Abstract
Calcitonin gene-related peptide (CGRP) acting within the bed nucleus of the stria terminalis (BNST) increases anxiety as well as neural activation in anxiety-related structures, and mediates behavioral stress responses. Similar effects have been described following intra-ventricular as well as intra-BNST infusions of the stress-responsive neuropeptide, corticotropin releasing factor (CRF). Interestingly, CGRP-positive terminals within the lateral division of the BNST form perisomatic baskets around neurons that express CRF, suggesting that BNST CGRP could exert its anxiogenic effects by increasing release of CRF from these neurons. With this in mind, the present set of experiments was designed to examine the role of CRFR1 signaling in the anxiogenic effects of CGRP within the BNST and to determine whether CRF from BNST neurons contributes to these effects. Consistent with previous studies, we found that 400 ng CGRP infused bilaterally into the BNST increased the acoustic startle response and induced anxiety-like behavior in the elevated plus maze compared to vehicle. Both of these effects were attenuated by 10 mg/kg PO of the CRFR1 antagonist, GSK876008. GSK876008 alone did not affect startle. An intra-BNST infusion of the CRFR1 antagonist CP376395 (2 μg) also blocked increases in acoustic startle induced by intra-BNST infusion of CGRP, as did virally-mediated siRNA knockdown of CRF expression locally within the BNST. Together, these results suggest that the anxiogenic effects of intra-BNST CGRP may be mediated by CRF from BNST neurons acting at local CRFR1 receptors.
Keywords: bed nucleus of the stria terminalis, calcitonin gene-related peptide, corticotropin releasing factor, anxiety, fear, startle
1. Introduction
The bed nucleus of the stria terminalis (BNST), particularly its lateral subdivision, is an important component of the neural circuitry that regulates anxiety [1–3]. We previously demonstrated that calcitonin gene-related peptide (CGRP) acting within the BNST evokes anxiety-like responses, increases neural activation in anxiety-related structures, and modulates behavioral responses to stressors in rats [4]. Intriguingly, the stress-responsive CGRP neurons within the parabrachial nucleus [5, 6] that project to the BNST form perisomatic baskets around BNST cells that express corticotropin releasing factor (CRF) [7], another peptide well known for its involvement in stress and anxiety [8, 9]. In a previous study, we demonstrated that CRF over-expression localized within the BNST increases sustained fear-potentiated startle, which models aspects of anxiety [4]. In addition to its CRF-expressing neurons, the BNST is also enriched with CRF receptors [10–13], and numerous studies have demonstrated anxiety-like behavioral effects of CRF infusions within the BNST in rodents. For example, intra-BNST CRF infusions enhance the acoustic startle response [14], evoke anxiety-like behavior in the elevated plus maze [15] and social interaction tests [16], enhance retention of an inhibitory avoidance task [17], and produce anorectic effects [18] and tachycardia [19]. The anxiety-like effects of CRF within the BNST seem to be mediated primarily by CRFR1 receptors, in that a CRFR1 but not a CRFR2 antagonist co-infused with CRF into the BNST inhibited CRF-induced anxiety behavior in the elevated plus maze [15], and the CRFR1 antagonist used in the present studies (GSK876008) prevented elevated acoustic startle induced by ICV CRF infusions [20].
Although the physical proximity of CGRP terminals with CRF cells within the BNST and the similar anxious behaviors evoked by manipulation of these systems suggest a functional interaction between CGRP and CRF within the BNST, to date there are no data that have directly demonstrated the behavioral ramifications of this CGRP-CRF connection. A few studies, however, do suggest that an interaction between CGRP and CRF (specifically, CRFR1 receptor) signaling forms part of the circuitry that regulates anxiety. For example, Kovacs et al. [21] demonstrated that CRF antiserum blocked increases in grooming and elevation of plasma corticosterone induced by intracerebroventricular (ICV) CGRP, and Bowe et al. [22] found that a CRFR1 but not a CRFR2 receptor antagonist blocked ICV CGRP-induced increases in plasma corticosterone.
With all of this evidence in mind, we hypothesized that CGRP within the BNST may affect anxiety in part by increasing CRF release from the BNST neurons onto local CRFR1 receptors. In order to examine whether downstream CRFR1 receptors form part of the pathway by which CGRP increases anxiety, we assessed anxiety behavior (acoustic startle and elevated plus maze) in response to pharmacological treatment with intra-BNST CGRP plus the CRFR1 antagonists GSK876008 and CP376395 administered systemically and locally, respectively. We also investigated whether CRF expressed within BNST neurons mediates CGRP-induced anxiogenesis, by measuring the acoustic startle following intra-BNST CGRP infusions in animals with virally mediated BNST-specific siRNA knockdown of CRF expression.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Charles River, Raleigh, NC), weighing between 275 and 350 g at the time of surgery and housed in groups of 4 in 45 × 20 × 24-cm (depth × width × height) polycarbonate cages, were maintained on a 12-h light/dark cycle (lights on at 0800 h) with food and water available ad libitum. A total of 126 animals were used in these experiments. All procedures were conducted under conditions consistent with U.S. Department of Agriculture, Emory University IACUC, and National Institutes of Health guidelines for the care and use of laboratory animals.
2.2 Surgery
Prior to surgery, rats were anesthetized with 75 mg/kg (i.p.) ketamine (Bionichepharma) and 0.5 mg/kg (i.p.) Dexdomitor (Orion Pharma) and also given an analgesic dose of 1.0 mg/kg (s.c.) meloxicam (Boehringer Ingelheim) to reduce postoperative discomfort. Once unresponsive to tailpinch, rats were placed in a Kopf Instruments stereotaxic frame with the nosebar set to −3.8 mm (flat-skull position). For BNST cannulations, 11 mm 26-gauge guide cannulae (Plastics One, Model C315G) were implanted bilaterally (20° coronal angle – to avoid the lateral ventricles, 0.3 mm caudal, 5.8 mm ventral, and 3.8 mm lateral to bregma. For AAV infusions (Experiment 4), injection cannulae (32-gauge; Plastics One, Model C315I) were inserted through the guide cannulae and virus infused bilaterally (0.2 μl/min, 1 μl total volume/BNST). The injectors remained in place for two min following viral infusion. For all surgeries, four jeweler screws were attached to the skull, and the entire assembly was cemented in place using Cranioplastic Powder (Plastics One). Stainless steel wires (i.e., stylets) were inserted into the guide cannula to maintain patency. The tip of each extended 1 mm past the end of the guide cannula. After surgery was complete, 0.5 mg/kg Antisedan (Orion Pharma) was given to reverse the anesthetic effects. A minimum of 7 days elapsed between cannula implantation and the onset of behavioral procedures.
2.3 Drugs and infusion procedure
CGRP (Tocris) was dissolved in a vehicle of 0.1% bovine serum albumin in phosphate buffered saline. The CRFR1 antagonist CP376395 was dissolved in a vehicle of 10% tween in distilled water. Both CGRP and CP376395 were frozen in aliquots at −80C until the day of use. Injection cannulae (32-gauge; Plastics One, Model C315I) were used for bilateral BNST infusions (0.25 μl/min, 0.5-μl total volume/BNST). After the infusions were completed, the injection cannulae were left in place for two min and then removed, whereupon the stylets were placed back into the guide cannula. The CGRP dose used in these studies (400 ng/side) was based on our own previously published data[4] as well as studies of intra-amygdala CGRP effects on fear-related behaviors [23]. CP376395 was infused into the BNST at a dose of 2 μg/side (chosen based on pilot data). The CRFR1 antagonist GSK876008 [24] was suspended at 5 mg/ml in 0.5 % hydroxypropylmethylcellulose and orally administered at a dose of 10 mg/kg (dose based on previous results from our laboratory [20]).
2.4 Elevated Plus Maze
The maze was a plus-shaped apparatus consisting of two 50 × 11 cm open arms, and two 50 × 11 × 40 cm enclosed arms, elevated at a height of 40 cm. Testing was conducted in a room illuminated by a single red light bulb over the center of the maze.
2.5 Startle Apparatus
Rats were trained and tested in four identical 8 × 15 × 15-cm Plexiglas and wire mesh cages, each suspended between compression springs within a steel frame located within a custom-designed sound-attenuating chamber. The floor of each cage consisted of four 6.0-mm diameter stainless steel bars spaced 18 mm apart. Startle responses were evoked by 50-msec 95-dB white-noise bursts (5-msec rise-decay time, 0–22 kHz) generated by a Macintosh G3 computer sound file, amplified by a Radio Shack amplifier (Model MPA-200; Tandy), and delivered through Radio Shack Supertweeter speakers located 4 cm in front of each cage. Background noise (60-dB wideband) was produced by an ACO Pacific white-noise generator (Model 3024) and was delivered through the same speakers as those used to provide background noise. Sound level measurements were made with a Brüel & Kjaer model 2235 sound-level meter (A scale; random input) with the microphone (Type 4176) located 10 cm from the center of the speaker, which approximates the distance of the rat’s ear from the speaker during testing. Startle response amplitudes were quantified using an accelerometer (model U321AO2; PCB Piezotronics) affixed to the bottom of each cage. Thus, cage movement (e.g., produced by the rats’ startle response) resulted in displacement of the accelerometer, which in turn produced a voltage output proportional to the velocity of cage movement. This output was amplified (PCB Piezotronics, Model 483B21) and digitized on a scale of 0–9.98 units by an InstruNET device (Model 100B; GW Instruments) interfaced to a Macintosh G3 computer. Startle amplitude was defined as the maximal peak-to-peak voltage that occurred during the first 200 msec after onset of the startle-eliciting white-noise burst. The presentation and sequencing of all stimuli were under the control of the Macintosh G3 computer using custom-designed software (The Experimenter; Glassbeads Inc.).
2.6 Cannula Placement Verification
Rats in Experiments 1–3 were sacrificed by chloral hydrate overdose and perfused intracardially with 0.9% (wt/vol) saline followed by 10% (vol/vol) formalin. The brains were removed and immersed in a 30% (wt/vol) sucrose-formalin solution for at least 3 d, after which 50-μm coronal sections were cut through the rostro-caudal extent of the BNST. Every other section was mounted on gelatinized slides and stained with cresyl violet. In Experiment 4, animals were rapidly decapitated following chloral hydrate administration, their brains extracted, snap-frozen on dry ice, and stored at −80°C until use. The fresh tissue was cut on a cryostat into 18-μm sections and mounted onto charged microscope slides. The mounted tissue was then immersed in 10% formalin for 30 min prior to cresyl violet staining. A scorer blind to the animal’s group assignment and behavioral data judged cannulae placements. For those animals treated with active drugs, only those with cannula tip placements within 0.5 mm of the lateral BNST, medial to the internal capsule, and not penetrating the lateral ventricle were included in the behavioral analyses.
2.7 Plasmid Preparation
In order to generate an AAV vector that expresses CRF siRNA, a 50-base custom oligo was designed to encode an shRNA against CRF mRNA. This oligo and its complement were designed using Invitrogen’s online RNAi Designer tool (Top strand sequence: CACCGGATCCAAGGAGGAAACCTTTCGAAAAAGGTTTCCTCCTTGGATCC, bottom strand sequence: AAAAGGATCCAAGGAGGAAAACCTTTTTCGAAAGGTTTCCTCCTTGGATCC). These oligos were designed to produce a 21-bp siRNA (target DNA sequence: GGATCCAAGGAGGAAACCTTT) against CRF mRNA. These top and bottom strand oligos were annealed and ligated into the pENTR/U6 plasmid using T4 DNA ligase per manufacturer’s protocol (Invitrogen). This plasmid (pENTR/U6-shCRF), containing a U6 promoter driving the CRF shRNA expression, was then tested for in vitro knockdown of CRF expression as described below.
The U6 promoter and the shRNA oligos were then excised from the pENTR/U6 plasmid at the KpnI and XbaI restriction sites and ligated into the polylinker region of an AAV backbone plasmid (pAAV-MCS; Addgene) modified to contain a KpnI site. An eGFP cDNA sequence bounded by SacII restriction sites was then PCR-generated and ligated into the SacII restriction site located just after the CMV promoter. The final viral vector expression plasmid, pAAV-CMV-eGFP-U6-CRF shRNA (hereafter referred to as pAAV-siCRF), was verified using restriction digests and DNA sequencing. This plasmid was used to generate the AAV1-siCRF viral vector.
2.8 AAV preparation
An AAV virus (serotype 1) containing CRF siRNA driven by a U6 promoter and the GFP gene driven by a CMV promoter (AAV1-siCRF) was generated by the Emory Viral Vector Core. Briefly, a total of 420ug of plasmids were co-transfected into HEK293T cells at a ratio of 2:1:1, (210ug of AAV helper plasmid/ 105ug of replication/capsid gene plasmid/ 105ug of inverted terminal repeat plasmid containing genes of interest) using polyethyleneimine. 72 hours post-transfection, supernatant and cells were collected. Virus in the supernatant was precipitated with 40% polyethylene glycol 8000 in 2.5 N NaCl, combined with the cell lysate, then treated with 10% deoxycholate and benzonase. The crude virus was then purified by a discontinuous iodixanol density gradient ultracentrifugation, then dialyzed and concentrated with an Amicon 15 100,000MWCO concentration unit. The genomic titer of the viral stock, was determined by qPCR with plasmid standards. Titer for AAV1-siCRF was 1.2 × 1013 infectious particles per mL. As a control, a stock AAV virus (serotype 1) expressing GFP (AAV1-CMV-GFP) was purchased from the University of North Carolina Chapel Hill Vector Core. Viral titer for AAV1-CMV-GFP was 4×1012 infectious particles per mL.
2.9 Cell Culture, Transfection, and Immunocytochemistry
All of the tissue culture media and related reagents were purchased from Invitrogen. HEK-293 cells were maintained in complete medium (Dulbecco’s modified Eagle’s medium plus 10% fetal bovine serum and 1% penicillin/streptomycin) at 37 °C with 5% CO2. 80–95% confluent cells in 2-cm2 wells were transfected with a total of 3μg plasmid cDNA mixed with 6 μL Lipofectamine 2000 in 100μL of serum-free medium (Optimem) per well. Four wells were assigned to each of the following three conditions: untransfected (negative control), CRF + scrambled siRNA, and CRF + CRF siRNA. Following overnight incubation, complete medium was added.
For examination of in vitro CRF gene expression, the cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, washed with PBS again, and then dryed with methanol. The cells were then blocked with 1% hydrogen peroxide followed by 30 min incubation in a blocking buffer of 5% non-fat milk with 0.01% triton in PBS. Cells were then incubated overnight with anti-CRF antibody in 1% bovine serum albumin (BSA) in PBS (1:2000; Abcam #11133) followed by incubation with biotinylated anti-rabbit secondary antibody in blocking buffer for 2 h at room temperature (1:500; Jackson Immuno Research Laboratory). Cells were then washed five times with PBS, and then incubated for 1 h in avidin-biotin complex in 1% BSA/PBS. Following 5 PBS washes, cells were stained with the chromagen diaminobenzidine (Sigma) for 10 min, rinsed with PBS, and then microscopic images were captured with a Microfire (Optronics) digital camera attached to an Olympus BX51 microscope (Japan) using a 10x objective lens. The optical density of the images from each well was calculated on the basis of gray values (GVs) between 0 (brightest) and 255 (darkest) obtained from the luminosity histogram feature of Image J (NIH).
2.10 In Situ Hybridization
Rats were euthanized by administration of chloral hydrate as described above and brains rapidly extracted, frozen on dry ice, and stored at −80°C. 18-μm thick sections from throughout the entire rostro-caudal extent of the BNST were cut on a cryostat at −20°C and mounted onto charged microscope slides. In situ hybridization was performed as previously described [25, 26]. Briefly, 35S-UTP (1250Ci/mmol, 12.5mCi/ml, Perkin-Elmer, Boston, MA) labeled riboprobes were prepared from linearized clones using T3 polymerase at high specific activity by only using radioactive UTP in the polymerase reaction, with approximately 20–40% incorporation. Following preparation of full-length antisense RNA strands, the RNA was base-hydrolyzed to average lengths of 50–100 bp and then purified using a sephadex spin column (Illustra ProbeQuant G-50 microcolumn, GE Healthcare, UK). 1×105 cpm/ml of radioactive probe was applied to each slide, which was immediately covered with parafilm to incubate at 52° C overnight. Slides were stringently washed, placed against Kodak (Rochester, NY) MR autoradiographic film for 4 days, and then developed in a Minolta SRX-101A film processor.
Autoradiography films were scanned using a high-resolution Epson 3700 flat-bed scanner at 6000 dpi. The signal intensities of brain regions were calculated on the basis of gray values (GVs) between 0 (brightest) and 255 (darkest) obtained from the luminosity histogram feature of Image J (NIH). For each animal, hybridization signal was analyzed bilaterally by measuring the most intense 500-pixel region within each dorsolateral BNST region, and adjacent background area with little or no hybridization signal (ventral striatum). Because the cannulations caused damage that appeared to affect the hybridization signal, every effort was taken to measure the hybridization signal from sections that were least affected by cannulation damage. The anatomical level of analysis was verified using the rat brain atlas of Paxinos & Watson [27]. A Mann-Whitney U-test was performed on the average BNST CRF mRNA levels measured bilaterally from two adjacent sections for all animals included in these experiments to determine if the AAV1-siCRF virus significantly decreased BNST CRF mRNA expression compared to animals treated with a control virus (AAV1-GFP).
2.11 Experimental Procedures
2.11.1 Experiments 1, 3, and 4: Acoustic Startle
On each of two days prior to data collection, rats were acclimated to the test chambers and stimuli. For these sessions, rats were placed into the chambers where, after 5 min, they were presented with 48 95-dB startle-eliciting white-noise bursts. For this and all other experiments and tests, the interstimulus interval [ISI] between noise bursts was 30 seconds. On each of the test days that followed, rats were tested twice – once before and once after vehicle or drug infusion. The pre-infusion test consisted of 60 startle-eliciting white-noise bursts, and the post-infusion test consisted of 120 startle bursts. The treatment conditions varied from one experiment to the next.
Rats in Experiment 1 (Vehicle n = 10, GSK876008 n = 12) were given either 10 mg/kg GSK876008 or vehicle (between subjects) by oral gavage 2 hours prior to testing. Just before testing, they were infused into the BNST with either vehicle or, on a separate day, CGRP (400 ng/side, n=11) in a counterbalanced within-subjects manner. Thus, for this experiment, there were two groups of rats (oral vehicle or GSK876008), with each being tested twice – once following intra-BNST infusion of vehicle and once following intra-BNST infusion of CGRP.
Rats in Experiment 3 were infused into the BNST with either vehicle or, on a separate day, the CRFR1 antagonist CP376395 (2 μg/side) 30 min prior to testing. Just before testing, they were infused with either vehicle (n = 11) or CGRP (n = 8; between subjects). Thus, as in Experiment 1, there were two groups of animals (intra-BNST vehicle or CGRP), each tested on two separate days—one time following intra-BNST infusion of vehicle, and another time following intra-BNST infusion of CP376395. For Experiments 1 and 3, at least 48 hrs elapsed between test days. This inter-test interval was chosen based on pilot data that found no lingering effect of CGRP on startle after 48 hrs.
In Experiment 4, rats were divided into three separate treatment groups that were tested only on one day. The first group was infected with AAV1-CMV-GFP (control virus) and then on the day of testing, infused with vehicle (n = 10). The second group was infected with control virus and infused with CGRP on test day (n = 9). The third group was infected with AAV1-siCRF and infused with CGRP on test day (n = 10). In all groups, testing occurred 14 days after viral infusion, when CRF knockdown was shown to be maximal based on previous studies.
For all experiments, startle responses elicited by the last 60 noise bursts (last 30 min of test session) were averaged and used in the statistical analysis, insofar as the onset latency for CGRP effects on startle was found in previous studies to be between 30 and 45 min [4].
2.11.2 Experiment 2: Elevated Plus Maze
In order to minimize the effects of handling stress on test outcomes, each rat was handled for approximately 2 min/day for each of at least 3 days prior to testing. Rats were assigned randomly to one of four different treatment conditions: intra-BNST vehicle + oral vehicle (n = 15), intra-BNST CGRP + oral vehicle (n = 15), intra-BNST vehicle + oral GSK876008 (n = 14), or intra-BNST CGRP + oral GSK876008 (n = 12). Oral drugs were administered 2 h prior to testing and intra-BNST infusions were given 30 min prior to testing. Immediately following intra-BNST infusions, rats were taken to the testing room and kept in the dark until testing commenced. The apparatus was novel to the subject at the time of testing, and each subject was tested only once. Between subjects, the maze was wiped with Quatricide (Pharmacal). All testing was conducted within two hrs after the beginning of the dark cycle (i.e. between 7 and 9 p.m.). A scorer blind to the experimental manipulation recorded four measures for each rat: (1) time spent in the open arms, (2) time spent in the closed arms, and (3) number of entries into the closed arms. A rat was considered to have entered or spent time in an arm only when all four paws were in the respective arm.
2.12 Statistical analyses
For Experiments 1, 3, and 4, the mean startle amplitude during the pre- and post-infusion test sessions was determined and a percent change score calculated ([mean post-infusion startle amplitude - mean pre-infusion startle amplitude]/[mean pre-infusion startle amplitude] × 100) [28]. Experiment 1 (Effects of systemic GSK876008 on intra-BNST CGRP-enhanced startle) was analyzed using 2 × 2 factorial ANOVA with repeated measures on CGRP vs. vehicle treatment. Experiment 3 (Effects of intra-BNST CP376395 on intra-BNST CGRP-enhanced startle) was also analyzed using 2 × 2 factorial ANOVA, but with repeated measures on GSK876008 vs. vehicle. Experiment 4 (Effect of BNST CRF knockdown on CGRP-enhanced startle) behavioral data was analyzed using one-way ANOVA. For Experiment 2 (elevated plus maze), the time spent in the open arms and the number of open-arm entries were expressed as a percentage of total arm activity (open arm time/(open arm time + closed arm time) × 100), and total arm entries (open arm entries/(open arm entries + closed arm entries) × 100), respectively. A lower percentage of open arm time or open arm entries are taken as indicating increased anxiety. The total number of closed arm entries was used as an index of general activity [29, 30]. These measures (chosen according to the ethopharmacological analysis of Cruz et al [31]) were analyzed using one-way ANOVA. For all behavioral experiments, if the overall ANOVA term was significant, Tukey post-hoc comparisons were used to compare each treatment condition with controls. Histological and in situ hybridization data were analyzed using non-parametric Mann-Whitney U-tests. All inferential statistics were performed using SPSS software (version 16.0.0; SPSS, Inc.).
3. Results
3.1 Cannulae placements
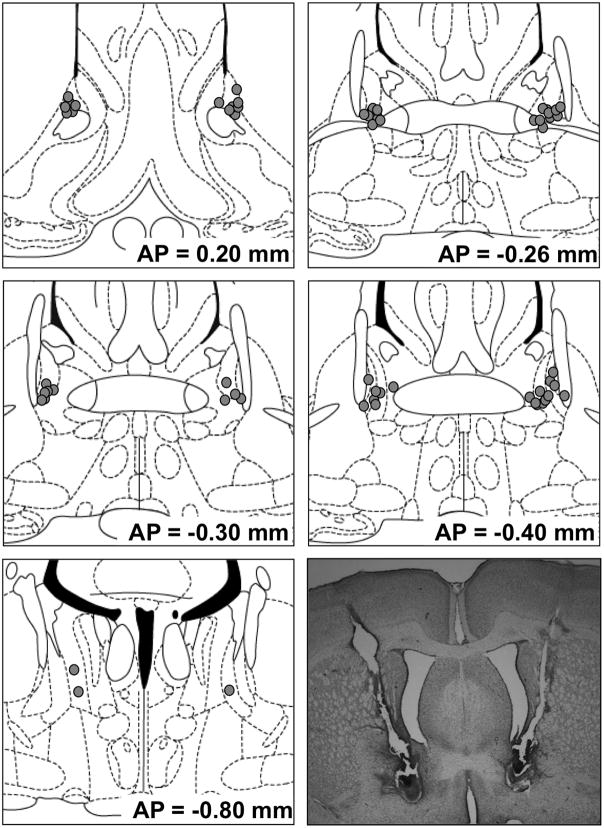
BNST cannulae placements for drug-infused animals from Experiments 1, 3, 4 and 5 are shown in Figure 1. All rats treated with drug that were included in behavioral analyses had cannula placements that met the inclusion criteria (i.e., both cannulae within 0.5 mm of the lateral BNST, medial to the internal capsule, and not penetrating the lateral ventricle). As there were no apparent differences between vehicle-infused rats with cannula which met or did not meet these criteria, data from vehicle-infused rats were included regardless of placement in order to reduce animal usage.
Figure 1.
Cannula tip placements of rats treated with CGRP or CP376395 whose data were included in behavioral analyses. The approximate distance posterior to bregma is millimeters is indicated to the lower right of each figure. Coronal sections are adopted from the atlas of Paxinos and Watson [33].
3.2 Experiment 1: Systemic CRFR1 antagonist GSK876008 blocked acoustic startle enhancement produced by intra-BNST CGRP
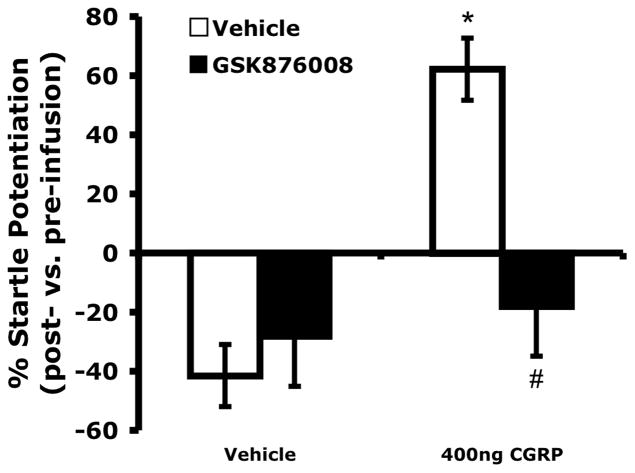
Figure 2 shows that vehicle-treated animals habituated to the startle stimulus, resulting in reduced acoustic startle response over the course of the test session. Startle response in those animals treated with vehicle plus GSK8760078 did not significantly differ from that of vehicle-treated animals. However, CGRP infused bilaterally into the BNST (400 ng/side) increased the acoustic startle response and this effect was blocked in animals pretreated systemically with the CRFR1 antagonist, GSK876008. A 2 x 2 ANOVA with repeated measures on CGRP found a significant within-subjects effect of CGRP on the average percent change in acoustic startle (F1,20 = 10.452, p = 0.004) and a significant interaction between CGRP and GSK876008 (F1,20 = 7.054, p =0.015). In those subjects infused into the BNST with vehicle, there were no significant differences in acoustic startle scores between animals pre-treated with vehicle vs. GSK876008 (F1,20 = 2.711, p = 0.115).
Figure 2.
Pretreatment with a systemic CRFR1 antagonist (10 mg/kg GSK876008, p.o.) blocked startle potentiation induced by intra-BNST infusions of CGRP (400 ng/side, bilaterally), but had no significant effect in the absence of CGRP administration. Values shown are means μ SEM. * p = 0.004 compared to vehicles only. # p = 0.015 compared to CGRP + vehicle.
3.3 Experiment 2: Systemic CRFR1 antagonist GSK876008 attenuated BNST CGRP-induced anxiety-like behavior
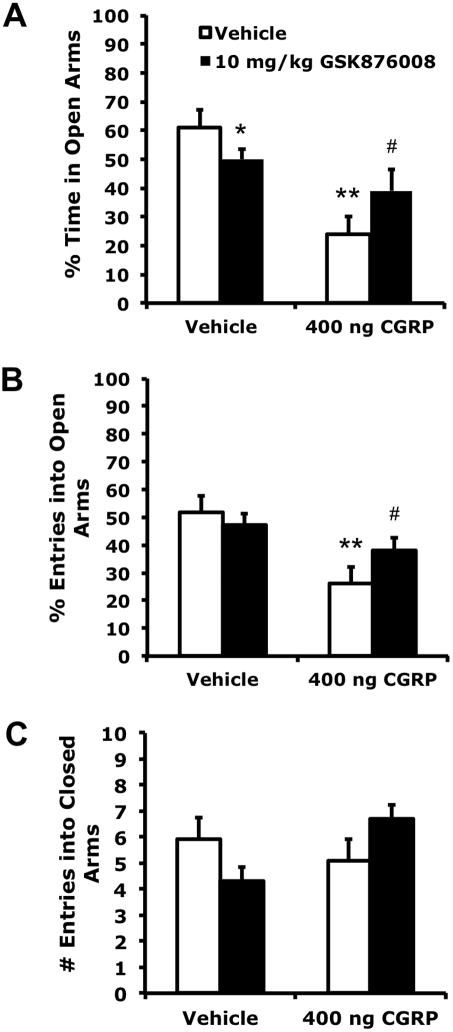
Similar to the effects of GSK876008 on CGRP-enhanced startle, GSK876008 attenuated the anxiogenic effect produced by intra-BNST CGRP in elevated plus maze behaviors, but also appeared slightly anxiogenic when administered alone (Figure 3). Thus, one-way ANOVA indicated a significant treatment effect on the open arm to total arm time ratio (F3, 55 = 9.013, p < 0.001) and open arm to total arm entries ratio (F3,55 = 5.927, p = 0.001). However, the number of closed-arm entries, taken as a measure of general locomotor activity, was not significantly affected (F3,55 = 0.924, p = 0.436). Tukey post-hoc comparisons revealed that CGRP significantly decreased the open arm time ratio (p < 0.001) and open arm entries ratio (p = 0.001) compared to vehicle, and pre-treatment with systemic GSK876008 reversed these effects (open arm time ratio: p = 0.012, open arm entries ratio: p = 0.030). GSK987008 alone also decreased the open arm time ratio (p = 0.028), but not the open arm entries ratio (p = 0.195).
Figure 3.
Anxiety-like behavior produced by intra-BNST infusion of CGRP (400 ng/side, bilaterally) was attenuated by systemic administration of a CRFR1 antagonist (10 mg/kg GSK876008, p.o.). GSK876008 alone also slightly decreased percent time spent in open arms (but not percent entries into open arms), possibly indicating a mild anxiogenic effect. A) Percentage of total time spent on the open arms. B) Percentage of total entries that were made into the open arms. C) Total closed arm entries. Values shown are means μ SEM. * p = 0.028 compared to vehicles only. ** p ≤ 0.001 compared to vehicles only. # p = 0.012 compared to CGRP + vehicle.
3.4 Experiment 3: Intra-BNST CRFR1 antagonist CP376395 blocked acoustic startle enhancement produced by intra-BNST CGRP
In order to assess whether CRFR1 receptors localized within the BNST might play a role in CGRP-enhanced startle, the potent CRFR1 antagonist, CP376395, was infused into the BNST prior to CGRP infusion. CP376395 (2 μg) infused in the BNST completely blocked CGRP-enhanced startle, but had no effect when administered alone (Figure 4). According to a 2 × 2 ANOVA with repeated measures on CP387396 treatment, there was a significant effect of CGRP (F1,17 = 27.55, p < 0.001), a significant effect of CP376395 (F1,17 = 6.697, p = 0.019), and, most importantly, a significant interaction between CP376395 and CGRP (F1,17 = 8.435, p = 0.010).
Figure 4.
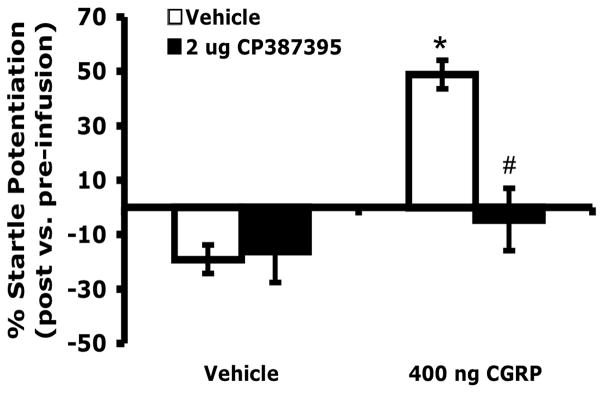
Pretreatment with an intra-BNST CRFR1 antagonist (CP376395, 2 μg/side bilaterally) blocked startle potentiation induced by intra-BNST infusions of CGRP (400 ng/side, bilaterally), but had no significant effect in the absence of CGRP administration. Values shown are means ± SEM. * p = 0.004 compared to vehicles only. # p = 0.015 compared to CGRP + vehicle.
3.5 Experiment 4: Intra-BNST CRF knockdown blocked acoustic startle enhancement produced by intra-BNST CGRP
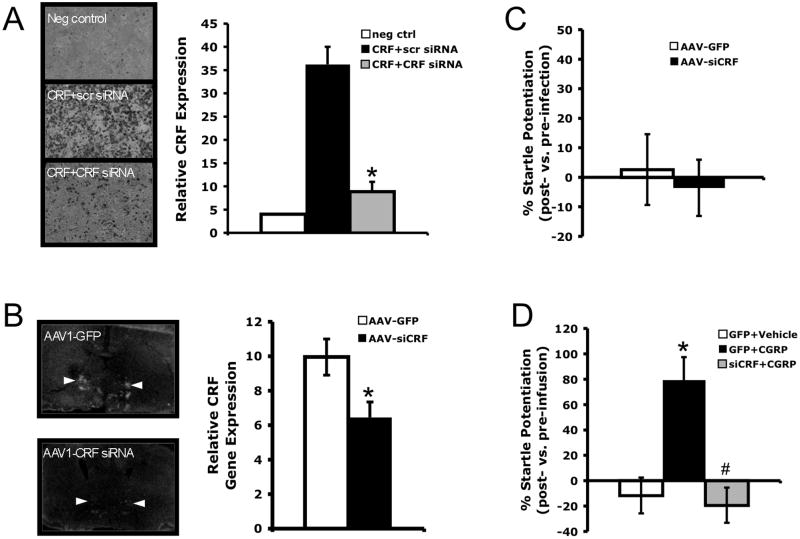
Prior to attempting CRF knock down within the BNST, the efficacy of the CRF siRNA to reduce CRF expression was tested in vitro as described in the Methods section above. Equally confluent HEK 293T cells were co-transfected with a CRF expression plasmid and either a plasmid containing the eGFP gene (control) or the pENTR/U6-shCRF plasmid. CRF peptide expression was then examined using ICC. Those cells co-transfected with shCRF had approximately 76% CRF knockdown compared to cells co-transfected with a control plasmid (Figure 5a). Animals infected within the BNST with the AAV1-siCRF viral vector expressing this CRF siRNA also had lower levels of BNST CRF mRNA expression compared to animals infected with the control AAV1-CMV-GFP virus (Figure 5b; p = 0.009).
Figure 5.
siRNA knockdown of CRF expression within the BNST prevents startle potentiation induced by intra-BNST CGRP (400 ng/side, bilaterally), but does not affect baseline acoustic startle levels. A) In vitro co-transfection of HEK293T cells with plasmids expressing CRF and CRF siRNA reduces CRF peptide expression compared to co-transfection with plasmids expressing CRF and scrambled siRNA. Relative CRF expression is taken as the difference between the average optical density for the entire image and the background level measured from a region without staining. Values are the average of four wells per treatment condition. B) The adeno-associated virus, AAV1-siCRF produces site-specific knockdown of CRF gene expression within the BNST. Relative CRF gene expression is taken as the average difference in optical brightness (± SEM) between the BNST and an adjacent background region of uniformly low expression (ventral striatum). *p = 0.009. At left, representative in situ hybridization autoradiographs of brain sections containing the lateral BNST (measurements taken from the dorsolateral BNST, indicated by arrows) from AAV1-GFP and AAV1-siCRF-infected animals, respectively. C) Baseline acoustic startle potentiation compared to pre-infection levels. Infection with AAV1-siCRF did not change baseline acoustic startle. D) Percent change of post-infusion startle levels compared to pre-infusion levels. * p < 0.001 compared to AAV1-GFP + vehicle. # p < 0.001 compared to AAV1-GFP + CGRP. All values shown are means ± SEM.
Although there were no differences in baseline acoustic startle after intra-BNST infection with AAV1-siCRF or AAV1-CMV-GFP (Figure 5c, p = 0.727), BNST infection with AAV1-siCRF blocked CGRP-enhanced startle (Figure 5d F2,28 = 14.64, p < 0.001). Tukey post-hoc comparisons showed that animals treated with AAV1-CMV-GFP (the control virus) plus CGRP had significantly higher startle potentiation than either rats treated with AAV1-CMV-GFP plus vehicle (p < 0.001) or rats treated with AAV1-siCRF plus CGRP (p < 0.001). Startle potentiation scores of animals treated with AAV1-siCRF plus CGRP were not significantly different from the scores of those animals treated with AAV1-CMV-GFP plus vehicle (p = 0.850).
4. Discussion
The present set of experiments was designed to examine the role of CRFR1 signaling in the anxiogenic effects of CGRP within the BNST and to determine whether CRF from BNST neurons contributes to these effects. Consistent with previous work, the present studies showed that intra-BNST administration of CGRP potentiated the acoustic startle response. In prior studies, CGRP administered immediately dorsal to the BNST had no effect on acoustic startle response or neuronal activation (c-Fos expression) in anxiety-related structures, evincing that the interaction of CGRP at receptors within the BNST is responsible for these effects [4].
A systemically administered CRFR1 antagonist (GSK876008) blocked potentiation of startle induced by intra-BNST administration of CGRP and also attenuated anxious behavior in the elevated plus maze. Although speculative, the fact that GSK876008 only partially reversed the pattern of anxiety-like behavior produced by BNST CGRP in the elevated plus maze compared to the nearly complete blockade of CGRP-enhanced startle may suggest that CGRP release within the BNST activates a wide array of neurotransmitters besides CRF that are more extensively involved in the control of complex anxiety-related behaviors compared to simple reflexes such as acoustic startle potentiation. It should also be noted that, although GSK876008 alone did appear to slightly reduce the percent of time spent on the open arms, these animals were still spending 50% of their time in the open arms, indicating that they were not avoiding the open arms or behaving anxiously. We believe that this apparent reduction is rather the result of an abnormally high mean for the vehicle-treated group (above 50%), due primarily to the value of a single individual animal (not excluded from analysis because it did not meet the outlier criterion), and does not reflect an anxiogenic effect of GSK876008 when administered alone.
Similarly, infusion of a different CRFR1 antagonist, CP376395, directly into the BNST blocked startle enhancement induced by intra-BNST CGRP. These results demonstrate that CRFR1 receptors (particularly those localized within the lateral BNST) are part of the pathway by which BNST CGRP evokes anxiety. Further, BNST CGRP-induced startle potentiation was prevented by site-specific CRF knockdown within the BNST by local infection with an AAV virus expressing siRNA targeting CRF. This result would imply that CRF from BNST neurons mediates the anxiety-like effects of CGRP within the BNST.
It has long been established that central CRF signaling facilitates anxiety-like responses (e.g., [32, 33]). Intracerebroventricularly administered CRF produces a constellation of anxious behavior including increased grooming [34–36] changes in exploratory or locomotor behavior [34, 37, 38], inhibition of feeding [39], decreased social interaction [35], defensive withdrawal [40, 41], enhanced acoustic startle [14, 42] and anxiety-like patterns of behavior in the elevated plus maze [43, 44]. The BNST, part of the extended amygdala thought to be particularly important for sustained conditioned fear, anxiety, and stress responses [1–3, 45–47], appears to be particularly important for many of these effects. For example, CRF over-expression localized within the BNST increases sustained fear-potentiated startle, which models aspects of anxiety [48], and intra-BNST CRF infusions enhance the acoustic startle response [14], evoke anxiety-like behavior in the elevated plus maze [15] and social interaction anxiety tests [16], enhance retention of an inhibitory avoidance task [17], and produce anorectic effects [18] and tachycardia [19].
Although the BNST contains both CRFR1 and CRFR2 receptors [10, 13], CRFR1 receptor expression predominates over CRFR2 expression within the lateral BNST, where the majority of CRF-containing cells are located [10, 49, 50]. Additionally, CRF has significantly greater affinity for the CRFR1 receptor subtype compared to CRFR2 [51], so it seems likely that at physiological levels, CRF interaction with CRFR1 receptors play a more important role in anxiety. Indeed, behavioral studies appear to support this hypothesis. The anxiety-like effects of CRF within the BNST seem to be mediated primarily by CRFR1 receptors, in that a CRFR1 but not a CRFR2 antagonist co-infused with CRF into the BNST inhibited CRF-induced anxiety behavior in the elevated plus maze [15], and the CRFR1 antagonist used in the present studies (GSK876008) prevented elevated acoustic startle induced by intra-BNST CRF infusions [20]. These results are in line with previous findings showing a more nuanced (and generally anxiolytic) influence of CRFR2 receptor signaling in anxiety [52–55].
Altogether, these results point to a potentially useful method of modulating CRF function and therefore stress-related anxiety. In fact, there is evidence that other neuropeptides may also work closely with CRF to affect anxiety. For example, pituitary adenylate cyclase-activating peptide (PACAP [56]) and oxytocin, like CGRP, are found in terminals apposed to CRF-containing neurons within the BNST [57, 58], suggesting a functional interaction between these neuropeptides. CGRP is not expressed within BNST neurons like PACAP is, but rather, is one of several neuropeptides (including PACAP) that is expressed in the external lateral parabrachial nucleus of the pons, which relays various visceral sensory information (e.g., nociceptive input [59, 60] from the nucleus of the solitary tract to various forebrain nuclei, possibly facilitating integration of peripheral inputs into regulation of emotional responses including anxiety [61]. Although highly speculative, this anatomical arrangement suggests that CGRP antagonists might be especially useful in treating the affective symptoms that are frequently associated with chronic pain (such as those that often accompany prolonged disease states).
While our results demonstrate that a relevant pool of CRFR1 receptors involved in CGRP-enhanced anxiety is located within the BNST itself, BNST CRF neurons project to more distal structures that contribute to anxiety including the ventral tegmental area [62], the parabrachial nucleus [63, 64], the nucleus of the solitary tract [65], the midbrain central gray [66] and the paraventricular hypothalamus [67]. Many of these structures also densely express CRFR1 receptors [10, 13]. Thus, although blockade of BNST CGRP enhanced startle with intra-BNST CRFR1 antagonist demonstrates an important role for local release of CRF onto CRFR1 receptors, the data do not exclude a role for CRF receptors in other structures targeted by CRF-containing terminals projecting from the BNST. Indeed, we have found that intra-BNST CGRP infusions significantly increase c-fos activation in a number of brain areas that receive CRF projections from the BNST [4]. Together, these findings suggest that the role of CRF within BNST neurons might not only be to modulate activity within the BNST, but also in structures that receive BNST efferents, including those essential for anxiety.
In summary, CGRP within the BNST interacts closely with CRF signaling to induce anxiety. We have previously shown that a CGRP antagonist is able to disrupt fear and anxiety responses [4], indicating that this system, at least under some circumstances, does mediate anxiety induced by environmental stressors and that therefore, CGRP receptors may be useful targets for anxiety reduction. Orally available CGRP antagonists are currently being investigated as migraine therapies, and as the case continues to build for involvement of CGRP in fear and anxiety, it is our hope that these compounds will be further evaluated for their effects on stress-related fear and anxiety in humans. With increasing evidence of anxiety-induced BNST activation from imaging studies [45, 46, 68] and provocative evidence from Greenberg et al. [69] that deep brain stimulation of the ventral internal capsule/ventral striatum (corresponding to the BNST) markedly reduces anxiety and co-morbid depression in patients with otherwise intractable obsessive-compulsive disorder, the further exploration of these possibilities is highly warranted.
Knockdown of CRF expression locally within the BNST disrupted BNST CGRP-enhanced startle.
Systemic or intra-BNST CRFR1 antagonists blocked BNST CGRP-induced anxiogenesis.
Anxiogenic effects of intra-BNST CGRP may be mediated by CRF acting at BNST CRFR1 receptors.
Acknowledgments
This research was supported by NIH National Service Research Award MH093023 to KSS, NIH awards MH47840 and MH069056 to MD, NIH award MH080330 to DLW, and a NARSAD Young Investigator Award to DLW, the National Center for Research Resources P51RR000165 and the Office of Research Infrastructure Programs/OD P51OD01132. This project was also supported in part by the Viral Vector Core of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077, and the Emory Custom Cloning Core Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–36. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 3.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–10. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- 6.Kainu T, Honkaniemi J, Gustafsson JA, Rechardt L, Pelto-Huikko M. Co-localization of peptide-like immunoreactivities with glucocorticoid receptor- and Fos-like immunoreactivities in the rat parabrachial nucleus. Brain Res. 1993;615:245–51. doi: 10.1016/0006-8993(93)90034-k. [DOI] [PubMed] [Google Scholar]
- 7.Kozicz T, Arimura A. Axon terminals containing CGRP-immunoreactivity form synapses with CRF- and Met-enkephalin-immunopositive neurons in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Res. 2001;893:11–20. doi: 10.1016/s0006-8993(00)03118-8. [DOI] [PubMed] [Google Scholar]
- 8.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–52. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–23. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286:459–68. [PubMed] [Google Scholar]
- 13.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–46. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186:122–32. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–94. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- 18.Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–51. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijsen MJ, Croiset G, Diamant M, De Wied D, Wiegant VM. CRH signalling in the bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 20.Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–42. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs A, Biro E, Szeleczky I, Telegdy G. Role of endogenous CRF in the mediation of neuroendocrine and behavioral responses to calcitonin gene-related peptide in rats. Neuroendocrinology. 1995;62:418–24. doi: 10.1159/000127031. [DOI] [PubMed] [Google Scholar]
- 22.Bowe JE, Li XF, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. The role of corticotrophin-releasing hormone receptors in the calcitonin gene-related peptide-induced suppression of pulsatile luteinising hormone secretion in the female rat. Stress. 2008;11:312–9. doi: 10.1080/10253890701801448. [DOI] [PubMed] [Google Scholar]
- 23.Kocorowski LH, Helmstetter FJ. Calcitonin gene-related peptide released within the amygdala is involved in Pavlovian auditory fear conditioning. Neurobiol Learn Mem. 2001;75:149–63. doi: 10.1006/nlme.2000.3963. [DOI] [PubMed] [Google Scholar]
- 24.Di Fabio R, St-Denis Y, Sabbatini FM, Andreotti D, Arban R, Bernasconi G, et al. Synthesis and pharmacological characterization of novel druglike corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:7370–9. doi: 10.1021/jm800744m. [DOI] [PubMed] [Google Scholar]
- 25.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 1997. [Google Scholar]
- 28.Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl) 2002;164:318–28. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- 29.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 30.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 31.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 32.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 33.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Hormones and behavior. 2006;50:550–61. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life sciences. 1982;31:363–7. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- 35.Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Hormones and behavior. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuis HD, De Wied D. Differential behavioral actions of corticotropin-releasing factor (CRF) Pharmacol Biochem Behav. 1984;21:707–13. doi: 10.1016/s0091-3057(84)80007-6. [DOI] [PubMed] [Google Scholar]
- 37.Berridge CW, Dunn AJ. CRF and restraint-stress decrease exploratory behavior in hypophysectomized mice. Pharmacol Biochem Behav. 1989;34:517–9. doi: 10.1016/0091-3057(89)90551-0. [DOI] [PubMed] [Google Scholar]
- 38.Spadaro F, Berridge CW, Baldwin HA, Dunn AJ. Corticotropin-releasing factor acts via a third ventricle site to reduce exploratory behavior in rats. Pharmacol Biochem Behav. 1990;36:305–9. doi: 10.1016/0091-3057(90)90408-a. [DOI] [PubMed] [Google Scholar]
- 39.Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res. 1988;443:63–9. doi: 10.1016/0006-8993(88)91598-3. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–54. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- 41.Yang XM, Dunn AJ. Central beta 1-adrenergic receptors are involved in CRF-induced defensive withdrawal. Pharmacol Biochem Behav. 1990;36:847–51. doi: 10.1016/0091-3057(90)90088-y. [DOI] [PubMed] [Google Scholar]
- 42.Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–12. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1997;22:297–309. doi: 10.1016/s0306-4530(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 44.Song C, Earley B, Leonard BE. Behavioral, neurochemical, and immunological responses to CRF administration. Is CRF a mediator of stress? Ann N Y Acad Sci. 1995;771:55–72. doi: 10.1111/j.1749-6632.1995.tb44670.x. [DOI] [PubMed] [Google Scholar]
- 45.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–24. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage. 2007;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. doi: 10.1038/mp.2011.188. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju G, Han ZS. Coexistence of corticotropin releasing factor and neurotensin within oval nucleus neurons in the bed nuclei of the stria terminalis in the rat. Neurosci Lett. 1989;99:246–50. doi: 10.1016/0304-3940(89)90454-0. [DOI] [PubMed] [Google Scholar]
- 50.Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. The American journal of anatomy. 1982;165:385–96. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- 51.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends in endocrinology and metabolism: TEM. 2002;13:436–44. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 52.Skorzewska A, Lehner M, Hamed A, Wislowska-Stanek A, Turzynska D, Sobolewska A, et al. The effect of CRF2 receptor antagonists on rat conditioned fear responses and c-Fos and CRF expression in the brain limbic structures. Behav Brain Res. 2011;221:155–65. doi: 10.1016/j.bbr.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi C, Ohata H, Shibasaki T. Corticotropin-releasing factor (CRF) receptor subtypes in mediating neuronal activation of brain areas involved in responses to intracerebroventricular CRF and stress in rats. Peptides. 2011;32:2384–93. doi: 10.1016/j.peptides.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, et al. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–54. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- 55.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcoholism, clinical and experimental research. 2004;28:865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 56.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- 57.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–26. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, et al. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. Journal of molecular neuroscience: MN. 2010;42:327–40. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Experimental physiology. 2002;87:251–8. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 60.Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Molecular pain. 2010;6:10. doi: 10.1186/1744-8069-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paxinos G. The Rat Nervous System. 3. Academic Press; 2004. [Google Scholar]
- 62.Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 63.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–32. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 64.Panguluri S, Saggu S, Lundy R. Comparison of somatostatin and corticotrophin-releasing hormone immunoreactivity in forebrain neurons projecting to taste-responsive and non-responsive regions of the parabrachial nucleus in rat. Brain Res. 2009;1298:57–69. doi: 10.1016/j.brainres.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262:365–74. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- 66.Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–60. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 67.Champagne D, Beaulieu J, Drolet G. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol. 1998;10:119–31. doi: 10.1046/j.1365-2826.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]