ± 41.6 | 112.48 ± 27.6 |
| Nic-DHβE precipitated | 95.86 ± 8.25 | 105.39 ± 12.62 |
| Nic-MLA precipitated | 103.36 ± 15.67 | 90.5 ± 8.82 |
| Saline spontaneous | 100 ± 7.78 | 100 ± 6.94 |
| Nicotine spontaneous | 61.66 ± 10.9a | 72.11 ± 7.85a |
denotes P < 0.05 vs. corresponding spontaneous saline control. Veh, vehicle; nic, nicotine
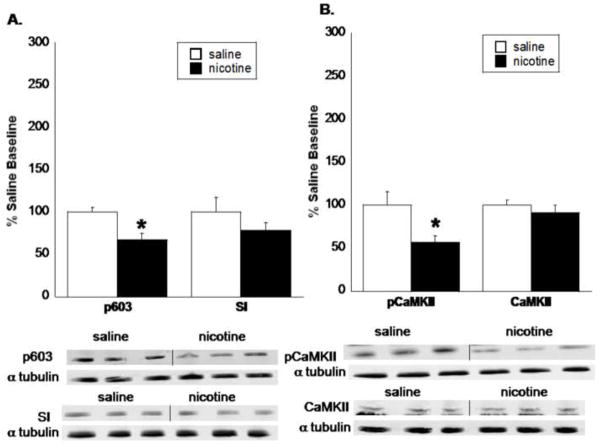
3.2. pCaMKII and p603 protein levels are decreased after spontaneous nicotine withdrawal
To determine if the reduction in protein levels also occurred after spontaneous nicotine withdrawal, a subset of C57Bl/6 mice was chronically infused with saline or nicotine for 14 days, and minipumps were removed the afternoon of day 14. Mice were withdrawn from nicotine for approximately 24 h prior to dissection of the nucleus accumbens. Results are shown in Fig. 3. There was a significant reduction in p603 level in the nucleus accumbens 24 hours after cessation of nicotine treatment (t(6) = 2.74, P = 0.04; Fig. 3A). Total synapsin I levels were not significantly different from saline baseline. Similarly, pCaMKII levels were significantly decreased in the nucleus accumbens after spontaneous withdrawal from nicotine (t(6) = 3.35, P = 0.03; Fig. 3B), while total CaMKII levels were unchanged. Table 1 reveals a significant decrease in the p603:syanpsin I and pCaMKII:CaMKII ratios, expressed as a percentage of saline baseline.
Fig. 3. CaMKII and synapsin I activity levels are reduced 24 hr after cessation of nicotine treatment.
Mice were chronically infused with nicotine for 14 days. Mini pumps were removed on the evening of day 14, approximately 24 hr prior to dissection of the nucleus accumbens. A. Synapsin I and B. CaMKII activity were significantly reduced 24 hr after cessation of nicotine treatment. Total protein levels were unchanged. Results from two independent blots were combined, normalized, and represented as a percentage of saline baseline. The x-axis represents the protein analyzed. The y-axis represents the percent expression compared to saline baseline. Each point represents the mean ± S.E.M of 4 mice per group. * denotes P < 0.05 vs. the corresponding saline group.
4. Discussion
The goal of these biochemical studies was to complement previously published behavioral pharmacology studies from our lab implicating an important role for calcium-dependent signaling in nicotine dependence and withdrawal (Damaj, 2005; Jackson et al., 2009a) and expand on previously published studies indicating the relevance of calcium-dependent mechanisms in acute nicotine responses (Damaj, 2000; Damaj, 2007; Jackson et al., 2009d) by examining the post-receptor calcium-dependent mechanisms that mediate nicotine responses after chronic nicotine exposure and nicotine withdrawal. We measured the phosphorylated, or activated, state of CaMKII (pCaMKII) and synapsin I (p603), which is an indication of protein activity, as well as the total protein levels (CaMKII, synapsin I), which reflect the total protein expression in the system after precipitated and spontaneous nicotine withdrawal. Together, our studies suggest that β2-containing, but not α7 nicotinic receptors are involved in nicotine induced changes in CaMKII and synapsin I function in the nucleus accumbens after nicotine withdrawal.
Our chronic nicotine assessment revealed a significant increase in phosphorylated and total CaMKII and synapsin I protein levels after 14 days of chronic nicotine exposure, suggesting that tolerance does not develop to the acute nicotine-induced increases in phosphorylated CaMKII and synapsin I levels in the nucleus accumbens (Jackson et al., 2009d). Both CaMKII and synapsin I are important markers for neurotransmitter release, and many studies support the vital role of the neurotransmitter dopamine in nicotine dependence. Indeed, the nicotine induced increase in ventral tegmental area dopamine neuron firing rate (Grenhoff et al., 1986), and subsequent dopamine release in the nucleus accumbens is a process thought to underlie the addictive properties of nicotine (Ponteiri et al., 1996). Compatible with the current studies, acute nicotine increases dopamine release in the nucleus accumbens, and tolerance does not develop to chronic-nicotine induced increases in dopamine release (Damsma et al., 1989; Nisell et al., 1997). In our withdrawal assessment, DHβE (2 mg/kg, s.c.) at a dose that precipitates significant nicotine withdrawal-induced aversion in mice (Jackson et al., 2009b), but not MLA, precipitated a significant decrease in CaMKII and synapsin I activity in the nucleus accumbens. In support of our precipitated studies, CaMKII and synapsin I activity were also significantly decreased 24 h after cessation of chronic nicotine, and the ratios of pCaMKII:CaMKII and p603:syanpsin I were decreased, though total protein levels were unchanged. A significant reduction in total protein levels may be observed after longer periods of withdrawal. Studies report decreased dopamine neuronal activity in the ventral tegmental area (Liu and Jin, 2004) and decreased dopamine output in the nucleus accumbens after precipitated and spontaneous nicotine withdrawal (Hildebrand et al., 1998; Rada et al., 2001). It has been proposed that this deficient dopamine release in the nucleus accumbens contributes to the mood disorders and overall negative affective state that accompanies nicotine withdrawal (Benowitz, 2008). Taken together, we propose that the nicotine withdrawal-induced decrease in CaMKII and synapsin I function in the nucleus accumbens may contribute in part to the decrease in dopamine release following nicotine withdrawal, as decreases in CaMKII function may also be relevant to affective nicotine withdrawal behaviors (Jackson et al., 2009a).
While both the precipitated and spontaneous models support a significant decrease in CaMKII and synapsin I function after nicotine withdrawal, in the spontaneous withdrawal study, pCaMKII and p603 levels were significantly reduced below saline baseline. One explanation is that this additional decrease in the spontaneous assessment may be attributed to contributions of additional non-β2 containing nicotinic receptor subtypes, which are not blocked by DHβE, such as the α3β4* nicotinic receptor subtype (where * denotes the possible inclusion of additional nAChR subunits). While this receptor combination is not present in the nucleus accumbens, it is expressed in small amounts in the ventral tegmental area (Perry et al., 2002), which contains dopaminergic projections to the nucleus accumbens, and in the medial habenula and interpeduncular nucleus, where α3β4*/α3β4α5* nicotinic receptors dominate function (Quick et al., 1999). Indeed, α3β4* nicotinic receptors in these two brain regions mediate nicotine-induced dopamine function in the nucleus accumbens (Taraschenko et al., 2007; McCallum et al., 2012), and incorporation of the α5 nicotinic receptor subunit in the α3β4* subtype increases calcium permeability (Gerzanich et al., 1998). Further, we cannot completely rule out the possibility of stress-related changes in pCaMKII and p603 resulting from mini pump removal surgery.
It was surprising that DHβE precipitated a significant decrease in CaMKII and synapsin I total protein level after 20 minutes. It is possible that rather than reducing total CaMKII and synapsin I protein level, blockade of β2-containing nicotinic receptors by DHβE in the presence of nicotine induces translocation of the proteins to a different brain region. In support of this hypothesis, activation of CaMKII is known to involve translocation of the protein to the postsynaptic density (Shen and Meyer, 1999; Shen et al., 2000). Withdrawal from the benzodiazepine flurazepam significantly reduces total CaMKII protein localization in the postsynaptic density due to removal of total protein from the region (Earl et al., 2012). Thus, future immunohistochemistry studies assessing CaMKII and synapsin I after nicotine withdrawal are necessary determine if translocation of the proteins out of the nucleus accumbens provides a valid explanation for the results. It is also noted that DHβE precipitated a significant reduction in total syanpsin I protein level below saline baseline, while appearing to precipitate a return to baseline saline levels for the other proteins assessed. The implications of this decrease in the synapsin I DHβE group are unclear; however, because there were no experimental differences between precipitated withdrawal groups, we speculate that this decrease is attributed to random variability between experiments. The p603:syanpsin I ratio was also unchanged in the nicotine-DHβE, further supporting this notion.
Previous studies from our lab revealed a role for β2-containing receptors, specifically α6β2* and/or α4α6β2* nicotinic receptor subtypes in affective nicotine withdrawal behaviors (Jackson et al., 2009b,c; Jackson et al., 2010). The α6β2* and α4α6β2* nicotinic receptor subtypes are also located on presynaptic dopamine and γ-aminobutyric acid neurons in the ventral tegmental area and are involved in nicotine-stimulated dopamine release in the striatum (Champtiaux et al., 2003; Salminen et al., 2004; Lai et al., 2005). Further, the increased spinal CaMKII activity observed after acute and chronic nicotine is mediated through β2-containing nicotinic receptors and is relevant to acute nicotine behaviors and nicotine tolerant behaviors in mice (Damaj, 2000; Damaj, 2005; Damaj, 2007). In a nicotine withdrawal behavioral assessment, the CaMKII inhibitor KN-93, significantly enhanced the nicotine withdrawal-induced anxiety-related response and showed a strong trend for enhancement of nicotine conditioned place aversion (Jackson et al., 2009a). The CaMKII antagonist-induced exacerbation of affective nicotine withdrawal signs suggests a decrease in CaMKII function after nicotine withdrawal. Based on our behavioral and biochemical results on β2-containing nicotinic receptors, CaMKII, and nicotine withdrawal, we propose that decreases in CaMKII and synapsin I function in the nucleus accumbens after nicotine withdrawal are mediated through β2-containing nicotinic receptors and are relevant to affective nicotine withdrawal behaviors. Overall, these studies identify receptor (β2-containing nicotinic receptors) and post-receptor calcium-dependent (CaMKII and synapsin I) mechanisms and brain regions (nucleus accumbens) which likely contribute to the nicotine withdrawal syndrome. Future studies will focus on clarifying a more direct correlation between our biochemical and behavioral findings by measuring the nicotine withdrawal syndrome in mice following intraaccumbal injections of CaMKII antagonists and DHβE, as well as assessment of CaMKII and synapsin I function following measurement of affective nicotine withdrawal signs.
Acknowledgments
This work was supported by National Institute on Drug Abuse grants DA-12610 and DA-05274 to MID.
Footnotes
There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Biala G, Weglinska B. Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res. 2005;51:483–488. doi: 10.1016/j.phrs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Mogg AJ, Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- Damaj MI. The involvement of spinal calcium/calmodulin-protein kinase II in nicotine-induced antinociception in mice. Eur J Pharmacol. 2000;404:103–110. doi: 10.1016/s0014-2999(00)00579-3. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Calcium-acting drugs modulate expression and development of nicotine-induced antinociception in mice. J Pharmacol Exp Ther. 2005;315:959–964. doi: 10.1124/jpet.105.092460. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Behavioral modulation of neuronal calcium/calmodulin-dependent protein kinase II activity: differential effects on nicotine-induced spinal and supraspinal antinociception in mice. Biochem Pharmacol. 2007;74:1247–1252. doi: 10.1016/j.bcp.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Benfenati F, Valtorta F, Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien R. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Earl DE, Das P, Gunning WT, 3rd, Tietz EI. Regulation of Ca2+/calmodulin-dependent protein kinase II signaling within hippocampal glutamatergic postsynapses during flurazepam withdrawal. Neural Plast. 2012;2012:405926. doi: 10.1155/2012/405926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Hewlett GH, Ferrell ST, Czernik AJ, Meiri KF, Gnegy ME. Increases in vivo phosphorylation state of neuromodulin and synapsin I in striatum from rats treated with repeated amphetamine. J Pharmacol Exp Ther. 1996;278:1428–1434. [PubMed] [Google Scholar]
- Iwata S, Hewlett GH, Gnegy ME. Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse. 1997a;26:281–291. doi: 10.1002/(SICI)1098-2396(199707)26:3<281::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Iwata S, Hewlett GH, Ferrell ST, Kantor L, Gnegy ME. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther. 1997b;283:1445–1452. [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Damaj MI. L-type calcium channels and calcium/calmodulin-dependent kinase II differentially mediate behaviors associated with nicotine withdrawal in mice. J Pharmacol Exp Ther. 2009a;330:152–161. doi: 10.1124/jpet.109.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology. 2009b;56:970–974. doi: 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009c;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Walters CL, Damaj MI. Beta 2 subunit-containing nicotinic receptors mediate acute nicotine-induced activation of calcium/calmodulin-dependent protein kinase II-dependent pathways in vivo. J Pharmacol Exp Ther. 2009d;330:541–549. doi: 10.1124/jpet.109.153171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment decreases striatal 6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol. 2005;67:1639–1647. doi: 10.1124/mol.104.006429. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioral memory. Nature. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Jin WQ. Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport. 2004;15:1479–1481. doi: 10.1097/01.wnr.0000126218.25235.b6. [DOI] [PubMed] [Google Scholar]
- Matus-Leibovitch N, Ezra-Macabee VE, Saya D, Attali B, Avidor-Reiss T, Barg J, Vogel Z. Increased expression of synapsin I mRNA in defined areas of the rat central nervous system following chronic morphine treatment. Mol Brain Res. 1995;34:221–230. doi: 10.1016/0169-328x(95)00166-p. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD. α3β4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63:434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm. 1997;104:1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Deigo: 2001. [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. α3β4-subunit containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal in extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schulman H, Hanson PI. Multifunctional Ca2+/calmodulin-dependent protein kinase. Neurochem Res. 1993;18:65–77. doi: 10.1007/BF00966924. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL. Involvement of the alpha3 subunit in central nicotinic receptor populations. J Neurosci. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]