Abstract
We previously reported improved survival and partial metabolic correction of a mouse intermediate maple syrup urine disease (iMSUD) model post allogenic hepatocyte transplant, confirming that a small number of enzyme proficient liver-engrafted cells can improve phenotype. However, clinical shortages of suitable livers for hepatocyte isolation indicate a need for alternative cell sources. Human amnion epithelial cells (hAEC) share stem cell characteristics while lacking many safety and ethical concerns, and differentiate to hepatocyte-like cells. Eight direct hepatic hAEC transplants were administered to iMSUD mice over the first 35 days beginning at birth; animals were provided a normal protein diet and sacrificed at days 35 and 100. Treatment at the neonatal stage is clinically relevant for MSUD, and may offer a donor cell engraftment advantage. Survival was significantly extended and body weight was normalized in iMSUD mice receiving hAEC transplants compared to iMSUD (severely cachectic; dead ≤28 days). Branched chain α-keto acid dehydrogenase enzyme activity was significantly increased in transplanted livers. Branched chain amino acids leucine, isoleucine, valine, and alloisoleucine were significantly improved in the sera and brain, as were other large neutral amino acids. Conclusion: Placental-derived stem cell transplantation lengthened survival and corrected many amino acid imbalances in a mouse model of iMSUD. This highlights the potential for their use as a viable alternative clinical therapy for MSUD and other liver-based metabolic diseases.
Keywords: Amnion, transplant, epithelium, metabolic, liver
Maple syrup urine disease (MSUD; OMIM 248600) is an inborn error of metabolism characterized by elevated branched-chain keto acids and branched-chain amino acids (BCAA; leucine, isoleucine, valine) resulting in severe brain injury and death unless treated. Mutation in any of the 4 branched chain α-keto acid dehydrogenase (BCKDH) subunits and varying residual enzymatic activity defines five MSUD variants (1). Higher enzyme activity translates to fewer crises and better long-term prognosis. Treatment consists of lifelong dietary BCAA restriction (2), though compliance in children and adolescents proves problematic. Noncompliance is associated with catabolic crisis, frequent hospital stays, and irreversible neurological dysfunction.
Orthotopic liver transplantation improves MSUD patient outcome (3). This is associated with high costs, severe complications including death, and a need for lifelong immunosuppression. Furthermore, critical donor shortages underscore a need for alternative therapies. Hepatocyte transplantation has been applied as a cell therapy for metabolic liver disease. Preclinical studies have shown significant improvement in animal models, some despite low levels of cell engraftment (4–6). Clinical hepatocyte transplantation has shown promise for a number of inherited metabolic diseases, such as Crigler-Najjar type 1, ornithine transcarbamylase deficiency, citrullinemia, glycogen storage disease, and others (7, 8). However, the availability of useful hepatocytes remains a limiting factor of clinical transplantation.
Amniotic membrane and AE cells are not immunogenic and have anti-inflammatory, anti-microbial, anti-viral, and anti-fibrotic properties (9). Human AEC do not express telomerase, are not immortal, and are immune privileged (10,11). Human amnion epithelial cells (hAEC) are readily obtained from placenta following live birth (12) and express molecular and surface markers characteristic of stem cells (10). Amniotic membranes have been used clinically for more than a century without immunosuppression or adverse effect (13,14) and human AE cells have been transplanted, without immunosuppression, into normal volunteers or patients with lysosomal storage diseases without reported adverse effects or evidence of rejection or tumorigenicity (14–17).
Methods to differentiate hAEC to hepatocyte-like cells have been described (18,19). Importantly, undifferentiated hAEC engrafted in the livers of mice were found to display hepatic morphology and expressed mature liver genes at levels similar to adult liver. Due to their documented plasticity and special characteristics regarding safety, we examined the hypothesis that hAEC transplantation could lengthen survival and correct amino acid imbalances in a mouse model of iMSUD.
Materials and Methods
All animal studies were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
iMSUD Mice
Line A iMSUD mice (4, 5, 20) and the PCR genotyping protocol used (21) have been previously described. All animals were provided standard (22% protein) mouse chow.
hAEC Isolation
Human placenta AEC isolation was previously described (12). IRB approval through the University of Pittsburgh and Magee-Women’s Hospital was acquired prior to human tissue use.
hAEC Transplant (Tx)
hAEC (10×10^6/mL in Hank’s Balanced Salt Solution; Lonza #04-315Q, Walkersville, MD) were transplanted as previously described (22), with some modifications. Each “Tx event” consisted of two direct hepatic transdermal injections into two sites in the liver. Two Tx events (0.5×106 hAEC each site) during days 1–10 were administered. Twice weekly Tx events (1×106 each site) were administered during days 21–35. Depending upon availability, freshly isolated or cryopreserved cells were used. Trypan blue exclusion determined hAEC viability (>90% in all cases) immediately prior to injection.
Mouse Sacrifice and Tissue Collection
Mice were sacrificed by cervical dislocation when animals appeared moribund, or at the experiment’s end (35 or 100 days). Whole blood collection (4mm animal lancet, MEDIpoint, Mineola, NY) for serum isolation (Microtainer serum separator tubes, BD #365959, Franklin Lakes, NJ) was done immediately prior to sacrifice. Liver and brains were immediately harvested post sacrifice and processed as previously reported (5, 22). Liver was separated into portions for (i) BCKDH activity, (ii) RNA expression (iii) DNA analysis. Tissue and sera were stored at −80°C until analysis.
Survival and Body Weight
iMSUD, iMSUD-hAEC Tx, and wildtype (WT) mouse date of birth, weekly body weight from weaning until death, and date of death was recorded. Data were analyzed: Kaplan–Meier method and log-rank test (survival), or one-way ANOVA and post-hoc Tukey’s test (body weight).
Human DNA Analysis
Liver DNA from mouse (iMSUD, iMSUD-hAE Tx, WT), human liver (HL), and hAEC were isolated using the DNeasy Blood and Tissue Kit (QIAGEN, Germantown, MD). Human DNA was quantified using the Taqman RNase P Detection Reagents (Applied Biosystems) following manufacturer instructions and compared to naïve hAEC. Data were presented as the mean ± SEM analyzed by one-way ANOVA and post-hoc Tukey’s test.
Serum/Brain Amino Acids
Amino acids from mouse sera and brains (iMSUD, iMSUD-hAE Tx, WT) were quantified as described previously (5). Data were presented as the mean ± SEM analyzed by one-way ANOVA and post-hoc Tukey’s test. Criteria for achieving “correction” or “partial correction” have been defined (5).
BCKDH Enzyme Activity
BCKDH enzyme activity was assessed as previously described (20). Data were analyzed by nonparametric Mann-Whitney test and compared to WT (100% activity).
Human RNA Expression
Liver RNA from mouse (iMSUD, iMSUD-hAE Tx, WT), human liver (HL), and hAEC was isolated and converted to cDNA as previously described (23). Human gene expression was assessed using Applied Biosystems TaqMan Assays-on-Demand Gene Expression kits [cyclophilin A (PPIA: Hs99999904_m1), BCKDHα (Hs00958109_m1), BCKDHβ (Hs00609053_m1), DBT (Hs01066445_m1), and DLD (Hs00164401_m1)] following the manufacturer’s protocol. The expression of human BCKDH subunit genes from donor hAECs present in the mouse liver was determined with human specific probes and by normalization to human cyclophilin A. Results are compared to the expression in normal adult human liver (HL;100%) and naïve hAEC. Data were presented as the mean ± SEM and analyzed by one-way ANOVA and post-hoc Tukey’s test.
Results
Human AEC expressing normal BCKDH were transplanted directly into the liver parenchyma of iMSUD mice as neonates and continuing until 35 days of age. Nontransplanted mice display adverse effects of MSUD prior to weaning (20), thus early treatment is clinically relevant to this disorder, and proliferating newborn livers may confer an engraftment/proliferative advantage to donor cells.
Human AEC transplant improved survival and normalized body weight
All experimental animals were provided normal (22% protein) diet. Untreated iMSUD mice were noticeably smaller than unaffected littermates at weaning (21 days), continued to lose weight, developed muscle weakness with intermittent recumbence, followed by seizures and death on or before 28 days of age (figure 1A, B) (5, 23, 24). Animals receiving hAEC transplants displayed significantly improved survival at 35 days (100% survival; n=11/11) and 100 days (81.2% survival; n=9/11) compared to iMSUD controls (figure 1A; p<0.001), and restored characteristics indicative of good health (i.e. smooth coat, active, bright eyes). Body weight was also normalized to WT levels (figure 1B). The two animals that perished prior to the 100 day time point were found dead in their cage without prodrome.
Figure 1.
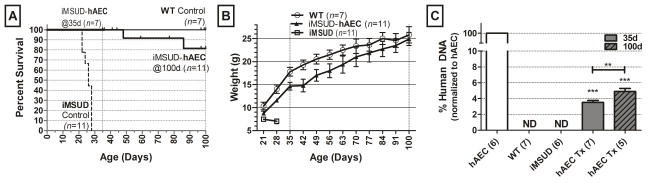
Survival and body weight was improved post transplant in intermediate maple syrup urine disease (iMSUD) mice. Vertical gridlines at 35 and 100 days indicates sacrifice points. (A) Survival of iMSUD mice given human amnion epithelial cell transplant (hAEC Tx) and untreated controls (WT and iMSUD). (B) Weekly body weight (g) beginning at weaning (21 days). (C) Human DNA quantified in mouse liver. WT, wildtype; ND, none detected; ***, p<0.001, one-way ANOVA, Tukey post-hoc compared to iMSUD. **p<0.01, unpaired t-test.
Donor hAEC engrafted at a low level
To verify hAEC engraftment, the presence of human DNA in recipient liver was determined by quantitative PCR of the human RNase P gene (figure 1C). Human DNA was undetectable in controls, but significantly increased in transplanted mice at both time points (3.50% +/−0.26% human DNA at 35 days and 4.9% +/−0.37% human DNA at 100 days), and significantly different at day 100 compared to day 35 (unpaired t-test, p<0.05).
Large neutral amino acids in the brain were significantly improved at 100 days with hAEC transplant
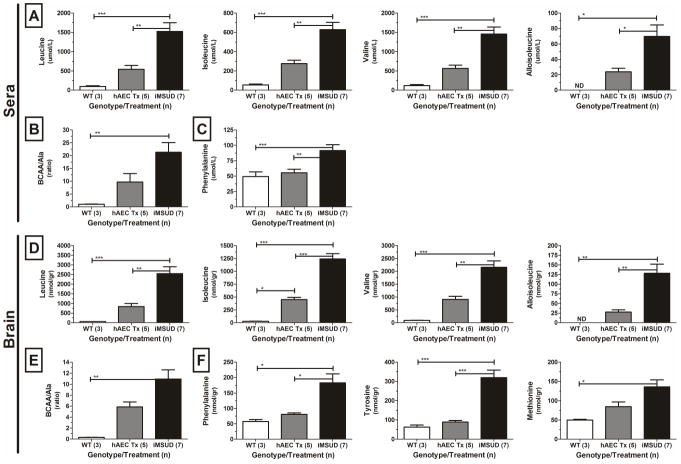
Large neutral amino acids (LNAAs), which includes the BCAAs (leucine, isoleucine, and valine), compete for transport into the brain. Elevated BCAAs disrupt other LNAA concentrations in the brain. Transplantation of hAEC normalized all three circulating BCAAs (leucine, isoleucine, valine) at 100 days (figure 2A), and importantly, the brain showed similar corrections (figure 2D, Table 1), though values still signified residual disease. Alloisoleucine was improved after transplant at 100 days in both sera (>65%, figure 2A) and brains (>80%, figure 2D, Table 1), to values not significantly different than WT. The ratio of total BCAA to alanine (25) was decreased by >50% indicating a significant partial correction in both the sera (figure 2B) and brains (figure 2E, Table 1). Alanine was completely corrected by hAEC transplants at 35 days in both sera and brain (data not shown). Additional LNAA corrections were observed (figure 2C, F, Table 1). At 100 days, the dopamine precursors phenylalanine and tyrosine were normalized, and methionine was partially corrected post transplant in the brain (figure 2F). Phenylalanine was also normalized in the sera at 100 days (figure 2C), though tyrosine was not different between groups (data not shown). Metabolite changes were maintained across time points (two-tailed “t”-test).
Figure 2.
Large neutral amino acids (LNAA) were corrected post transplant (Tx) in intermediate maple syrup urine disease (iMSUD) mouse sera and brains at 100 days. (A, D) branched chain amino acids (BCAA) and alloisoleucine. (B, E) BCAA to alanine ratio. (C, F) selected LNAA. hAEC, human amnion epithelial cell; WT, wildtype; *, p<0.05; **, p<0.01; ***, p<0.001, one-way ANOVA with Tukey post-hoc analysis.
Table 1.
Metabolic changes in brains of iMSUD mice following hAEC transplant.
| iMSUD vs. WT | iMSUD-hAEC vs. WT | iMSUD-hAEC vs. iMSUD | |
|---|---|---|---|
| Leucine | ↑ >40-fold | = | ↓ >60% |
| Isoleucine | ↑ >40-fold | ↑ 15-fold | ↓ >60% |
| Valine | ↑ >20-fold | = | ↓ >60% |
| Alloisoleucine | ↑ >120 fold | = | ↓ 80% |
| BCAA/alanine | ↑ >10-fold | = | ↓ >50% |
| Phenylalanine | ↑ >3-fold | = | ↓ >55% |
| Tyrosine | ↑ >5-fold | = | ↓ >70% |
| Methionine | ↑ 2.75-fold | = | ↓ >35%, NS |
An equals sign (=) indicates either a correction or partial correction (see criteria in Methods section). BCAA, branched chain amino acids (leucine, isoleucine, and valine); iMSUD, intermediate maple syrup urine disease; hAEC, human epithelial cell; WT, wildtype.
BCKDH enzyme activity doubled following hAEC transplant
BCKDH activity in transplanted animals was significantly increased at 35 days (13.5% of WT activity; p<0.01) and 100 days (12.5% of WT; p<0.01) compared to iMSUD (~5–6% of WT; figure 3A). There was no significant difference between time points (two-tailed “t”-test).
Figure 3.
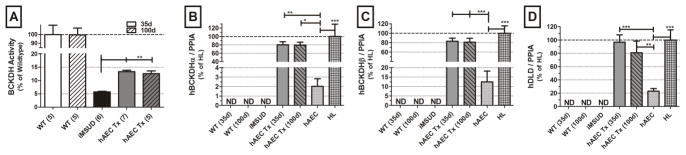
Human branched chain keto acid dehydrogenase (BCKDH) enzyme activity and subunit gene expression following human amnion epithelial cell transplant (hAEC Tx) in a mouse intermediate maple syrup urine disease (iMSUD) model. Mouse samples (WT, iMSUD, and hAEC_Tx) were collected at sacrifice; human samples were collected immediately after cell isolation (hAEC) or tissue receipt (HL). (A) BCKDH activity normalized to wildtype (WT). Human BCKDH subunit expression in the human component of the chimeric livers (B) BCKDHα (C) BCKDHβ and (D) DLD normalized to human cyclophillin A (PIAA). Expression of transplanted hAEC in the mouse liver was compared to adult human liver (HL), represented as 100% expression, and naive hAEC. ND, none detected; *, p<0.05; **, p<0.01; ***, p<0.001, one-way ANOVA, Tukey post-hoc analysis.
Human BCKDH gene expression was detectable in iMSUD-hAEC transplanted mice
To verify hAEC engraftment, expression of three of the four human BCKDH subunits (BCKDHα, BCKDHβ, and DLD) in mouse liver was determined by real time RT-PCR and normalized to human cyclophilin A (PIAA). Human DBT expression was not reported, as all animals were human DBT transgenic, as it was the gene used to rescue the classic MSUD knockout mouse and create the iMSUD model (20). Data from control animals (WT, iMSUD) and iMSUD-hAEC transplanted mice were compared to both naïve hAEC and human liver (figure 3B–D). Using human specific probes, it was determined that, post transplant, human BCKDH genes were expressed at a levels not significantly different than human liver (HL). Expression of human BCKDHα post hAEC Tx was 80.2% +/−7.1% of human liver expression at 35 days (p<0.01 vs hAEC) and 79.5% +/−6.8% at 100 days (p<0.05; figure 3B). BCKDHβ expression was 83% +/−6.75% of human liver at 35 days and 81.1% +/−8.3% at 100 days (p<0.001 vs hAEC; figure 3C). DLD was 96.7% +/−11.1% of human liver at 35 days (p<0.001 vs hAEC) and 81% +/−17.4% at 100 days (p<0.01; figure 3D). There was no difference in expression between transplant time points (two-tailed “t”-test). As expected, human BCKDHα, BCKDHβ, and DLD expression was not detectable in WT or iMSUD control animals.
Discussion
Orthotopic liver transplant has recently become a more common treatment for many inborn errors of metabolism when more conventional (i.e. dietary therapies) prove insufficient. Cell transplantation offers many advantages for therapy if these diseases (26). Of significant importance for metabolic disease patients, the native liver remains intact thereby, eliminating the need for transplanted cells to provide complete liver support. Poor cell function or rejection would only return the recipient to their pretransplanted state, and orthotopic liver transplantation would remain a viable treatment option. Organ shortage is the primary barrier for clinical cell Tx. There is a need for alternative cell sources. Human AE “stem” cells are easily accessible, free of the ethical and safety concerns common to ESC, efficiently cryopreserve, and exhibit many beneficial and immune privileged characteristics (11, 27–29).
Amnionic tissue was first used clinically more than a century ago; common applications include ocular surface reconstruction and skin injury repair (9, 13, 14). Human AEC were transplanted for Niemann-Pick and other lysosomal storage diseases without adverse effects (16, 30). Human AE like other placenta-derived cells have been reported to reduce inflammation and fibrosis and repair and preserve function in lung injury (31–33), and liver cirrhosis (34–36). Differentiated dopamine-expressing hAECs were able to survive and function in the brain of a rat model of Parkinson’s disease, which resulted in prevention of neuron degradation (37, 38). Finally, human AEC was used to target liver and deliver a transgene to treat the metabolic disease familial hypercholesterolemia in a rabbit model (39).
Transplants of human AEC converted iMSUD from a lethal disease; to one where mice survived long-term, recovered normal growth rate, and showed all the characteristics of good health (i.e. smooth coat, bright eyes, normal activity). These preclinical studies employed six intrahepatic transplants during the first few weeks of life, a protocol that has been achieved clinically (40). Transplants as early as possible are preferred because (1) recipients would require fewer cells, and inherited metabolic disease onset is most often at birth, and (2) delays in initial treatment or poor dietary control over time have been associated with serious and permanent side effects in MSUD and other metabolic liver diseases. In our experience and in reports where human AEC were transplanted or used on patients, there are no reports of tumorigenicity, immune rejection or any adverse effects (10, 14–16, 27, 41). Indeed, hAEC have been reported to escape immune recognition post transplant, and have been shown to have immunomodulatory properties similar to those described in mesenchymal stem cells (11) (9, 28). Xenotransplanted human mesenchymal stem cells were able to engraft long term and undergo site-specific differentiation in sheep (42), and were shown to improve a variety of diseases in a number of species (43), all without immunosuppression or the development of graft-vs-host disease. Accordingly, no immunosuppression was administered to test subjects despite the transplantation of human cells, and there was no evidence of cell rejection. However, in the current study, the initial hAE transplants were conducted in the early neonatal period, and this experimental design does not exclude the possibility that the iMSUD mice were induced to become tolerant to the human cells by such early exposure (44, 45).
In MSUD, leucine toxicity is the primary cause of brain injury (1), and when competing with other LNAA for brain access, leucine is able to cross the blood-brain barrier more efficiently than other amino acids (46). Pathology from MSUD occurs in multiple compartments (i.e. liver, kidney, muscle and brain) (47–48), and in the current protocol, cell transplants were liver directed. Nonetheless, transplanted animals survived and actually thrived, which allowed temporal comparison of metabolites at later time points. Improved brain LNAA levels observed in this study demonstrate that hepatic correction provided metabolic correction to distal organs. Liver-directed hAEC transplants resulted in greater than a 60% correction in BCAAs (leucine, isoleucine, valine) and alloisoleucine, as well as additional LNAA normalizations (Figure 2). Brain isoleucine was not completely corrected at 100 days despite significant improvement. Alloisoleucine, an isoleucine analogue and pathognomonic marker for MSUD (49), was improved after hAEC transplant in both sera and brains (figure 2A, D) while isoleucine was only significantly improved by day 100. Therefore, improvements in isoleucine were not due to an enhanced conversion to alloisoleucine. Metabolic improvements were maintained across time points suggesting stable engraftment.
Perinatal hAEC transplants were equivalent in cell number to approximately 8–12% of the total number of hepatocytes in the mouse liver and resulted in a chimeric organ with 3.5–5% human DNA (figure 1C). It must be stated, that in these studies we cannot definitively exclude the possibility that cell fusion between hAE and mouse hepatocytes had occurred. This level of engraftment more than doubled liver hepatic BCKDH activity from 5–6% to 12.5–13.5% (figure 3A). This large increase in BCKDH activity relative to cell engraftment was similar to results obtained after hepatocyte transplantation (5, 23), and corresponds with clinical observations where incremental differences in enzyme activity significantly impact disease phenotype and patient outcome (1). Although human DNA was significantly different between 35 and 100 days, the modest increase, suggests that hAEC are at least maintained throughout the study or that some level of proliferation occurred between 35 and 100 days. Human BCKDH subunit gene expression of the engrafted donor cells was not significantly different from human liver (HL; Figure 3 B–D) suggesting that hAEC mature post transplant (18), It is also important to note that human BCKDH subunit expression reported here do not represent total BCKDH expression in mouse liver, but only that the human component of these chimeric livers, expressed BCKDH subunits at levels similar to hepatocytes in adult human liver.
In sum, transplanted human amnion epithelial stem cells significantly increased BCKDH enzyme activity in a mouse model of iMSUD resulting in lengthened survival, normalized body weight, and improved circulating and brain amino acids. Our study supports the concept that placental-derived stem cells such as hAEC may provide a safe and abundant cell source for the treatment of MSUD, and other liver-based metabolic diseases.
Acknowledgments
Financial Support: This work was supported in part by the National Institutes of Health. N01-DK-7-0004/HHSN26700700004C and RC1DK086135 (SCS) and the National PKU Alliance (RG and KJS), HD58553 (KMG)
Abbreviations List
- BCAA
branched chain amino acid
- BCKDH
branched chain alpha-keto acid dehydrogenase
- hAEC
human amnion epithelial cells
- iMSUD
intermediate maple syrup urine disease
- LNAA
large neutral amino acid
- MSUD
maple syrup urine disease
- Tx
transplant
- WT
wildtype
Footnotes
Potential Conflicts: SCS has stock in Stemnion, LLC.
Contribution of Authors
Study concept and design KJS, RG, KMG, SCS
Data acquisition KJS, RG, VT, KD, SCS
Analysis and interpretation of data, KJS, KMG, SCS.
Drafting manuscript, KJS, RG, SCS,
Critical review of manuscript, KJS, KD, FM, VT, MCH, RG, KMG, SCS
Obtaining funding, KJS, RG, MCH, KMG, SCS
Technical or material support, KJS, KD, FM, VT, MCH, KMG, SCS
References
- 1.Chuang DT, Shih VE. Maple syrup urine disease (branched-chain ketoaciduria) In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. Vol. 2. New York: McGraw-Hill; 2001. pp. 1971–2005. [Google Scholar]
- 2.Snyderman SE, Norton PM, Roitman E, LEH Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics. 1964;34:454–472. [PubMed] [Google Scholar]
- 3.Strauss KA, Mazariegos GV, Sindhi R, Squires R, Finegold DN, Vockley G, Robinson DL, et al. Elective liver transplantation for the treatment of classical maple syrup urine disease. Am J Transplant. 2006;6:557–564. doi: 10.1111/j.1600-6143.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 4.Skvorak KJ. Animal models of maple syrup urine disease. J Inherit Metab Dis. 2009;32:229–246. doi: 10.1007/s10545-009-1086-z. [DOI] [PubMed] [Google Scholar]
- 5.Skvorak KJ, Hager EJ, Arning E, Bottiglieri T, Paul HS, Strom SC, Homanics GE, et al. Hepatocyte transplantation (HTx) corrects selected neurometabolic abnormalities in murine intermediate maple syrup urine disease (iMSUD) Biochim Biophys Acta. 2009;1792:1004–1010. doi: 10.1016/j.bbadis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamman K, Clark H, Montini E, Al-Dhalimy M, Grompe M, Finegold M, Harding CO. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strom S, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, Miki T. Hepatocyte Transplantation: Clinical Experience and Potential for Future Use. Cell Transplantation. 2006;15:S105–S110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 9.Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 10.Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Niederkorn JY, Neelam S, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophtalmol Visual Science. 2005;46:900–907. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 12.Miki T, Marongiu F, Dorko K, Ellis EC, Strom SC. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2010;Chapter 1(Unit 1E):3. doi: 10.1002/9780470151808.sc01e03s12. [DOI] [PubMed] [Google Scholar]
- 13.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Medical Journal. 1910;15:307. [Google Scholar]
- 14.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Yeager AM, Singer HS, Buck JR, Matalon R, Brennan S, O’Toole SO, Moser HW. A therapeutic trial of amniotic epithelial cell implantation in patients with lysosomal storage diseases. Am J Med Genet. 1985;22:347–355. doi: 10.1002/ajmg.1320220219. [DOI] [PubMed] [Google Scholar]
- 16.Cerneca F, Andolina M, Simeone R, Boscolo R, Ciana G, Bembi B. Treatment of patients with Niemann-Pick type is using repeated amniotic epithelial cells implantation: correction of aggregation and coagulation abnormalities. Clin Pediatr (Phila) 1997;36:141–146. doi: 10.1177/000992289703600304. [DOI] [PubMed] [Google Scholar]
- 17.Akle CA, Adinolfi M, Welsh KI, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 18.Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Paola Serra M, Doratiotto S, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53:1719–1729. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki T, Marongiu F, Ellis EC, Dorko K, Mitamura K, Ranade A, Gramignoli R, et al. Production of hepatocyte-like cells from human amnion. Methods Mol Biol. 2009;481:155–168. doi: 10.1007/978-1-59745-201-4_13. [DOI] [PubMed] [Google Scholar]
- 20.Homanics GE, Skvorak K, Ferguson C, Watkins S, Paul HS. Production and characterization of murine models of classic and intermediate maple syrup urine disease. BMC Med Genet. 2006;7:33. doi: 10.1186/1471-2350-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.JAX. Genotyping Protocols Database. Bar Harbor: JAX; 2009. Dbttm1Geh Genotyping Protocol, Version 2.1. [Google Scholar]
- 22.Skvorak KJ, Paul HS, Dorko K, Marongiu F, Ellis E, Chace D, Ferguson C, et al. Hepatocyte transplantation improves phenotype and extends survival in a murine model of intermediate maple syrup urine disease. Mol Ther. 2009;17:1266–1273. doi: 10.1038/mt.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skvorak KJ, Paul HS, Dorko K, Marongiu F, Ellis E, Chace D, Ferguson C, et al. Hepatocyte Transplantation Improves Phenotype and Extends Survival in a Murine Model of Intermediate Maple Syrup Urine Disease. Mol Ther. 2009 doi: 10.1038/mt.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, et al. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain. 2009;132:903–918. doi: 10.1093/brain/awp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton D, Strauss K, Robinson D, Puffenberger E, Kelley R. Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics. 2002;109:999–1008. doi: 10.1542/peds.109.6.999. [DOI] [PubMed] [Google Scholar]
- 26.Skvorak K, Gramignoli R, Hansel MC, Uraz S, Tahan V, Dorko K, Marongiu F, et al. Cell Transplantation: a possible alternative to orthotopic liver transplant (OLT) In: Abdeldayem H, Allam NAA, editors. Liver Transplantation -Technical Issues and Complications. InTech; 2012. [Google Scholar]
- 27.Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 28.Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Human immunology. 2008;69:321–328. doi: 10.1016/j.humimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, et al. CONCISE REVIEW: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 30.Scaggiante B, Pineschi A, Sustersich M, et al. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44:59–61. doi: 10.1097/00007890-198707000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Murphy S, Lim R, Dickinson H, Acharya R, Rosli S, Jenkin G, Wallace E. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20:909–923. doi: 10.3727/096368910X543385. [DOI] [PubMed] [Google Scholar]
- 32.Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, Jenkin G, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182:643–651. doi: 10.1164/rccm.201001-0014OC. [DOI] [PubMed] [Google Scholar]
- 33.Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, Lombardi G, et al. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell transplantation. 2009;18:405–422. doi: 10.3727/096368909788809857. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, et al. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 2010;111:1453–1463. doi: 10.1002/jcb.22873. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011;6:e16789. doi: 10.1371/journal.pone.0016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, Williams ED, et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl(4)-treated mice. Cell transplantation. 2010;19:1157–1168. doi: 10.3727/096368910X504496. [DOI] [PubMed] [Google Scholar]
- 37.Kakishita K, Elwan MA, Nakano N, Itakura T, Sakuragawa N. Human amnionic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson’s disease: a potential source of donor for transplantation therapy. Experimental Neurology. 2000;165:27–34. doi: 10.1006/exnr.2000.7449. [DOI] [PubMed] [Google Scholar]
- 38.Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980:48–56. doi: 10.1016/s0006-8993(03)02875-0. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Ohsugi K, Yamamoto T, Shiomi M, Sakuragawa N. A novel approach to ex vivo gene therapy for familial hypercholesterolemia using human amniotic epithelial cells as a transgene carrier. Tohoku J Exp Med. 2001;193:279–292. doi: 10.1620/tjem.193.279. [DOI] [PubMed] [Google Scholar]
- 40.Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481–1486. doi: 10.1097/01.tp.0000113809.53415.c2. [DOI] [PubMed] [Google Scholar]
- 41.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 42.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 43.Lin CS, Lin G, Lue TF. Allogeneic and Xenogeneic Transplantation of Adipose-Derived Stem Cells in Immunocompetent Recipients Without Immunosuppressants. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touraine JL, Sanhadji K. Transplantation tolerance induced in humans at the fetal or the neonatal stage. J Transplant. 2011;2011:760319. doi: 10.1155/2011/760319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Touraine JL, Roncarolo MG, Raudrant D, Bacchetta R, Golfier F, Sembeil R, Gebuhrer L. Induction of transplantation tolerance in humans using fetal cell transplants. Transplant Proc. 2005;37:65–66. doi: 10.1016/j.transproceed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 47.Escobar J, Frank JW, Suryawan A, Nguyen HV, Horn CGV, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutrition. 2010;140:1418–1424. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT, Hutson SM. Branched-chain amino acid metabolon: interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase (BCATm) J Biol Chem. 2010;285:265–276. doi: 10.1074/jbc.M109.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schadewaldt P, Bodner-Leidecker A, Hammer H-W, Wendel U. Significance of l-Alloisoleucine in Plasma for Diagnosis of Maple Syrup Urine Disease. Clinical Chemistry. 1999;45:1734–1740. [PubMed] [Google Scholar]