Abstract
There is both extensive species-specificity and critical experience-dependence in the recognition of own species songs in many songbird species. For example, female zebra finches Taeniopygia guttata raised by their parents show behavioral preferences for the songs of the father over unfamiliar conspecific males and for unfamiliar songs of conspecifics over heterospecifics. Behavioral discrimination between different species’ songs is also displayed by females raised without exposure to any male songs but it is diminished in females raised by heterospecific foster parents. We tested whether neural responses in the female auditory forebrain paralleled each of these known behavioral patterns in song-class discrimination. We analyzed spike rates, above background levels, recorded from single units in the L2a subregion of the field L complex of female zebra finches. In subjects raised by genetic parents, spike rates were similar to songs of fathers and unfamiliar male zebra finches, and higher to unfamiliar conspecific over unfamiliar heterospecific songs. In females raised in isolation from male songs, we also found higher spike rates to unfamiliar conspecific over heterospecific songs. In females raised by heterospecific foster parents, spike rates were similar in response to songs of the foster father and unfamiliar males of the foster species, similar between unfamiliar songs of conspecifics and the heterospecific foster species, and higher to unfamiliar songs of the foster species over a third finch species. Thus, in parallel to the experience-dependence of females’ behaviors in response to different male song classes, differences in social experiences can also alter neural response patterns to male song classes in the auditory forebrain of female zebra finches.
Keywords: Bengalese finch, black-throated finch, female perception, oscines, recognition template, spike rates
Introduction
Species recognition and mate choice have critically shaped the evolutionary diversity of acoustic displays in diverse lineages of birds, whose songs often function as the primary mate attraction signals to advertize and identify suitable sexual partners (Beecher and Brenowitz 2005; Seddon 2005). In turn, it remains less clear how social experience shapes the sensory and cognitive bases of avian song recognition behaviors; for example, what is the neural basis of behavioral discrimination between socially salient classes of auditory communication signals in birds with different early experiences (Brenowitz and Beecher 2005, Bolhuis and Gahr 2006)?
In the zebra finch Taeniopygia guttata, an Australian estrildid songbird used as a model species for the neuroethology of auditory recognition (Zann 1996), species identity and early social experience both modify the behavioral patterns of conspecific song discrimination behaviors (Hauber et al. 2010). Accordingly, on the one hand, female and male zebra finches show operant and spatial preferences for unfamiliar conspecific over heterospecific songs, even when raised without exposure to male zebra finch songs (Braaten and Reynolds 1999, Lauay et al. 2004, Campbell and Hauber 2009). On the other hand, such behavioral discrimination between conspecific and heterospecific songs is still dependent on early social experience because it is diminished when zebra finches are raised by heterospecific Bengalese finch Lonchura striata vars. domestica foster parents (Clayton and Prove 1989, Campbell and Hauber 2009).
Similarly, experience with specific songs during ontogeny also plays a critical role in the behavioral discrimination of different classes within conspecific songs by female zebra finches; even after months of separation, females can show behavioral preferences for familiar songs of fathers or brothers over songs of unfamiliar adult males, including the acoustically similar songs of unfamiliar brothers (Miller 1979, Riebel et al. 2002, Riebel and Smallegange 2003). Such extensive early experience dependence is also seen in the behavioral ontogeny of species and song discriminations in many other songbird species (reviewed in Goth and Hauber 2004, Beecher and Brenowitz 2005).
In the long-standing search for identifying the neural basis of species recognition templates in songbirds (Marler 1980, Hauber and Sherman 2001), investigating neurophysiological parallels of behavioral patterns in the discrimination of different song classes should allow us to begin to investigate whether and how sensory systems are dependent on both species-specific stereotypy and experience-dependent plasticity for selectively processing complex acoustic stimuli (Riebel 2003a, Riebel et al. 2002). Importantly, female zebra finches, unlike males, do not sing (Zann 1996), and so they can be used to test the role of early experience and species identity in shaping song preferences, without the confound of self-referencing from own song production (Pytte and Suthers 1999, Hauber and Sherman 2001, Woolley et al. 2010).
Previous neurobiological work, using genomic activational, immediate early gene (IEG) data, pointed to the caudomedial nidopallium (NCM) in male (Terpstra et al. 2004) and the caudiomedial mesopallium (CMM) in female zebra finches (Terpstra et al. 2006), as potential neural substrates of song familiarity discrimination (Mello et al. 1995, Dong et al. 2009). To parallel these results, several studies based on neurophysiological recordings from males of different songbird species, also revealed differential responses to familiar songs vs. unfamiliar songs, using repeated presentations of songs of both unrelated individuals and songs fathers or tutors, in CMM (Gentner and Margoliash 2003) and NCM (Amin et al. 2004). Similarly, prior work using lesioning, physiological, and/or IEG approaches on female zebra finches specifically also implied that these secondary auditory forebrain areas (CMM and NCM) underlie behavioral discrimination between conspecific and heterospecific songs (MacDougall-Shackleton et al. 1998, Stripling et al. 2001, Bailey et al. 2002, Phan and Vicario 2010) and its experience-dependent modulation (Lauay et al. 2005, Tomaszycki et al. 2006, Terleph et al. 2008).
However, both CMM and NCM receive direct inputs from the field L complex, which is the primary auditory forebrain area activated by hearing natural sounds (Theunissen et al. 2004), including in female zebra finches (Hauber et al. 2007a). This connectivity of the ascending auditory pathway, therefore, points to a need for further analyses of the neural selectivity within the field L complex. Until recently, IEG techniques were not available to detect auditory activation in the oscine field L, although this important step has finally been accomplished (Horita et al. 2010). However, in several reports, neurophysiological data from field L and its subregions have revealed only limited selectivity between different classes of complex acoustic stimuli (Nagel and Doupe 2008, Meliza and Margoliash 2012), beyond the well known tonotopy of this area (Gehr et al. 1999). In contrast, ongoing work from our own laboratories on field L neurons, especially in the L2a subregion in zebra finches of both sexes, raised by genetic parents, has shown different neural responses, indicated by higher spike rates, for conspecific songs over acoustically-matched synthetic sounds (males: Grace et al. 2003, Woolley et al. 2005, females: Hauber et al. 2007a,b).
Field L neurons from male zebra finches, however, do not show spike rate differences in response to conspecific over heterospecific songs, irrespective of whether raised by conspecifics or fostered by Bengalese finches, whereas spike train patterns in field L neurons do encode both species identity in male zebra and Bengalese finches and are modified by social experience in male zebra finches raised by Bengalese finch foster parents (Woolley et al. 2010). Our study, therefore, was also motivated to begin the fill the existing gender gap in developmental aspects of birdsong research (Yamaguchi 2001), because no extensive analyses of parallel ontogenetic influences on neurophysiological data from female zebra finches have been reported to date; to this aim we used experimental manipulations of female subjects’ social experiences with conspecific or heterospecific songs (Campbell and Hauber 2009).
Here we report on the outcome of several experiments to begin to test the hypothesis that early social-acoustic experience plays a critical role in shaping neural responses to different classes of male songs in the primary auditory forebrain area of female songbirds. Specifically, based on published behavioral data from female zebra finches of the discrimination between songs of fathers over unfamiliar males (Miller 1979a, b, Riebel et al. 2000, 2002, Riebel and Smallegange 2003) and our prior work from single unit records from field L of female zebra finches in response to conspecific songs and artificial sounds (Hauber et al. 2007a,b), we predicted that females raised in the presence of adult males would show different neural responses within Field L to the foster/father’s songs over unfamiliar males’ songs, irrespective of the species identity of the foster-/father. In turn, also based on published behavioral data (Clayton and Prove 1989, Braaten and Reynolds 1999, Lauay et al. 2004, Campbell and Hauber 2009, 2010), we predicted that females raised by conspecifics, whether in the presence or absence of hearing adult male songs, would show different neural responses between unfamiliar exemplars of conspecific over heterospecific songs, but show no differences between songs of different heterospecific finches. Finally, to parallel the reported lack of behavioral discrimination between conspecific and heterospecific songs in zebra finches fostered by Bengalese finches (Campbell and Hauber 2009), we predicted similar spike rates in response to songs of different finch species in adult female zebra finches fostered by Bengalese finches.
In addition to using foster/father’s and unfamiliar exemplars of zebra and Bengalese finches, in some of our experiments we also incorporated the playbacks of unfamiliar songs of a third bird species, the Parson’s finch (or black-throated finch) Poephilia cincta, a close relative and sympatric of the zebra finch in Australia (Zann 1976, 1996). This was done to assess the potential generality of drawing conclusions on neural parallels of species discrimination between just two species of birds, the zebra finches and Bengalese finches, alone (Woolley et al. 2010). Specifically, based on our recent behavioral experiments with song playbacks of several different species of sympatric Australian finches to cross-fostered zebra finches (Campbell and Hauber 2009), we predicted that neurons from female zebra finches raised by Bengalese finches would show similar responses to songs of all three different estrildid finch species used in our study as playback stimuli. Our experimental treatments and statistical analyses are structured so as to address specific components of these predictions driven by published behavioral data.
Methods
Study Species and Ontogenetic Treatments
Subjects were sourced from our breeding colony at the University of California, Berkeley, USA, operated under university and government research permits, where all birds were given ad lib food and water. There, in two different single-species animal rooms, breeding cages housed a pair of adult zebra finches and their genetic progeny, ~ 92 days following hatching to allow full imprinting on the father’s species-specific vocalizations (Zann 1996, Woolley et al. 2010). Sexually mature young were then transferred to single-sex cages in the same colony room until used in the experiments; this constituted the “control treatment” (following Hauber et al. 2007a). All cages were visually isolated from immediate neighbors but birds inside each colony room could hear one another.
Because our experiments were conducted during the course of 2 years (2003–2005), and the individuals in each breeding and housing cage, and the colony room overall, were constantly changing, we assumed that raising and housing zebra finches in the same colony rooms did not constitute pseudoreplication of the subjects’ early social and acoustic experience. Our additional ontogenetic treatments (see below) included differences from the control treatment in the species-specific acoustic stimuli of subjects both during prior and after the onset of adulthood, and these manipulations also varied in many other biological and physical aspects, including colony room location, breeding population size, and immediate family composition and size. Therefore, our study is an experimental test of the role of social experience overall, and not solely of species-specific acoustic exposure during the parental-dependent stage of development per se (see Campbell and Hauber 2010 for a further discussion of these issues).
For the second, “father-absent” treatment (following Hauber et al. 2007b), individual breeding pairs of zebra finches were isolated in cages placed in different sound-attenuation chambers (Acoustic Systems, Austin Texas) in a different room from the breeding colonies. These chambers provided 60 dB SPL attenuation at 4 kHz (Hauber et al. 2007b). Pairs were allowed to nest and incubate their own clutches but fathers were removed from the breeding cage 0–2 days after hatching to eliminate the exposure of female young to adult male songs. Acoustic sensitivity of altricial birds is typically poor until several days after hatching, justifying our treatment to remove fathers with nestlings ≤ 2 days of age (Lock and Hauber 2012 but see Colombelli-Negrel et al. 2012). Isolated mothers and young were monitored daily to assure adequate provisioning and growth of the juveniles. Between 25–35 days of age and before the onset of singing behavior, juvenile males were identified based on sexually dimorphic plumage (Zann 1996) and removed from the chambers to prevent the exposure of the juvenile females to male song until experimentation. Daughters were kept together with their mothers until ~ 110 days of age, and then moved to single-sex cages of isolation-raised females in different chambers.
For the third, “cross-fostered” treatment, we established individual cages of pairs of Bengalese finches in a separate colony room in a design paralleling our published behavioral (Campbell and Hauber 2009) and neurophysiological (Woolley et al. 2010) studies using a cross-fostering protocol. Bengalese finch pairs were allowed to complete one full breeding cycle to assure experience with parenting. Pairs were then allowed to start to incubate their second clutches but prior to hatching we replaced them with entire clutches of hatching-day zebra finch eggs or broods of 0–2 day old hatchling zebra finches from our colony rooms. Zebra finch young were kept in the cages of Bengalese finch foster parents until ~ 100 days of age, at which point they were moved into single sex cages within the same foster-species colony room until experimentation. As seen in previous work (Clayton and Prove 1988), male zebra finch siblings of our subject females in this treatment learned to produce Bengalese finch-like songs (Woolley et al. 2010). However, we also note here that male zebra finches do not copy foster species’ songs perfectly and retain several zebra finch like elements in their vocalizations, including its timing and introductory notes (Clayton and Prove 1988); therefore, cross-fostered female zebra finch subjects in this treatment cannot be considered naïve with respect to all aspects of conspecific songs. Nevertheless, the early social and acoustic experiences of the cross-fostered subjects were dramatically different from the subjects compared to the control or father-absent treatments, therefore allowing us to test our central hypothesis regarding experience-dependence of neural responses of field L neurons to different classes of male songs.
Neurophysiological Experiments
Anesthetized adult female zebra finch subjects (mean ± SD: 241 ± 200 days old) were used for extracellular neurophysiological recordings. The playback and data collection protocol is described in detail by Hauber et al. (2007a,b). In brief, two days before the experiment, females were anesthetized with equithesin intramuscularly and a small section (< 1 mm2) of the upper layer of the skull was removed at around a mark made always 1.4 mm lateral (on either side of the midline) and 1.2 mm rostral from the midsagittal sinus (Grace et al. 2003). Stereotaxic coordinates were then marked directly on the lower layer of the skull with ink for later guidance of the initial electrode penetrations for recordings. A stainless steel pin was also glued with dental cement to immobilize the bird’s head to the stereotaxic apparatus during recording. Recovered subjects were moved into individual cages either in their respective colony room (control and cross-fostered) or in a sound-attenuation chamber (father-absent) for two days.
On the day of the electrophysiological experiment, each subject was anesthetized with three intramuscular injections of 20% urethane administered 30 min. apart (75 µL total). A small hole was made in the lower layer of the skull and through the dura mater prior to lowering a 1–4 MΩ resistance tungsten electrode into the brain using micro-drives. The bird and the stereotax were then moved into an anechoic chamber with a calibrated speaker positioned 20 cm in front of the bird’s head to deliver free-field sound playbacks.
We used a digital library of prerecorded, band-passed (500–8000Hz) auditory stimuli for our playback experiments. Each playback stimulus was standardized for power to deliver sounds at a peak amplitude of 75 dB SPL at the head of the subject, lasted ~ 2 s, and was separated with an inter-stimulus silent interval drawn from a uniform distribution of 7–8 s of duration. To isolate auditory excitatory units, the set of search stimuli included one randomly chosen exemplar of an unfamiliar conspecific (zebra finch) song, a heterospecific (always Bengalese finch) song, and a band-pass white noise stimulus exemplar (Fig. 1), repeated in a random order 10 times per stimulus type. Spikes from a single unit, as assessed by the visual inspection of the generated waveform, were isolated using a window discriminator. Statistically higher spike rates, as calculated online by a student’s t-test for data collected during the 10-repeated presentations of any one of the three different types of search stimuli compared to data collected during the preceding 2 s of silence, were taken as indicators of an isolated auditory excitatory unit. We then recorded spike responses to three different song exemplars drawn from several different song classes and used only those data in the analyses presented here where each song exemplar was presented 10 times during the randomized order of playback stimulus class presentations.
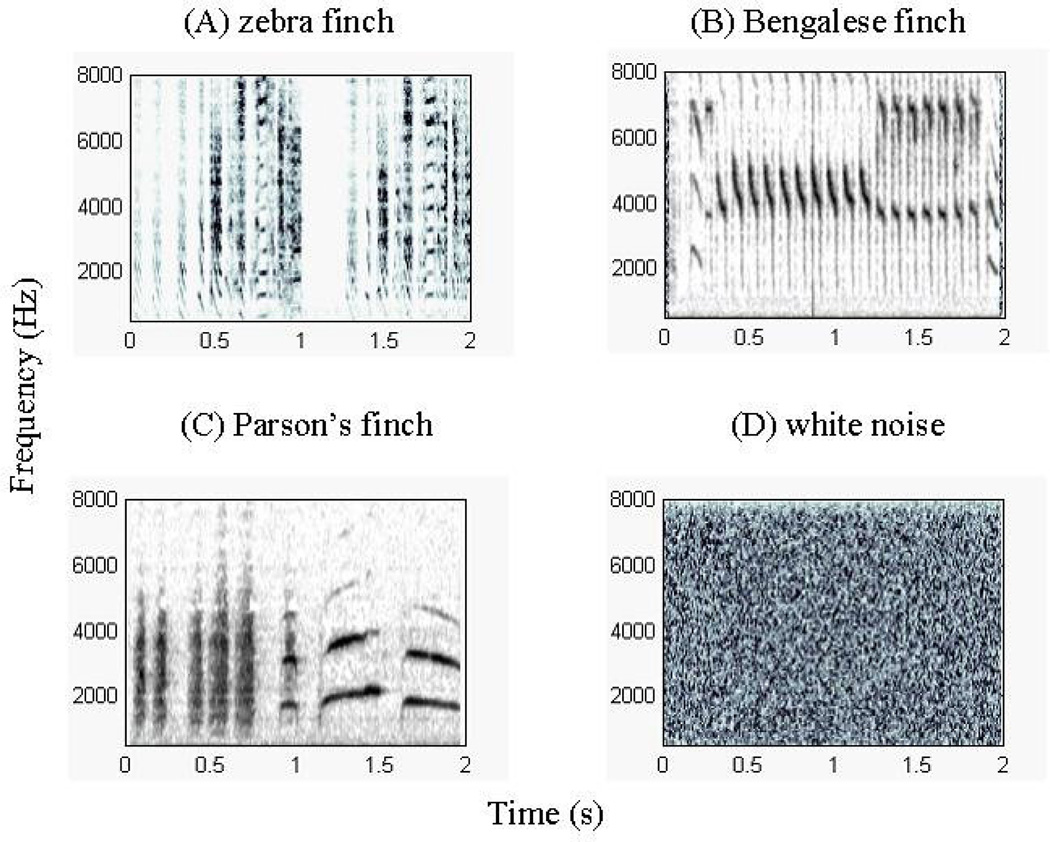
Fig. 1.
Spectrograms of representative male song stimulus types presented to anesthetized female zebra finches during searching for (A, B, & D) and recording (A, B, & C) from single auditory units in the L2a of the field L complex.
In this report we analyze results from the following classes of sound playback stimuli: (A) 3 unfamiliar conspecific songs (chosen randomly from a library of 20 songs, from 17 different zebra finches); (B) 3 different songs of the foster/father (the zebra finch father for the control treatment and the Bengalese finch foster father in the cross-fostered treatment); (C) 3 unfamiliar heterospecific songs of Bengalese finches (chosen randomly from a library of 40 songs, from 9 different males); and (D) 3 unfamiliar heterospecific songs of Parson’s finches (chosen randomly from 6 songs, from 4 different males (Fig. 1). All unfamiliar songs were obtained from genetically distinct lines of males raised and kept outside of our own breeding colonies. Due to logistical constraints and changes in personnel availability during the course of our study, the genetic father’s songs was not presented to subjects in the father-absent treatment and in the cross-fostered treatment, and we also did not present Parson’s finch songs to the father-absent treatment. Data in response to unfamiliar conspecific songs recorded from the same subjects were previously analyzed and reported for the control and father-absent treatments only (Hauber et al. 2007a,b); all other data and analyses reported here are novel.
Male zebra finches and, in part, Bengalese finches, sing a stereotyped song (Woolley et al. 2010), therefore the playback of 3 different songs from the (foster) father represents an over-representation of the same individual’s vocalizations, and we consider the comparison of these data with the playbacks of 3 different unfamiliar songs from 3 different males, as a preliminary analysis.
We recorded responses along electrode passes that penetrated field L in the auditory forebrain (following Hauber et al. 2007a). To ensure that a different unit was recorded at each point along a pass, each recording site was at least 100 µm apart from the neighboring sites. At the end of the experiment, we made electrolytic lesions at known coordinates to allow the anatomical reconstruction of recording locations using standard histological techniques (Amin et al. 2004, Fortune and Margoliash 1992). Following recording, birds were terminally anesthetized with 0.06 ml equithesin and transcardially perfused with 0.9% saline, followed by 3.7% formalin in 0.025 mol/L phosphate buffer.
Statistical Analyses
Sample sizes were too small for statistical comparisons for the L1 (n ≥ 4) and L3 (n ≥ 0) subregions of field L within and between treatment groups (Amin et al. 2004); and so we report on data collected from within only the most selective of the field L subregions, L2a (n ≥ 25 units for each treatment group) (Fortune and Margoliash 1992, Hauber et al. 2007a, Meliza and Margoliash 2012). We obtained response strengths for each stimulus exemplar by taking the spike rate (spike / s) during the 10 repeated stimulus presentations minus the spike rate during 2 s of silence that immediately preceded each presentation (i.e. spike rate above background). We then averaged the response strengths across the 3 different exemplars of each stimulus class to generate a single spike-rate response variable for each auditory unit for each stimulus type.
We followed the recommendations of a recent guide to statistical techniques applicable to repeated-measures data from neurophysiological experiments (Nakagawa and Hauber 2011); specifically, we accounted for the biological and statistical non-independence of data recorded from units within the same subjects by applying generalized linear mixed models (GLMM) in JMP 8.0. In these analyses, we used subject identity as the random factor (i.e. repeated measure), initially included laterality, age, treatment, and playback stimulus type and their interactions as fixed factors (i.e. predictors), and response strength (spike rate above background rate) as the dependent factor (i.e. response variable), and fitted the model parameters using a restricted maximum likelihood (REML) approach. We then used a step-wise procedure to remove non-significant predictors to generate a final model including only the random factor and the significant fixed factors and interaction terms (if any) (Grim et al. 2011). To control for the heterogeneity in our data due to the different treatment/experience of individual subjects and the data collection protocol/personnel between the treatments, we then conducted post-hoc pairwise comparisons of spike rates, using two-tailed paired t-tests which also accounted for differences in the intrinsic physiological properties of different units at different recording sites. Following additional recommendations about a common error in the statistical interpretation of significant vs. non-significant comparisons by treatment in neuroscience data (Nieuwenhuis et al. 2011), we specifically tested for the effect of ontogenetic treatment on the relative patterns of spike rate responses to different song class playbacks, and used additional GLMMs and post hoc t-tests on the resulting least square mean estimates. We used JMP 9.0 for all of analyses and set α < 0.05, and did not use Bonferroni corrections to avoid overly conservative conclusions in the statistical analyses of datasets involving multiple comparisons (Nakagawa 2004).
Results
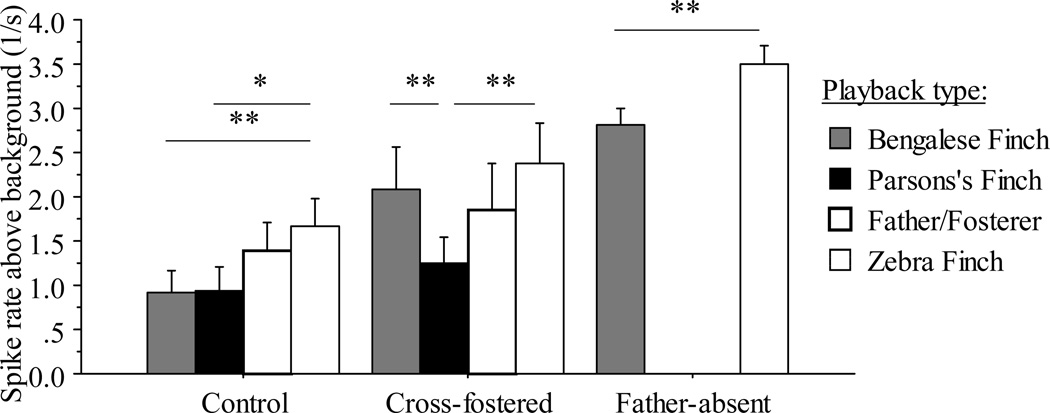
We recorded electrophysiological responses of 39 L2a neurons from 13 control females, 76 units from 6 father-absent females, and 25 units from 6 cross-fostered females. Overall, the GLMM analysis showed no statistical differences due to subject age or recording-site laterality, or their interactions in spike rates above background (all p ≥ 0.06), whereas both treatment (F2,14.5 = 8.8, p = 0.0031) and playback stimulus type (F3,371 = 4.7, p = 0.0032) were significant predictors in the final model (Fig 2).
Fig. 2.
Spike rates (above background levels during silence) recorded in response to paternal (genetic father’s or foster father’s, denoted as: Father/Fosterer) songs or unfamiliar conspecific or heterospecific songs presented to single auditory units in L2a of female zebra finches in the control, father-absent, and cross-fostered treatments. Spike rates are averaged for each single unit across all exemplars of the same stimulus class, with mean ± SE shown. Paternal and Parson’s finch songs were not presented in the father-absent treatment. Bars connected with * and ** are different at p < 0.06 and p < 0.03 levels (two-tailed, paired t-tests).
In our post hoc comparisons, spike rates to unfamiliar zebra finch songs and to the father’s songs were similar within control females (paired t37 = 1.5, p = 0.15), and spike rates were also not statistically different to unfamiliar Bengalese finch songs and the foster father’s songs in cross-fostered female zebra finches (t64 = 1.9, p = 0.06) (Fig. 2). These patterns of non-significant differences in spike rates for subjects between these two treatments were statistically confirmed in an additional GLMM between the two treatments by comparing spike rates in response to unfamiliar and foster/father songs, with subject identity as random effect (post hoc student’s t-test on least square means: control or cross-fostered treatment, t = 0.28, p = 0.61).
In turn, in our post hoc comparisons, pairwise differences in spike rates to unfamiliar zebra finch were significantly greater over unfamiliar Bengalese finch songs in both the control treatment (paired t37 = 3.8, p = 0.0005) and in the father-absent treatment (t75 = 5.5, p < 0.0001), but spike rates were not statistically different between unfamiliar zebra and Bengalese finch songs in the cross-fostered treatment (t24 = 1.3, p = 0.22) (Fig 2). These relative patterns were statistically confirmed in a GLMM between all three treatments by comparing pairwise spike rate differences in response to zebra finch minus to Bengalese finch songs with subject identity as random effect (control and father-absent treatment: t = 1.2, p = 0.11; control or cross-fostered treatment: t = 2.5, p = 0.0071; cross-fostered or father-absent treatment: t= 2.2, p = 0.015).
Regarding responses to unfamiliar Parson’s finch songs, spike rates were greater to zebra finch over Parson’s finch songs in control females, but not significantly so (t33 = 1.9, p = 0.057), and significantly greater in cross-fostered females (t24 = 3.6, p = 0.0013). However, these relative patterns were statistically similar to each other in a GLMM between the two treatments when comparing spike rate differences in response to zebra finch minus to Parson’s finch songs with subject identity as random effects (t= 0.61, p = 0.25).
Finally, spike rates to Bengalese finch and Parson’s finch songs were similar in control females (t34= 0.042, p = 0.97), but significantly higher to Bengalese over Parson’s finch songs different in cross-fostered females (t24= 2.4, p = 0.027). These relative patterns in differences were statistically confirmed in a GLMM between the two treatments when comparing spike rate differences in response to Bengalese finch minus to Parson’s finch songs with subject identity as random effects (t= 1.8, p = 0.034).
Discussion
Responses from L2a units within the field L complex of anesthetized female zebra finches showed no experience dependent modulation between foster/father’s and unfamiliar males’ songs, as we detected similar spike rates to these different classes of male songs in female subjects raised by genetic parents or by Bengalese finches (Fig. 2). It still remains to be explored what, if any, potential neural parallels may be detected with known behavioral patterns discrimination of other classes of familiar vocalizations (e.g. songs of male mates by female zebra finches: Miller 1979b, calls of mates: Vignal et al. 2004) in field L, and its subregions, in the female zebra finch forebrain. However, our data are consistent with the proposed mechanism that increasing selectivity between different classes of salient vocal stimuli, including familiarity discrimination, is mediated hierarchically along the ascending auditory input pathway from field L to CMM of female songbirds (Bolhuis and Gahr 2006).
Regarding the data on the spike rate differences to unfamiliar songs of different estrildid finch species, single L2a neurons of adult female zebra finches here showed higher spike rates in response to conspecific over heterospecific songs in both the control and father-absent treatments, and showed no differences between genetic and foster species’ songs in Bengalese finch cross-fostered females (Fig. 2). We detected no differences between songs of two different heterospecific finches in control females, but recorded higher spike rates to the foster species’ songs over a third species’ songs, in cross-fostered female zebra finches (Fig. 2). Our findings thus establish a neural parallel between the known impacts of both species identity, and experience dependence with different foster/parental species, and the impact of both these factors on modulating behavioral patterns of species discrimination of male songs in female zebra finches (Clayton and Prove 1988, Braaten and Reynolds 1999, Campbell and Hauber 2009, 2010).
The first of the several limitations we acknowledge in our work is that these experiments were conducted under anesthesia, and so similarities and differences in neural response strength and selectivity in awake/behaving versus anesthetized birds (Cardin and Schmidt 2003) must be revisited in future work. Second, as young adults our subjects were housed in the same physical environment as during early development, and so early social experience of juveniles was matched by the typical acoustic experience of adult subjects immediately prior to experimentation, thereby confounding potential effects of sensitive periods during early in development relative to recent, adult-stage experiences and familiarity (sensu Riebel 2003b, Terleph et al. 2008, Campbell and Hauber 2010). Beyond these social differences in the composition of the different breeding colony rooms, we also note that the overall sound levels and song rate exposures are different as zebra finches tend to be more often and more loudly vocal and active than Bengalese finches (Zann 1996), Woolley et al. 2010).
Third, in this study, we only examined the treatment effects on above background spike rates averaged over the entire stimulus presentation, with the underlying assumption that species-specific and experience-dependent neural discrimination would be manifest in this particular neuronal response metric (Hauber et al. 2007a,b). However, even using this relatively simple measure of neural response strengths, we immediately detected spike rate differences above the background between subjects from different treatments, in that females from the father-absent treatment had the highest, the cross-fostered treatment had intermediate, and the control treatment had the lowest overall spike rates, in response to any stimulus class. These relative patterns are opposite to the predicted pattern of song exposure by our subjects (not analyzed here): control females would have heard the most songs in the typically loud and active zebra finch colony, cross-fostered females would have heard fewer songs in the relatively quieter Bengalese finch colony, and father-absent females would have heard only female contact calls and no male songs in their isolation booths (Woolley et al. 2010). Nevertheless, to account for the implications of this potential physiological effect and auditory confound, our primary statistical comparisons were designed to be restricted to within subjects and within treatments using repeated measures analyses of data obtained from within each recording site, thereby accounting for the differences in overall spike rates between units, subjects, and also treatments.
Fourth, the L2a subregion of field L, in several bird species, including zebra finches, is tonotopically organized (Gehr et al. 1999). Therefore, one proximate explanation for our findings is the differential response of the particular auditory units sampled to the variation in the frequency, and other spectro-temporal components of the different classes of auditory stimuli presented here. This mechanism, however, does not appear to apply across our experimental paradigm because the overall frequency content and span are both highly similar between our zebra and Bengalese finch species’ songs (Fig. 1; also see Woolley et al. 2010) and also because control birds showed no response strength differences between Bengalese and Parson’s finch songs, despite the many acoustic differences in these two species’ songs in tonality, frequency range, average power, and pattern of amplitude modulation (Fig. 1; Zan 1976). The use of three randomly chosen representative exemplars for each stimulus type from a library of 6–40 song stimuli per species further reduced the likelihood of response differences being due to the frequency spectrum of individual males’ song variants only, or other aspects of song stimulus pseudoreplication (Kroodsma et al. 2001). Finally, the x and y coordinates for the initial electrode penetrations were the same for subjects in both the control and the cross-fostered treatments bilaterally (see Methods), whereas the z coordinates of the recording sites did not differ significantly between these two treatments (t = −0.06, p > 0.95), further implying a lack of technical confounds yielding tonotopy-related differences in our spike-rate data for these treatments. Nonetheless, species-specific auditory neuronal responses reported here might still be mediated by several acoustic differences typical of different species’ songs, including motif and syllable durations, syllable numbers, inter-syllable intervals, and, amplitude-frequency spectra (Williams 1990), Williams et al. 1993, Woolley et al. 2010) (Fig. 1). Critically, however, our experiment did not set out to determine which of the many, covarying acoustic components of each of the three different finch species song yielded the detected spike rate differences; that would have been a different study, where one acoustic parameter is carefully altered at a time, while keeping all the other acoustic parameters constant. Instead, we set out to determine whether social experience treatment per se modifies the relative spike rate differences to each of the different finch species’ songs; and we have shown this to be the case (Fig. 2). Thus, a detailed description of each of the finch species songs is beyond the scope of this study because it would not help us to explain the social experience dependence of our results.
Fifth, the average spike rate measures per stimulus analyzed here may not be sensitive enough to be used to identify consistent variation in spiking patterns in which acoustic trait(s) differences between stimulus song types explain the different neurophysiological responses obtained in our experiments (sensu Woolley et al. 2010). Designing experimental protocols and using response metrics to specifically estimate neuronal spectrotemporal receptive fields (STRFs) in response to natural stimuli and applied spike-rich electrophysiological data (e.g., Theunissen et al. 2000; Woolley et al. 2005; Woolley et al. 2006) from female zebra finches are needed to address these questions more fully in future work.
Irrespective of these limitations, our study clearly demonstrates that single units within field L in anesthetized female zebra finches showed greater spike rates to unfamiliar conspecific songs compared to unfamiliar songs of each of two heterospecific estrildid finches in control subjects, one of which (the Parson’s finch) is both ecologically and evolutionarily salient for zebra finch species recognition behaviors in the wild, as a sympatric and phylogenetically close relative species (Zann, 1996, Campbell et al. 2009).
Overall, the results confirm that the early social and acoustic environments can alter relative neural response patterns of primary auditory forebrain neurons in response to conspecific over heterospecific songs in female zebra finches (also see Hauber et al. 2007b, Maul et al. 2010). Our results form an important gender-specific comparison of the role of ontogeny in neural responses of females relative to published data from male zebra finches (Phan et al. 2006, Horita et al. 2010, Phan and Vicario 2010, Woolley et al. 2010) and other songbird species (Cousillas et al. 2004; Cousillas et al. 2006). Specifically, in contrast to our findings in females, Woolley et al. (2010) showed that spike rates did not differ within field L between control and cross-fostered male zebra finches in response to unfamiliar zebra finch or Bengalese finch songs; this may imply sex differences in the neuronal coding of species-specific songs in field L of zebra finches. However, that study differed critically in the methodological details of song stimulus playbacks (i.e., more exemplars with more repetitions were used for each unit recorded; Woolley et al. 2010). Yet, when that study used a more detailed analysis of spike train data, the information coding capacity of field L neurons was shown to be different between control and cross-fostered male zebra finches in response to conspecific over heterospecific songs (Woolley et al. 2010), demonstrating experience-dependence in song-coding of the male field L complex, in parallel to the known experience dependence of behavioral song discrimination by male zebra finches (Campbell and Hauber 2009). Furthermore, spike rates themselves were different within field L of male Bengalese finches in response to conspecific over heterospecific songs, suggesting that species identity of the subjects also matters (Woolley et al. 2010).
Here, we did not study neural responses in L2a of control or cross-fostered female Bengalese finches. Accordingly, future work should focus on conducting parallel studies with similar experimental paradigms between females of different species of estrildid finches and between females and males of zebra finches, to test for both species-specificity and sexual dimorphism of neuronal responses along the ascending auditory inputs of the mid- and forebrain (Terleph et al. 2008, Poirier et al. 2009, Maul et al. 2010). Nonetheless, our results here and future approaches should combine critically to contribute to the continued exploration of whether the experience dependence of neural responses to different natural and synthetic stimuli arises along the ascending auditory pathway between the cochlear nuclei through the auditory midbrain and the thalamus, and to field L and beyond (Wild 1995; Maney et al. 2003, Hsu et al. 2004; Woolley et al. 2005; Woolley et al. 2006, Poirier et al. 2009, Horita et al. 2010, Woolley et al. 2010, Amin et al. 2010), and how it may be further modified in downstream secondary auditory areas in the telencephalon of female zebra finches and other songbirds (Bailey and Wade 2003; Lauay et al. 2005, Cousillas et al. 2006, Terleph et al. 2008, Maul et al. 2010, Phan and Vicario 2010).
Highlights.
Female zebra finches are selective to male songs in the L2a forebrain region.
Females raised without males also show higher spike rates to conspecific songs.
Females raised by another species show reduced selectivity between songs.
Songbirds’ experiences alter both behavioural and neural song selectivity.
Acknowledgements
We improved on earlier drafts of our manuscripts based on several reviewers’ comments. This study was funded by grants from the National Institutes of Health (NIH) and the UC Berkeley Field Station for Behavioral Research (to FET), the NIH and the National Science Foundation (to SMNW), the UC Berkeley Miller Institute for Basic Research in Science and the PSC-CUNY faculty grants program (to MEH). PC is an Australian ARC Future Fellow. All protocols followed the guidelines of the Association for the Study of Animal Behaviour and were approved by the institutional animal ethics committee at UC Berkeley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adret P. Operant conditioning, song learning, and imprinting to taped song in the zebra finch. Animal Behaviour. 1993;46:149–159. [Google Scholar]
- Amin N, Grace GA, Theunissen FE. Neural response to bird’s own song and tutor song in the Zebra Finch Field L and caudal mesopallium. Journal of Comparative Physiology A. 2004;190:469–489. doi: 10.1007/s00359-004-0511-x. [DOI] [PubMed] [Google Scholar]
- Amin N, Gill P, Theunissen FE. Role of the zebra finch auditory thalamus in generating complex representations for natural sounds. Journal of Neurophysiology. 2010;104:784–798. doi: 10.1152/jn.00128.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. Journal of Neurobiology. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Molecular Brain Research. 2003;116:147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- Beecher MD, Brenowitz EA. Functional aspects of song learning in birds. Trends in Ecology and Evolution. 2005;20:143–149. doi: 10.1016/j.tree.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature Reviews Neuroscience. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Braaten RF, Reynolds K. Auditory preference for conspecific song in isolationreared zebra finches. Animal Behaviour. 1999;58:105–111. doi: 10.1006/anbe.1999.1134. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends in Neuroscience. 2005;28:127–132. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Campbell DLM, Hauber ME. Cross-fostering diminishes song discrimination in zebra finches (Taeniopygia guttata) Animal Cognition. 2009;12:481–490. doi: 10.1007/s10071-008-0209-5. [DOI] [PubMed] [Google Scholar]
- Campbell DLM, Shaw RC, Hauber ME. The strength of species recognition in captive female zebra finches (Taeniopygia guttata): a comparison across estrildid heterospecifics. Ethology. 2009;115:23–32. [Google Scholar]
- Campbell DLM, Hauber ME. Conspecific-only experience during development reduces the strength of heterospecific song discrimination in zebra finches (Taeniopygia guttata): a behavioural test of the optimal acceptance threshold hypothesis. Journal of Ornithology. 2010;151:379–389. [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. Journal of Neurophysiology. 2003;90:2884–2899. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Prove E. Song discrimination in female zebra finches and Bengalese finches. Animal Behaviour. 1989;38:352–362. [Google Scholar]
- Colombelli-Negrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Evans C, Kleindorfer S. Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Current Biology. 2012;22:2155–2160. doi: 10.1016/j.cub.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Cousillas H, Richard J-P, Mathelier M, Henry L, George I, Hausberger M. Experience-dependent neuronal specialization and functional organization in the central auditory area of a songbird. European Journal of Neuroscience. 2004;19:3343–3376. doi: 10.1111/j.0953-816X.2004.03376.x. [DOI] [PubMed] [Google Scholar]
- Cousillas H, George I, Mathelier M, Richard J-P, Henry L, Hausberger M. Social experience influences the development of a central auditory area. Naturwissenschaften. 2006;93:588–596. doi: 10.1007/s00114-006-0148-4. [DOI] [PubMed] [Google Scholar]
- Dong S, Replogle KL, Hasadsri L, Imai BS, Yau PM, Rodriguez-Zas S, Southey BR, Sweedler JV, Clayton DF. Discrete molecular states in the brain accompany changing responses to a vocal signal. Proceedings of the National Academy of Sciences USA. 2009;106:11364–11369. doi: 10.1073/pnas.0812998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata) Journal of Comparative Neurology. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Gehr DD, Capsius B, Grabner P, Gahr M, Leppelsack H-J. Functional organisation of the field-L-complex of adult male zebra finches. Neuroreport. 1999;10:375–380. doi: 10.1097/00001756-199902050-00030. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal Populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth A, Hauber ME. Ecological approaches to species recognition in birds through studies of model and non-model species. Annales Zoologici Fennici. 2004;41:823–842. (Special Issue on Recognition Systems). [Google Scholar]
- Grace JA, Amin NA, Singh NC, Theunissen FE. Selectivity for conspecific song in the zebra finch auditory forebrain. Journal of Neurophysiology. 2003;89:472–487. doi: 10.1152/jn.00088.2002. [DOI] [PubMed] [Google Scholar]
- Grim T, Samas P, Moskat C, Kleven O, Honza M, Moksnes A, Roskaft E, Stokke BG. Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? Journal of Animal Ecology. 2011;80:508–518. doi: 10.1111/j.1365-2656.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Sherman PW. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends in Neurosciences. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Cassey P, Woolley SMN, Theunissen FE. Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. Journal of Comparative Physiology A. 2007a;193:765–774. doi: 10.1007/s00359-007-0231-0. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Woolley SMN, Theunissen FE. Experience-dependence of neural responses to social versus isolate conspecific songs in the forebrain of female Zebra Finches. Journal of Ornithology. 2007b;148:S231–S239. [Google Scholar]
- Hauber ME, Campbell DLM, Woolley SMN. Functional role and female perception of male song in zebra finches. Emu – Austral Ornithology (Special Issue Honouring Richard Zann) 2010;110:209–218. [Google Scholar]
- Horita H, Wada K, Rivas MV, Hara E, Jarvis ED. The dusp1 immediate early gene is regulated by natural stimuli predominantly in sensory input neurons. Journal of Comparative Neurology. 2010;518:2873–2901. doi: 10.1002/cne.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Woolley SMN, Fremouw T, Theunissen FE. Modulation and phase spectrum of natural sounds enhance neural discrimination performed by single auditory neurons. Journal of Neuroscience. 2004;24:9201–9221. doi: 10.1523/JNEUROSCI.2449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male Zebra Finch song system. Journal of Neuroscience. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroodsma DE, Byers BE, Goodale E, Johnson S, Liu W-C. Pseudoreplication in playback experiments, revisited a decade later. Animal Behaviour. 2001;61:1029–1033. [Google Scholar]
- Lauay C, Gerlach NM, Adkins-Regan E, Devoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Animal Behaviour. 2004;68:1249–1255. [Google Scholar]
- Lauay C, Komorowski RW, Beaudin AE, DeVoogd TJ. Adult female and male zebra finches show distinct patterns of spine deficits in an auditory area and in the song system when reared without exposure to normal adult song. Journal of Comparative Neurology. 2005;487:119–126. doi: 10.1002/cne.20591. [DOI] [PubMed] [Google Scholar]
- Lock J, Hauber ME. A predation risk-and-avoidance model of nestling responses to parental vocalizations. Evolutionary Ecology Research. 2012;14:235–245. [Google Scholar]
- MacDougall-Shackleton SA, Hulse SH, Ball GF. Neural bases of song preferences in female zebra finches (Taeniopygia guttata) Neuroreport. 1998;9:3047–3052. doi: 10.1097/00001756-199809140-00024. [DOI] [PubMed] [Google Scholar]
- Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. Journal of Comparative Physiology A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- Marler P. Actis XVII Congressus Internationalis Ornithologici. Berlin: Deutsche Ornithologen-Gesellschaft; 1980. Song learning, dialects and auditory templates: An ethological viewpoint; pp. 637–641. [Google Scholar]
- Maul KK, Voss HU, Parra LC, Salgado-Commissariat D, Ballon D, Tchernichovski O, Helekar SA. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Developmental Neurobiology. 2010;70:28–40. doi: 10.1002/dneu.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in Zebra Finch telencephalon. Journal of Neuroscience. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliza CD, Margoliash D. Emergence of selectivity and tolerance in the avian auditory cortex. Journal of Neuroscience. 2012;32:15158–15168. doi: 10.1523/JNEUROSCI.0845-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979a;280:3890–3891. [Google Scholar]
- Miller DB. The acoustic basis of mate recognition by female zebra finches (Taeniopygia guttata) Animal Behaviour. 1979b;27:376–380. [Google Scholar]
- Nagel KI, Doupe AJ. Organizing principles of spectro-temporal encoding in the avian primary auditory area field L. Neuron. 2008;58:938–955. doi: 10.1016/j.neuron.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioral Ecology. 2004;15:1044–1045. [Google Scholar]
- Nakagawa S, Hauber ME. Great challenges with few subjects: statistical strategies for neuroscientists. Neuroscience and Biobehavioral Reviews. 2011;35:462–473. doi: 10.1016/j.neubiorev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers E-J. Erroneous analyses of interactions in neuroscience: a problem of significance. Nature Neuroscience. 2011;9:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proceedings of the National Academy of Sciences USA. 2010;107:2301–2306. doi: 10.1073/pnas.0900091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proceedings of the National Academy of Sciences USA. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A. Own-song recognition in the songbird auditory pathway: selectivity and lateralization. Journal of Neuroscience. 2009;29:2252–2258. doi: 10.1523/JNEUROSCI.4650-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytte CL, Suthers RA. A bird's own song contributes to conspecific song perception. Neuroreport. 1999;10:1773–1778. doi: 10.1097/00001756-199906030-00027. [DOI] [PubMed] [Google Scholar]
- Riebel K. Early exposure to song leads to repeatable preferences for male song in female zebra finches. Proceedings of the Royal Society of London B. 2000;267:2553–2558. doi: 10.1098/rspb.2000.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K. The ‘mute’ sex revisited: vocal production and perception learning in female songbirds. Advances in the Study of Behaviour. 2003a;33:49–86. [Google Scholar]
- Riebel K. Developmental influences on song perception in female zebra finches, Taeniopygia guttata : is there a sensitive phase for song preference learning? Animal Biology. 2003b;53:73–87. [Google Scholar]
- Riebel K, Smallegange IM. Does zebra finch (Taeniopygia guttata) preference for the (familiar) father’s song generalize to the songs of unfamiliar brothers? Journal of Comparative Psychology. 2003;117:61–66. doi: 10.1037/0735-7036.117.1.61. [DOI] [PubMed] [Google Scholar]
- Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: Evidence for a dissociation between song recognition and production learning. Proceedings of the Royal Society of London B. 2002;269:729–733. doi: 10.1098/rspb.2001.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K, Hall ML, Langmore NE. Female songbirds still struggling to be heard. Trends in Ecology and Evolution. 2005;20:419–420. doi: 10.1016/j.tree.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Seddon N. Ecological adaptation and species recognition drives vocal evolution in Neotropical suboscine birds. Evolution. 2005;59:200–215. [PubMed] [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proceedings of the Royal Society of London B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. Journal of Neurobiology. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Terleph TA, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS ONE. 2008;3:e2854. doi: 10.1371/journal.pone.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of bird song memory. Journal of Neuroscience. 2004;24:4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JMM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. Journal of Neurobiology. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Sen K, Doupe AJ. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. Journal of Neuroscience. 2000;20:2315–2331. doi: 10.1523/JNEUROSCI.20-06-02315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen FE, Amin N, Shaevitz SS, Woolley SMN, Fremouw T, Hauber ME. Song selectivity in the song system and in the auditory forebrain. In: Zeigler HP, Marler P, editors. Behavioral neurobiology of bird song. Vol. 1016. Albany: Annals of the New York Academy of Sciences; 2004. pp. 222–245. [DOI] [PubMed] [Google Scholar]
- Tomaszycki ML, Sluzas EM, Sundberg KA, Newman SW, DeVoogd TJ. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: Effects of rearing environment. Journal of Neurobiology. 2006;66:1175–1182. doi: 10.1002/neu.20275. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Audience drives male songbird response to partner’s voice. Nature. 2004;430:448–451. doi: 10.1038/nature02645. [DOI] [PubMed] [Google Scholar]
- Wild JM. Convergence of somatosensory and auditory projections in the avian torus semicircularis, including the central auditory nucleus. Journal of Comparative Neurology. 1995;358:465–486. doi: 10.1002/cne.903580402. [DOI] [PubMed] [Google Scholar]
- Williams H. Models for song learning in the zebra finch: fathers or others. Animal Behaviour. 1990;39:745–757. [Google Scholar]
- Williams H, Kilander K, Sotanski ML. Untutored song, reproductive success and song learning. Animal Behaviour. 1993;45:695–705. [Google Scholar]
- Woolley SMN, Casseday JH. Response properties of single neurons in the zebra finch auditory midbrain: response patterns, frequency coding, intensity coding and spike latencies. Journal of Neurophysiology. 2004;91:136–151. doi: 10.1152/jn.00633.2003. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Casseday JH. Processing of modulated sounds in the zebra finch auditory midbrain: responses to noise, frequency sweeps and sinusoidal amplitude modulations. Journal of Neurophysiology. 2005;94:1143–1157. doi: 10.1152/jn.01064.2004. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Gill P, Theunissen FE. Stimulus-dependent auditory tuning results in synchronized population coding of vocalizations in the songbird midbrain. Journal of Neuroscience. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SMN, Fremouw TE, Hsu A, Theunissen FE. Spectro-temporal modulation tuning as a mechanism for auditory discrimination of natural sounds. Nature Neuroscience. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Hauber ME, Theunissen FE. Developmental experience alters information coding in auditory midbrain and forebrain neurons. Developmental Neurobiology. 2010;70:235–252. doi: 10.1002/dneu.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A. Sex differences in vocal learning in birds. Nature. 2001;411:257–258. doi: 10.1038/35077143. [DOI] [PubMed] [Google Scholar]
- Zann RA. Variation in the songs of three species of Estrildine Grassfinches. Emu. 1976;76:97–108. [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; 1996. [Google Scholar]