Abstract
Gender specific effects on human eating have been previously reported. Here we investigated sex-based differences in neural activation via whole-brain blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) in response to high energy-dense (high-ED) vs. low-ED visual and auditory food cues in obese men vs. women in both fed and fasted states. The results show that in response to high vs. low ED foods in the fed state, obese men (vs. women), had greater activation in brain areas associated with motor control regions (e.g. supplementary motor areas) whereas women showed greater activation in cognitive-related regions. When fasted, obese men had greater activation in a visual-attention region whereas obese women showed greater activation in affective and reward related processing regions (e.g. caudate). Overall the results support our a priori hypothesis that obese women (vs. men) have greater neural activation in regions associated with cognition and emotion-related brain regions. These findings may improve our understanding of sex specific differences among obese individuals in eating behavior.
Keywords: Gender, Obese, fMRI, High-energy Dense, Neural Activation, Food-related Cues
Given that obesity and eating disorders are more prevalent in women than men [1,2], sex-based differences in eating behavior patterns are an important subject of ongoing investigation [3,4]. Sex-dependent differences have been observed in neural responses to food cues [5–7], ingestion [8], behavioral interventions [9], and taste [10]. Moreover, studies show enhanced responsivity to cues (i.e. sight of food) during the fasted vs. fed states [11], and that fasted women rate food images higher on valence than women who are satiated [4]. In addition, higher neural activation in affective-cognitive processing brain regions when hungry to food vs. non-food cues has been reported indicating an interaction effect between energy states (hunger and satiety) and food cue types on neural activation [12]. Thus, eating behavior patterns differ in men vs. women, and are affected by motivational state (i.e. fed vs. fasted), alongside neural differences in cognitive, emotional, and reward processing. Although several structural brain differences have been identified between obese and lean persons [13,14], and cerebral white matter changes related to elevated body weight in men vs. women [15], fundamental questions remain about sexual dimorphic neural mediators of appetite and weight regulation. Using whole-brain functional magnetic resonance imaging (fMRI), we sought to elucidate the effect of gender on neural responsivity to high vs. low energy-dense (ED) food cues with visual and auditory modalities in fed and fasted conditions. We hypothesized that obese women (vs. men) would have higher neural activation in emotion-related regions in response to external food stimuli.
Thirty-one obese men (Nm=17) and women (Nw=14) with similar body mass index (BMI) (men=36.2±5.5 and women=36.9±5.6; mean ± SD) and age (m=35±9.0 and w=35±6.9; mean ± SD) were recruited. All participants were right-handed, weight-stable (± 5%) ≤ 3 months, nonsmoking, premenopausal, not pregnant (urine pregnancy test), with no history of neurological, psychiatric, or medical (i.e. diabetes) conditions and not taking any medication or are not obesity treatment (e.g. exercise > 5h/week). The protocol was approved by the Institutional Review Boards of Columbia University and St. Luke’s Roosevelt Hospital Center.
Participants’ height, weight, and BMI were measured. Binge eating status was assessed via the Eating Disorder Examination [16] diagnostics, and used as a covariate in the imaging analysis to control for its potential effect on neural activation [17]. Participants rated their preferred flavor: chocolate, vanilla, or strawberry for Boost (Mead Johnson), a nutritionally complete shake.
The experimental procedures were scheduled on two non-consecutive days. On separate days of each procedure participants had a pre-fast meal of approximately 4180 kJ (1000 kcal) around 7–8 pm followed by a 12-h overnight fast. They ingested 750 ml Boost (fed condition) or 750 ml of water (fasted condition) in a randomized counterbalanced sequence 95 min prior to scans, and rated their hunger and fullness (0–100) on a visual analog scale (VAS) 10 min prior to scans. Participants were exposed to high-ED food ≥ 3.5 kcal/g (e.g. pepperoni pizza), low-ED foods < 1 kcal/g (e.g. raw vegetables), and non-foods (e.g. office supplies) stimuli in both visual (photos) and auditory (spoken words) modalities during the fMRI. Visual cues were transmitted through eye goggles and auditory cues were transmitted through a headset. Stimuli of each type did not repeat, and were presented in two separate runs, resulting in a total of twelve runs (2 visual high-ED, 2 visual low-ED, 2 visual non-food, 2 auditory high-ED, 2 auditory low-ED and 2 auditory non-foods), each lasting 2 min, 12 sec. Within each run, 10 different stimuli of the same type were presented consecutively resulting in a total block duration of 40 s (4s each picture presentation, or a spoken word repeated twice, e.g. “chocolate brownie, chocolate brownie”) with a 52-sec pre- and 40-sec post-stimulus baseline (white crosshair centered in a black background for visual, and tone for auditory conditions). The six runs containing visual cues were followed by the six runs containing auditory cues, which were presented in pseudorandom order within each set of six runs, and any stimulus type could not be followed by a run of the same stimulus type (e.g. for the set of visual runs: 1) high-ED, 2) low-ED, 3) non-food, 4) lowED, 5) non-food, 6) high-ED).
A 1.5-Tesla twin-speed fMRI scanner (GE) with quadrature RF head coil and 65 cm bore diameter was used. Participants wore a headset and goggles with their head placed in a passive restraint (pads and tape around the head) to minimize motion. Three-plane localization was used to verify head position. A head coil (MRI Devices Corporation, Gainesville, FL) was used to improve the signal to noise ratio. Total time in the scanner was about 60 min. In each run, 36 axial scans of the whole brain were acquired, consisting of 25 contiguous slices (4 mm thick), with a 19 × 19 cm field of view, an acquisition matrix size of 128 × 128, and 1.5 mm × 1.5 mm in plane resolution. The first three scans of each run (12 sec) were discarded to attain magnetic equilibration. The axial slices were parallel to the AC/PC line. T2*-weighted images with a gradient echo pulse sequence (echo time = 60 ms, repetition time = 4 sec, flip angle = 60°) were acquired with matched anatomic high resolution T1-weighted scans.
1st level analysis: Functional data were analyzed with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/spm8.html). Prior to statistical analyses, the realigned T2*-weighted volumes were slice-time corrected, spatially transformed to a standardized brain (Montreal Neurologic Institute) and smoothed with a 8-mm full-width half-maximum Gaussian kernel. Each of the 12 runs in each session were concatenated together to create a single run per session (396 total time points × 2 sessions). Block regressors were included in each subject’s 1st level model to account for the mean of each run within each session. Additional nuisance covariates included motion, as well as global signal and spikes as computed using the scn_session_spike_id.m script available in Diagnostics Tools for MATLAB (http://wagerlab.colorado.edu/tools). Both scan sessions (fed and fasted conditions) were included in the 1st level model for each participant. 1st-level regressors-of-interest were created by convolving the onset of each trial (visual and auditory high-ED, low-ED, and non-food) with the canonical HRF with a duration of 40 sec. Given our specific hypotheses, we first examined the neural activation in response to visual high-ED relative to visual low-ED food cues in fed and fasted conditions. The following contrasts of beta parameters were then submitted to 2nd level analyses: 1) Fed visual high-ED > Fed visual low-ED, and 2) Fed auditory high-ED > Fed auditory low-ED, 3) Fasted visual high-ED > Fasted visual low-ED and 2) Fasted auditory high-ED > Fasted auditory low-ED.
2nd level analyses: The above contrasts maps for each modality (auditory or visual) and condition (fed or fasted) were submitted to group random effects models using multiple regression with the following factors: age and binge-eating disorder status (DSM-IV TR) as covariates-of-no-interest, and gender as a covariate of interest. Main effects of high vs. low-ED averaged over all participants were derived from these models (see supplementary material). We did not perform a 2nd level 2×2 ANOVA analysis between gender and food cue type (using high and low-ED beta images), but would expect the results would be similar to performing a multiple regression with gender as the independent variable and the high-ED > low-ED contrast as the dependent variable. For display purposes, statistical maps for the main effects of gender, (i.e. fed visual for women > men) with a threshold of p < 0.005 uncorrected, were combined with a cluster-size threshold of 50 contiguous voxels. In addition, contiguous clusters with k ≥ 147 were deemed significant at p < 0.05 corrected (denoted by asterisk in the tables). This number was determined by 2000 Monte Carlo simulations of whole-brain fMRI data with respective data parameters of the present study according to AFNI 3dClustSim [18].
VAS scores showed that when fed (vs. fasted), participants scored higher on fullness (mean=56.42 vs. 29.83; p < 0.0001), and when fasted (vs. fed), they scored higher on hunger (mean=57.83 vs. 29.82; p < 0.0001). When fed, higher neural activation across all participants for high-ED (vs. low-ED) food cues was observed in primary and association sensory cortices (i.e. visual cortex for visual cues, auditory cortex for auditory cues). In the fasted state, higher neural activation across all participants was observed in the above areas, as well as brainstem, cognitive and reward processing areas in response to high vs. low-ED food cues (see Supplementary Figures and Tables 1–4).
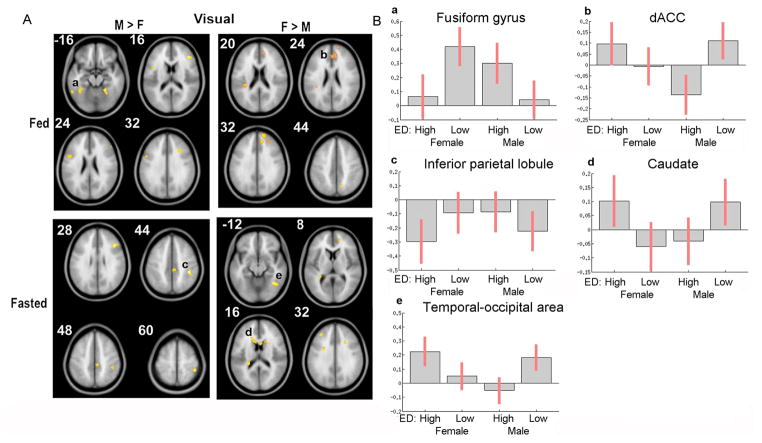
Figure 1 shows the sex-based differential BOLD responses to visual stimuli. In response to visual high-ED (vs. low-ED) food cues, when fed, men (vs. women) showed greater activation in the left fusiform gyrus (FFG), whereas women (vs. men) had greater activation in the right dorsal anterior cingulate cortex (dACC) (Table 1). When fasted however, men showed greater activation in right inferior parietal lobule (IPL) and women had greater activation in the bilateral caudate nucleus and right temporal-occipital area (Table 1).
Figure 1.
Effects of gender in response to visual high-ED vs. low-ED food cues. A) In fed state obese men had higher response to high-ED foods in fusiform gyrus (a), whereas women had higher responses in dACC (b). When fasted obese men had responses to high-ED foods in inferior parietal lobule (c), whereas women had higher responses in caudate (e) and temporal-occipital area (f). B) Bar graphs depicting average beta estimates for each group: visual high-ED and low-ED responses in both males and females. Error bars represent 90% confidence intervals. (M=men, F=females)
Table 1.
Visual food cues in fed and fasted states.
| Region | Hemisphere | Cluster Size | Coordinates | t-value | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Visual-Fed | |||||||
| M > F | Fusiform Gyrus* | Left | 220 | −56 | −50 | −22 | 4.4735 |
| Right | 71 | 22 | −46 | 14 | 4.1064 | ||
| Precentral Gyrus | Left | 133 | −46 | 6 | 20 | 3.8713 | |
| dlPFC | Right | 89 | 42 | 34 | 16 | 4.7532 | |
| Right | 68 | 30 | 22 | 34 | 3.9047 | ||
| Superior Parietal Gyrus | Left | 78 | −22 | −56 | 52 | 3.6619 | |
| F > M | dACC* | Right | 214 | 14 | 40 | 30 | −5.5479 |
| dmPFC | Right | 114 | 16 | 56 | 32 | −4.739 | |
| Insula | Left | 77 | −32 | −34 | 20 | −3.6618 | |
| dlPFC | Right | 52 | 30 | 40 | 34 | −3.5415 | |
| Precuneus | Right | 50 | 12 | −64 | 44 | −4.6123 | |
| Visual-Fasted | |||||||
| M > F | Inferior Parietal Lobule* | Right | 147 | 42 | −36 | 44 | 4.4639 |
| Inferior Frontal Gyrus | Right | 78 | 42 | 24 | 28 | 4.4333 | |
| Middle Cingulate | Right | 52 | 10 | −28 | 46 | 3.5146 | |
| Precentral Gyrus | Left | 59 | −20 | −12 | 70 | 3.9924 | |
| F > M | Temporal-occipital area/Fusiform Gyrus* | Right | 170 | 36 | −54 | 4 | −5.0847 |
| Caudate * | Left | 160 | −24 | −36 | 16 | −4.143 | |
| Right | 203 | 22 | 12 | 22 | −4.0614 | ||
| Right | 54 | 6 | 12 | 14 | −4.2567 | ||
| Left | 96 | −10 | 16 | 14 | −4.1069 | ||
| Insula | Left | 124 | −28 | 0 | 30 | −3.7361 | |
| ACC | Right | 65 | 16 | 48 | 10 | −3.812 | |
| dlPFC | Left | 65 | −34 | 38 | 32 | −4.5 | |
| Cingulate Gyrus | N/A | 53 | 16 | 16 | 40 | −3.583 | |
All results above thresholded at p < 0.005 uncorrected, k ≥ 50.
p < 0.05 corrected, k ≥ 147. (see methods).
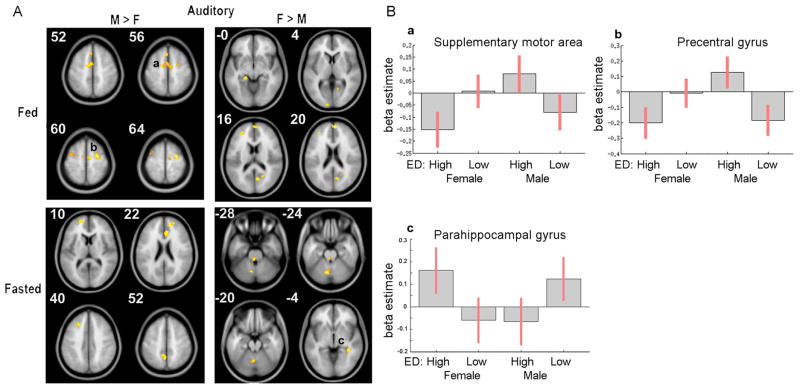
Figure 2 shows the sex-based differential BOLD responses to auditory stimuli. In response to auditory high-ED (vs. low-ED) food cues, when fed, men (vs. women) showed greater activation in bilateral supplementary motor areas (SMA) and right precentral gyrus. Women (vs. men) did not show significant differential neural activation although sub-threshold clusters were observed in posterior cingulate, dorsomedial prefrontal cortex (dmPFC) and hippocampus (Table 2). Moreover, when fasted, no significant differences were found for men with sub-threshold clusters in ACC and precuneus (Table 2). Women showed greater activation in right parahippocampal gyrus, with a sub-threshold cluster in cerebellum (Table 2).
Figure 2.
Effects of gender in response to auditory high-ED vs. low-ED food cues. A) In fed state obese men had higher response to high-ED foods in supplementary motor area (a) and precentral gyrus (b), When fasted obese women had higher responses in parahippocampal gyrus (c). B) Bar graphs depicting average beta estimates for each group: auditory high-ED and low-ED responses in both males and females. Error bars represent 90% confidence intervals. (M=men, F=females)
Table 2.
Auditory food cues in fed and fasted states.
| Region | Hemisphere | Cluster Size | Coordinates | t | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Auditory-Fed | |||||||
| M > F | Supplementary motor area (SMA)* | Left | 281 | −14 | −6 | 54 | 4.5532 |
| Precentral Gyrus* | Right | 184 | 28 | −10 | 62 | 5.4876 | |
| SMA | Left | 61 | −4 | 22 | 54 | 4.0101 | |
| Precentral Gyrus | Left | 61 | −34 | −2 | 60 | 3.374 | |
| F > M | Posterior Cingulate | Right | 138 | 8 | −68 | 16 | −3.9021 |
| dmPFC | Right | 127 | 8 | 58 | 20 | −4.7184 | |
| Hippocampus | Left | 65 | −18 | −32 | −6 | −4.2075 | |
| Middle Occipital Gyrus | Left | 52 | −10 | −98 | 2 | −3.5001 | |
| Middle Frontal Gyrus | Left | 61 | −28 | 44 | 14 | −3.6191 | |
| Auditory-Fasted | |||||||
| M >F | mPFC | Left | 105 | −12 | 56 | 14 | 3.813 |
| ACC | Right | 124 | 6 | 30 | 22 | 4.2531 | |
| Precuneus | Left | 111 | −2 | −52 | 54 | 3.784 | |
| mPFC | N/A | 95 | 14 | 48 | 22 | 4.8052 | |
| dlPFC | Left | 61 | −22 | 26 | 38 | 3.8666 | |
| F > M | Cerebellum | Left | 142 | −8 | −68 | −26 | −5.2901 |
| Parahippocampal Gyrus* | Right | 148 | 38 | −40 | −6 | −4.7578 | |
| Pons | N/A | 60 | −2 | −34 | −28 | −3.7671 | |
All results above thresholded at p < 0.005 uncorrected, k ≥ 50.
p < 0.05 corrected, k ≥ 147. (see methods).
ACC processes cognitive and emotion-related information as well as interacts with other cortical and sub-cortical regions to regulate behavior [19]. We found greater neural activation in obese women (vs. men) when fed in dACC, which was specifically associated with error-related negativity and detection [20], and conflict processing [21] in humans, and selective attention as well as novel response selection during emotional states [22] and for tasks [19,23] requiring effortful control [24]. Thus, when fed, obese women may be exhibiting more cognitive-emotional processing than men, for high vs. low-ED visual food cues. This finding is consistent a previous report [6] on fed, healthy weight women (vs. men) showing greater activation in dorsolateral PFC (dlPFC)–an area implicated in cognitive inhibition and control [25], in response to food cues. On the other hand, we found that when fed, obese men (vs. women) had higher neural activation in SMA, which agrees with studies reporting higher neural activation in motor-related areas in persons who binge-eat [17,26]. Brooks et al. [27] found that women with bulimia nervosa (vs. binging-purging subtype of anorexia nervosa) have higher neural activation in precentral gyrus and SMA. This may be related to binge-like eating in bulimia patients which may be present in obese persons that may explain the similar neural activation pattern. Moreover, we also found that obese men (vs. women) when fed, in response to visual high vs. low-ED food stimuli, showed higher activation in left fusiform gyrus (FFG) –a region associated with the perception of faces, emotional expression, salient [28] and appetitive stimuli [5,29]. Frank et al. [7] reported higher activation in FFG in response to high vs. low-ED food cues in lean women (vs. men) when hungry (Nm=6, Nw=6 ). Thus our finding of higher FFG activation in obese men when fed, indicates that the sex-based effects on neural responsivity to food cues may also be dependent on body weight and energy state.
When fasted, we found that in response to high vs. low-ED food cues (auditory), obese women had higher bilateral caudate nucleus, right parahippocampal gyrus activation, as well as right temporal-occipital area in response to high vs. low-ED visual food cues. Caudate nucleus is a brain region associated with reward processing and craving [30]. It has been suggested that obese persons have similar patterns of craving for food as seen with other types of cravings (i.e. drugs, alcohol). Thus our results suggest that when fasted, obese women have higher neural responses associated with craving than men. Moreover, activation in parahippocampal gyrus, an area related to affective evaluation of stimuli [28], has been previously shown in response to high vs. low calorie food cues in obese women-only group, in an energy state between hungry and satiated [29] and in an obese men-only group, when hungry [31]. Uher et al. [5] also reported that during fasting, lean women (vs. men) had stronger activation in visual processing neural areas (i.e. inferior temporal occipital) in response to visual high vs. low-ED food cues. In fasted obese men (vs. women) however, the only supra-threshold region was right inferior parietal lobule (IPL), a region related to visuospatial attention, responding to salient new or alerting stimuli [32], as well as judgment and knowledge manipulation (e.g. grasp-to-use) [33]. Taken together, these results suggest that, in response to food cues, obese men (vs. women) have greater neural activation in regions associated with motor execution and planning, whereas obese women have higher neural responses in areas associated with cognitive and affective processes.
This study examines the sexually dimorphic neural activity in obese persons in response to food cues. When fed, obese men (vs. women) showed greater activation in motor planning and control regions (e.g. SMA) whereas women showed greater activation in cognitive-affective processing regions (i.e. dACC). When fasted, obese men had greater activation in visual and attention regions (i.e. IPL) whereas obese women showed greater activation in affective and reward related processing regions (e.g. caudate), and visual perception region (i.e. temporal-occipital). Overall, these results support our a priori hypothesis that obese women (vs. men) have greater neural activation in regions associated with cognition and emotion.
There were several study limitations. We did not 1) control for the time of menstrual cycle, 2) match for the colors of the high vs. low-ED food items and instead selected commonly preferred high-ED foods [9], or 3) include a lean control group. However, our results with obese individuals are in line with other findings [5] which showed that lean women had higher activations in emotion-related areas than men in response to food cues Taken together, greater affective neural processing in obese women could be a contributing factor for the higher prevalence of obesity in women. Further research comparing lean and obese individuals for sexually dimorphic neural activation and functional connectivity may help better elucidate the neural mediators of overeating and obesity.
Supplementary Material
Highlights.
Obesity and eating disorders are more prevalent in women compared to men.
Gender-based differences in neural function in response to food are investigated.
Neural basis of eating is examined by functional magnetic resonance imaging -fMRI.
Effect of gender on neural responsivity to food cues in fed and fasted conditions.
Women had higher emotional responsivity than men in response to food cues.
Acknowledgments
This study is supported by R01DK080153 and R01DK074046S2 (PI: Allan Geliebter). We would like to thank Joy Hirsch for advice and contributions in designing the fMRI task design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 3.Bates CJ, Prentice A, Finch S. Gender differences in food and nutrient intakes and status indices from the National Diet and Nutrition Survey of people aged 65 years and over. Eur J Clin Nutr. 1999;53(9):694–9. doi: 10.1038/sj.ejcn.1600834. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckel LE, Cox JE, Cook EW, 3rd, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48(2):139–44. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 5.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–9. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–66. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E, et al. Sex differences in the human brain’s response to hunger and satiation. Am J Clin Nutr. 2002;75(6):1017–22. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 9.Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. PNAS. 2009;106 (4):1249–54. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83(6):1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET, Rolls BJ, Rowe EA. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983;30(2):185–92. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- 12.Führer D, Zysset S, Stumyoll M. Brain activity in hunger and satiety: an exploratory visually stimulated fMRI study. Obesity. 2008;16(5):945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 13.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31(4):1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, et al. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS One. 2011;6(4):e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairburn CG, Cooper Z. The Eating Disorder Examination (12th edition) In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. New York: Guilford Press; 1993. pp. 317–60. [Google Scholar]
- 17.Schienle A, Schäfer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65(8):654–61. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages computers and biomedical research. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 19.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cogn Sci. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 20.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and on-line monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 21.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action inanterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 22.Gusnard DA, Akbudak E, Sulman GL, Raichle M. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC, Raichle M. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognit Emotion. 1998;12:353–85. [Google Scholar]
- 24.Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: A review of 107 PET activation studies. Neuroreport. 1998;9:37–47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 25.Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann NY Acad Sci. 1990;608:637–69. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- 26.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsh J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–5. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Brooks SJ, O’Daly OG, Uher R, Friederich H-C, Giampietro V, Brammer M, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS ONE. 2011;6(7):e22259. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Parigi A, Gautier JF, Chen K, Sable AD, Ravussin E, Remain E, et al. Mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–97. [PubMed] [Google Scholar]
- 29.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Tataranni PA, Gautier J-F, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci. 1999;96:4569–74. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi R, Lambon RMA, Saito S, Pobric G. Different roles of lateral anterior temporal lobe and inferior parietal lobule in coding function and manipulation tool knowledge: Evidence from an rTMS study. Neuropsychologia. 2011;49(5):1128–35. doi: 10.1016/j.neuropsychologia.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:6, 1434–48. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.