Abstract
Purpose
Population-based studies have shown improved survival for patients diagnosed with metastatic breast cancer (MBC) over time, presumably due to the availability of new and more effective therapies. The objective of this analysis was to determine if survival improved for patients who developed distant recurrence of breast cancer after receiving adjuvant therapy.
Methods
Adjuvant chemotherapy trials coordinated by the Eastern Cooperative Oncology Group (ECOG) that accrued patients between 1978–2002 were reviewed. Survival following distant recurrence was estimated for progressive time periods, and adjusted for baseline covariates in a Cox proportional hazards model.
Results
Of the 13,785 patients who received adjuvant chemotherapy in 11 trials, 3447 (24.4%) developed distant recurrence; median survival following recurrence was 20 months (95% confidence intervals: 19, 21). Factors associated with inferior survival included shorter distant recurrence free interval (DRFI), ER- and PR-negative disease number of positive axillary nodes at diagnosis and black race (p<0.0001 for all). When time-period of recurrence was added to the model, it was not significantly associated with survival for the general population with recurrence. Survival improved over time only in hormone-receptor negative patients with a DRFI ≤ 3 years, both among the 5 recent and entire trial datasets (p=0.01 and p=0.05 respectively).
Conclusions
In contrast to reports from population-based studies, we did not observe general improvement in survival over the last three decades for patients who developed distant recurrence after adjuvant chemotherapy after adjusting for DRFI. Improved survival for hormone-receptor negative patients with a short DRFI suggests benefit from trastuzumab.
Keywords: breast cancer, distant recurrence, survival, distant recurrence free interval, metastatic disease
Introduction
MBC remains the second leading cause of cancer death in women, with over 40,000 dying in the United States (US) and over 400,000 dying globally each year.1 Although breast cancer mortality rates have declined in the US due to screening and improved systemic adjuvant therapy,2 the disease remains incurable for those with distant metastases.3 Evidence suggests survival has modestly improved in the era of modern systemic therapy,4–6 implying further improvement may be possible with new therapeutic approaches. A potential secondary benefit is that identification of effective new agents for MBC may produce survival gains when used as adjuvant therapy for localized disease: notable examples include anthracyclines, taxanes, and trastuzumab.7–9
In order to determine whether survival has improved for patients who developed distant recurrence after receiving adjuvant therapy, we undertook a review of adjuvant phase III trials conducted by the Eastern Cooperative Oncology Group (ECOG) over a period of approximately three decades.
Methods
Population
Eleven phase III adjuvant breast cancer trials conducted by the ECOG that treated patients with adjuvant chemotherapy, and with at least 5 years of followup were identified and included in this analysis (Table 1). Participants who did not receive adjuvant chemotherapy were excluded from this analysis.
Table 1.
ECOG-coordinated adjuvant breast cancer trials included in analysis
| Trial | Treatment Arms | Key Characteristics | Enrollment Dates (month/year) | Enrolled (n) | Received Chemo-therapy (n) | Distant Recurrence (n) |
|---|---|---|---|---|---|---|
| E517720 | CMF/P/PT | Premenopausal | 2/78 to 2/82 | 662 | 662 | 284 |
| E617721, 22 | CMFP vs. CMFPT vs. Observation | Postmenopausal | 2/78 to 7/81 | 265 | 170 | 92 |
| E118023, 24 | CMFP vs. Observation | Node-negative | 5/81 to 5/88 | 541 | 276 | 47 |
| E418125 | CMFPT 4 vs. 12 vs. 12 + continued T | Postmenopausal | 2/82 to12/86 | 961 | 961 | 392 |
| E518126 | CMFPT vs. Alternating chemo with Continued T vs. Observation | Premenopausal | 2/82 to 7/87 | 658 | 658 | 224 |
| E318127 | CAFTH with RT vs. Observation | Locally Advanced Resectable | 1/82 to 2/87 | 332 | 332 | 171 |
| “Recent” adjuvant trials | ||||||
| * E318928 | CAF vs Multi-drug | ER-negative | 8/89 to 4/93 | 646 | 646 | 212 |
| * E518829 | CAF vs. CAF+Z vs. CAF+ZT | Premenopausal, ER-positive, Node-positive | 7/89 to 2/94 | 1536 | 1536 | 533 |
| * E219030 | CAF vs. CAF plus high dose chemotherapy | 10+ positive axillary nodes | 8/91 to 8/98 | 540 | 540 | 246 |
| * E219731 | AC vs AD | 0–3 positive axillary nodes | 7/98 to 1/00 | 2952 | 2952 | 293 |
| * E119932 | AC + Taxanes | Positive nodes or high-risk node-negative | 10/99 to 1/02 | 5052 | 5052 | 953 |
| Total numbers | 14,145 | 13,785 | 3,447 | |||
A: Adriamycin (doxorubicin), C: cyclophosphamide, M: methotrexate, F: 5-fluorouracil, T: tamoxifen, Z: Zoladex (goserelin), D: docectaxel, P: prednisone, H: Fluoxymesterone.
All patients received “modern” chemotherapy: anthracyclines, or sequential/concurrent anthracycline-taxane therapy
Analysis
The primary study endpoint was survival following distant recurrence, defined as time from date of distant recurrence to date of death (or date last known alive). Survival after distant recurrence was determined with adjustment for baseline covariates in a Cox proportional hazards model. The models included calendar year of (distant) recurrence, age at diagnosis of recurrence, estrogen receptor (ER) and progesterone receptor (PgR) status, number of positive axillary lymph nodes at diagnosis, primary tumor size at diagnosis, race and DRFI. HER2 was not available to include in the model. DRFI was defined as the time from study entry to the date of distant recurrence. Because DRFI is strongly associated with survival after recurrence and the potential for “gap time” bias,10 logrank tests for other covariates were computed stratified on DRFI (0–3, >3–6, >6 years). Estimates of survival after distant recurrence were weighted averages of the Kaplan Meier estimates computed within DRFI groups. Therapeutic intervals of interest were identified between the first on-study date (1978) and the last point at which data was censored (2010). Recurrences were assigned to one of six time periods: 1978–1983, 1984–1988, 1989–1993, 1994–1998, 1999–2003, and 2004–2010. The most recent time period (2004–2010) was used as the comparator. Analyses are based on all 3447 patients with distant recurrence. We additionally examined the outcomes for 2237 patients receiving “recent” chemotherapy regimens (E3189, E5188, E2190, E2197, E1199) between 1989 and 2002, given that the type of adjuvant chemotherapy might influence resistance in the metastatic setting.
Results
Characteristics of Included Trials
The study population included 13,785 patients enrolled on 11 adjuvant ECOG trials between 1978 and 2002 (Table 1). Among the 13,785 enrolled, 3447 (24.4%) had distant recurrences, 814 (5.9%) had local recurrences only, and 20 (0.1%) had unknown sites of recurrence. Table 2 shows the characteristics of both all adjuvant participants and the subset with distant recurrence. Median survival following distant recurrence (n=3447) was 20 months (95% confidence intervals: 19, 21 months). Estimated 5 and 10-year survival rates were 16.3% and 6.4%.
Table 2.
Characteristics of Adjuvant Trial Population and Subsets with Distant Recurrence
| Entire Adjuvant Study Population Treated with Chemo | Cases with Distant Recurrence from Adjuvant Study Population | Cases with Distant Recurrence from Recent Trials Only | |
|---|---|---|---|
| Number of adjuvant trials | 11 | 11 | 5 most recent: E3189, E5188, E2190, E2197, E1199 |
| Number of Patients | 13,785 | 3,447 | 2,237 |
| Age at Initial Diagnosis, Median (range) | 49 (19, 85) | 48 (19, 80) | 47 (19, 80) |
| Age at Distant Recurrence, Median (range) | -- | 52 (21, 85) | 50 (22, 83) |
| Hormone Receptor Status | |||
| ER Positive | 8822 (64%) | 2093 (61%) | 1388 (63%) |
| PgR Positive | 7977 (62%) | 1794 (59%) | 1359 (62%) |
| Tumor Size at Diagnosis | |||
| Unknown | 121 | 19 | 16 |
| 0–2.0 cm | 5261 (39%) | 933 (27%) | 613 (28%) |
| 2.1–5.0cm | 7103 (52%) | 1994 (58%) | 1285 (58%) |
| 5.1cm or greater | 1300 (10%) | 501 (15%) | 323 (15%) |
| Number of Positive Axillary Nodes at Diagnosis | |||
| Unknown | 76 | 12 | 6 |
| 0 positive | 2771 (20%) | 275 (8%) | 225 (10%) |
| 1–3 positive | 6400 (47%) | 1281 (37%) | 843 (38%) |
| 4–9 positive | 2746 (20%) | 981 (29%) | 560 (25%) |
| 10 or more positive | 1792 (13%) | 898 (26%) | 603 (27%) |
| Race | |||
| Unknown | 301 | 45 | 43 |
| While | 11817 (88%) | 2972 (87%) | 1873 (85%) |
| Black | 1137 (8%) | 298 (9%) | 221 (10%) |
| Other | 530 (4%) | 132 (4%) | 100 (5%) |
| DRFI | |||
| ≤3 years | -- | 1781 (52%) | 1144 (51%) |
| 3–6 years | -- | 987 (29%) | 700 (31%) |
| > 6 years | -- | 679 (20%) | 393 (18%) |
Note: Information was missing for some variables in some patients in the entire cohort and relapsed cohort, including age at initial diagnosis (33 entire adjuvant/4 recurrent/0 recent study and recurrent), age at distant recurrence (--/4/0); ER expression (85/22/21), PR expression (932/386/33), tumor size (121/22/19), nodal status (76/12/6) and race (301/45/43).
Analysis of Distant Recurrence
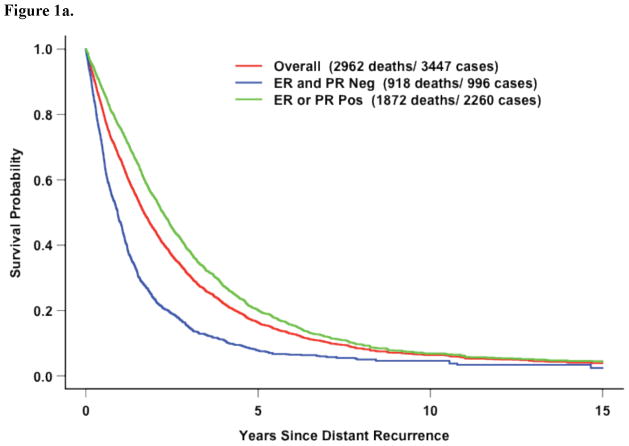
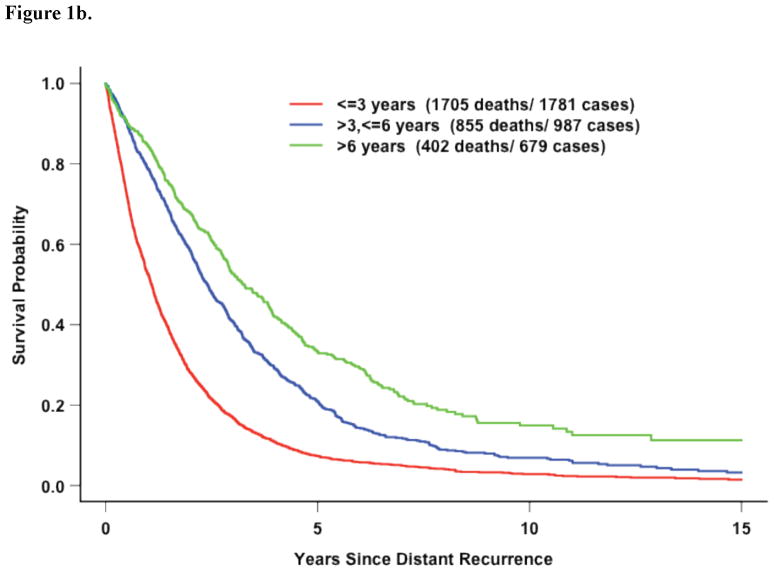
Covariates significantly associated with inferior survival after distant recurrence included ER- and PR-negative disease (Figure 1a). However, DRFI was the most strongly associated with survival after recurrence (Figures 1b–c). Black race and increasing number of positive axillary nodes at diagnosis were also significant.
Figure 1.
Figure 1a. All Eleven Trials: Survival after distant recurrence
Figure 1b. All Eleven Trials: Survival following recurrence by DRFI (p<0.0001)
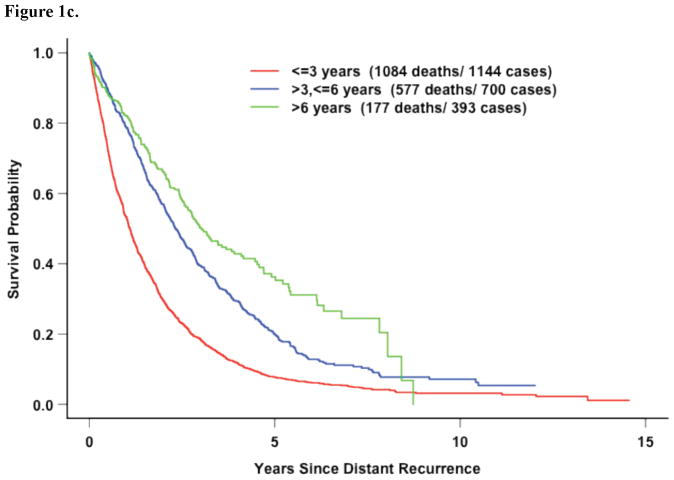
Figure 1c. Five Recent Trials: Survival following recurrence by DRFI (p<0.0001)
Table 3 shows the estimated hazard ratio (HR) from the Cox proportional hazards model. Patients with a shorter DRFI fared significantly worse than those with a longer DRFI (HR of 2.44 DRFI > 6 vs. ≤ 3 years, p<0.0001; and a HR of 1.43 DRFI of >6 years vs >3–6 years, p <0.0001.) Patients with ER- and PR-negative disease also had significantly shorter survival following recurrence (HR 1.35, p<0.0001; 1.33, p<0.0001 respectively), as did those of black race (HR 1.34, p<0.0001) and with more positive nodes at diagnosis (HR 1.17, 1–3 vs. 0 nodes; HR 1.35, 4–9 vs. 0 nodes; HR 1.33, >9 vs. 0 nodes; p<0.0001). Age, primary tumor size and year of recurrence were not significant.
Table 3.
Estimated hazard ratios from Cox proportional hazards multivariate models
| All 11 studies (n=3431) | 5 Recent Studies (n=2231) | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
| DRFI (years) | ≤3 vs. >6 | 2.44 | 2.16–2.76 | <0.0001 | 2.22 | 1.87–2.63 | <0.0001 |
| >3, ≤6 vs. >6 | 1.43 | 1.26–1.62 | 1.38 | 1.16–1.63 | |||
| ER status | Negtv vs. Postv | 1.35 | 1.23–1.49 | <0.0001 | 1.34 | 1.16–1.56 | <0.0001 |
| Unk vs. Postv | 0.88 | 0.52–1.48 | 0.54 | 0.24–1.21 | |||
| PR status | Negtv vs. Postv | 1.33 | 1.20–1.47 | <0.0001 | 1.27 | 1.09–1.47 | 0.007 |
| Unk vs. Postv | 1.04 | 0.90–1.21 | 1.43 | 0.78–2.62 | |||
| Number of positive axillary nodes* | 1–3 vs. 0 | 1.17 | 1.01–1.36 | <0.0001 | 1.05 | 0.89–1.25 | 0.15 |
| 4–9 vs. 0 | 1.35 | 1.16–1.58 | 1.16 | 0.97–1.40 | |||
| >9 vs. 0 | 1.33 | 1.14–1.56 | 1.17 | 0.97–1.40 | |||
| Race | Black vs. White | 1.34 | 1.18–1.53 | <0.0001 | 1.38 | 1.19–1.60 | <0.0001 |
| Other vs. White | 0.88 | 0.72–1.07 | 0.82 | 0.65–1.03 | |||
| Unk vs. White | 0.95 | 0.68–1.33 | 1.06 | 0.75–1.50 | |||
| Age at Distant Recurrence (years) | 40–49 vs. <40 | 0.95 | 0.85–1.07 | 0.30 | 0.96 | 0.83–1.11 | <0.0001 |
| 50–59 vs. <40 | 0.99 | 0.88–1.11 | 1.06 | 0.91–1.23 | |||
| 60–69 vs. <40 | 1.01 | 0.89–1.16 | 1.20 | 1.00–1.43 | |||
| >69 vs. < 40 | 1.13 | 0.95–1.33 | 1.68 | 1.31–2.15 | |||
| Tumor Size (cm) | 2.1–5.0 vs. ≤2.0 | 1.11 | 1.01–1.21 | 0.12 | 1.13 | 1.02–1.27 | 0.07 |
| >5.0 vs. ≤2.0 | 1.07 | 0.95–1.21 | 1.19 | 1.03–1.39 | |||
| Unk vs. ≤2.0 | 0.87 | 0.53–1.44 | 0.96 | 0.56–1.64 | |||
| Time period of Distant Recurrence | 1978–83 vs. 2004–10 | 0.93 | 0.76–1.14 | 0.24 | -- | -- | |
| 1984–88 vs. 2004–10 | 0.99 | 0.85–1.15 | -- | -- | |||
| 1989–93 vs. 2004–10 | 1.02 | 0.87–1.18 | 1.29 | 1.06–1.58 | 0.005 | ||
| 1994–98 vs. 2004–10 | 0.97 | 0.84–1.12 | 1.13 | 0.97–1.31 | |||
| 1999–2003 vs. 2004–10 | 0.89 | 0.77–1.02 | 0.98 | 0.84–1.14 | |||
Abbreviations: NS = not significant, HR = Hazard Ratio, CI = confidence interval, Negtv = negative, Postv = positive, Unk = unknown
Cases with age or number of positive nodes unknown excluded (16 cases for all 11 studies, 6 cases for 5 recent studies); ‘unknown’ included as a separate category for other factors with unknown cases.
Interaction test for Time Period of Distant Recurrence vs. Five Recent Studies = 0.38
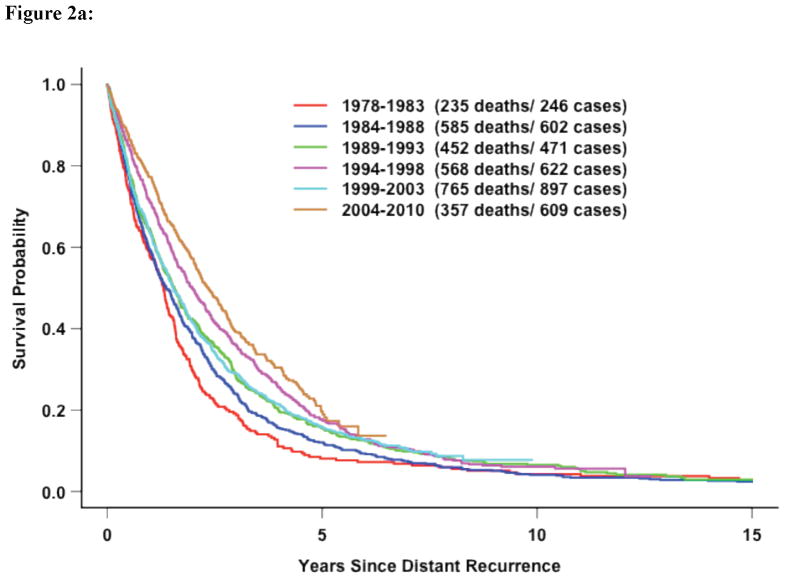
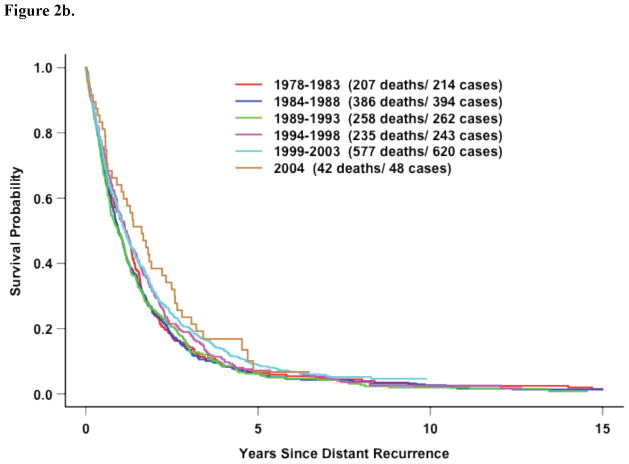
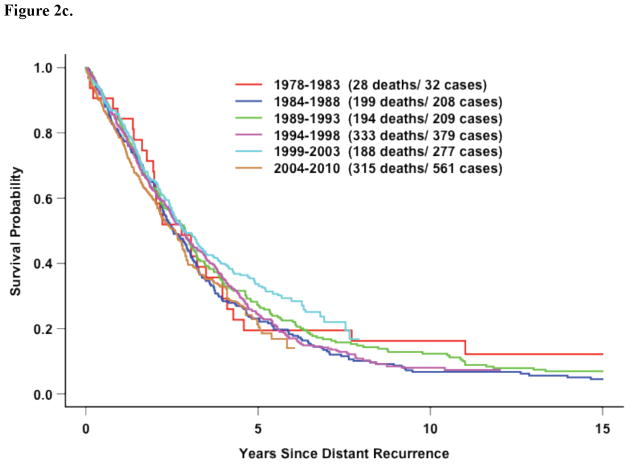
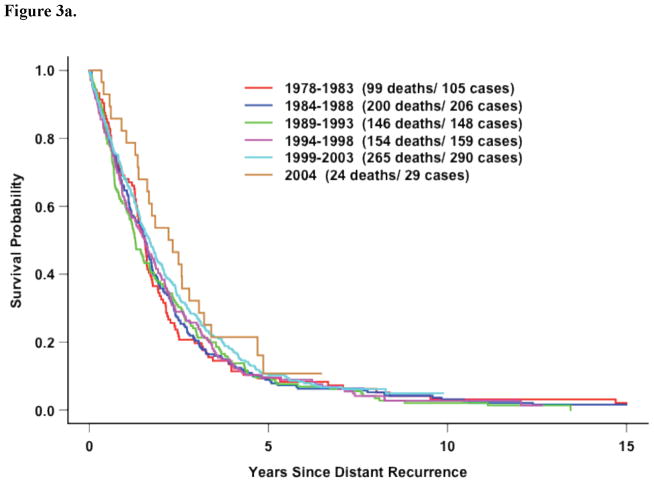
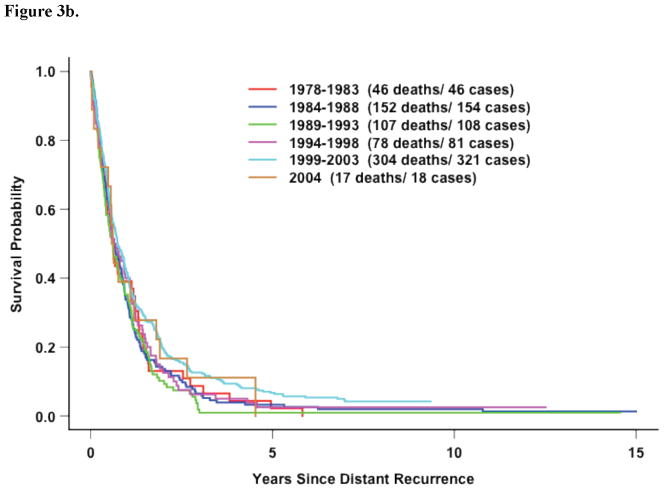
If survival after distant recurrence was evaluated without stratification for DRFI for all 11 studies, survival did significantly improve over time (Figure 2a). However, once stratified by DRFI, there was not significant improvement in survival over time (Figure 2b). The improvement observed in the unadjusted analysis for the entire population is likely reflects more favorable patients recruited by later adjuvant trials–recent adjuvant trials selected for more ER-positive disease. Table 4 illustrates this point. Survival after distant recurrence by time period of recurrence stratified by DRFI and receptor status is shown in Figures 3a–d. Only among hormone-receptor negative participants who recurred within 3 years does there appear to be any improvement in survival over time.
Figure 2.
Figure 2a: All Eleven Trials: Survival following distant recurrence by time period WITHOUT adjustment for DRFI (p<0.0001, logrank test without stratification)
Figure 2b. All Eleven Trials: Survival following recurrence by time period of recurrence in patients WITH DRFI≤ 3 Years (p=0.04)
Figure 2c. All Eleven Trials: Survival following recurrence by time period in patients WITH DRFI> 3 Years (p=0.47)
Table 4.
Characteristics of patients with distant recurrence based on time period of recurrence
| Calendar Year of Recurrence | |||||||
|---|---|---|---|---|---|---|---|
| 1978–1983 | 1984–1988 | 1989–1993 | 1994–1998 | 1999–2003 | 2004–2010 | ||
| No. Patients | 246 | 602 | 471 | 622 | 897 | 609 | |
| Age at Initial Diagnosis, Median (range) | 47 (23, 73) [4 Unk] | 52(20,79) | 47(25,78) | 44(24,77) | 48(24,80) | 50(19,77) | |
| Age at Distant Recurrence, Median (range) | 49 (25, 74) [4 Unk] | 55(21,81) | 50(25,83) | 48(28,85) | 52(25,82) | 57(22,83) | |
| ER status | ER Negative | 129(52%) | 278(46%) | 191(41%) | 217(35%) | 394(44%) | 123(20%) |
| ER Positive | 117(48%) | 323(54%) | 278(59%) | 403(65%) | 493(56%) | 479(80%) | |
| Unknown | 0 | 1 | 2 | 2 | 10 | 7 | |
| PR status | PgR Negative | 61(56%) | 266(58%) | 194(45%) | 198(33%) | 411(47%) | 137(23%) |
| PgR Positive | 48(44%) | 192(42%) | 238(55%) | 394(67%) | 461(53%) | 461(77%) | |
| Unknown | 137 | 144 | 39 | 30 | 25 | 11 | |
| Tumor Size | ≤ 2cm | 59(24%) | 156(26%) | 110(23%) | 194(31%) | 235(26%) | 179(30%) |
| >2, ≤ 5 cm | 140(57%) | 354(59%) | 279(59%) | 337(55%) | 523(59%) | 361(60%) | |
| >5 cm | 45(18%) | 91(15%) | 80(17%) | 85(14%) | 134(15%) | 66(11%) | |
| Unk | 2 | 1 | 2 | 6 | 5 | 3 | |
| Number of positive axillary nodes at diagnosis | 0 | 1(0%) | 31(5%) | 11(2%) | 5(1%) | 147(16%) | 80(13%) |
| 1–3 | 83(34%) | 199(33%) | 159(34%) | 214(34%) | 354(40%) | 272(45%) | |
| 4–9 | 94(39%) | 205(34%) | 185(39%) | 174(28%) | 172(19%) | 151(25%) | |
| >9 | 65(27%) | 165(28%) | 116(25%) | 229(37%) | 220(25%) | 103(17%) | |
| Unk | 3 | 2 | 0 | 0 | 4 | 3 | |
| Race | White | 219(89%) | 543(90%) | 398(85%) | 520(84%) | 767(88%) | 525(89%) |
| Black | 18(7%) | 40(7%) | 48(10%) | 64(10%) | 76(9%) | 52(9%) | |
| Other | 8(3%) | 19(3%) | 22(5%) | 36(6%) | 32(4%) | 15(3%) | |
| Unk | 1 | 0 | 3 | 2 | 22 | 17 | |
| DRFI | < 3 yrs | 214(87%) | 394(65%) | 262(56%) | 243(39%) | 620(69%) | 48(8%) |
| 3–6 yrs | 32(13%) | 167(28%) | 97(21%) | 246(40%) | 125(14%) | 320(53%) | |
| >6 yrs | 0(0%) | 41(7%) | 112(24%) | 133(21%) | 152(17%) | 241(40%) | |
Unk= unknown
Figure 3.
Figure 3a. All Eleven Trials: Survival following recurrence by time period for hormone-receptor positive patients with DRFI≤ 3 Years (p=0.41)
Figure 3b. All Eleven Trials: Survival after recurrence by time period in hormone-receptor negative patients with DRFI≤ 3 Years (p=0.05)
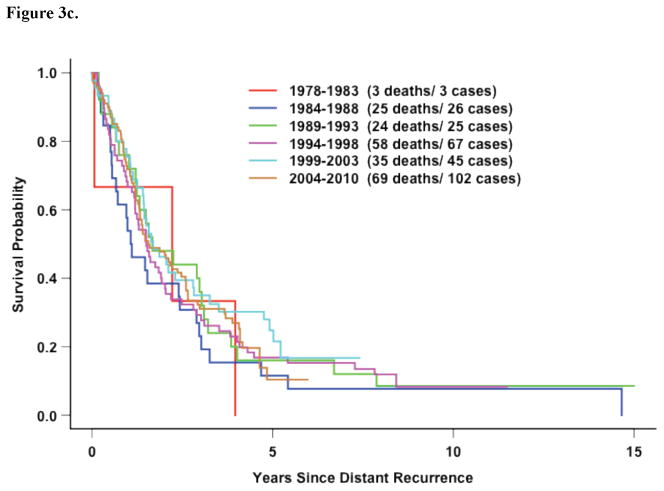
Figure 3c. All Eleven Trials: Survival following recurrence by time period in hormone-receptor negative patients with DRFI > 3 Years. (p=0.94)
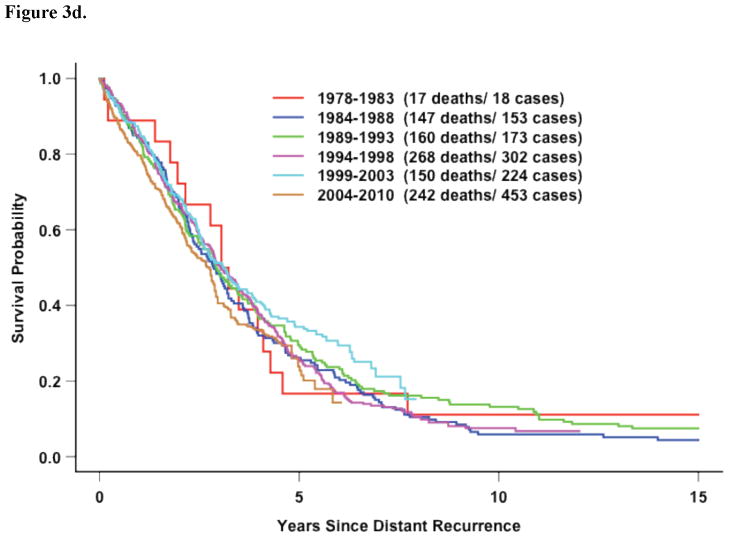
Figure 3d. Five Recent Trials: Survival following distant recurrence by time period in hormone-receptor positive patients with DRFI > 3 Years (p=0.46)
Analysis of Recent Trials Only
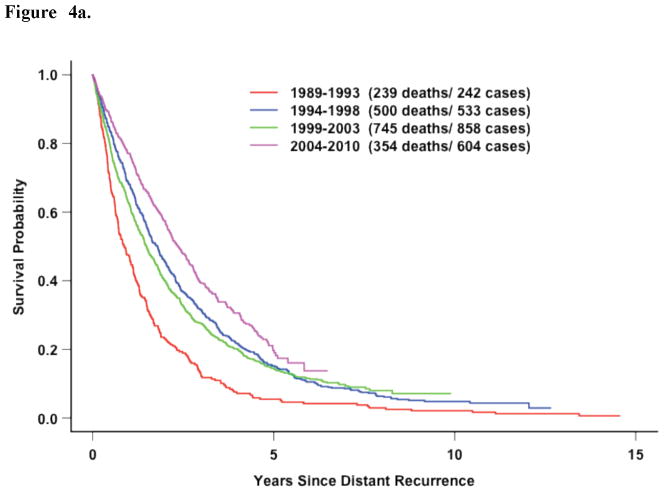
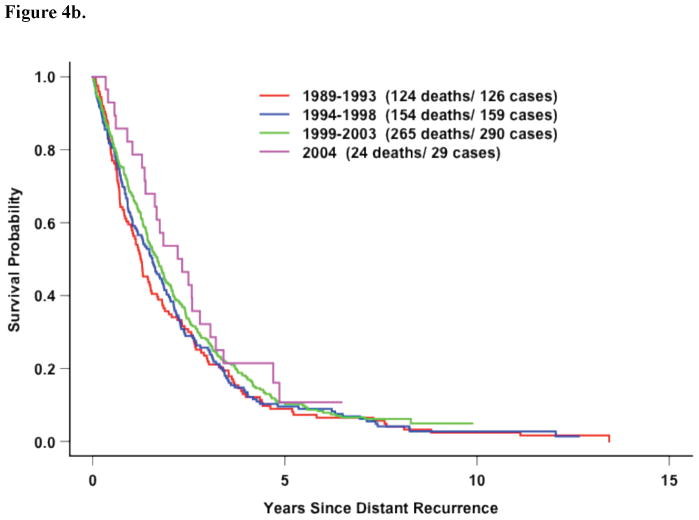
In the 5 most recent trials, factors associated with survival after distant recurrence included DRFI, ER/PR expression, and race (Figure 2c, Table 3). However, in contrast to the entire study population, older age at recurrence and time period of recurrence beginning in 1999 (compared with before 1994) were significantly associated with improved survival. However, this improvement over time was again confined to those patients with hormone-receptor negative disease who recurrent within 3 years of diagnosis (Figure 4a–e).
Figure 4.
Figure 4a. Five Recent Trials: Survival following distant recurrence by time period WITHOUT adjustment for DRFI (p<0.0001, logrank test without stratification)
Figure 4b. Five Recent Trials: Survival following recurrence by time period of distant recurrence in hormone-receptor positive patients with DRFI≤ 3 Years (p=0.22)
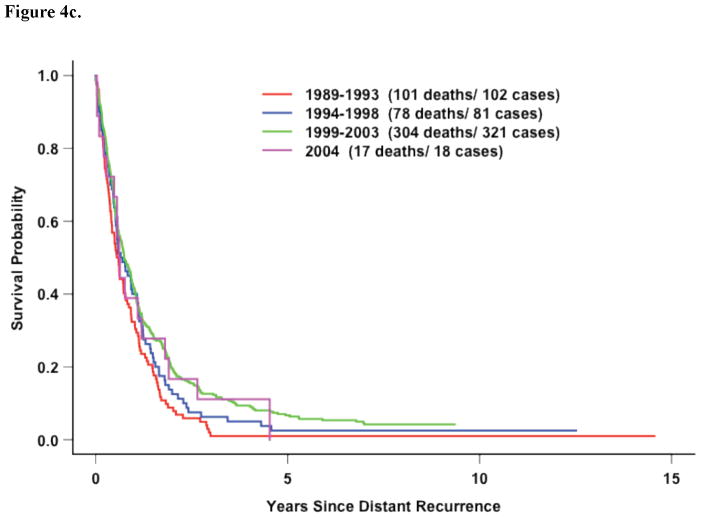
Figure 4c. Five Recent Trials: Survival following recurrence by time period hormone-receptor negative patients with DRFI≤ 3 Years (p=0.01)
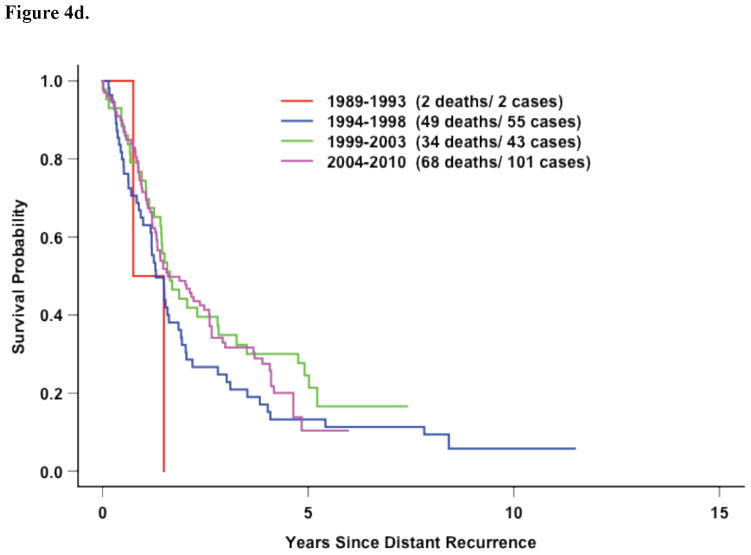
Figure 4d. Five Recent Trials: Survival following recurrence by time period in hormone-receptor negative patients with DRFI > 3 Years (p=0.64)
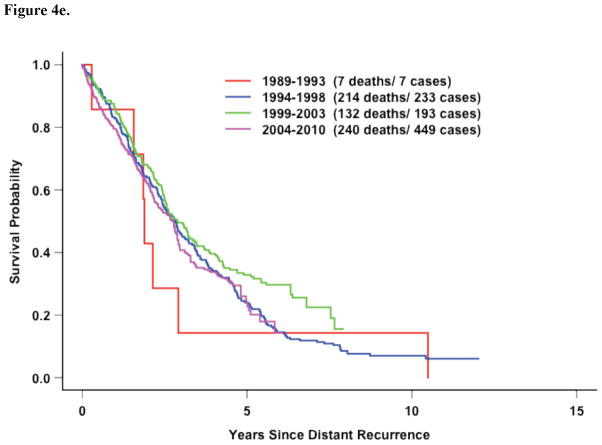
Figure 4e. Five Recent Trials: Survival after recurrence by time period in hormone-receptor positive patients with DRFI > 3 Years (p=0.57)
Discussion
Among the many phase III MBC trials performed over the past three decades, survival significantly improved in only of handful.11–14 Despite difficulty in demonstrating improved survival in individual trials, population-based studies suggest that MBC patients now survive modestly longer than in the past.4–6 This improvement observed in population-based studies could be due to the increased availability of drugs that when used individually have minimal effect in prolonging survival, but when used sequentially may produce modest survival gains. Other suggested explanations include the impact of improved imaging.4 Better imaging may lead to apparent prolongation in the interval between diagnosis of recurrence and death by identifying MBC at earlier time points. Better imaging also increases the percentage of women with de novo MBC (women with recurrent breast cancer have inferior survival compared to women with de novo disease.15)
To determine whether the perceived improvement in survival demonstrated by population-based studies was also evident in clinical trial populations, we evaluated survival following recurrence among ECOG clinical trial participants who received adjuvant chemotherapy. Our analysis suggests that for women who develop distant recurrence following adjuvant chemotherapy, the availability of new cytotoxic and biologic agents has not broadly translated into improved survival. The exception appears to be among hormone-receptor negative patients who relapse within 3 years of diagnosis, where survival improved for the period of recurrence beginning in 1999 (compared with before 1994). We hypothesize that this may reflect an effect of trastuzumab rather than improved cytotoxic therapy: trastuzumab became commercially available in 1998, and HER2-positive disease is associated with early recurrence.
Table 5 summarizes characteristics of this and other reports evaluating trends in metastatic survival. There are several key differences between our analysis and the other reports.4, 5, 16, 17 First, we included only patients who recurred after receiving adjuvant chemotherapy for early stage breast cancer and thus were more likely to have drug resistant disease (de novo disease was included in other analyses.) Second, we adjusted for multiple prognostic covariates in multivariate models, including DRFI. Giordano et al 4 noted large differences in outcome by the year of recurrence, which was not evident when adjusted for DRFI. However, not all prior analyses shown in Table 5 adjusted for DRFI and other covariates. This may contribute to the improved survival over time observed by others. Indeed, survival was improved over time in our dataset if the survival analysis was not stratified by DRFI, highlighting the importance of controlling for this variable when evaluating survival over time.
Table 5.
Comparison of trials examining survival over time
| Author | Number with MBC* (and % with de novo Stage IV) | Time Period Examined | Source | Median Survival | DFI or DRFI in model | Survival over time |
|---|---|---|---|---|---|---|
| Chia et al5 | 2,150 (21.4%) | 1991–2001 | Population- based registry | 15–22 months# | No | Improved |
| Dabakuyo et al33 | 1459 (12%) | 1982–2005 | Population- based registry | Relative survival reported | No | Improved |
| Dawood et al16 | 2,091 (22.4%) | 1991–2007 | Single institution database | 28.6 months | No | Improved$ |
| Dawood et al6 | 15,438 (100%) | 1988–2003 | Population- based registry | 23 months | No | Improved |
| Giorodano et al4 | 834 (0%) | 1974–1994 | Single institution database | 21 months | Yes | Not Improved^ |
| Largillier et al17 | 1,038 (0%) | 1975–2005 | Single institution database | 23.1 months | Yes | Not improved |
| This analysis | 3477 (0%) | 1978–2010 | Multi- institution database | 20 months | Yes | Not improved overall |
DFI = disease-free interval; MBC=metastatic breast cancer
MBC includes both de novo Stage IV and recurrence after operable disease;
Based on time period of recurrence, 30-day/month;
HER2 status and trastuzumab use known and included in model;
In multivariate analysis adjusting for tumor size, nodes, stage, DFI, ER status and site of metastases, p-value =0.09. In an alternate, unstratified model including DFI, p=0.04.
This analysis has several strengths and limitations. Strengths include the large sample size, long followup, conduct by the same group of investigators, and standardized treatment regimens and prospective data collection. The heterogeneity of the population over time is a limitation imposed by evolving eligibility criteria for the 11 adjuvant trials. To account for this, analysis was not based on time period of enrollment, but rather on time period of recurrence, while controlling for factors such as age, ER/PR expression and race. Other limitations include lack of information regarding sites of recurrence, treatments used after recurrence, and the potential for lead-time bias due to improved imaging. Additionally, information is absent about HER2 status and anti-HER2 therapy. Although we hypothesize that the survival benefit for those diagnosed with an early recurrence after 1999 was due to the availability of trastuzumab, it is not possible for us to determine with certainty that this reflects the improved survival for HER2+ disease demonstrated in prospective clinical trials.11, 18, 19
In conclusion, we found evidence over a 30-year time period for an improvement in survival among hormone-receptor negative patients who recurred within 3 years following adjuvant chemotherapy for localized disease, but not for the population as a whole. Survival seemed to improve over the past 30 years for the entire population, but this effect was not persistent when the survival analysis was adjusted for DRFI and other covariates. This suggests that survival improvements observed among population-based studies may not reflect outcomes for all subsets of women recurring after adjuvant chemotherapy. There remains a critical need for developing more effective therapies for patients with MBC, especially those who have recurred after receiving adjuvant chemotherapy.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA21076, CA49883, CA17145, CA16116, CA39229, CA27525, CA14958 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
All authors report no financial disclosures.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 1(11):2169–74. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100(1):44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 5.Chia SK, Speers CH, D’Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–9. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 6.Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(30):4891–8. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 8.Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of HER-2-positive early breast cancer: a meta-analyses of published randomized trials. BMC Cancer. 2007;7(1):153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer. 2006;106(11):2337–44. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 10.Lin D, Sun W, Ying Z. Nonparametric estimation of the gap time distribution for serial events with censored data. Biometrika. 1999;86(1):59–70. [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(5):1413–24. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- 13.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–23. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 14.O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(12):2812–23. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2010;21(11):2169–74. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(1):92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2008;19(12):2012–9. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(7):1124–30. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 19.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SGt, Knuiman MW, Sleeper LA, et al. Six-year results of the Eastern Cooperative Oncology Group trial of observation versus CMFP versus CMFPT in postmenopausal patients with node-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7(7):879–89. doi: 10.1200/JCO.1989.7.7.879. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SGt, Kalish LA, Olson JE, et al. Adjuvant CMFP versus CMFP plus tamoxifen versus observation alone in postmenopausal, node-positive breast cancer patients: three-year results of an Eastern Cooperative Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1985;3(2):144–54. doi: 10.1200/JCO.1985.3.2.144. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SGt, Knuiman MW, Sleeper LA, et al. Six-year results of the Eastern Cooperative Oncology Group trial of observation versus CMFP versus CMFPT in postmenopausal patients with node-positive breast cancer. J Clin Oncol. 1989;7(7):879–89. doi: 10.1200/JCO.1989.7.7.879. [DOI] [PubMed] [Google Scholar]
- 23.Mansour EG, Gray R, Shatila AH, et al. Efficacy of adjuvant chemotherapy in high-risk node-negative breast cancer. An intergroup study N Engl J Med. 1989;320(8):485–90. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- 24.Mansour EG, Gray R, Shatila AH, et al. Survival advantage of adjuvant chemotherapy in high-risk node-negative breast cancer: ten-year analysis--an intergroup study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(11):3486–92. doi: 10.1200/JCO.1998.16.11.3486. [DOI] [PubMed] [Google Scholar]
- 25.Falkson HC, Gray R, Wolberg WH, et al. Adjuvant trial of 12 cycles of CMFPT followed by observation or continuous tamoxifen versus four cycles of CMFPT in postmenopausal women with breast cancer: an Eastern Cooperative Oncology Group phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990;8(4):599–607. doi: 10.1200/JCO.1990.8.4.599. [DOI] [PubMed] [Google Scholar]
- 26.Tormey DC, Gray R, Abeloff MD, et al. Adjuvant therapy with a doxorubicin regimen and long-term tamoxifen in premenopausal breast cancer patients: an Eastern Cooperative Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10(12):1848–56. doi: 10.1200/JCO.1992.10.12.1848. [DOI] [PubMed] [Google Scholar]
- 27.Olson JE, Neuberg D, Pandya KJ, et al. The role of radiotherapy in the management of operable locally advanced breast carcinoma: results of a randomized trial by the Eastern Cooperative Oncology Group. Cancer. 1997;79(6):1138–49. [PubMed] [Google Scholar]
- 28.Fetting JH, Gray R, Fairclough DL, et al. Sixteen-week multidrug regimen versus cyclophosphamide, doxorubicin, and fluorouracil as adjuvant therapy for node-positive, receptor-negative breast cancer: an Intergroup study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(7):2382–91. doi: 10.1200/JCO.1998.16.7.2382. [DOI] [PubMed] [Google Scholar]
- 29.Davidson NE, O’Neill AM, Vukov AM, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(25):5973–82. doi: 10.1200/JCO.2005.05.551. [DOI] [PubMed] [Google Scholar]
- 30.Tallman MS, Gray R, Robert NJ, et al. Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med. 2003;349(1):17–26. doi: 10.1056/NEJMoa030684. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein LJ, O’Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(25):4092–9. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabakuyo TS, Bonnetain F, Roignot P, et al. Population-based study of breast cancer survival in Cote d’Or (France): prognostic factors and relative survival. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2008;19(2):276–83. doi: 10.1093/annonc/mdm491. [DOI] [PubMed] [Google Scholar]