Abstract
CD45 is a receptor-like tyrosine phosphatase that positively regulates BCR signaling by dephosphorylating the inhibitory tyrosine of the Src family kinases (SFKs). We have previously shown that a single point mutation, E613R, introduced into the cytoplasmic membrane-proximal ‘wedge’ domain of CD45 is sufficient to drive a lupus-like autoimmune disease on a susceptible genetic background. To clarify the molecular mechanism of this disease, here we took advantage of a unique allelic series of mice in which the expression of CD45 is varied across a broad range. Although both E613R B cells and those with supraphysiologic CD45 expression exhibited hyper-responsive B cell receptor (BCR) signaling, they did so by opposite regulation of the SFK Lyn. We demonstrated that the E613R allele of CD45 does not function as a hyper- or hypo-morphic allele, but rather alters the substrate specificity of CD45 for Lyn. Despite similarly enhancing BCR signaling, only B cells with supraphysiologic CD45 expression became anergic, while only mice harboring the E613R mutation developed frank autoimmunity on a susceptible genetic background. We showed that selective impairment of a Lyn-dependent negative regulatory circuit in E613R B cells drove autoimmunity in E613R mice. This demonstrates that relaxing negative regulation of BCR signaling rather than enhancing positive regulation is critical for driving autoimmunity in this system.
Keywords: autoimmunity, lupus, signal transduction, B cells, tolerance
Introduction
Hyper-responsive B cell receptor (BCR) signaling has been implicated in the pathogenesis of lupus-like autoimmunity in mice and humans (1, 2). Dysregulated BCR signaling in mice can produce systemic autoimmunity, whereas human genome-wide association studies have identified a role for BCR signaling genes in lupus pathogenesis (1–3). However, the molecular and cellular mechanisms by which abnormal BCR signaling breaks tolerance and drives autoimmune disease remain uncertain.
Antigen receptor (AgR) signaling relies upon the sequential activation of the Src and Syk family kinases (4, 5). In response to receptor ligation, the Src family kinases (SFKs) phosphorylate tyrosines within cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) of the BCR or the T cell receptor (TCR). Doubly phosphorylated ITAMs in B cells and T cells recruit the tandem-SH2 domain-containing Syk family kinases, Syk and Zap70, respectively (4, 6). Once activated, Src and Syk family kinases phosphorylate critical downstream targets, generating extensive signal diversification, and driving diverse cellular outcomes.
Importantly, numerous inhibitory coreceptors containing immunoreceptor tyrosine-based inhibitory motifs (ITIMs) are expressed on B cells and serve as critical negative regulators of BCR signaling (6). The B cell SFK Lyn phosphorylates ITIMs, which in turn recruit the inhibitory effector protein and lipid phosphatases SHP-1 and SHIP1, respectively (7, 8). Unlike the SFKs Fyn and Blk which play exclusively positive regulatory roles during BCR signaling, Lyn thus plays a non-redundant negative regulatory role in addition to its positive regulatory role (7, 8). Importantly, loss or impairment of SHP-1, SHIP1, or Lyn function have each been shown to produce autoimmunity in mice, suggesting that this negative regulatory circuit is essential in order to maintain tolerance (2, 9–14).
Situated at the top of antigen receptor signaling cascades, the SFKs are themselves tightly regulated. Phosphorylation of the c-terminal tyrosine of the SFKs stabilizes an autoinhibitory conformation, while phosphorylation of the activation loop tyrosine of the SFKs is required for full enzymatic activity (15). The receptor-like tyrosine phosphatase CD45 serves as a critical positive regulator of AgR signaling by dephosphorylating the inhibitory tyrosine of the SFKs (15, 16). The inhibitory tyrosine of the T-cell-specific SFK Lck (Y505) is hyper-phosphorylated in CD45-deficient primary T cells (15, 17). Consequently, TCR signaling and T cell development are severely disrupted in mice deficient for CD45 (17–20). BCR signaling and B cell development are also impaired in CD45-deficient mice, but this phenotype is much milder than that of T cells because of the expression of a partially redundant phosphatase, CD148 (21).
Although CD45 is the most abundantly expressed phosphatase on the surface of nucleated hematopoietic cells (15), the function of its large, heavily glycosylated extra-cellular domain remains unknown. Alternate splicing of this extracellular domain generates multiple CD45 isoforms whose expression are tightly regulated in a cell-type, activation, and developmental stage-specific manner (15, 22). A genetic polymorphism that alters such splicing but not amino acid sequence is associated with human autoimmune disease (23). These data imply that the extracellular domain of CD45 must have an important function. However, a bona-fide ligand has yet to be identified, raising the possibility that the extracellular domain of CD45 may regulate its activity by other means. Postulated functions for this domain have included association with the CD4 coreceptor or dimerization (24, 25).
Our lab has previously explored the importance of a cytoplasmic juxtamembrane wedge domain in CD45 that may function to mediate dimerization-induced inhibition of phosphatase activity. Introduction of a point mutation (E613R) at the tip of this structural wedge domain relieves its inhibitory function in cell line studies of forced dimerization (26, 27). In vivo, this mutation produces a lupus-like phenotype on susceptible genetic backgrounds and renders B cells hyper-responsive to BCR signaling (28, 29). Both thymic positive selection and TCR signaling in thymocytes are enhanced in E613R mice (30). Although this mutation was predicted to generate a hypermorphic allele of CD45, E613R T and B cells exhibit hyper-phosphorylation of the inhibitory tyrosine of the SFKs, suggesting that an alternative mechanism might account for functional and cellular phenotypes in these mice (29, 30). The normal function of the wedge domain and the precise biochemical mechanism by which E613R alters CD45 function remains unclear.
We have recently generated an allelic series of mice in which CD45 splicing is intact but expression is varied across a broad range (0–180% in B cells) (31, 32). In T and B cells, increasing CD45 expression across this series reduces phosphorylation of the inhibitory tyrosines of Lck and Lyn, respectively (31, 32). We and others have demonstrated, through studies of this series, that CD45 plays a dual positive and negative regulatory role in response to in vitro TCR stimulation, such that even very low CD45 expression is sufficient to reconstitute TCR signaling, but supraphysiologic CD45 expression inhibits TCR signaling (32, 33). The unexpected negative regulatory role of CD45 in T cells is thought to be mediated by dephosphorylation of the activation loop tyrosine of the SFKs. By contrast, in B cells, CD45 plays a purely positive regulatory role (31). Because the mechanism by which the E613R allele of CD45 produces dysregulated AgR signaling and autoimmunity remains unclear, we decided to compare the CD45 E613R allele with our allelic series. In particular, we have focused attention on so-called HE (H/− or H/H) mice in which one or two copies, respectively, of normally spliced CD45 are overexpressed on top of endogenous CD45. This represents a bona-fide CD45 “hyper-morph” and serves as a critical contrast to E613R mice.
We find that the CD45 E613R allele is neither a pure hypo-morphic nor a hyper-morphic variant of CD45, but instead alters specificity for individual SFK substrates, suggesting that the wedge domain of CD45 normally plays a role in substrate selectivity. We showed that both E613R and H/H mice exhibited hyper-responsive proximal BCR signaling but do so through opposing regulation of Lyn. We further showed that these differences have important functional consequences such that H/H but not E613R B cells were partially anergic. On a susceptible genetic background, E613R but not H/− mice exhibited frank autoimmunity. Finally, we show that impairment of a Lyn-dependent negative regulatory circuit in E613R but not H/H B cells accounts for these phenotypes. This demonstrates that relieving negative regulation of BCR signaling rather than enhancing positive regulation is critical for driving systemic autoimmunity in this model.
Materials and Methods
Mice
Lightning mice were generated directly on the C57BL/6 genetic background during N-ethyl-N-nitrosourea mutagenesis screen conducted at Australian National University (32). Lightning mice were backcrossed to C57BL/6 at least 6 generations. H/H (HE) mice and CD45−/− mice were previously described (19, 34). CD45 E613R and Lyn−/− mice were previously described (9, 28). All knockout and transgenic strains were fully backcrossed to C57BL/6 genetic background. Mice were used at 5–9 weeks of age for all functional and biochemical experiments. All mice were housed in a specific pathogen free facility at UCSF according to the University Animal Care Committee and National Institutes of Health (NIH) guidelines.
Antibodies and Reagents
Antibodies were obtained as follows: murine CD3, CD4, CD5, CD8, CD19, CD21, CD22, CD23, CD44, panCD45, B220, CD45.1, AA4.1, IgM, IgD antibodies conjugated to FITC, PE, PerCP-Cy5.5, PE-Cy5.5, PE-Cy7, Pacific Blue, APC, or Alexa 647 (eBiosciences or BD Biosciences); pErk1/2 T202/Y204 (197G2) antibody and phospho-S6 ribosomal protein (S235/236) Al-488 (2F9) antibody for intracellular staining; pSrc Y416, pLyn Y507, pSHP-1 Y564, pSHIP1 Y1020, SHP-1 and Lyn antibodies (Cell Signaling); SHIP1, Erk 1 and 2 antibodies (Santa Cruz Biotechnology); pLck Y505 and CD45 antibodies (BD Transduction); Lck (1F6), pY (4G10) and Zeta (6B10) antibodies were prepared in our laboratory; unconjugated CD3ε (2C11) antibody (Harlan); goat anti-armenian hamster IgG(H+L) antibody, goat anti-mouse IgM, and goat anti-rabbit IgG antibody conjugated to either PE or APC (Jackson Immunoresearch).
Flow Cytometry and data analysis
Cells were stained with indicated antibodies and analyzed on FACSCalibur, or Fortessa (Becton Dickson) as previously described (29). Data analysis was performed using FlowJo (v8.8.4) software (Treestar Incorporated, Ashland, OR). Statistical analysis and graphs were generated using Prism v4c (GraphPad Software, Inc). Densitometry measurements were performed using Kodak Image station/Molecular Imaging software.
Lymph node T and B cell stimulation, and intracellular phospho-Erk and phospho-S6 staining
Performed as previously described (35). Phospho-S6staining performed using same protocol as phospho-Erk except phospho-S6 antibody is directly conjugated to fluorophore. Phospho-Erk assay also carried out after 15 minute preincubation with U0126 Mek1/2 inhibitor (Cell Signaling) at 10nM concentration in order to confirm specificity of antibody.
Calcium measurements
Assays were performed as previously described(35) except Indo-1 dye (Invitrogen) was used to load cells, and a UV laser on the BD Fortessa was used for detection. Prior to stimulation and analysis, lymphocytes were surface stained for expression of CD23 and AA4.1 to identify B cell subsets. Stimulation was carried out using varying doses of anti-IgM Fab’2.
Immunoprecipitation and Immunoblotting
Performed as previously described (21). Purified cell populations of splenic B cells and CD4+ T cells were obtained by sorting with MACS kits as per manufacturer’s instructions (Miltenyi). Blot densitometry was performed using Kodak Imagestation software.
BAFF ELISA
Kit obtained from R&D biosystems and used per manufacturer’s instructions
dsDNA ELISA, proteinuria, and histopathology: assessed as previously described (36)
Results
Differential regulation of the SFKs Lck and Fyn by the CD45 E613R allele in T cells
SFK phosphorylation and antigen receptor signaling are tightly regulated by CD45 in T cells. We sought to identify a biochemical ‘signature’ of hyper-morphic and hypo-morphic CD45 function in T cells against which to compare the E613R allele of CD45. To do so, we took advantage of an allelic series of mice in which CD45 expression is varied across a broad range. The series includes mice harboring one or two copies of the Lightning (L) allele in which surface levels of CD45 expression are reduced relative to wild type (L/− = 7%; L/L = 15%) (32). In addition the series includes mice with either one or two copies of the ‘H’ CD45 transgene (H/− and H/H mice, respectively) that express supraphysiologic levels (140% and 180%, respectively) of the protein (32, 34).
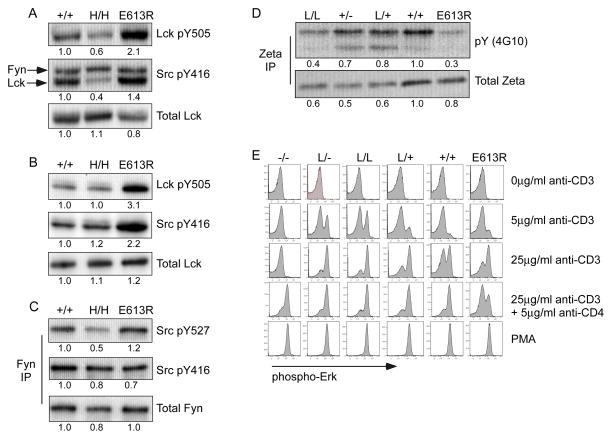
T cells express two SFKs, Lck and Fyn, whose inhibitory and activating sites of tyrosine phosphorylation serve as CD45 substrates (32, 33, 37, 38). We assessed tyrosine phosphorylation of Lck and Fyn in thymocytes and peripheral T cells from E613R and allelic series mice (Fig 1A, B, S1A). As reported previously, E613R thymocytes and peripheral T cells exhibit hyper-phosphorylation at both of these tyrosines in Lck (Fig 1A, B, S1A; (30)). Interestingly, this biochemical phenotype most closely resembles that of L/− and L/L mice with low levels of CD45 expression, suggesting that CD45 E613R might be a hypomorphic allele (31, 32). In contrast, T cells with high CD45 expression exhibit reduced phosphorylation of both Lck regulatory tyrosines (Fig 1A, B; (31, 32)). However, regulation of Fyn in these mice differed markedly from that of Lck (Fig 1A, B, C). In contrast to Lck, phosphorylation of the activation loop tyrosine of Fyn was not appreciably altered in E613R T cells relative to wild type (Fig 1A, B, C). This suggests that access to Lck tyrosine phosphorylation sites but not of Fyn’s are specifically impaired in E613R T cells.
Figure 1. Selective dysregulation of Lck phosphorylation in E613R T cells.
(A) Whole-cell lysates of resting LN CD4 T cells from +/+, H/H, and E613R mice were blotted with Ab to the inhibitory and activating tyrosines of Lck (Lck pY505/Src pY416). Src416 Ab binds activating tyrosines of all SFKs. The lower band represents p56 Lck; the upper band, p59 Fyn. Densitometry was performed on the lower Lck band. Total Lck is detected as a loading controls.
(B) Whole-cell lysates of resting thymocytes from +/+, H/H, and E613R mice were blotted with Ab to the inhibitory and activating tyrosines of Lck (Lck pY505/Src pY416). The single band detected by Src416 in thymocytes represents Lck. Total Lck is detected as a loading control.
(C) Fyn was immunoprecipitated from whole-cell lysates of resting thymocytes from +/+, H/H, and E613R mice. Immunoprecipitates (IPs) were subsequently blotted with Ab to the inhibitory and activating tyrosines of Fyn (pSrc527/pSrc416). Total Fyn is detected as loading control.
(D) TCR-associated ζ chain was immunoprecipitated from whole-cell lysates of resting thymocytes from CD45 allelic series and E613R mice. IPs were subsequently blotted for total ζ and total phosphotyrosine (pY) levels.
Data in 1A–D are representative of at least two independent experiments.
(E) TCR-stimulated, fixed, and permeabilized thymocytes were stained for phospho-Erk and costained for CD4 and CD8 so that DP subsets could be identified. Data were collected by flow cytometry. Histograms depict intracellular phospho-Erk in DP subsets from allelic series and E613R thymi. Data are representative of at least three independent experiments.
Densitometry measurements in this and all subsequent figures were normalized to wild type.
It has been suggested that dual phosphorylation of both the activation loop and inhibitory tyrosines of Lck can enhance its enzymatic activity (39). To clarify the activity of Lck in E613R T cells, we next assessed phosphorylation of TCR-associated zeta chain, a direct Lck substrate, in E613R and allelic series thymocytes (40, 41). Basal zeta chain phosphorylation in thymocytes requires non-selecting MHC/peptide, CD45, and Lck, but not Fyn (32, 40, 41). We observe markedly reduced basal zeta phosphorylation in E613R thymocytes, most closely resembling L/− thymocytes with low CD45 expression (Fig 1D). This suggests that Lck is less active in E613R thymocytes in the basal state, similar to cells with low CD45 expression.
In addition to its well-characterized role as a positive regulator of TCR signaling, we and others have previously reported that low CD45 expression in thymocytes results in TCR hyper-responsiveness, unmasking an additional negative regulatory role for CD45 in TCR signaling, most likely mediated by dephosphorylation of the activation loop tyrosine of Lck (Fig 1E; (32, 33, 37). E613R thymocytes are also hyper-responsive to TCR stimulation (Fig 1E; (30). Here we show that E613R thymocytes appear to functionally resemble L/+ thymocytes most closely (Fig 1E). However, in response to CD3/CD4 co-ligation, E613R thymocytes are uniquely hypo-responsive, in contrast to low CD45-expressing mice. This suggests that E613R in T cells is not a simple hypomorphic allele of CD45. Rather, the CD4-associated pool of Lck in E613R thymocytes appears to be hypo-active, suggesting that the E613R mutation at the wedge domain may alter access to this particular substrate pool.
Differential regulation of the SFKs Lyn and Fyn by the CD45 E613R allele in B cells
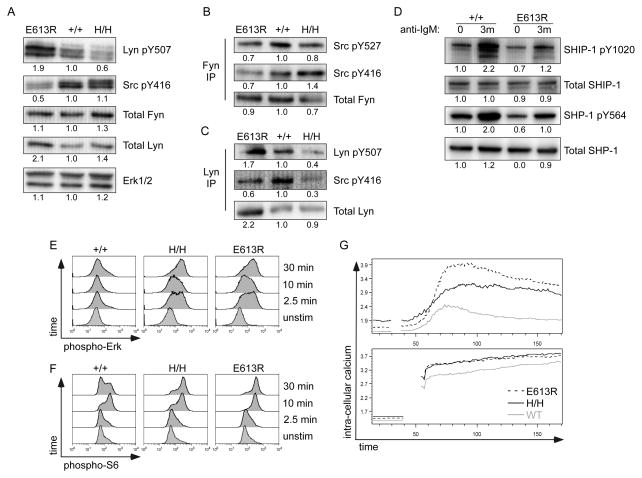
SFK phosphorylation and antigen receptor signaling in B cells are also tightly regulated by CD45. It has been previously shown that E613R B cells are markedly hyper-responsive to BCR stimulation (29). Studies of the CD45 allelic series have shown that increasing CD45 expression produces increasing BCR signaling, suggesting a similarity between E613R and high CD45-expressing H/H B cells (31). To determine whether E613R might function as a hyper-morphic allele of CD45 in B cells, we compared SFK phosphorylation in B cells from E613R and allelic series mice. B cells express three predominant SFKs: Lyn, Fyn, and Blk (6). We observed increased phosphorylation of the inhibitory tyrosine of Lyn (Y507) and reduced phosphorylation of the activation loop tyrosine of the SFKs (detected with mAb against Src pY416) in E613R B cells suggesting reduced Lyn kinase activity (Fig 2A, S1B; (29). By contrast, increasing CD45 expression in allelic series B cells results in progressive dephosphorylation of the inhibitory tyrosine of Lyn (Fig 2A, S1B, (31). In contrast to B cells expressing either low or high CD45, E613R B cells appear to exhibit a unique ‘biochemical signature’.
Figure 2. Selective dysregulation of Lyn phosphorylation in E613R B cells.
(A) Whole-cell lysates of resting B cells from E613R, +/+, and H/H mice were blotted with Ab to the inhibitory and activating tyrosines of Lyn (Lyn pY507/Src pY416). Src pY416 Ab binds activating tyrosines of all SFKs while Lyn pY507 is relatively specific. Total Lyn, Fyn, and Erk1/2 are detected as loading controls. Data are representative of at least three independent experiments.
(B, C) Fyn (B) and Lyn (C) were immunoprecipitated from whole-cell lysates of resting B cells from E613R, +/+, and H/H mice. IPs were subsequently blotted with Ab to the inhibitory and activating tyrosines of Fyn (B) and Lyn (C). Total Fyn (B) and Lyn (C) are detected as loading controls.
Data in 2B, C are representative of at least two independent experiments.
(D) Whole-cell lysates of resting and anti-IgM stimulated B cells from +/+ and E613R mice were blotted with Ab to SHP-1 pY564 and SHIP1 pY1020 along with total SHP-1 and total SHIP1.
(E, F) BCR-stimulated, fixed, and permeabilized lymphocytes were stained for phospho-Erk (E) or phospho-S6 Kinase (F) and costained for CD23 and B220 so that B cells could be identified. Data were collected by flow cytometry. Histograms depict intracellular phospho-Erk (E) or phospho-S6Kinase (F) in LN B cells from +/+, H/H, and E613R mice. Data are representative of at least three independent experiments.
(G) CD45.1+ BJ, E613R, and H/H lymphocytes were loaded with Indo-1 dye, stained for CD23, and stimulated with 10μg/ml anti-IgM or ionomycin. Ratio-metric assessment of intra-cellular calcium in CD23+ gate was carried out by flow cytometry. Data in 2D, E, F, G are representative of at least three independent experiments.
Interestingly, we observed a consistent increase in the amount of Lyn but not Fyn protein expressed in E613R B cells, comparable to that observed in CD45−/− B cells (Fig 2A, S1B). It has been reported that constitutively active Lyn kinase results protein degradation and reduced Lyn protein expression (42). Consistent with the phosphorylation pattern of Lyn in E613R B cells, this suggested to us that Lyn kinase was less enzymatically active in E613R B cells. Importantly, immunoprecipitation of individual SFKs revealed that the altered SFK phosphorylation and expression patterns observed through analysis of whole cell lysates corresponded primarily to Lyn rather than Fyn kinase (Fig 2B, C). We conclude that the E613R allele has disparate effects on Lyn and Fyn in B cells.
Because both phosphorylation and expression of Lyn appeared to be altered in E613R B cells, we assessed Lyn-specific substrates in order to clarify the activity level of Lyn. To do so, we next evaluated phosphorylation of the Lyn-specific substrates, SHP-1 and SHIP1 phosphatases which in turn serve as negative regulators of BCR signaling (Fig 2D). We observed a reduction in basal phosphorylation of both phosphatases in E613R B cells relative to wild type. In addition, inducible phosphorylation of these phosphatases in response to BCR stimulation was also reduced in E613R B cells. These data are consistent with partial inhibition of Lyn kinase activity in E613R B cells in both the basal and inducible state. Since SHP-1 and SHIP1 play negative regulatory roles in the BCR signaling pathway, these results suggest selective impairment of the Lyn-dependent negative regulatory circuit in E613R B cells.
We next probed more downstream BCR signaling events in E613R and allelic series B cells. We observed enhanced and prolonged Erk phosphorylation, S6 ribosomal protein phosphorylation downstream of PI3 kinase, and intra-cellular calcium increase both in E613R B cells and in H/H B cells with supraphysiologic CD45 expression (Fig 2E, F, G). Importantly, Erk phosphorylation in all genotypes is lost upon pretreatment with the Mek1/2 inhibitor U0126, confirming specificity of antibody staining (Fig S2A). These data confirm that BCR signaling in both E613R and H/H B cells is hyper-responsive as previously shown (29, 31). However, our studies of SFK phosphorylation suggest that the mechanism for hyper-responsiveness is biochemically distinct in these two lines. Low CD45 expression is associated with impaired BCR signaling, further confirming that E613R does not appear to function as a simple hypomorphic allele of CD45 (43). This is consistent with the selective dysregulation of Lyn but not Fyn phosphorylation observed in E613R B cells (Fig 2B, C).
Opposing functional effects of supraphysiologic CD45 expression and the E613R allele on B cell development and activation
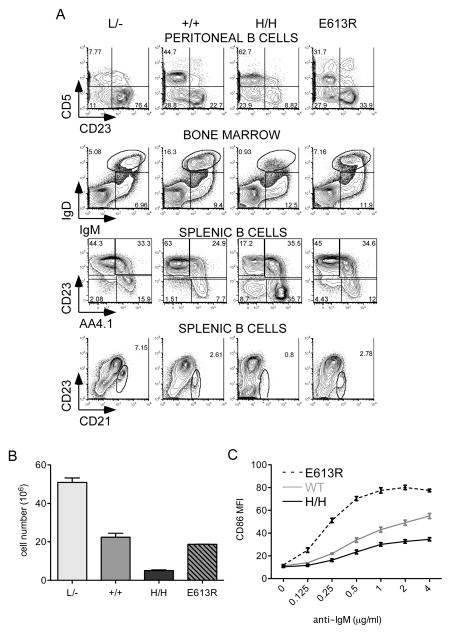
Since BCR signaling is perturbed in both E613R and allelic series mice, we assessed B cell development, a BCR-dependent process (Fig 3A). As previously reported, increasing CD45 expression to supraphysiologic levels in H/H mice results in relative expansion of the B1 cell lineage (CD5+CD23−), and loss of mature follicular (CD23+AA4.1−) and marginal zone (MZ;CD21hi) B cells (Fig 3A, B; (31). Further, surface coreceptors are downregulated by increasing CD45 expression (Fig S2). By contrast, E613R mice do not exhibit these changes (Fig 3A, S2), reinforcing biochemical data suggesting that E613R B cells do not phenocopy either high, or low CD45-expressing B cells (Fig 2A, B, C, S1B).
Figure 3. Distinct phenotypic and functional consequences of CD45 titration and E613R mutation.
(A) Representative plots of peritoneal, BM, or splenic B cells from allelic series and E613R mice stained to identify developmental B cell subsets (B1a = CD5+ CD23− PL B cells; B2 = CD5− CD23+ PL B cells; Hardy fraction E/immature B cells = IgM+IgD-BM; Hardy fraction F/mature recirculating BM B cells = IgM+IgD+; T1 = AA4.1+CD23− splenic B cells; T2/3 = AA4.1+CD23+ splenic B cells; Fo = AA4.1−CD23+ splenic B cells; MZ = CD21+CD23low splenic B cells). Data are representative of at least three biological replicates.
(B) Quantification of absolute numbers of splenic B cells in allelic series and E613R mice. Values are the mean of three biological replicates ± SEM.
(C) Graph representing MFI of CD86 expression on LN B cells from CD45+/+, E613R and H/H mice stimulated for 16 hours with varying doses of anti-IgM. Values are mean ± SEM of three biological replicates.
Perhaps the most notable functional consequence of the E613R allele in B cells, as previously reported, is markedly enhanced activation in response to BCR stimulation, and as assessed here by CD86 upregulation (Fig 3C; and ref. (29)). This is thought to be due to enhanced proximal BCR signaling. However, H/H B cells with similarly enhanced proximal BCR signaling (Figure 2E–G) nevertheless exhibit impaired upregulation of activation markers following BCR stimulation (Figure 3C;(31)). These data imply that H/H, but not E613R, B cells are functionally unresponsive or ‘anergic’ as we have previously demonstrated (31). This suggests that impaired anergy and failure of tolerance is a unique feature of E613R B cells that is not simply attributable to enhanced proximal BCR signaling.
Lyn mediates anergic phenotype of H/H B cells with supraphysiologic CD45 expression
In our quest to define the mechanism for autoimmunity in E613R animals, we wanted to understand why H/H but not E613R B cells are rendered anergic. Our biochemical studies of E613R and allelic series B cells identified dysregulation of Lyn phosphorylation in E613R B cells (Fig 2A, B, C, S1B). Lyn is the only SFK in B cells to subserve a negative regulatory role downstream of inhibitory ITIM-containing receptors (6, 8). Since E613R B cells exhibit hyper-phosphorylation of the inhibitory tyrosine of Lyn, we proposed that inhibition of Lyn and release of such negative regulation might account for enhanced signaling and activation of E613R B cells. Indeed, Lyn−/− B cells exhibit increased Erk phosphorylation and activation in response to BCR ligation, similar to E613R but unlike H/H B cells ((44); Fig S3). Conversely, since the inhibitory tyrosine of Lyn is dephosphorylated in H/H B cells, we proposed that hyper-activation of Lyn and its associated negative regulatory pathways might lead to anergy in these cells.
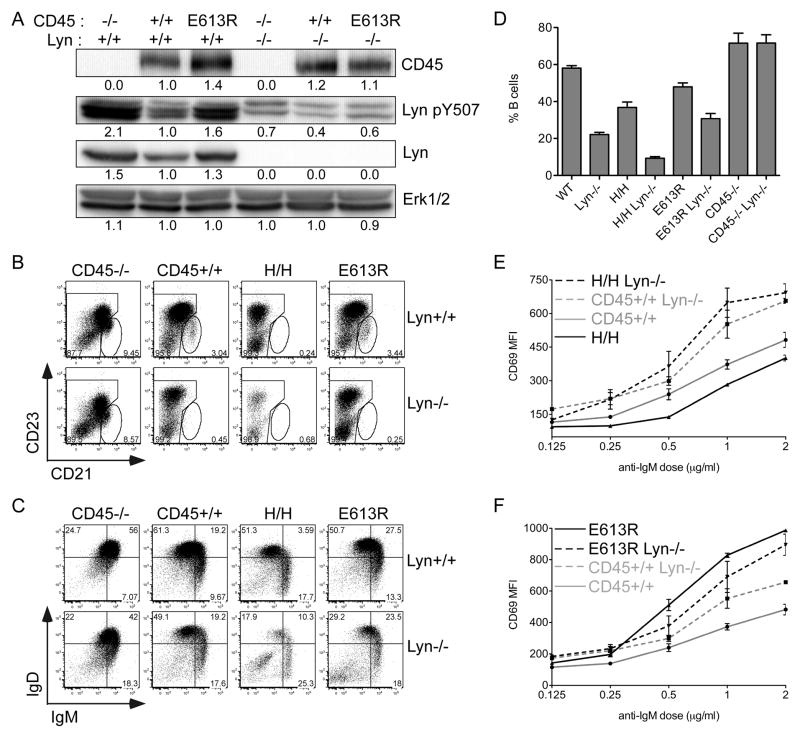
To test this hypothesis, we genetically deleted Lyn from E613R and allelic series mice. We found that hyper-phosphorylation of Lyn Y507 as detected by a phospho-specific antibody was indeed absent in E613R B cells deficient for Lyn, confirming the identity of the hyper-phosphorylated band as Lyn (Fig 4A). Other bands detected with the Lyn Y507 antibody in this range presumably represent the other major SFKs, Fyn and Blk, present in these cells,
Figure 4. Lyn is required to mediate phenotype of H/H but not E613R B cells.
(A) Whole-cell lysates of resting B cells from E613R, +/+, and CD45−/− mice genetically sufficient or deficient for Lyn were blotted with Ab to the inhibitory tyrosine of Lyn (Lyn Y507). Total CD45, Lyn, and Erk1/2 are detected as loading controls.
(B, C) Representative plots of splenic B cells from E613R, +/+, CD45−/− and H/H mice genetically sufficient or deficient for Lyn stained to identify B cell developmental stages (MZ = CD21+CD23low splenic B cells; T1=IgMhiIgDlo; T2/3/FO=IgDhi).
(D) Graph representing % B cells in the spleens of mice from E613R, +/+, CD45−/− and H/H mice genetically sufficient or deficient for Lyn. Values are mean ± SEM of three biological replicates.
Data in 4B–D are representative of at least 3 biological replicates.
(E, F) Graph representing MFI of CD69 expression on LN B cells from E613R and allelic series mice genetically sufficient or deficient for Lyn stimulated for 16 hours with varying doses of anti-IgM. Values are mean ± SEM of three biological replicates. Data in 4B–F are representative of at least three independent experiments.
We next compared B cell development and activation in allelic series mice deficient for Lyn in order to define the genetic relationship between CD45 and Lyn in these animals. Lyn-deficient B cells, as described above, are characterized by enhanced BCR signaling (9, 12, 44). In addition, B cell development is perturbed and mature B cell numbers are strikingly reduced in Lyn−/− mice (9, 12). We found that CD45−/− Lyn−/−double mutant animals phenocopy CD45−/− animals and effectively rescue the Lyn−/−phenotype (Fig 4B, C, D, S4). In contrast, H/H Lyn−/− double mutant animals exhibit a synergistic phenotype with a reduction in B cells more severe than either mutant alone (Fig 4B, C, D, S4). Importantly, deletion of Lyn rescues H/H B cells from functional unresponsiveness, arguing that Lyn is required to impose functional inhibition on H/H B cells (Fig 4E). We wanted to understand how the E613R allele of CD45 genetically interacts with Lyn. E613R Lyn−/− double mutant animals resemble Lyn−/− single mutants (Fig 4B–D, F, S4). We did not observe either rescue of the Lyn−/− phenotype or synergistic increase in B cell activation in double mutant animals (Figure 4F). Our data are most consistent with inhibition of Lyn but not other SFKs in E613R B cells. Taken together, our genetic data strongly argue that E613R represents neither a loss-of-function, nor a gain-of-function allele of CD45. Our results further suggest that impaired Lyn function in E613R B cells accounts for loss of anergy.
E613R but not H/− mice exhibit frank autoimmunity on a susceptible genetic background
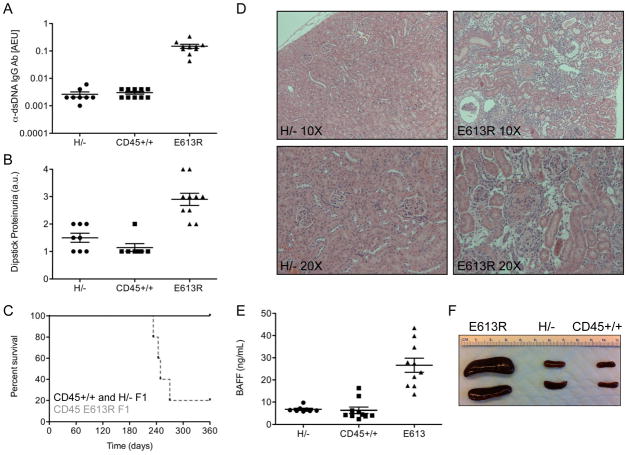
We next determined whether differences in AgR signaling and activation of E613R and H/H B cells had functional consequences in vivo. On susceptible genetic backgrounds, E613R mice develop lupus-like autoimmune disease (28, 29) unpublished data). We compared the disease phenotype of E613R to that of H/− mice with supraphysiologic CD45 expression on the susceptible B6/129 F1 genetic background (Fig 5A–D). Although E613R mice develop a robust autoimmune disease with high titer dsDNA Ab, proteinuria, renal inflammation, and premature mortality, H/− animals do not differ significantly from those bearing wild type CD45 (Fig 5A–D). These results reinforce biochemical and genetic data suggesting that E613R allele of CD45 does not represent a CD45 gain-of-function variant.
Figure 5. E613R but not H/− mice develop autoimmunity on a susceptible genetic background.
(A) Graph representing anti-dsDNA IgG antibodies assessed by ELISA in serum of 6 month old H/−, CD45+/+, and E613R B6/129 F1 mice. AEU = arbitrary elisa units. Values are mean ± SEM of 8–10 mice.
(B) Graph representing urine protein assessed by dipstick in 6 month old H/−, CD45+/+, and E613R B6/129 F1 mice. Values are mean ± SEM of 8–10 mice.
(C) Kaplan-Meier curve depicts survival of a cohort of H/−, CD45+/+, and E613R B6/129 F1 mice, N=10 per genotype.
(D) Hematoxylin and eosin staining of formalin fixed kidney sections from H/− and E613R B6/129 F1 mice at 8 months of age. Representative specimens at 10X and 20X magnification are depicted.
(E) Graph representing serum BAFF (ng/ml) assessed by ELISA in 6 month old H/−, CD45+/+, and E613R B6/129 F1 mice. Values are mean ± SEM of 8–10 mice.
(F) Representative spleens from 8 month old H/−, CD45+/+, and E613R B6/129 F1 mice.
Like E613R animals, Lyn−/− B cells develop a lupus-like autoimmune disease, albeit on the B6 genetic background (9). It has been recently reported that although Lyn−/− B cells are hyper-responsive, lupus-like autoimmunity in these animals is driven partly by the myeloid compartment and BAFF hyper-production (45). To test whether disease pathogenesis in E613R mice indeed resembles that in Lyn−/− mice, we assessed serum BAFF levels in these animals. We found elevated BAFF levels in E613R but not in H/−animals along with concomitant splenomegaly and myeloid expansion (Fig 5E, F; data not shown). These results suggest that E613R mice share a common disease pathogenesis features with the well-studied Lyn−/− model of lupus-like disease, while mice with supraphysiologic CD45 expression were protected from disease despite hyper-responsive proximal signaling.
Discussion
Introduction of the E613R mutation into the membrane-proximal wedge domain of CD45 drives systemic autoimmune disease in mice, but the molecular mechanism by which it does so has been unclear. Here we have taken advantage of the CD45 allelic series to establish that the E613R allele of CD45 produces neither a pure hypomorphic nor a hypermorphic variant of the phosphatase, but rather appears to alter CD45 substrate selectivity for specific SFKs. The E613R variant selectively impairs dephosphorylation of CD4-associated Lck in T cells and Lyn in B cells, but not Fyn. The E613R mutation thereby appears to limit access of the phosphatase to some but not all SFK substrates in lymphocytes. This is incompatible with a simple model in which the wedge domain exerts a general inhibitory effect on CD45 phosphatase activity. We speculate that these observations may be due to disrupted association of the wedge domain of CD45 with the N-terminal unique domains of the SFKs Lyn and Lck in E613R mice. Indeed, it has been previously argued that the unique domains of the SFKs are important in regulating CD45 association (46–48). Alternatively, the E613R mutation may alter CD45 substrate access indirectly by affecting CD45 localization at the membrane and/or association with surface co-receptors such as CD4. It has been shown that CD45 and CD4 preferentially associate (49). However, alternate splicing of the extracellular domain has been shown to regulate this association, suggesting that it may be the extracellular rather than the cytoplasmic domain of CD45 that mediates this association (49). It is also possible that differential effects of the E613R allele of CD45 on distinct SFKs may be due to differential localization of the SFKs within lymphocytes. For instance, Fyn preferentially associates with the TCR while Lck is associated with CD4/8 coreceptors (50, 51). It has also been suggested that intracellular localization of Lck and Fyn differs (52).
Although H/H B cells with supraphysiologic CD45 expression and E613R B cells both exhibit enhanced Erk phosphorylation, PI3K signaling, and calcium signaling in response to BCR ligation, the biochemical mechanisms underlying this hyper-responsiveness are distinct. Lyn inhibitory tyrosine (Y507) phosphorylation is increased in E613R B cells but reduced in H/H B cells, suggesting that Lyn is inactivated in the former, but overactive in the latter. Importantly, E613R B cells do not phenocopy CD45−/−B cells, nor do they resemble B cells with low CD45 expression. Consistent with biochemical evidence in T cells, these results support the notion that the E613R mutation does not simply enhance or impair phosphatase activity of CD45.
We establish through both biochemical and genetic epistasis experiments that Lyn, but not other SFKs, is uniquely inactivated in E613R mice. B cells from Lyn−/− mice and B cells harboring constitutively active Lyn (Y507F; so-called Lynup/up mice) have been previously described, and both exhibit hyper-responsive BCR signaling, reminiscent of E613R and H/H mice (42). These phenotypes have been attributed to dual positive and negative regulatory roles for Lyn via its phosphorylation of ITAM and ITIM-containing receptors, respectively. Interestingly, like H/H B cells, Lynup/up mice are characterized by downregulation of multiple cell surface co-receptors and paradoxically impaired activation of B cells, suggestive of anergy (42). By contrast, neither Lyn−/− nor E613R B cells exhibit such functional unresponsiveness. We conclude that E613R B cells resemble Lyn−/− B cells while H/H B cells phenocopy Lynup/up B cells.
Lyn regulates signaling by ITIM-containing inhibitory receptors both by phosphorylation of the ITIM tyrosine which leads to recruitment of the inhibitory effectors SHP-1 and SHIP1 and, by direct phosphorylation of these effector phosphatases (8). We have demonstrated that both SHP-1 and SHIP1 phosphorylation are impaired in E613R B cells consistent with selective Lyn inhibition. Importantly, both of these phosphatases have been implicated in enforcing B cell anergy (53). We show through genetic epistasis that the functional unresponsiveness of H/H B cells is mediated by Lyn. We speculate that H/H B cells recruit SHP-1 and SHIP1 to phosphorylated ITIM receptors more efficiently than wild type, thereby impairing downstream signaling events despite enhanced proximal BCR signaling. We propose that impaired activation of of SHP-1/SHIP1 phosphatases via Lyn contributes to autoimmunity in E613R mice, while the converse maintains anergy in mice with supraphysiologic CD45 expression. Consistent with this model, several different mouse mutants characterized by enhanced BCR signaling have selective defects in this negative regulatory circuit and develop systemic autoimmunity (e.g. CD22−/−, FcgRIIβ−/−, Shp-1Mev/Mev, and Lyn−/−) (1, 2). It is incompletely understood how Lyn, SHP-1 and SHIP1 enforce anergy. One possible mechanism is reduction in PtdIns(3–5)P3 levels and selective dampening of the PI3K signaling pathway pathway (14). Which ITIM-bearing surface receptors are most critical in this process and whether additional inhibitory effectors are recruited via Lyn remains to be determined. Finally, it is uncertain whether the anergic state is enforced by transcriptional or by post-translational changes (14, 54).
What role does this pathway play in spontaneous as opposed to genetically engineered autoimmune disease? B cells from both the polygenic NZB/W lupus model and from patients with lupus exhibit hyper-responsive BCR signaling (1). It is uncertain whether selective impairment of ITIM-pathways are responsible for these signaling phenotypes and for subversion of anergy. Altered expression of Lyn and genetic polymorphisms in Lyn as well as the ITIM-containing receptor FcγRIIb are associated with human lupus, suggesting that this may be the case (3, 55–59). Indeed it has been recently shown that haploinsufficiency for Lyn in mice is sufficient to produce lupus-like disease and that this phenotype cooperates genetically with CD22, SHP-1 and SHIP-1 haploinsufficiency suggesting that disease is a quantitative trait and partial dysregulation of this inhibitory circuit is sufficient to produce disease (60, 61). In the current study we have identified a molecular mechanism for the CD45 E613R autoimmune disease that converges on this pathway and thereby highlights the importance of the Lyn-mediated negative regulatory circuit in maintaining B cell tolerance.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (POI AI035297, to AW and MH; K08 AR059723 to JZ), the Arthritis National Research Foundation (to JZ), the American College of Rheumatology REF Rheumatology Investigator Award (to JZ), and the Rosalind Russell Medical Research Foundation Bechtel Award (to JZ).
We would like to thank Al Roque for assistance with animal husbandry and Marianne Mollenauer and James Mueller for help with experiments. This work was supported in part by the NIH (POI AI035297, to AW and MH; K08 AR059723 to JZ), the Arthritis National Research Foundation (to JZ), the American College of Rheumatology REF Rheumatology Investigator Award (to JZ), and the Rosalind Russell Medical Research Foundation Bechtel Award (to JZ).
References
- 1.Zikherman J, Weiss A. Antigen receptor signaling in the rheumatic diseases. Arthritis Res Ther. 2009;11:202. doi: 10.1186/ar2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zouali M, Sarmay G. B lymphocyte signaling pathways in systemic autoimmunity: implications for pathogenesis and treatment. Arthritis Rheum. 2004;50:2730–2741. doi: 10.1002/art.20487. [DOI] [PubMed] [Google Scholar]
- 3.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 5.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 7.Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–8006. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 10.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 12.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell MJ, Duan M, Armes JE, Anderson GP, Tarlinton DM, Hibbs ML. Genetic segregation of inflammatory lung disease and autoimmune disease severity in SHIP-1−/− mice. J Immunol. 2011;186:7164–7175. doi: 10.4049/jimmunol.1004185. [DOI] [PubMed] [Google Scholar]
- 14.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunol Rev. 2010;237:249–263. doi: 10.1111/j.1600-065X.2010.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 17.Stone JD, Conroy LA, Byth KF, Hederer RA, Howlett S, Takemoto Y, Holmes N, Alexander DR. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-zeta, and ZAP-70. J Immunol. 1997;158:5773–5782. [PubMed] [Google Scholar]
- 18.Byth KF, Conroy LA, Howlett S, Smith AJ, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas ML, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 20.Mee PJ, Turner M, Basson MA, Costello PS, Zamoyska R, Tybulewicz VL. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur J Immunol. 1999;29:2923–2933. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28:183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeill L, Cassady RL, Sarkardei S, Cooper JC, Morgan G, Alexander DR. CD45 isoforms in T cell signalling and development. Immunol Lett. 2004;92:125–134. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–153. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Dornan S, Sebestyen Z, Gamble J, Nagy P, Bodnar A, Alldridge L, Doe S, Holmes N, Goff LK, Beverley P, Szollosi J, Alexander DR. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 26.Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 27.Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 28.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, Weiss A. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 29.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23:635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Hermiston ML, Zikherman J, Tan AL, Lam VC, Cresalia NM, Oksenberg N, Goren N, Brassat D, Oksenberg JR, Weiss A. Differential impact of the CD45 juxtamembrane wedge on central and peripheral T cell receptor responses. Proc Natl Acad Sci U S A. 2009;106:546–551. doi: 10.1073/pnas.0811647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A. 2012;109:E3–12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, Weiss A. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Virts EL, Diago O, Raschke WC. A CD45 minigene restores regulated isoform expression and immune function in CD45-deficient mice: therapeutic implications for human CD45-null severe combined immunodeficiency. Blood. 2003;101:849–855. doi: 10.1182/blood-2002-07-1969. [DOI] [PubMed] [Google Scholar]
- 35.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. The Journal of Immunology. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashwell JD, D’Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 38.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 39.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker AM, DeFord-Watts LM, Wuelfing C, van Oers NS. The constitutive tyrosine phosphorylation of CD3zeta results from TCR-MHC interactions that are independent of thymic selection. J Immunol. 2007;178:4120–4128. doi: 10.4049/jimmunol.178.7.4120. [DOI] [PubMed] [Google Scholar]
- 41.van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbs ML, Harder KW, Armes J, Kountouri N, Quilici C, Casagranda F, Dunn AR, Tarlinton DM. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002;196:1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A. 2011;109:E3–12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gervais FG, Veillette A. The unique amino-terminal domain of p56lck regulates interactions with tyrosine protein phosphatases in T lymphocytes. Mol Cell Biol. 1995;15:2393–2401. doi: 10.1128/mcb.15.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gervais FG, Veillette A. Reconstitution of interactions between protein-tyrosine phosphatase CD45 and tyrosine-protein kinase p56(lck) in nonlymphoid cells. J Biol Chem. 1997;272:12754–12761. doi: 10.1074/jbc.272.19.12754. [DOI] [PubMed] [Google Scholar]
- 48.Ng DH, Watts JD, Aebersold R, Johnson P. Demonstration of a direct interaction between p56lck and the cytoplasmic domain of CD45 in vitro. J Biol Chem. 1996;271:1295–1300. doi: 10.1074/jbc.271.3.1295. [DOI] [PubMed] [Google Scholar]
- 49.Leitenberg D, Novak TJ, Farber D, Smith BR, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 51.Samelson LE, Phillips AF, Luong ET, Klausner RD. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990;87:4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of Lck and Fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, Dal Porto JM, Li QZ, Cambier JC. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35:746–756. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 55.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 56.Huck S, Le Corre R, Youinou P, Zouali M. Expression of B cell receptor-associated signaling molecules in human lupus. Autoimmunity. 2001;33:213–224. doi: 10.3109/08916930109008048. [DOI] [PubMed] [Google Scholar]
- 57.Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Investig Med. 2001;49:157–165. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- 58.Lu R, Vidal GS, Kelly JA, Delgado-Vega AM, Howard XK, Macwana SR, Dominguez N, Klein W, Burrell C, Harley IT, Kaufman KM, Bruner GR, Moser KL, Gaffney PM, Gilkeson GS, Wakeland EK, Li QZ, Langefeld CD, Marion MC, Divers J, Alarcon GS, Brown EE, Kimberly RP, Edberg JC, Ramsey-Goldman R, Reveille JD, McGwin G, Jr, Vila LM, Petri MA, Bae SC, Cho SK, Bang SY, Kim I, Choi CB, Martin J, Vyse TJ, Merrill JT, Harley JB, Alarcon-Riquelme ME, Nath SK, James JA, Guthridge JM. Genetic associations of LYN with systemic lupus erythematosus. Genes Immun. 2009;10:397–403. doi: 10.1038/gene.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenks SA, Sanz I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmun Rev. 2009;8:209–213. doi: 10.1016/j.autrev.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsantikos E, Maxwell MJ, Kountouri N, Harder KW, Tarlinton DM, Hibbs ML. Genetic interdependence of lyn and negative regulators of B cell receptor signaling in autoimmune disease development. J Immunol. 2012;189:1726–1736. doi: 10.4049/jimmunol.1103427. [DOI] [PubMed] [Google Scholar]
- 61.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.