Abstract
Breast cancer is the most common invasive cancer among women in developed countries. Obesity is a major risk factor for breast cancer recurrence and mortality in both pre-and postmenopausal women. Co-morbid medical conditions are common among breast cancer survivors. The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) study is a 4-year randomized clinical trial of 693 overweight/obese women aged ≥21 years diagnosed with any early stage breast cancer (stages I[≥1 cm]-III) within the previous five years, designed to demonstrate the feasibility of achieving sustained weight loss and to examine the impact of weight loss on quality of life and co-morbidities, and to enable future exploration of biochemical mechanisms linking obesity to lower likelihood of disease-free survival. This trial is strategically designed as a vanguard for a fully-powered trial of women who will be evaluated for breast cancer recurrence and disease-free survival. Participants were recruited between 2010 and 2012 at four sites, had completed initial therapies, and had a body mass index between 25 and 45 kg/m2. The intervention featured a group-based cognitive-behavioral weight loss program with telephone counseling and tailored newsletters to support initial weight loss and subsequent maintenance, with the goal of 7% weight loss at two years. This study has high potential to have a major impact on clinical management and outcomes after a breast cancer diagnosis. This trial initiates the effort to establish weight loss support for overweight or obese breast cancer survivors as a new standard of clinical care.
Keywords: Obesity, Weight Reduction, Breast Cancer, Quality of Life, Co-Morbidities
1. Introduction
Breast cancer is the most common invasive cancer among women in developed countries and is the second most common cause of cancer death among women in the United States [1, 2]. The high incidence of breast cancer, coupled with earlier diagnosis and more effective therapies, have led to over 2.7 million women in the United States who are now breast cancer survivors [3].
About 70% of women are already overweight at the time of their breast cancer diagnosis, and additional weight gain often results from common forms of treatment [4]. Obesity is an important risk factor for postmenopausal breast cancer incidence and is a major risk factor for breast cancer recurrence and morbidity in both pre- and postmenopausal women [5–7]. In a meta-analysis of 43 studies of women diagnosed with breast cancer, Protani et al. [8] found a significant increase in all-cause and breast cancer specific mortality in obese compared to non-obese women. Higher body mass index (BMI) remains a significant independent predictor of breast cancer mortality even in multivariate analysis that includes numerous tumor characteristics [9].
Obesity, particularly when characterized by increased visceral fat, is associated with an adverse metabolic and cardiovascular disease risk profile and co-morbidities such as type 2 diabetes, hypertension, and cardiovascular disease [10, 11]. These and other co-morbid medical conditions are common among breast cancer survivors and of concern in their long-term care [12–14].
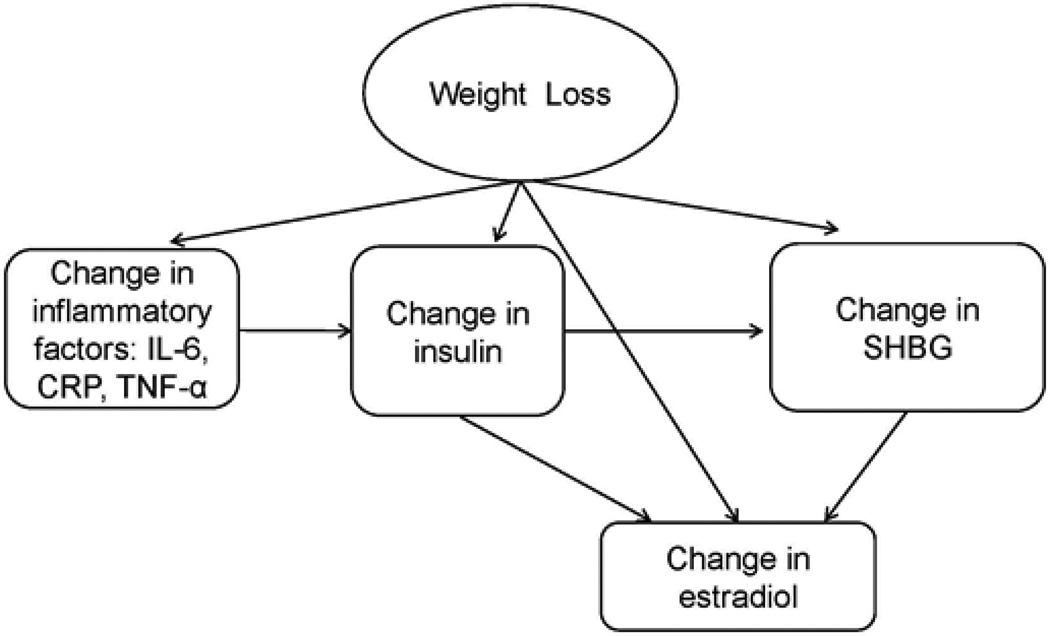
Excess adipose tissue results in an increased production of estrogens, insulin, leptin, and pro-inflammatory cytokines, and a decreased production of sex hormone binding globulin (SHBG), all of which are plausibly linked to breast cancer recurrence and progression [15–19]. Estrogenic stimulation promotes breast cancer pathogenesis and tumorigenesis, and insulin and leptin exhibit proliferative, mitogenic, and anti-apoptotic activities in mammary cells, thus promoting tumor growth [17, 20]. High circulating estrogen levels are associated with increased risk of breast cancer recurrence [21]. Increased adiposity is accompanied by alterations in cytokines, hyperinsulinemia, and reduced levels of binding proteins [22–24]. Figure 1 illustrates the potential effect of weight loss on proposed hormonal and biological factors linking obesity to breast cancer recurrence.
Fig. 1.
Potential effect of weight loss on hormonal and biological mediators linking obesity to breast cancer recurrence (IL-6, interleukin-6; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; SHBG, sex hormone binding globulin)
The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) study is a 4-year randomized controlled trial of 693 overweight or obese women who have been diagnosed with early stage breast cancer, designed to demonstrate the feasibility of achieving sustained weight loss in this target population and to examine the impact of weight loss on quality of life (QOL) and co-morbidities. The primary aim is the achievement of a 7% sustained weight loss among breast cancer survivors randomized to the intervention arm at two years post-randomization. Secondary aims are to evaluate weight loss at 24 months according to time since diagnosis and type of tumor and therapy; to assess the impact of the intervention on QOL; and to prospectively collect biological samples that will enable analysis of the effects on hormones and other factors to explain the mechanism and probable differential response across subgroups.
The trial is strategically designed as a vanguard component of a fully-powered trial of women who will be evaluated for breast cancer recurrence and disease-free survival. The vanguard approach, which involves enrolling and committing to an initial cohort of participants, produces data and evidence to support expansion into a study that is fully-powered to examine cancer-specific outcomes. Further, this strategy allows the opportunity to further tailor and streamline our efforts for a larger trial to follow, while at the same time accomplishing important scientific aims.
2. Methods
2.1. Study population
2.1.1. Eligibility criteria
Inclusion criteria were: Age 21 years and older; a history of breast cancer (stages I [≥1 cm], II, or III) diagnosed within the previous 5 years; completion of initial therapies; BMI between 25 and 45 kg/m2; and able to comply with all required study procedures and schedule.
Exclusion criteria were: Serious medical condition, including but not limited to renal or hepatic insufficiency, congestive heart failure, angina pectoris, myocardial infarction, stroke resulting in neurologic sequelae, claudication, or acute limb ischemia, history of malignancies other than initial breast cancer with the exception of non-melanoma skin cancer, paralysis, untreated stage 3 hypertension, pulmonary conditions that require hospitalization or oxygen within 6 months, arteriosclerotic cardiovascular disease procedure within the past 6 months, degenerative neurological conditions, diabetes treated with insulin, anti-coagulation treatment such as coumadin; serious psychiatric illness (e.g., lifetime history of bipolar disorder, schizophrenia or other psychosis; bulimia nervosa and anorexia nervosa; current serious personality disorder; borderline severe major depressive disorder), recent (previous 6 months) suicide attempt or current active suicidal ideation, recent hospitalization due to psychiatric illness; any medical condition substantially limiting moderate physical activity such as severe orthopedic conditions; obesity of known endocrine origin (e.g., untreated hypothyroidism, Cushing’s syndrome, established polycystic ovary syndrome); currently enrolled in a weight loss program or use of weight loss medication or supplements and unwilling to discontinue; have had previous surgical bariatric procedures for weight reduction or plan such surgery in the next two years; chronic use (at least past 6 months) of medications likely to cause weight gain or prevent weight loss (e.g., corticosteroids, lithium, olanzapine, risperidone, clozapine); planned surgical procedure that could impact the conduct of the study; currently pregnant or breastfeeding or planning to become pregnant within the next two years; plans to relocate from the area within two years; family relative or close friend is a trial staff member or a study participant; and any condition which in the opinion of the investigator makes the subject unsuitable for inclusion in this study.
2.1.2. Participant recruitment
Participants were recruited at four study sites (University of California, San Diego [UCSD]; University of Colorado Denver; University of Alabama at Birmingham; and Washington University in St. Louis [WUSTL]) between the Fall of 2010 through May, 2012. Participants were recruited through local or regional cancer registries, which provided the names of potentially eligible women to whom letters were sent, informing them of the study and providing contact information, as well as from clinics, television and radio media coverage, local print media, and community support groups, events and organizations, such as local chapters of the Susan G. Komen Foundation. Electronic announcements of the study were sent to the subscribers to the Army of Women (www.armyofwomen.org), a non-profit breast cancer organization which serves as a recruitment source to researchers, adhering to all HIPAA guidelines. The study was reviewed and approved by the local institutional review boards (IRB), as well as the IRB of the local or regional cancer registry if appropriate.
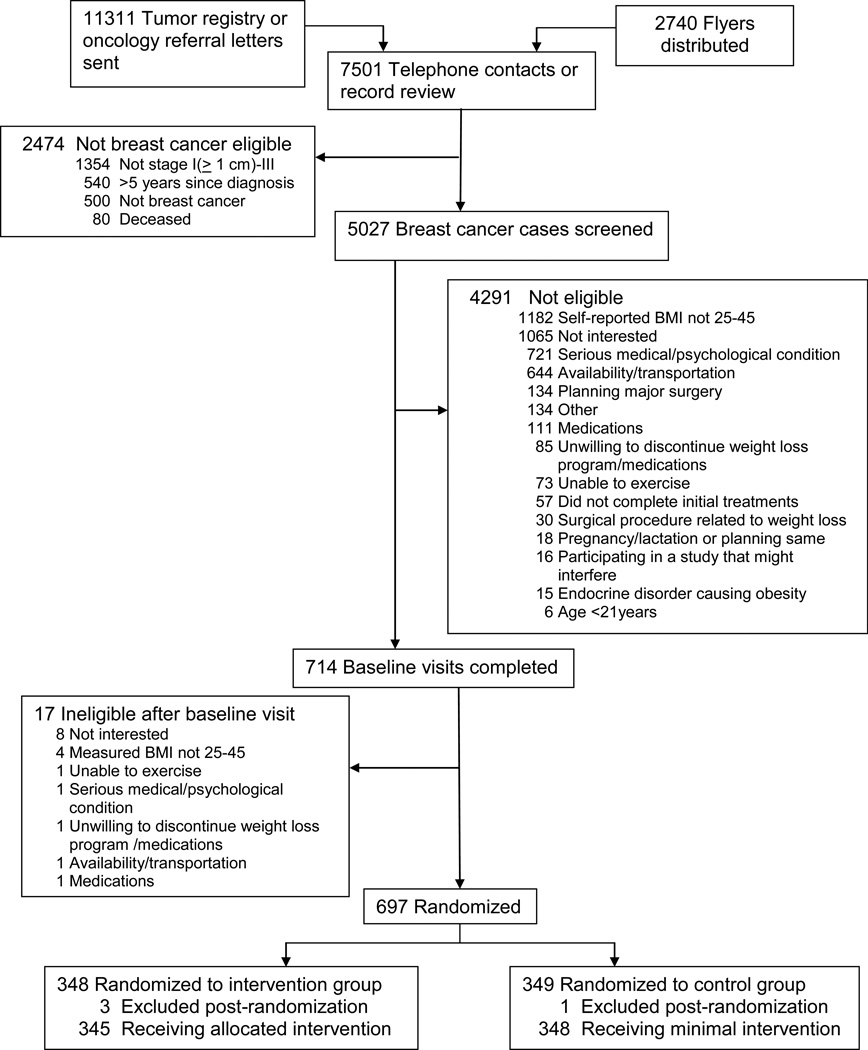
Potential participants were screened for study eligibility using a standardized, structured form. Figure 2 presents the participant flow during enrollment and exclusions during screening. Of the 5027 women with a diagnosis of stage I (≥1 cm), II, or III breast cancer within the previous five years who were screened, 697 (approximately 14%) met all eligibility criteria and were enrolled in the study. Numbers of randomized participants across the four sites are shown in Table 1.
Fig. 2.
CONSORT diagram
Table 1.
Number of randomized study participants by site
| Site | Control | Intervention | Total |
|---|---|---|---|
| Birmingham | 59 | 58 | 117 |
| Denver | 96 | 91 | 187 |
| San Diego | 106 | 109 | 215 |
| St Louis | 87 | 87 | 174 |
| TOTAL | 348 | 345 | 693 |
2.1.3. Screening and randomization
There was a screening and evaluation process that occurred in the 1–1.5 months prior to randomization. Final eligibility was determined at the baseline clinic visit, during which written informed consent was obtained. Subjects were asked to perform specific tasks in order to qualify for randomization. Tasks included completing an assessment call with the study coordinator to confirm interest and potential eligibility; attending and completing all procedures at the baseline clinic visit, including all questionnaires and blood collection; contacting the primary care physician (PCP) and/or oncologist to facilitate release of medical records, or signing a medical release form so that medical records could be requested and obtained by the research staff; and relevant medical record review to confirm eligibility.
Randomization was performed by a centralized computer process coordinated by the WUSTL data analysis center, randomly assigning subjects in a ratio of 1:1 to either the intervention or less intensive intervention control group. The parameters that were used for blocking were age (older/younger than 55 years), stage (I vs all others [II and III]), and study site. Table 2 shows the distribution of characteristics of the study groups.
Table 2.
Comparability of study groups
| Control (n = 348) | Intervention (n = 345) | |
|---|---|---|
| Age, years (mean [SD]) | 56.5 (9.5) | 56.1 (9.4) |
| <50 (%) | 25.0 | 28.1 |
| 50–64 (%) | 54.0 | 54.2 |
| ≥65 (%) | 21.0 | 17.7 |
| Education, years (mean [SD]) | 15.5 (2.4) | 15.6 (2.5) |
| Hispanic (%) | 5.8 | 7.5 |
| Race (%) | ||
| White | 84.5 | 83.2 |
| African-American | 10.6 | 10.4 |
| Asian-American | 2.0 | 1.5 |
| American Indian | 0.3 | 0.6 |
| Hawaiian/Pacific Islander | 0 | 0.3 |
| Mixed/Other | 2.0 | 3.8 |
| Missing or refused | 0.6 | 0.3 |
| Postmenopausal at study entry (%) | 85.0 | 85.8 |
| Weight, kg (mean [SD]) | 84.7 (13.8) | 85.0 (14.3) |
| Body mass index, kg/m2 (mean [SD]) | 31.4 (4.6) | 31.6 (4.7) |
| 25–29.9 (%) | 45.1 | 46.4 |
| 30–34.9 (%) | 35.1 | 29.6 |
| 35–45 (%) | 19.8 | 24.1 |
| Years between diagnosis and study entry (mean) | 2.83 | 2.72 |
| Breast cancer stage (%) | ||
| I | 31.9 | 32.2 |
| II | 51.7 | 48.4 |
| III | 16.4 | 19.4 |
2.2. Study intervention
2.2.1. Development of the intervention
The intervention features a group-based cognitive-behavioral weight loss program that was developed and pilot-tested in a previous randomized clinical trial at UCSD [25–27]. Overweight or obese women (N = 85, BMI 25–40 kg/m2) with early stage breast cancer had 16 weeks of weekly group meetings plus telephone counseling. Intent-to-treat analysis showed significant differences in weight loss at both 16 weeks and one year (P < 0.02). For the completers (82% of the study group), the average weight loss was −6.6 kg (8% of initial weight) at one year in the intervention group, and due to weight gain among the controls, there was a 8.4 kg weight difference between the two study arms. Within-group differences for intervention group completers were also significant for percent body fat (from 47.4 [4.8] at baseline to 42.9 [7.1] % at 16 weeks) and step test (60.0 [7.9] at baseline to 53.4 [10.4] at 16 weeks and 50.6 [6.8] heart rate/30 sec) at 12 months, associated with an average level of moderate plus vigorous physical activity of 7.4 (4.6) hours/week (P < 0.05). This weight loss intervention was subsequently tested in a randomized clinical trial of overweight or obese early stage breast cancer survivors (N = 259) [28, 29]. In that study, average weight loss for women assigned to immediate intervention was 5.3% (vs. <1% for the wait list control group) at six months and 4.5% (vs. 1.9% for the wait list control group) at 18 months. Weight loss of ≥5% of initial weight decreased leptin and insulin compared with those who did not achieve that amount of weight loss (P < .0001) [29].
Tailored print materials used in the intervention were adapted from the Fresh Start (N = 543) and RENEW (N = 641) trials, which used home-based methods to promote behavior change in cancer survivors [30–34]. Participants in the RENEW study, which included a goal of modest weight loss, were ≥65 years of age, overweight, and at least 5 years out from diagnosis of breast, prostate or colorectal cancer. At one year, participants in the immediate intervention arm lost −2.45 kg (3% of initial weight) [34].
In a trial designed as a pilot for this study, 28 Denver-area overweight or obese breast cancer survivors were randomized to the Colorado Weigh® program, a community-based comprehensive behavioral weight loss program that consists of three unique 12-week curricula taught by registered dietitians or a control group that received standard health information. After 6 months, women in the intervention group weighed 9.8% less than women in the control group (unpublished data).
2.2.2. The intervention
The goal of the intervention was to achieve a modest weight loss of at least 7% body weight that would be sustained, and behavioral goals to achieve that outcome were reduced energy intake relative to expenditure and increased physical activity. The intervention began with an intensive phase that consisted of four months of weekly one-hour group sessions for closed-group contingents of an average of 15 women, tapering to every other week for two months. The group sessions had 1–2 leaders, who had backgrounds in dietetics, psychology and/or exercise physiology, and who remained assigned to the closed group throughout the intervention. Sessions were scheduled at convenient times, including evening and daytime hours, to facilitate attendance. From 6 months onward, the groups met monthly for the remainder of the year. The core content was standardized, although group leaders had flexibility in adapting the material and discussions to be relevant to the needs and characteristics of a given group.
The group program was supported by personalized guidance delivered by telephone and/or email to individualize the feedback, goal-setting, planning and follow-through for specific behavioral goals. These individualized contacts were highly focused and brief (10–15 minutes) and were designed to reinforce goal-setting and follow-through. The goal was for each participant in the intervention arm to receive a total of approximately 14–16 counseling calls or contacts in the first study year and a total of 24–38 calls or messages (depending on need for support and feedback) during the two-year period of the intervention. Completed contacts were noted in the database, and inability to contact triggered follow-up by the site project coordinator.
Tailored print newsletters provided additional support and reinforced self-management when the groups met less frequently. These were produced by a health communications consulting and development firm with expertise in health communication and individually-tailored messaging (People Designs, Durham, NC). Newsletters were provided quarterly from 6–24 months and were individually tailored on information about physical activity (minutes/day and pedometer counts), dietary intake, and weight, and provided suggestions for overcoming common barriers to exercise and weight management and reinforcement for progress and appropriate goal setting.
The Coordinating Center at UCSD provided continuing oversight for quality assurance (QA) for intervention sessions and related activities across sites. These activities included standardization of the intervention program by providing session materials, training and oversight of the group sessions across sites; structuring of overall content of group sessions; monitoring group sessions by logging attendance and a post-group summary; and teleconferencing with staff across sites at least once per month (weekly during the initial phase of the study) to promote uniformity of the delivery and responses to issues that arise.
The foundation of the intervention was based on several theoretical models. The primary theory that forms the basis is the behavioral determinants model [35], which is based on Social Cognitive Theory [36–38]. This model posits there are personal, social, and physical environmental antecedents and consequences of behavior that affect one’s motivation and self-efficacy for behavior change. According to this theory, participant goal-setting is encouraged to provide direction. Self-efficacy is built and maintained by having the participant commit to explicit, proximal subgoals, which are instrumental in achieving the larger behavioral goals. Self-efficacy is reinforced when the participant overcomes perceived barriers and has successfully achieved the short-term subgoal. Another central concept of Social Cognitive Theory is one of self-monitoring, which was an important focus of the intervention, as participants were instructed to actively monitor their weight, dietary intake and pedometer counts daily throughout the intervention.
One-to-one interactions between group leaders and participants, and the telephone and email counseling component, utilized strategies of motivational interviewing techniques [39], which have been successfully incorporated into behavioral weight loss programs [40]. Finally, the cognitive restructuring component of the intervention is derived from a concept that the maintenance of a problem is promoted by cognitive processes [41, 42]. A major barrier to the acquisition of weight maintenance behavior has been proposed to be cognitive in nature [43, 44], so a particular focus on cognitive factors, such as a tendency to evaluate self-worth in terms of shape and weight, is incorporated into the intervention. Most behavioral weight loss programs currently include a few sessions on topics such as negative thinking patterns, but this particular intervention aimed to more specifically include cognitive therapy techniques with the goal of optimizing maintenance of weight loss [45]. As proposed by Wilson [46], self-acceptance is appropriately included in the cognitive restructuring component of the treatment of obesity. Overweight or obese individuals may achieve a clinically meaningful degree of weight loss in a comprehensive behavioral program, but not an unrealistic goal weight, so self-acceptance at a healthier (although perhaps not ideal) weight is theorized to promote better weight maintenance.
2.2.3. Content of the intervention
The overall content of the intervention consisted of both standard and new elements of cognitive-behavioral therapy for obesity, increased physical activity, and individualized diet modification that promotes an energy deficit. The cognitive behavioral aspect in this intervention incorporates the key aspects of a behavioral approach to obesity treatment. Briefly, strategies and approaches that were applied in this intervention included self-monitoring of food intake and exercise; realistic goal-setting, using behavior-specific goals and a step-wise approach to progress to promote self-efficacy; addressing body image concerns; training and role-playing in problem-solving; and relapse prevention.
Compared to the general population, breast cancer survivors have specific issues and problems that can influence response to weight loss strategies and guidance. These include body image issues related to cancer and cancer treatments; enduring psychosocial symptoms, such as depression and fatigue; changes in family dynamics that may affect social support; arthralgias and pain related to anti-estrogen therapy; and changes in body composition associated with cancer treatment. Thus, the intervention information specific to breast cancer-related problems and symptoms, including fatigue, symptoms of estrogen withdrawal, body image concerns and lymphedema, were addressed in the intervention, so it was tailored to this population.
The main goal of dietary guidance was to promote a reduction in energy intake relative to expenditure, aiming for a 500–1000 kcal/day deficit relative to expenditure to promote a weight loss of 1–2 pounds/week. Lower energy density of the diet was accomplished by advocating high-fiber vegetables, whole grains, and fruit to add bulk and weight to the diet, as these changes promote maximal satiety while reducing energy intake. Behavioral strategies that enable conscious eating were also used, such as stimulus control and planning meals. The curriculum included discussion of myths and realities about food, diet, weight control, popular diets, and other popularized strategies that are incorrectly believed to promote long-term weight loss.
The physical activity component emphasized planned aerobic exercise, increased physical activity in the lifestyle, and strength training. A priority was placed on regular planned aerobic exercise because it creates an energy deficit that is much greater per unit of time than strength training. The long-term goal prescribed was an average of at least 60 min/day of purposeful exercise at a moderate level of intensity, which is consistent with current recommendations for weight management [47]. As a component of the group discussion and notebook material, participants were taught how to recognize perceived level of exertion to enable them to gauge whether they were achieving the goal level of intensity when exercising. Current evidence suggests that this level of physical activity (approximately 2500 kcal/week) is associated with better long-term weight loss than the standard levels prescribed in behavioral weight loss interventions and in cardiovascular disease prevention guidelines [48, 49]. Notably, the vast majority of women who have been diagnosed with breast cancer have very low levels of physical activity, and age-related problems such as degenerative joint disease and therapy-related arthralgias impose some restrictions on the pace of increase in intensity and duration that can be achieved in many middle-aged and older women. Thus, the initial goal communicated in the program was to plan and implement daily purposeful mild to moderate exercise for a minimum of at least 10 minutes/day with a step-wise increase in time and intensity that was evaluated and modified on a twice per week (initially) and weekly basis. Subjects who exercised at a higher level than 10 minutes/day at enrollment were instructed to aim for an incrementally greater goal of daily exercise (e.g., weekly increases of 5 minutes more per day until the goal of 60 minutes/day is reached) and to plan to increase intensity as tolerated. This approach results in an individualized plan of specific activities and goals, based on capabilities, lifestyle pattern and preferences, and the participants were encouraged to set realistic, achievable goals at each step of the process of working toward the long-term goal.
A second aspect of the physical activity component was strength training. These exercises were demonstrated in the group sessions, and participants were encouraged to perform this activity on their own at home or at an exercise facility 2–3 times per week. The specific training exercises began with a focus on the core, and new resistance training exercises were introduced and demonstrated at specific intervals. The intervention did not include a large resistance training component because of concerns with arm and shoulder morbidity associated with unsupervised resistance training in women who have been treated for breast cancer [50]. Finally, increased lifestyle activity was strongly encouraged as a third aspect of physical activity, and pedometers were distributed in the first group session. Participants were encouraged to work toward achieving 10,000 steps per day. In all aspects of increased physical activity, standard behavioral elements such as convenience, enjoyment, time management, managing the environment, and social support were addressed as well as overconcern with weight and problems and body image. Also, small but important barriers to exercise in middle-aged and older sedentary women were identified and addressed, such as planning around showering, hair styling, and bathroom accessibility.
Even though the intervention material emphasized weight loss, there was a significant focus on teaching skills for long-term weight loss maintenance. Relapse prevention techniques and cognitive restructuring to promote weight maintenance were therefore woven throughout the curriculum to reduce the likelihood of weight regain, with increasing emphasis on relapse prevention later in the program. For example, participants learned to recognize their own high-risk situations and were taught how to handle them or avoid them, and to develop coping strategies for those that were unavoidable.
Materials and other items were provided to all intervention group participants to facilitate behavioral changes and strategies for promoting weight reduction. A participant notebook with worksheets, handouts and illustrations was developed and provided at the beginning of the group sessions. At the first group session, participants also were provided food and exercise journals, in which they were encouraged to monitor intake, and a pedometer (Classic Step Only Pedometer, Walk4Life, Plainfield, IL) to measure steps per day. At the second group session, participants received books with caloric content of food (CalorieKing Publications, Costa Mesa, CA) and were instructed to record calorie content of foods and drinks consumed and to plan a daily caloric deficit. Recommended web-based resources for monitoring intake and expenditure were also discussed as an alternative approach to self-monitoring. Participants also received a digital scale (EatSmart Precision Digital Scale, Eat Smart-HEALTH TOOLS, Wyckoff, NJ) and were advised to weigh themselves daily at a similar time, record their daily weight in their journal, and graph their weight. In subsequent sessions, all participants were provided two digital video discs for walking three and five miles (Leslie Sansone’s Walk at Home, Anchor Bay Entertainment, Beverly Hills, CA).
2.3. Less intensive intervention control group
Participants in the less intensive intervention control group were provided weight management resources and materials that were based on standard weight loss and maintenance guidelines available for the general public. Materials provided included brochures and information available to the public from the National Institutes of Health, the American Heart Association, the American Institute for Cancer Research, the United States Department of Health and Human Services, and the United States Department of Agriculture (e.g., ChooseMyPlate.gov). An individualized weight loss counseling session was provided at baseline and at 6 months, during which a calorie level appropriate for weight loss was prescribed (1200–2000 kcal/day, based on estimated energy requirements) and current physical activity recommendations (at least 30 minutes/day) were advised. In addition, participants in the control arm received monthly telephone calls from the study coordinator. These calls had a standardized script and served the purpose of staying in touch with the participants and promoting retention as well as updating health and personal information. As with the intervention participants, completed contacts were noted in the database, and inability to contact triggered follow-up by the site project coordinator.
Finally, these participants were also invited to attend informational optional seminars every other month during the first study year. The topics for these seminars focused on aspects of healthy living other than weight control, such as supplement use, food safety and healthy cooking. These seminars were tailored to the unique nature of the study population of breast cancer survivors.
2.4. Measures
Participants completed five clinic visits during the course of the study, at baseline and six, 12, 18, and 24 months, and received a reimbursement for time and effort for each visit. To enable an exploration of the effect of the intervention in various subgroups of participants, data on demographic and other characteristics (i.e., race/ethnicity, age, marital status, educational attainment, smoking status, menopausal status) were obtained at baseline.
Study-specific questionnaires were developed and were completed by participants at every clinic visit to assess any changes in medical conditions; any emergency room visits, hospitalizations, injuries and medications; and general and gastrointestinal symptoms and pain. These data will allow examination of effects of the intervention on co-morbidities.
Follow-up visits were conducted by staff who were blinded to participants’ group assignment. Table 3 summarizes the timing of measurements across these clinic visits.
Table 3.
Measurement and data collection points
| Measurement | Time Point | ||||
|---|---|---|---|---|---|
| Baseline | 6 Mos. | 12 Mos. | 18 Mos | 24 Mos. | |
| Weight | x | x | x | x | x |
| Waist circumference | x | x | x | ||
| Medical record review | x | ||||
| Blood pressure | x | x | x | x | x |
| Blood sample | x | x | x | x | |
| Questionnaires | x | x | x | x | |
| Step test | x | x | x | x | x |
| Dietary recall (UCSD only) | x | x | x | ||
2.4.1. Clinic visit measurements
Height was measured at baseline, and weight was measured at baseline and at 6-, 12-, 18- and 24-month follow-up visits, using a calibrated scale. Height and weight were used to calculate BMI (kg/m2), a widely-used index of adiposity. Waist (abdominal) circumference was obtained at baseline and 12 and 24 months. Standardized procedures for anthropometric measurements [51] were outlined in the Operations Manual.
Blood pressure was measured at all clinic visits following standardized procedures [52] outlined in the Operations Manual. Effort was made to measure blood pressure before other potentially stressful procedures such as phlebotomy or weighing. Two systolic and diastolic blood pressure measurements using a conventional mercury sphygmomanometer were obtained with the participant seated.
At the baseline and 6-, 12-, and 24-month clinic visits, a total of 30 mL of blood was collected by a licensed phlebotomist using standard protocols. Blood was collected after a minimum of six hours of fasting, which was verified prior to phlebotomy. Bar-coded labels for cryovials were provided to all sites by the UCSD Coordinating Center. Following appropriate processing, blood was stored in −80 degree C freezers, and cryovials were sent at regular intervals to the Coordinating Center on dry ice. Aliquots of serum, plasma (both heparin- and EDTA-preserved), buffy coat, and buffy coat with an RNA preservative were stored to enable conducting assays of mediating factors in relevant ancillary studies.
2.4.2. Medical record review
At baseline, medical record review was conducted to obtain information on breast cancer diagnosis and treatment and to verify eligibility. During the study, all new breast cancer event endpoints were reviewed and verified by the oncologist investigator at the study site and the study oncologist.
2.4.3. Diet, cardiopulmonary fitness, and physical activity measurements
The 3-minute step test was conducted during the clinic visit at baseline and six, 12, 18 and 24 months to detect possible changes in cardiopulmonary fitness. This test measures heart rate by taking the pulse for 30 seconds immediately after a 15-second recovery period from stepping. Step tests are useful for field-testing subjects, and although they are less accurate than measuring maximal oxygen uptake, they have high reliability and are sensitive to change [53].
Physical activity was measured with the Godin Leisure-Time Exercise Questionnaire (GLTEQ) at baseline, six, 12, 18 and 24 months. The GLTEQ consists of three questions that record the frequency and duration of mild, moderate, and strenuous exercise performed during free time in a typical week. It is a validated self -report measure of exercise that has been reliably used in previous cancer research studies [54].
Dietary data were collected from a subsample of participants (those enrolled at the UCSD site) and were based on a single day recall collected at baseline, six and 24 months by Automated Self-administered 24-hour Dietary Recall (ASA24). Participants were encouraged to make note of foods and beverages consumed prior to the clinic visit, at which time the recall was completed with guidance from trained research staff. Developed by the National Cancer Institute, ASA24 is a software tool that enables automated and self-administered 24-hour dietary recalls [55]. These data will be useful for examining group differences in dietary intake, although this approach will not permit accurate characterization of the dietary intake of a given individual, due to day-to-day variations in intake.
2.4.4. Psychosocial measures
Psychosocial data were collected using the following instruments at the baseline and specified follow-up clinic visits:
The SF-36
A multi-purpose, short-form health survey, the Short Form Health Survey (SF-36) was used as a general measure of QOL [56–59] at baseline and six, 12 and 24 months. It yields an 8-scale profile of functional health and well-being scores as well as psychometrically-based physical and mental health summary measures. Considerable evidence for the reliability of the SF-36 (Cronbach’s α>0.85, reliability coefficient >0.75) and for construct validity, in terms of distinguishing between groups, has been published [60, 61].
Center for Epidemiologic Studies Depression Scale (CES-D)
Risk for depression was assessed using the CES-D, which is comprised of 20 items and was developed to assess depression in the general population [62], at baseline and six, 12 and 24 months. Measures of internal consistency are high in the general population (0.85) and in psychiatric samples (0.90). Test-retest correlations are reported to be in the moderate range (0.45–0.70). Validity has been established with other self-report measures, correlations with clinical ratings of depression, and by construct validity [63, 64].
Breast Cancer Prevention Trial (BCPT) Symptom Scales
The BCPT was used to measure concurrent and late side effects of medical interventions to prevent and treat breast cancer [65] at baseline and 6, 12 and 24 months. Factor analysis with this instrument has revealed eight factors corresponding to physical symptoms associated with cancer treatment, chemoprevention, menopause, and normal aging: hot flashes, nausea, bladder control, vaginal problems, musculoskeletal pain, cognitive problems, weight problems, and arm problems.
Impact of Cancer Scale (IOCv2)
The refined IOCv2 was used to measure the impact of cancer on QOL [66] at baseline and 12 and 24 months. Results of analysis of this instrument in breast cancer survivors has yielded a factor structure relating IOC items to psychosocial impact domains that exhibited high factor loadings (factor-item correlations of 0.59–0.94) and high internal consistency (Cronbach’s α=0.76–0.89). The scales consist of a Positive Impact Summary scale with four subscales (Altruism and Empathy, Health Awareness, Meaning of Cancer, and Positive Self-Evaluation), a Negative Impact Summary scale with four subscales (Appearance Concerns, Body Change Concerns, Life Interferences, and Worry), and subscales for Employment and Relationship Concerns.
Lifetime Medical Conditions Questionnaire, and the Participant Questionnaire
These questionnaires were developed for the study and were used to assess the presence and the severity of co-morbid conditions at baseline and follow-up clinic visits. The information obtained was similar to that obtained from the Self-Administered Comorbidity Questionnaire [67].
2.5. Retention strategies
Coordinators at each site oversaw the cohort maintenance efforts directed at participants in both study arms. These activities included monthly standardized contacts, invitation to bimonthly seminars, mailing personalized cards, including birthday and secular holiday cards, as well as distribution of donated items (e.g., massage vouchers, coupons, beauty and clothing items) at regular intervals and monthly newsletters. Approximately two-thirds of the participants attended the seminars, which were offered as 1–2 sessions per topic (if two were offered, one was in the morning and one was in the afternoon) and were attended by 20–30 participants per session topic. In addition, staff at each site were trained to develop and maintain good rapport with participants, and an effort was made to schedule clinic visits and intervention sessions at times that were convenient to the participants, including week nights, early weekdays and weekends. The study manager at the Coordinating Center provided ongoing monitoring of activities at each site and provided assistance with retention activities.
Every attempt was made to complete the entire assessment battery, especially the body weight, which was the most critical outcome measure. Weight obtained within a three-month window of the clinic visit was used as the outcome of that assessment point. If the participant could not complete a follow-up clinic visit, they were asked to mail in completed study questionnaires in provided preaddressed postage-paid envelopes. For follow-up visits, participants also were given the option for a home visit at some sites.
In order to prevent loss to follow-up, participants were asked to provide multiple contact information (e.g., home, cell and work telephone numbers and email address) at study enrollment and follow-up time points. Additionally, contact information of up to three close relatives/friends who are not in the same household, as well as the contact information for the primary care physician and oncologist, was obtained at study entry.
2.6. Quality assurance (QA) and monitoring
The QA efforts for data collection included providing an Operations Manual that described the working procedures for the study protocol in detail. This ensured that all sites used the same visit procedures, participant management, follow-up schedules, definitions, and to the extent possible, the same type of equipment. In addition, site internal procedures (SIP) at each site were developed that included policies and procedures unique to the site and provided site-specific reference for the Coordinating Center. The SIPs facilitated routine operations, especially when staff members were absent and back-up was required. Regular meetings were held with site coordinators to discuss key trial issues (e.g., eligibility, enrollment, data entry) and update the Operations Manual and the SIPs as needed. QA was further assured by centralizing the study-wide training needs at the Coordinating Center. Certification was required to assess each staff member’s general understanding of study procedures and basic job functions. Because the study used a central data repository, various QA procedures for data cleaning also were conducted. Finally, study equipment was routinely calibrated.
A Certificate of Confidentiality was obtained from the Department of Health and Human Services to protect the privacy of participants by protecting the Investigator(s) from being forced to release any research data. Serious adverse events (SAE) were monitored at regularly scheduled intervals via clinic visits. Procedures for reporting the SAEs were included in the Operations Manual.
Most routine procedures associated with the conduct of the study had minimal risk. The physical activity recommendations targeted moderate physical activity. The risk associated with this component was managed by enrolling individuals for whom the level of activity recommended was highly unlikely to pose a medical problem and training all staff in appropriate guidelines, including providing information on lymphedema in order to facilitate timely referrals. There was no risk involved in the dietary guidance provided in the intervention materials or communications. These guidelines were identical to those recommended for all women for disease prevention and optimum weight control.
Critical values were identified for routine procedures (e.g., blood pressure, CES-D scores) that were performed as part of the clinic visit. The trial staff members were instructed to act upon immediately if and when these values were observed.
A Data and Safety Monitoring Board (DSMB) was established to monitor the progress of the trial, including safety-related matters. The DSMB met approximately twice per year and the members have access to unblinded outcome data during the trial. The committee included a statistician, an oncologist, a physician with expertise in weight loss studies and obesity management, and a patient advocate cancer survivor. The committee reviewed post-randomization exclusion requests, adherence rates, trends by group in the psychosocial measures, details of any study withdrawals, and health symptoms. Following a consideration of these data, the committee minutes were prepared and submitted to the UCSD IRB prior to the annual review.
2.7. Data management and statistical analysis
2.7.1. Data management
Project coordinators at each site were responsible for supervision of data gathering, editing and entry. Study data were managed using REDCap electronic data capture tools hosted at WUSTL. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources. The database was maintained as part of a HIPAA compliant network which is protected by a firewall and backed up regularly. The access to the database was restricted and users needed authorization and a user-specific password in order to log in the system. Data were coded at each site using unique identifiers and offsite users could only view their site-specific data. Dietary recall data were collected and maintained at UCSD and downloaded monthly to be sent to WUSTL.
2.7.2. Sample size justification
To address the primary hypothesis, change in weight at 24 months, we used the Diabetes Prevention Trial (DPT) baseline measures to estimate the standard deviation (SD) for weight (20.3) in planning this trial [68]. For power estimates, we assumed 2-tailed testing at an α = 0.05. Mean weight loss in the DPT was 6%, or 5.6 kg. For the present study, we assumed a 6.6 kg reduction in mean weight for the intervention group (estimated to be 7%) and a 0 kg reduction for the control arm. We also assumed that we will have data from the 24-month time point for 90% of participants in each arm, consistent with the range of completion in the DPT and Premier weight loss interventions [69] and in other large diet modification trials [28, 29]. The 10% of participants who do not complete the trial were assumed to have a mean weight change of 0 over 24 months. Hence, we assumed completers in the intervention arm would achieve a 7.3 kg weight loss yielding a net weight loss of 6.6 kg overall, and 0 kg among completers for the control group.
We also assumed a cross-sectional σ= 20.3 kg SD, based on DPT. Based on Nurse’s Health Study (NHS) data, we estimated the Pearson correlation between repeated weight measures over 2 years, i.e., from 2000 to 2002 and from 2002 to 2004, respectively, among women who had a BMI ≥ 25 and ≤50 kg/m2 at baseline. The estimated age-specific correlation (5-year age groups) over 2 years was 0.85.
Power = (− Z0.975 + (Sqrt (n )* Δ)) / (sqrt 2σ2 change)
where σ2change = 2 σ2baseline (1 – ρ) = 123.6, σ2baseline = 20.32,
ρ= 0.85, Z0.975 = 1.96, Δ = change over 2 years
With a sample of size 345 per group, we have 90% power to detect a mean change of 2.75 kg at 24 months.
2.7.3. Primary analysis
The primary analysis is based on change over 24 months without considering weight at the intermediate time points using mixed effects regression models with PROC MIXED of SAS [70]. The model is
| (1) |
where Yit = weight for subject i at time t, where t = 0 for baseline, t = 1 for 24 months.
Xi1 = 1 if the subject is in the intervention group,
= 0 otherwise
eit ~ N(0, σ2)
Of primary interest is β3A = mean difference in weight change between the intervention and control groups. The coefficients β2A allows for mean differences in weight between groups at baseline. The coefficient β4 allows the change in weight to depend on initial level.
2.7.4. Missing data
All missing weights will be treated as “missing at random” (MAR; see definition and justification below). Specifically, for administrative censoring (individual is still in the study and there has not been time for more follow-up), “moved out of area,” or for missed interior visits (the 24-month weight is available) use the analysis program’s missing data indicator (in SAS use “.”, in R use “NA”) so that each participant has either a recorded weight or an indication of missing data at each potential visit. For dropouts (not administratively censored, but missing the 24-month weight), also treat the missing weight as produced by an MAR process.
High-leverage events: recurrence and death
These events may have high impact on subsequent weights. Therefore, in our analysis recorded weights up to the event would be used and we would treat subsequent weights as missing at random. Similarly, our analysis would use recorded weights up to the visit before death, and treat subsequent weights as missing at random.
Justification for the MAR assumption
Our primary analysis would treat all missing weights as resulting from a “Missing at Random” (MAR) process. MAR denotes a missing data process through which the probability of missing can depend on all observed information (measured weights, covariates) but does not depend on the weight or weights that are not recorded. Importantly, MAR is by no means “Missing Completely at Random;” strong dependence of the missing data process on observed outcomes and on measured covariates is allowed. If available data are analyzed by a correct model (correct mean structure and covariance structure), a MAR missing data process is “ignorable” in that the analyst doesn’t need to model that process in order to produce valid inferences. Therefore, it is very important to build an approximately correct model for the observed outcome data, weight and BMI.
Even though MAR is almost never strictly correct, commonly it is close to correct [71]. This is especially the case with an analysis model that includes baseline covariates that are associated with dropout and for a measurement process with very high longitudinal correlations (our weight sequence will have high correlations). Therefore, even when MAR is not strictly correct, the situation would be close to MAR because of accurate prediction of missing data by the observed data. For these reasons and because the MAR-based analysis is straightforward to communicate, it is good practice to build a primary analysis on the MAR assumption and conduct sensitivity analyses around that assumption [72].
2.7.5. Outlier detection
Outliers (e. g., exceedingly large weight gains or losses) would be identified using the extreme Studentized deviate (ESD) approach of Rosner [73]. Outlier detection was planned to be performed separately for the three dependent variables (change in weight, BMI [detection will be based on change in 1/BMI], and log[weight]).
2.7.6. Secondary outcomes
Subgroup analysis
As a secondary outcome, fixed effect regression models are planned to compare 24-month weight loss change in the following subgroups: time since diagnosis (binarized on median time), type of tumor, and type of therapy.
Disease-free survival analysis
For the expanded cancer outcome trial, disease-free survival (DFS) will be defined as the time from randomization to breast cancer recurrence or death. The distribution of DFS time was to be estimated using the Kaplan-Meier method for intervention and control groups.
Time to event was planned to be analyzed with a Cox proportional hazards model to identify appropriate risk factors. Model building will follow the approach outlined by Harrell [70] with close collaboration between the statistician and clinicians.
Impact of the intervention on QOL
When we assess the impact of weight loss on overall health-related QOL, as reported by Stanton and colleagues [65], the focus will be on the vitality subscale since we believe that it will be most responsive to weight loss. We plan to assess change in scores from baseline. Baseline mean and SD (50.2; 21.8) is drawn from previous data from women with breast cancer, and change from baseline to 12 months ranged from 6 to 9.38 units in that study, depending on intervention arm (control arm 12-month mean change 6.06). In previous prospective studies of weight loss and increase in vitality measured from the SF-36, we observed in the NHS among women 25 kg or greater and under 65 years of age that a 9 kg or greater weight loss was associated with a mean increase in vitality of 4 units [74].
Thus, using the approach to power estimation outlined for the primary endpoint, we estimated that with at least 345 participants per arm we have 98% power to detect a difference in vitality between groups of 2.0 units. Analysis was planned to follow the approach for the primary aim and control for baseline SF-36. Additional control for depression (from the CES-D) at baseline is planned to be included in secondary analyses.
Per protocol analysis
For the per protocol analysis, a post hoc secondary analysis, we planned to perform a dose response analysis based on the number of contacts made with each participant as the treatment variable. Two types of contrast would be considered. The first is based on the number of contacts for each intervention participant vs. a value of zero for the number of contacts for each control group participant. The second contrast would be based on the number of contacts for each intervention subject vs. the number of contacts for each control group subject.
3. Discussion
Despite the potential benefit, no clinical trial has yet been conducted to determine whether weight loss and maintenance of that loss increases disease-free survival in overweight or obese women with a history of breast cancer. Although evidence suggests that benefits are likely, the effect of intentional weight loss on QOL or co-morbidities in this population also has not previously been examined. In the general population, intentional weight loss has been observed to favorably affect many breast cancer-relevant risk factors and potential mediators, such as circulating estrogens, SHBG, inflammatory markers and insulin sensitivity [75].
The proposed project has several unique features that make it an innovative and unique approach to the problem. First, we see value in a vanguard study approach. If the favorable effects on QOL and co-morbid conditions we expect to find can be documented in this trial, this finding could itself change the norms of clinical practice, making this type of intervention a standard of care after breast cancer treatment. Identifying effects of healthy weight management and increased physical activity on health and QOL, which is a specific focus of this study, may result in reduced utilization and costs of health care services by breast cancer survivors. Another specific outcome of this study is the biological sample repository that can facilitate exploration of potential mechanisms and increased knowledge of how genetic factors may explain differential responsiveness to change in lifestyle behaviors. Using the vanguard approach, we also have the opportunity to further tailor and streamline our efforts for the larger trial to follow, which will be fully-powered to examine breast cancer recurrence and disease-free survival.
We see value in applying wide inclusion criteria, particularly given the historical perspective provided by trials such as the Women’s Intervention Nutrition Study, which found the greatest impact of a low-fat diet in the subgroup in which the effect was hypothesized to be the least, i.e., in women with estrogen receptor negative tumors [76]. Furthermore, evidence to date also supports benefits of weight loss for overweight and obese breast cancer survivors across all age groups and tumor types. Importantly, the strategies, process and components of the intervention have been purposely conceived to be readily disseminable to broad clinical practice and community-based programs, rather than being relevant only in the context of major tertiary cancer care centers.
Studies testing whether diet counseling or prescribing increased physical activity can prevent weight gain in women during the immediate post-diagnosis period have produced mixed results. Women provided intensive diet counseling to achieve energy-restricted diets did not exhibit differences in weight gain during initial treatment compared to control subjects in a randomized trial of 104 women with early stage breast cancer [77]. In another small randomized controlled study involving early stage breast cancer patients receiving adjuvant chemotherapy (N = 24), prescribing aerobic exercise did not have a significant effect on weight gain, although significant differences were observed in the change in percent body fat (averaging −0.51% in the treatment group versus +2.19% in the control group) [78]. Both diet and physical activity were the behavioral targets in two small studies that found a significant reduction in body weight (or weight maintenance in those not overweight) in women recently diagnosed with breast cancer [79, 80]. Follow-up analysis in one of these studies revealed that the strongest predictor of the program’s success was increased physical activity [79]. In a small pilot study of 10 premenopausal breast cancer patients receiving adjuvant chemotherapy, a six-month program focused on supervised strength training (and also included aerobic exercise, and guidance toward a low-fat, high-vegetable and -fruit diet) resulted in significant changes in total body weight of –2.0 (1.3) kg and percent body fat of –1.3 (1.2) %, compared to historic controls who experienced gains of 2.2 (0.4) kg and 1.8 (1.6) %, respectively [81].
The effect of a weight loss intervention provided after the completion of initial treatments has been examined in a few small previous studies. In 48 obese breast cancer survivors, Djuric et al. [82] examined the effect of individualized weight-loss counseling with or without participation in a commercial group-based program versus participation in the program alone. After 12 months of participation in the study, the average weight change was 0.85 (6.0) kg (mean [SD]) for the control group, −2.6 (5.9) kg for the commercial program only group, −8.0 (5.5) kg for the individualized counseling only group, and –9.4 (8.6) kg for the combined treatment group. Compared with the control group, the combined group exhibited statistically significant differences at 3, 6, and 12 months, while the individualized counseling group was significantly different only at 12 months. Thomson et al. [83] examined the effect of prescribing a low-fat or a low-carbohydrate diet to promote weight loss in overweight postmenopausal breast cancer survivors (N = 40) on body weight and metabolic factors. At six months, weight loss averaged 6.1 (4.8) kg and did not differ by diet group, and improvements in total/HDL cholesterol ratio, HbA1c, and insulin were observed in association with weight loss. A nonrandomized 6-month weight loss intervention for obese breast cancer survivors (N = 34) involving an energy-reduced diet incorporating prepackaged entrees and replacement meal beverages, increased physical activity, and weekly group telephone sessions promoted a reduction in weight (−12.5 [5.8] kg), waist circumference (−9.4 [6.3] cm), and insulin and leptin concentrations [84]. Notably, these previous studies demonstrate that weight loss, at least in the short-term, can be achieved in this target group. The ENERGY study, which is the largest weight loss randomized controlled trial to date, moves beyond the previous studies with a design, methodology and data collection that enables expansion to address sustained weight loss and QOL, and ultimately, breast cancer recurrence and disease-free survival.
In summary, the ENERGY study is a unique randomized controlled trial of the effect of a cognitive-behavioral weight loss intervention specifically designed for overweight or obese breast cancer survivors on body weight, QOL and co-morbidities. Overall, this study has a high potential to have an impact on the clinical management and outcomes after a diagnosis of breast cancer. Whether or not the expansion of this trial is approved and funded to examine cancer-specific outcomes will depend on data relating to recruitment, retention, and response to the intervention. If the eventual full trial can document lower recurrence risk and greater likelihood of survival with this same intervention, this trial initiates the effort to establish weight loss support for overweight or obese breast cancer survivors as a new standard of clinical care.
A key step needed to translate this type of program into standard of care is to conduct the larger trial in which effects on cancer outcomes and disease-free survival are examined. Based on several recently published sources of data on recurrence rates [76, 85, 86] and the cancer characteristics of the vanguard study participants, we estimate that a total sample size of approximately 2500 will be necessary for sufficient statistical power to examine effects on cancer outcomes. Further, we propose to carry out the trial in the most cost-effective way while integrating it into systems of clinical care and health care delivery organizations so that translation of positive findings might be enabled. This venue would suit our goal of testing the strategies, process and components of the intervention that are purposely conceived to be readily disseminable to broad clinical practice and community-based programs.
Acknowledgments
This study was supported by NCI grant CA148791. Preliminary studies to develop the intervention components were supported by NCI grants CA90413, CA101489, CA81191, and CA106919. Management of stored biological samples at the UCSD Coordinating Center was facilitated by the Nutrition Shared Resource of the Moores UCSD Cancer Center (NCI Cancer Center Support Grant CA23100).
The authors thank the data and safety monitoring committee: Bernard Rosner, PhD, Joanne Mortimer, MD, Ken Fujioka MD, and Linda Litzau. The authors thank Catherine Alfano, PhD (Program Officer) and Julia Rowland, PhD, NIH Office of Cancer Survivorship, and also Robert Croyle, PhD, NIH Division of Cancer Control and Population Sciences, for their assistance, guidance and support. The authors also thank Shirley Flatt, MS, Chris Zoumas, MS, RD, and Lea Jacinto for their assistance in manuscript preparation and submission.
This publication also was made possible by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or NIH. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Tissue Procurement Core, which provided sample storage and processing (supported in part by an NCI Cancer Center Support Grant CA91842).
Abbreviations
- ASA24
Automated Self-administered 24-hour Dietary Recall
- BMI
body mass index
- BCPT
Breast Cancer Prevention Trial
- CES-D
Center for Epidemiologic Studies Depression Scale
- DSMB
Data and Safety Monitoring Board
- DPT
Diabetes Prevention Trial
- DFS
disease-free survival
- EDTA
ethylenediaminetetraacetic acid
- ENERGY
Exercise and Nutrition to Enhance Recovery and Good Health for You
- GLTEQ
Godin Leisure-Time Exercise Questionnaire
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HIPAA
Health Insurance Portability and Accountability Act
- IOCv2
Impact of Cancer Scale
- IL-6
interleukin-6
- IRB
institutional review board
- MAR
missing at random
- NIH
National Institutes of Health
- NHS
Nurse’s Health Study
- PCP
primary care physician
- QA
quality assurance
- QOL
quality of life
- REDCap
Research Electronic Data Capture
- RENEW
Reach out to Enhance Wellness
- RNA
ribonucleic acid
- SAE
serious adverse events
- SHBG
sex hormone binding globulin
- SF-36
Short form Health Survey
- SD
standard deviation
- SIP
site internal procedures
- TNF-α
tumor necrosis factor α
- UCSD
University of California, San Diego
- WUSTL
Washington University in St. Louis.
Appendix
The ENERGY Trial Group
University of California, San Diego: Cheryl Rock, PhD, RD, Bilge Pakiz, EdD, Barbara Parker, MD, Chis Zoumas, MS, RD, Shirley Flatt, MS, Hava Shoshana Barkai, MS, RD, Dennis Heath, MS, Lea Jacinto, Mila Pruitt.
University of California, Los Angeles: Patricia Ganz, MD.
University of Colorado Denver: Tim Byers, MD, MPH, Rebecca Sedjo, PhD, Holly Wyatt, MD, Anthony Elias, MD, James Hill, PhD, Jhenny Hernandez, MBA, Kim Gorman, MS, RD, Carmen Faust, MPH, Anna Van Pelt.
Washington University in St. Louis: Graham Colditz, MD, Kathleen Wolin, ScD, Jingxia Liu, PhD, Michael Naughton, MD, Casey Fagin, MA, Jennifer Tappenden, Sonya Izadi.
University of Alabama at Birmingham: Wendy Demark-Wahnefried, PhD, RD, Helen Krontiras, MD, Maria Azrad, PhD, RD, Cindy Blair, PhD, Lahnor Powell, DO, Laura Lee Goree, MS, RD, Karen Kubas, MS, RD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
All authors state that they have no actual or potential conflict of interest including any financial, personal or other relationship with other people or organizations within three years of beginning the work submitted that could have inappropriately influenced their work.
Contributor Information
Tim E. Byers, Email: Tim.Byers@ucdenver.edu.
Graham A. Colditz, Email: colditzg@wudosis.wustl.edu.
Wendy Demark-Wahnefried, Email: demark@uab.edu.
Patricia A. Ganz, Email: pganz@mednet.ucla.edu.
Kathleen Y. Wolin, Email: wolink@wudosis.wustl.edu.
Anthony Elias, Email: anthony.elias@ucdenver.edu.
Helen Krontiras, Email: hkrontir@uab.edu.
Jingxia Liu, Email: esther@wubios.wustl.edu.
Michael Naughton, Email: mnaughto@im.wustl.edu.
Bilgé Pakiz, Email: bpakiz@ucsd.edu.
Barbara A. Parker, Email: baparker@ucsd.edu.
Rebecca L. Sedjo, Email: Rebecca.Sedjo@ucdenver.edu.
Holly Wyatt, Email: holly.wyatt@ucdenver.edu.
References
- 1.AICR/WCRF. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. London: World Cancer Research Fund; 2007. [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; [Google Scholar]
- 3.Ries L, Melbert D, Krapcho M, Stinchcomb D, et al. SEER Cancer Statistics Review 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 4.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;1188(Suppl):2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;204:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 6.Majed B, Moreau T, Senouci K, Salmon RJ, et al. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;1112:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 7.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;661:5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;1233:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 9.Daling JR, Malone KE, Doody DR, Johnson LG, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;924:720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;23:17–31. doi: 10.1016/s1098-3597(99)90002-9. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Center for Disease Control and Prevention. Overweight and Obesity. 2009 Available from: http://www.cdc.gov/nccdphp/dnpa/Obesity/.
- 12.Bines J, Gradishar WJ. Primary care issues for the breast cancer survivor. Compr Ther. 1997;239:605–611. [PubMed] [Google Scholar]
- 13.Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;8512:979–987. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]
- 14.Ahern TP, Lash TL, Thwin SS, Silliman RA. Impact of acquired comorbidities on all-cause mortality rates among older breast cancer survivors. Med Care. 2009;471:73–79. doi: 10.1097/MLR.0b013e318180913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaaks R, Rinaldi S, Key TJ, Berrino F, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;124:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 16.Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;134:371–379. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 17.Ray A. Adipokine leptin in obesity-related pathology of breast cancer. J Biosci. 2012;372:289–294. doi: 10.1007/s12038-012-9191-9. [DOI] [PubMed] [Google Scholar]
- 18.Key TJ, Appleby PN, Reeves GK, Roddam A, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;9516:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 19.Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2008;669:506–516. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 20.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;291:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 21.Rock CL, Flatt SW, Laughlin GA, Gold EB, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;173:614–620. doi: 10.1158/1055-9965.EPI-07-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastard JP, Maachi M, Lagathu C, Kim MJ, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;171:4–12. [PubMed] [Google Scholar]
- 23.Festa A, D'Agostino R, Jr, Williams K, Karter AJ, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;2510:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 24.Lithgow D, Covington C. Chronic inflammation and breast pathology: a theoretical model. Biol Res Nurs. 2005;72:118–129. doi: 10.1177/1099800405280823. [DOI] [PubMed] [Google Scholar]
- 25.Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007;1042:145–152. doi: 10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 26.Yeter K, Rock CL, Pakiz B, Bardwell WA, et al. Depressive symptoms, eating psychopathology, and physical activity in obese breast cancer survivors. Psychooncology. 2006;156:453–462. doi: 10.1002/pon.974. [DOI] [PubMed] [Google Scholar]
- 27.Pakiz B, Flatt S, Zoumas-Morse C, Rock C. AICR Research Conference on Food, Nutrition, Physical Activity and Cancer. Washington, D.C.: 2008. Risk factors after participating in a one-year cognitive-behavioral therapy weight management program for obese breast cancer survivors. [Google Scholar]
- 28.Taylor DL, Nichols JF, Pakiz B, Bardwell WA, et al. Relationships between cardiorespiratory fitness, physical activity, and psychosocial variables in overweight and obese breast cancer survivors. Int J Behav Med. 2010;174:264–270. doi: 10.1007/s12529-010-9076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock CL, Pande C, Flatt SW, Ying C, et al. Favorable changes in serum estrogens and other biological factors after weight loss in overweight or obese breast cancer survivors. Clinical Breast Cancer. doi: 10.1016/j.clbc.2012.12.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;2519:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W, Clipp EC, McBride C, Lobach DF, et al. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Med Sci Sports Exerc. 2003;353:415–424. doi: 10.1249/01.MSS.0000053704.28156.0F. [DOI] [PubMed] [Google Scholar]
- 32.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, et al. Promoting Healthy Lifestyles Among Older Cancer Survivors to Improve Health and Preserve Function. Journal of American Gerontological Association. 2009 doi: 10.1111/j.1532-5415.2009.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder DC, Morey MC, Sloane R, Stull V, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;184:429–439. doi: 10.1002/pon.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morey MC, Snyder DC, Sloane R, Cohen HJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;30118:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallis JF, Calfas KJ, Alcaraz JE, Gehrman C, et al. Potential mediators of change in a physical activity promotion course for university students: Project GRAD. Ann Behav Med. 1999;212:149–158. doi: 10.1007/BF02908296. [DOI] [PubMed] [Google Scholar]
- 36.Bandura A. Social Learning Theory. Englewood Cliffs: Prentice-Hall; 1977. [Google Scholar]
- 37.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 38.Bandura A. Self-Efficacy: The Exercise of Self-Control. New York: W.H. Freeman; 1997. [Google Scholar]
- 39.Miller WR, Rollnick S. Motivational Interviewing: preparing People to Change Addictive Behavior. New York: Guilford Press; 1991. [Google Scholar]
- 40.DiLillo V, Siegfried NJ, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cognitive and Behavioral Practice. 2003;10:120–130. [Google Scholar]
- 41.Beck AT. Cognitive therapy and the emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- 42.Beck JS. Cognitive therapy: Basics and beyond. New York: Guildford; 1995. [Google Scholar]
- 43.Cooper Z, Fairburn CG. A new cognitive behavioural approach to the treatment of obesity. Behav Res Ther. 2001;395:499–511. doi: 10.1016/s0005-7967(00)00065-6. [DOI] [PubMed] [Google Scholar]
- 44.Fairburn CG, Cooper Z. New perspectives on dietary and behavioural treatments for obesity. Int J Obes Relat Metab Disord. 1996;20(Suppl 1):S9–S13. [PubMed] [Google Scholar]
- 45.Cooper A, Fairburn CG, Hawker DM. Cognitive-Behavioral Treatment of Obesity. New York: Guilford Press; 2003. [Google Scholar]
- 46.Wilson Acceptance and change in the treatment of eating disorders and obesity. Behavior Therapy. 1996;273:417–439. [Google Scholar]
- 47.IOM. Crossing the quality chasm: A new health system for the 21st Century. Washington, DC: Committee on Quality of Health Care in America. Institute of Medicine; 2001. [Google Scholar]
- 48.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;29010:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 49.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;784:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 427:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 51.NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY III. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf.
- 52.NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY III CYCLE 2. Revised July 1993; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/pressure.pdf.
- 53.McArdle WD, Katch FI, Pechar GS, Jacobson L, et al. Reliability and interrelationships between maximal oxygen intake, physical work capacity and step-test scores in college women. Med Sci Sports. 1972;44:182–186. [PubMed] [Google Scholar]
- 54.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;1082:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 55.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): A Resource for Researchers, Clinicians, and Educators from the National Cancer Institute. J Acad Nutr Diet. 2012;1128:1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brazier J, Usherwood T, Harper R, Thomas K. Deriving a Single Index Value for Health From the UK SF-36® Health Survey. J Clin Epidemiol. 1998;5111:1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 57.Brazier J, Roberts J, Deverill M. The Evaluation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 58.Ware J. SF-36 Health Survey update. In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3rd ed. Mahwah (NJ): Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 59.Ware J, Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructions. Med Care. 1992;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Brazier JE, Harper R, Jones NM, O'Cathain A, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;3056846:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ. 2009;338:a3006. doi: 10.1136/bmj.a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;268:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Murphy J. Psychiatric instrument development for primary care research: patient self-report questionnaire, report on contract 80M01428101D. Bethesda, Md.: National Institute of Mental Health; 1982. [Google Scholar]
- 64.Bardwell WA, Ancoli-Israel S, Dimsdale JE. Comparison of the effects of depressive symptoms and apnea severity on fatigue in patients with obstructive sleep apnea: a replication study. J Affect Disord. 2007;971-3:181–186. doi: 10.1016/j.jad.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;976:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 66.Crespi CM, Ganz PA, Petersen L, Castillo A, et al. Refinement and psychometric evaluation of the impact of cancer scale. J Natl Cancer Inst. 2008;10021:1530–1541. doi: 10.1093/jnci/djn340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sangha O, Stucki G, Liang MH, Fossel AH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;492:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 68.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;3466:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;1447:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 70.Harrell F. Regression modeling strategies: with applications to linear models, logistic regression and survival analysis. Springer; 2001. [Google Scholar]
- 71.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;43:175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 72.Daniels MJ, Hogan JW. Missing Data in Longitudinal Studies, Strategies for Bayesian Modeling and Sensitivity Analysis. Boca Raton, FL: Chapman & Hall/CRC; 2008. [Google Scholar]
- 73.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;252:165–172. [Google Scholar]
- 74.Fine JT, Colditz GA, Coakley EH, Moseley G, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;28222:2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 75.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;1312:1063–1072. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 76.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;9824:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 77.Loprinzi CL, Athmann LM, Kardinal CG, O'Fallon JR, et al. Randomized trial of dietician counseling to try to prevent weight gain associated with breast cancer adjuvant chemotherapy. Oncology. 1996;533:228–332. doi: 10.1159/000227565. [DOI] [PubMed] [Google Scholar]
- 78.Winningham ML, MacVicar MG, Bondoc M, Anderson JI, et al. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol Nurs Forum. 1989;165:683–689. [PubMed] [Google Scholar]
- 79.Goodwin P, Esplen MJ, Butler K, Winocur J, et al. Multidisciplinary weight management in locoregional breast cancer: results of a phase II study. Breast Cancer Res Treat. 1998;481:53–64. doi: 10.1023/a:1005942017626. [DOI] [PubMed] [Google Scholar]
- 80.McTiernan A, Ulrich C, Kumai C, Bean D, et al. Anthropometric and hormone effects of an eight-week exercise-diet intervention in breast cancer patients: results of a pilot study. Cancer Epidemiol Biomarkers Prev. 1998;76:477–481. [PubMed] [Google Scholar]
- 81.Demark-Wahnefried W, Kenyon AJ, Eberle P, Skye A, et al. Preventing sarcopenic obesity among breast cancer patients who receive adjuvant chemotherapy: results of a feasibility study. Clin Exerc Physiol. 2002;41:44–49. [PMC free article] [PubMed] [Google Scholar]
- 82.Djuric Z, DiLaura NM, Jenkins I, Darga L, et al. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;107:657–665. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 83.Thomson CA, Stopeck AT, Bea JW, Cussler E, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;628:1142–1152. doi: 10.1080/01635581.2010.513803. [DOI] [PubMed] [Google Scholar]
- 84.Befort CA, Klemp JR, Austin HL, Perri MG, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;1322:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierce JP, Natarajan L, Caan BJ, Parker BA, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;2983:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng L, Swartz MD, Zhao H, Kapadia AS, et al. Hazard of recurrence among women after primary breast cancer treatment--a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;215:800–809. doi: 10.1158/1055-9965.EPI-11-1089. [DOI] [PubMed] [Google Scholar]