Abstract
The management of type 2 diabetes is comprised of a complex series of medical decisions regarding goals of care, self-care behaviors, and medical treatments. The quality of these medical decisions is critical to determining whether an individual diabetes patient is treated appropriately, overtreated, or under-treated. It is hypothesized that the quality of these medical decisions can be enhanced by personalized decision support tools that summarize patient clinical characteristics, treatment preferences, and ancillary data at the point of care. We describe the current state of personalized diabetes decision support based on 13 recently described tools. Three tools provided support for personalized decisions based on preferences, while the remaining 10 provided support for treatment decisions designed to achieve standard diabetes goals. For the tools that supported personalized decisions, patient participation in medical decisions improved. Future decision support tools must be designed to account for both clinical characteristics and patient preferences.
Keywords: Type 2 diabetes mellitus, decision support, decision support tool, decision aid, personalized decision support
INTRODUCTION
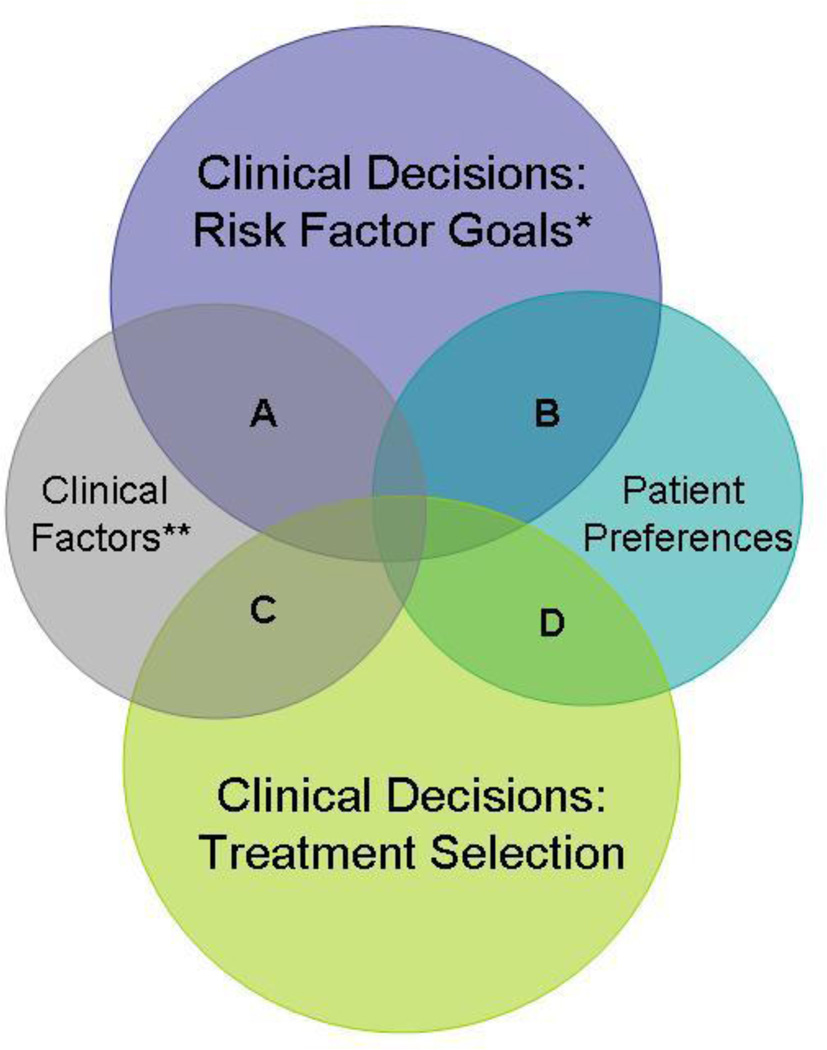
Management of chronic diseases, such as type 2 diabetes mellitus, requires repeated follow-up, with frequent re-calibration of goals and treatments. Setting the goals of diabetes care and then deciding which treatments are optimal for an individual patient requires patients and physicians to weigh multiple factors and data points. Personalization of diabetes care depends on clinical factors as well as patient preferences and can occur at the level of the goal or the treatment (Fig. 1) [1].
Fig. 1.
Conceptual framework for describing potential areas of personalization of diabetes care within the clinical decision making process. A = Consideration of clinical factors when determining risk factor goals. B = Consideration of patient preferences when determining risk factor goals. C = Consideration of clinical factors when making treatment decisions. D = Consideration of patient preferences when making treatment decisions. *Examples of risk factor goals: hemoglobin A1c, blood pressure, and cholesterol targets. **Examples of clinical factors: pharmacogenomics, comorbidity, life expectancy, stage of disease
Now more than ever, clinical practice guidelines are encouraging active personalization of diabetes care goals for glucose, blood pressure, and cholesterol targets [2]. For example, current guidelines from the American Diabetes Association regarding glycemic control in diabetes encourage personalization of treatment goals based on factors such as co-morbidity, life expectancy, and diabetes duration [3]. The call for greater personalization of treatment goals is based on divergent results emerging from diabetes clinical trials. While the United Kingdom Prospective Diabetes Study found that intensive glucose control reduced microvascular and cardiovascular complications for middle-aged patients with new-onset diabetes [4–6], more recent trials such as the Action to Control Cardiovascular Disease in Diabetes Trial found that very intensive glycemic control may actually cause harm in older patients with long-standing diabetes [7, 8]. These findings have motivated recent recommendations to personalize the goals and treatments for individual patients with diabetes.
Apart from purely clinical considerations, patient-centered diabetes care involves finding ways of helping to bring patients into the decision making process. Decision support tools may provide one way of accomplishing this goal. A recent Cochrane review that was not limited to trials involving diabetes mellitus found that the use of decision aids led to significant improvements in many aspects of the decision making process. Decision aids helped patients to be more involved in the decision making process, have greater knowledge regarding the available options, make choices that were more reflective of their values, and better understand potential harms and benefits [9].
The goal of our review is to describe the features of recently developed decision support tools in diabetes mellitus with a focus on the extent to which tools are designed to personalize diabetes care goals and treatments.
REVIEW OF RECENT PUBLICATIONS ON DECISION SUPPORT IN DIABETES
Our review of the literature was performed using PubMed and Google Scholar. We limited our search to articles in English, published within the past 5 years. In our final review, we included only studies involving decision support tools for type 2 diabetes mellitus. This meant excluding studies of decision aids for type 1 diabetes mellitus, glycemic management in the intensive care setting, and one study of a decision support tool for impaired fasting glucose screening. We also excluded any studies describing the development or piloting of a tool for which a more recent trial involving the tool was also available.
There were 13 tools with 16 publications. For each tool we describe the target audience, goal of intervention, medical decisions and outcomes affected, and, where applicable, the method and degree of personalization (Tables 1, 2). To organize the tools, we created a conceptual framework of personalized diabetes care that illustrates the extent to which diabetes care can be personalized within the clinical decision making process (Fig. 1). There are two main areas of clinical decision making, setting risk factor goals (e.g., HbA1c, blood pressure, cholesterol) and making treatment selections. These two areas of decision making can be personalized based on clinical factors (e.g., pharmacogenomics, comorbidity, life expectancy, stage of disease), and patient preferences. Within this framework, there are four main areas of overlap where a decision can be personalized. In categories A and C, clinical decisions are personalized based on clinical factors while for categories B and D, clinical decisions are personalized based on patient preferences. We define decision support as personalized when a decision aid or tool incorporates patients’ clinical characteristics and/or treatment preferences into the clinical decision making process.
Table 1.
Studies of decision support tools for type 2 diabetes mellitus which provide support for personalization
| Primary author, study year |
Target audience |
Study characteristics | Goal | Method and degree of personalization |
Decisions and outcomes affected |
|---|---|---|---|---|---|
| Corser, et al. 2007 | Physicians, patients | 58 Patients, single-group, pretest-posttest study, 15-month study period. | Improve outcomes, documentation of management goals, and patient knowledge and empowerment regarding diabetes goals. | Encouraged patients to set goals to be considered in shared-decision making process. | Addressed care decisions using shared decision-making that incorporates patient's goals. Significantly increased patient goal-setting and knowledge (P= .001). Did not have a significant impact on HbA1c, weight, or BP. |
| Mullan, et al. 2009 | Physicians, patients | 40 Clinicians and 56 patients, cluster randomized trial, 12-month enrollment period. | Improve adherence and glycemic control. | Printed tool used to help personalize pharmacologic therapy for diabetes based on patient and physician preferences. | Affected decision of how to medically manage diabetes. Increased patient involvement and aspects of knowledge and acceptability. Did not improve adherence or HbA1c at 6 months. |
| Weymiller, et al. 2007 | Physicians, patients | 98 Patients and 21 physicians, 2×2 clustered factorial design randomized trial, 4-month enrollment period. | Improve patient decision-making process. | Initiation of statin therapy was largely dependent on patient knowledge and preference. | Decision of whether to take statin. Increased patient knowledge and decreased decisional conflict. Increased medication adherence. |
| Nannenga, et al. 2009 | Physicians, patients | 16 Clinicians and 98 Patients, 2×2 clustered factorial design randomized trial, 4 month enrollment period. | Measure effect of the tool on patient knowledge, decisional conflict, participation and trust. | Initiation of statin therapy was largely dependent on patient knowledge and preference. | Decision of whether to take statin. Trend toward increased total trust in physician. Improved patient knowledge, decisional conflict, and participation, each of which increased the likelihood of total trust. |
| Abadie, et al. 2009 | Physicians, patients | 98 Patients, factorial-design randomized trial. | Examine decision aid use patterns by physicians. | Initiation of statin therapy was largely dependent on patient knowledge and preference. | Decision of whether to take statin. Tool was used as intended by physicians in 64% of the interventions. |
| Mann, et al. 2010 | Physicians, patients | 150 Patients, randomized trial. | Improve elements of patient decision-making process in a largely minority population and determine effect of tool on medication adherence. | Initiation of statin therapy was largely dependent on patient knowledge and preference. | Decision of whether to take statin. Improved patient perception of risk, beliefs regarding the medication, and decisional conflict. Did not affect medication adherence. |
HbA1c = hemoglobin A1c; BP = blood pressure.
Table 2.
Studies of decision support tools for type 2 diabetes mellitus which provide support for standard diabetes care without personalization
| Primary author, study year |
Target audience |
Study characteristics | Goal | Decisions and outcomes affected |
|---|---|---|---|---|
| Cleveringa, et al. 2008 | Physician, nurse | 3,391 Patients, cluster-randomized trial. | Improve clinical markers (A1c, BP, cholesterol). | Targeted at overall management. Decreased total cholesterol, LDL, BP. No significant change in HbA1c. |
| Holbrook, et al. 2009 | Physicians, patients | 46 Clinicians, 511 Patients, cluster-randomized trial, 1 year enrollment period. | Improve frequency and ease of assessing diabetes markers. | Targeted at overall management and frequency of certain assessments. Improved quality of monitoring. Resulted in lower BP and HbA1c. |
| Hunt, et al. 2009 | Physician | 4,265 continuously enrolled patients. Pre-post intervention, two year study period. | Improve clinical markers (HbA1c, BP, cholesterol), and process of care. | Targeted at overall management. Decreased LDL, BP. Improved LDL and HbA1c testing. Did not reduce mean HbA1c, but did improve percent of patients at HbA1c goal. |
| MacLean, et al. 2009 | Physicians, patients | 7,412 patients, cluster-randomized trial, 32 month study period. | Evaluate the effect of support system on processes of care and outcomes. | Targeted at overall management. Improved likelihood of testing for cholesterol, creatinine, and proteinuria, but not HbA1C. Did not impact HbA1c or LDL levels. |
| Augstein, et al. 2010 | Physician | 359 Patients, retrospective, observational study. | Improve glycemic control. | Targeted at overall management, emphasis on glycemic control. Decreased HbA1c, mean sensor glucose, and glucose variability. |
| O'Connor, et al. 2011 | Physician | 41 Clinicians, 2,556 Patients, cluster-randomized trial, 9 month study period. | Reduce HbA1C, BP, LDL | Targeted at overall management. Improved HbA1c and SBP, not LDL. |
| Quinn, et al. 2011 | Physicians, patients | 163 Patients, cluster-randomized trial, 1-year treatment period. | Reduce HbA1c. | Targeted at overall management. Certain forms of the intervention reduced HbA1c over 1 year compared with usual care. |
| Saenz, et al. 2012 | Physician | 66 Clinicians and 697 Patients, Cluster-randomized trial, 18-month study period. | Reduce HbA1c | How to use insulin in type II diabetes. Reduced HbA1c. |
| Leal, et al. 2009 | Not specified. | Development of life expectancy tables based on the United Kingdom Prospective Diabetes. | Develop a tool to help predict life expectancy. | Study describes tool. Presumably the decision relates to addressing modifiable risks in an attempt to improve life expectancy. |
| Rodbard, et al. 2011 | Physician, patients | Development of computerized clinical decision support tool for patients with type 2 diabetes. | Improve glycemic control. | Targeted at glycemic management. Currently being tested. |
HbA1c = hemoglobin A1c; BP = blood pressure; SBP = systolic blood pressure; LDL = low-density lipoprotein cholesterol.
REVIEW OF PERSONALIZED DECISION SUPPORT TOOLS
We found that 3 out of 13 tools actively provided support for purposes of personalized decisions, while the remaining 10 tools provided decision support for purposes of achieving standard diabetes management goals. Among the three personalized decision support tools, one of the interventions attempted to personalize risk factor goals based on patient preferences (category B) while the other two interventions attempted to personalize medical treatment decisions based on patient preferences (category D) (Table 1) (Fig. 1).
A decision support tool that was recently developed by Corser and colleagues emphasized shared-decision making and used a unique combination of patient and physician interventions, including a patient workbook and educational sessions for patients. The overall intent of the intervention was to improve risk factor levels, documentation of diabetes management goals, and patient knowledge and empowerment regarding diabetes goals. The tool did not attempt to personalize risk factor goals based on clinical characteristics, but rather emphasized the important role of patients in setting personal goals (category B). The study used a single-group, pre-post test design and during a 15 month study period enrolled 58 patients with type 2 diabetes from one clinic. The tool improved the degree to which patients with diabetes mellitus identified a specific diabetes management goal. The tool did not have a significant effect on hemoglobin A1c, blood pressure, or weight [10].
The Diabetes Mellitus Medication Choice Decision Aid was designed to improve patient involvement in glucose lowering medication selection (category D). The clinical goal of the decision aid was to improve medication adherence and glycemic control. The decision aid consisted of cards, each of which describes the characteristics of different diabetes medications, including effect on weight, side effects, and hemoglobin A1c lowering. The pilot study of this decision aid was a cluster-randomized trial with a total of 56 patients and 40 clinicians enrolled over a 12-month period. This decision aid improved patient involvement in the medical decision, treatment acceptability, and diabetes knowledge. There was no effect of the aid on medication adherence or hemoglobin A1c at 6 months [11••].
Recently, much work has been done using a decision aid called Statin Choice. We reviewed 4 publications which involve use of the Statin Choice tool. Like the previously described Diabetes Mellitus Medication Choice Decision Aid, the decision aid consisted of informational cards displaying the benefits and side effects of statins. The intent of the decision aid was to involve diabetes patients in the decision to use a statin (category D); of note, the decision aid was intended for use with both patients using and not using a statin. In the original Statin Choice trial published in 2007, the tool was found to be well liked by patients, improved knowledge, and decreased decisional conflict. It was also associated with higher adherence to statins at 3 months [12]. Another study of Statin Choice examined its effect on patient trust. This study found a trend toward increased total trust in the physician with use of the decision aid, and found that the likelihood of total trust increased with improvements in patient knowledge, decisional conflict, and participation. Each of these aspects of the patient decision-making process was found to be improved with use of the decision aid [13]. Use of the Statin Choice decision aid was also studied with the intent of examining use patterns by physicians. By videotaping interactions between physicians and patients the study found that during most encounters the tool was used as intended, but there were also several examples of unintended uses of the aid by physicians, including not using the tool at all and presenting probabilities regarding risks and benefits in a way other than was intended. For example, in some cases the aid was used to advance a physician’s notion that all patients with diabetes should take a statin [14]. Finally, a follow-up study similar to the original Statin Choice trial, but conducted in a different clinical setting, did not demonstrate an improvement in adherence to statins, but found similar benefits of the tool with regard to its effects on the patient decision-making process. 108 providers were trained to use the tool and 150 patients were randomly assigned to their usual primary care visit either with or without the tool. The results indicated that participants assigned to the intervention arm had increased risk perception accuracy for heart attack with and without taking a statin [15••].
REVIEW OF DECISION SUPPORT TOOLS FOR STANDARDIZED DIABETES MANAGEMENT
What follows are descriptions of recent publications involving use of decision support tools in diabetes which are designed to achieve standardized diabetes management goals for hemoglobin A1c, blood pressure, or cholesterol (Table 2). None of these interventions attempt to personalize management goals and none attempt to incorporate patient preferences. However, some of the interventions do attempt to personalize medical treatment decisions in achieving standardized goals based on data derived from the individual patient. These tools may be considered as part of category C.
The Diabetes Care Protocol (DCP) included use of a computerized decision support system that produced treatment recommendations based on algorithms derived from Dutch diabetes guidelines. The goal of the tool was to improve the degree to which patients with diabetes achieved standard goals for glucose, blood pressure, and cholesterol. This trial found no difference between study and control groups with regard to hemoglobin A1c, but did find significant improvement in BP, total cholesterol, and LDL cholesterol [16].
The COMPETE II trial utilized multiple forms of decision support, including a web-based tool, targeted at physicians and patients with the intent of improving the process of diabetes care. The trial enrolled 511 patients and 46 clinicians using a cluster-randomized design. This primary outcome was measured in terms of the frequency with which certain aspects of diabetes health were assessed, such as blood pressure, LDL cholesterol, and hemoglobin A1c. While risk factor level control was not the primary outcome of the study, the support tool did lead to significant reductions in blood pressure (−3.95 mm Hg Systolic BP, 95% CI −7.64 to −0.26, p = 0.036; and −2.38 mm Hg Diastolic BP, 95% CI −4.60 to 0.17, p = 0.035) and hemoglobin A1c (−0.20%, 95% CI −0.38% to −0.02%, p = 0.029) [17].
The CareManager™ tool is a physician-targeted EMR-based decision support tool that was recently tested for its effects on the process of diabetes care such as frequency of certain lab measurements, outcomes (LDL-cholesterol, hemoglobin A1c, and blood pressure), and patient satisfaction. The tool generates summaries of an individual patient’s data, and highlights aspects of management that the physician may need to address in order to better meet clinical goals. Additionally, the tool includes features such as providing monthly physician and clinic level information on how well process and outcome goals are being met, as well as access to diabetes guidelines. The pre-post intervention took place over a period of two years and 4,265 patients were continuously enrolled throughout the study period. The tool was found to significantly improve testing of LDL cholesterol, reduce mean LDL cholesterol (−13 mg/dL (0.33 mmol/l, P=0.002), and increase the number of patients with LDL-cholesterol at goal (32% to 56%, P=0.002). It significantly improved mean systolic and diastolic blood pressure, and also increased the number of patients with blood pressure at goal (from 30% to 52%). It increased hemoglobin A1c testing and the number of patients with hemoglobin A1c < 7%, but did not significantly reduce the mean hemoglobin A1c. It had no effect on patient satisfaction [18].
In Vermont, an automated system was tested which increased the accessibility of lab data to providers and patients, and provided both groups with automated reminders. This system was found to improve monitoring of cholesterol, creatinine, and proteinuria but not hemoglobin A1c. The intervention did not have a significant effect on hemoglobin A1c or cholesterol levels [19].
A computerized decision support aid (KADIS) was developed in Germany, and the impact of its use on glycemic control was studied in 359 patients in a retrospective, observational analysis. The tool uses data, including the results of continuous glucose monitoring, to simulate a patient’s metabolic profile (a so called, “in silico” model), which it then uses to simulate the effects of various treatment options. A report is generated for the physician identifying treatments linked with the best possible outcomes according to the simulation. Patients that used the tool were found to significantly improve glycemic control by reducing hemoglobin A1c (p<.01), mean sensor glucose (p=.003), and glucose variability (p=.001) [20].
The Diabetes Wizard is a decision support tool that was integrated into an electronic health record system with the goal of reducing hemoglobin A1c, blood pressure, and LDL cholesterol among patients with type 2 diabetes. In this cluster-randomized trial, 41 clinicians and 2,556 patients were enrolled over a 9 month period. The tool uses algorithms designed by the authors to produce treatment, testing, and follow-up recommendations based on variables entered into the patient chart. Use of the tool did produce a significant reduction in mean hemoglobin A1c within the intervention group compared with the control group, and the intervention group had a greater percentage of patients with systolic blood pressure below 130 mmHg post-intervention. There was no effect on LDL cholesterol [21].
A tool developed by Quinn and colleagues using both a mobile phone interface and web-based component to provide feedback and support to patients was tested for its effect on hemoglobin A1c lowering among other factors. The most intensive intervention group within this cluster-randomized trial included access to patient data and diabetes management guidelines for physicians online and by fax. When compared to usual care, patients within this most intensive arm of the intervention were found to have a hemoglobin A1c reduction after 1 year that was significantly better than that seen within the control arm. Interestingly, the “coach only” group, which involved use of the mobile phone interface and web-portal for patients but only included physician access to data if allowed by the patient, also achieved hemoglobin A1c reductions at 1 year that were better than the usual care group. The tool did not produce significant effects on other secondary outcomes in the study, including clinical measures of blood pressure and lipids, as well as other factors such as distress, diabetes symptoms, and depression [22].
Finally, a computer-based decision support tool was developed by Saenz and colleagues with the goal of helping primary care providers make decisions regarding insulin therapy in patients with type 2 diabetes. The program uses patient blood glucose data to generate possible insulin regimens from which the physician may choose. The study used a cluster-randomized trial in an 18 month study period, and enrolled 66 clinicians and 697 patients. Within the intervention group, use of the tool was associated with increased total daily doses of insulin and a significant reduction in hemoglobin A1c when compared with the control group. However, the hemoglobin A1c within the intervention group did not reach the goal of < 7% [23].
Publications which describe the development of decision support tools for diabetes include one in which life-expectancy tables were developed for patients with type 2 diabetes based on modifiable risk factors (hemoglobin A1c, smoking, systolic blood pressure, total: high-density lipoprotein cholesterol ratio) and the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model. While this publication describes only the tool, presumably these life-expectancy tables could be used by physicians, and possibly patients, to help visualize the potential improvements in life-expectancy that might be achieved by addressing these risk factors [24].
A comprehensive computer-based decision support tool was recently developed by Rodbard and colleagues and is undergoing further testing. The tool analyzes patient data, including self-monitored blood glucose, to generate treatment recommendations to improve glycemic control. For individual patients or groups of patients, physicians are able to set the hemoglobin A1c goal as well as goal ranges for preprandial and postprandial glucose. Importantly, this aspect of the tool introduces the potential for personalization, for example, if the physician using the tool were to personalize the hemoglobin A1c or other glycemia goals based on consideration of individual patient clinical factors or preferences. Of note, with regard to consideration of patient clinical factors, the system is designed to account for comorbid conditions [25•].
CONCLUSION
Our review of personalized decision support in diabetes mellitus suggests that, for the most part, recently developed decision support tools do not incorporate personalization of treatment goals or treatment selection based on clinical characteristics or patient preferences. We found only three decision support tools designed to involve the patient in diabetes decision-making. These tools attempted to elicit and incorporate patient preferences regarding the selection of treatments (category D) and, in one case, the selection of management goals (category B). In general, these tools improved patient knowledge, reduced decisional conflict, and increased patient involvement in decisions. To the extent that patient-centered care is critical to diabetes management, the fact that these three tools improved measures of patient engagement suggest that personalized decision support tools have significant potential for positively impacting the field of type 2 diabetes care.
The remaining 10 studies which we reviewed provide examples of decision aids which provide support for achieving standard diabetes management goals without explicit consideration for patient preferences. Most of these tools attempted to harness the data from electronic medical records and in several cases, the tools did make recommendations regarding the optimal treatment selection for a given patient in order to achieve a common management goal which is consistent with category C in our framework. Many of these decision support tools were able to improve clinical markers of diabetes management, such as hemoglobin A1c, blood pressure, and cholesterol. It remains to be seen whether or not the standardized goals selected for these patients will lead to better long-term outcomes and whether patients are satisfied with the treatments selected without actively acknowledging their preferences.
This review has highlighted the fact that the study of personalized decision support tools is highly complex both in terms of the design of new tools and their evaluation. To have a truly personalized decision support tool for diabetes requires the integration of very diverse perspectives and data sources. Patients bring their preferences and beliefs to the decision-making process which we believe, based on ethical and clinical grounds, should be integrated into goal setting and management decisions. Also, every individual patient differs in important ways with regard to a wide-range of clinical factors including duration of diabetes, history of complications, responsiveness to treatments, and overall prognosis. Each of these characteristics may affect the optimal goals and treatments for diabetes. Undoubtedly an effort to integrate all of these variables makes the management of a complex chronic disease like type 2 diabetes mellitus potentially more challenging.
Apart from the challenge of integrating diverse data sources, another challenge for personalized decision support might occur when different aspects of personalization are considered for the same decision, thereby potentially producing different goals. For example, personalizing care by considering treatments and patient preferences might produce a very different management strategy than personalizing care based on clinical factors. Consider a situation in which a patient’s clinical factors suggest that insulin is not an ideal treatment choice, yet after seeing insulin work in friends and family a patient’s treatment preference is for insulin therapy only. Presumably conflicts such as these should be managed by physician-patient discussion, leading to further refinement of personalized medical decisions.
The importance of personalization in diabetes care is gaining wide acceptance and is now formally endorsed by multiple clinical organizations. The components of a truly personalized decision support tool do exist but have yet to be fully integrated and studied as a whole. Whether these tools ultimately improve patients’ quality of life and satisfaction with care will be the motivating research questions for this field for the next decade.
Acknowledgments
A.G. Nathan: has received grant support from Retirement Research Foundation and the American Diabetes Association; E.S. Huang: has received grant support from Retirement Research Foundation, the American Diabetes Association (Clinical Research Award), and the NIDDK (NIDDK P30 DK092949-01)
Footnotes
Disclosure
Conflicts of interest: M.J. Wilkinson: none;
Contributor Information
Michael J. Wilkinson, University of Chicago, 5841 S. Maryland Ave., Chicago, IL 60637, 773-702-1000, michael.wilkinson@uchospitals.edu.
Aviva G. Nathan, University of Chicago, 5841 S. Maryland Ave., MC 2007, Chicago, IL 60637, 773-702-9521, 773-834-2238 (fax), anathan@bsd.uchicago.edu.
Elbert S. Huang, University of Chicago, 5841 S. Maryland Ave., MC 2007, Chicago, IL 60637, 773-834-9143, 773-834-2238 (fax), ehuang@medicine.bsd.uchicago.edu.
REFERENCES
Recently published publications of particular interest are indicated as:
• Of importance
•• Of outstanding importance
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing Glycemic Targets in Type 2 Diabetes Mellitus: Implications of Recent Clinical Trials. Ann Intern Med. 2011;154:554–559. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) The Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, et al. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.The ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intesive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD001431.pub3. CD001431. [DOI] [PubMed] [Google Scholar]
- 10.Corser W, Holmes-Rovner M, Lein C, Gossain V. A shared decision-making primary care intervention for type 2 diabetes. Diabetes Educ. 2007;33:700–708. doi: 10.1177/0145721707304086. [DOI] [PubMed] [Google Scholar]
- 11. Mullan RJ, Montori VM, Shah ND, et al. The Diabetes Mellitus Medication Choice Decision Aid. Arch Intern Med. 2009;169:1560–1568. doi: 10.1001/archinternmed.2009.293. This article describes use of a personalized decision support tool which considered patient preferences in treatment decisions. Its use increased patient involvement as well as aspects of knowledge and acceptability.
- 12.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions. Arch Intern Med. 2007;167:1076–1082. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 13.Nannenga MR, Montori VM, Weymiller AJ, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The Statin Choice randomized trial. Health Expect. 2009;12:38–44. doi: 10.1111/j.1369-7625.2008.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abadie R, Weymiller AJ, Tilburt J, et al. Clinician's use of the Statin Choice decision aid in patients with diabetes: a videographic study nested in a randomized trial. J Eval Clin Pract. 2009;15:492–497. doi: 10.1111/j.1365-2753.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 15. Mann DM, Ponieman D, Montori VM, et al. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80:138–140. doi: 10.1016/j.pec.2009.10.008. This article describes recent use of the Statin Choice tool, an important example of a personalized decision support tool which considers patient preferences in treatment decisions.
- 16.Cleveringa FG, Gorter KJ, van den Donk M, Rutten GE. Combined Task Delegation, Computerized Decision Support, and Feedback Improve Cardiovascular Risk for Type 2 Diabetic Patients. Diabetes Care. 2008;31:2273–2275. doi: 10.2337/dc08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holbrook A, Thabane L, Keshavjee K, et al. Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ. 2009;181:37–44. doi: 10.1503/cmaj.081272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt JS, Siemienczuk J, Gillanders W, et al. The impact of a physician-directed health information technology system on diabetes outcomes in primary care: a pre- and post-implementation study. Informatics in Primary Care. 2009;17:165–174. doi: 10.14236/jhi.v17i3.731. [DOI] [PubMed] [Google Scholar]
- 19.Maclean CD, Gagnon M, Callas P, Littenberg B. The Vermont diabetes information system: a cluster randomized trial of a population based decision support system. J Gen Intern Med. 2009;24:1303–1310. doi: 10.1007/s11606-009-1147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augstein P, Vogt L, Kohnert KD, et al. Translation of Personalized Decision Support into Routine Diabetes Care. J Diabetes Sci Technol. 2010;4:1532–1539. doi: 10.1177/193229681000400631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connor PJ, Sperl-Hillen JM, Rush WA, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med. 2011;9:12–21. doi: 10.1370/afm.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn CC, Shardell MD, Terrin ML, et al. Cluster-Randomized Trial of a Mobile Phone Personalized Behavioral Intervention for Blood Glucose Control. Diabetes Care. 2011;34:1934–1942. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saenz A, Brito M, Moron I, et al. Development and Validation of a Computer Application to Aid the Physician's Decision-Making Process at the Start of and during Treatment with Insulin in Type 2 Diabetes: A Randomized and Controlled Trial. J Diabetes Sci Technol. 2012;6:581–588. doi: 10.1177/193229681200600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30:834–839. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodbard D, Vigersky RA. Design of a Decision Support System to Help Clinicians Manage Glycemia in Patients with Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2011;5:402–411. doi: 10.1177/193229681100500230. From the perspective of personalization, this article is important because the tool allows physicians to set hemoglobin A1c and other glycemia goals. This introduces the potential for personalization, if physicians using the tool set glycemic goals based on a consideration of patient clinical factors or preferences.