Abstract
The incidence and prevalence of diabetes mellitus are each increasing rapidly in societies around the globe. The majority of patients with diabetes succumb ultimately to heart disease, much of which stems from atherosclerotic disease and hypertension. However, the diabetic milieu is itself intrinsically noxious to the heart, and cardiomyopathy can develop independent of elevated blood pressure or coronary artery disease. This process, termed diabetic cardiomyopathy, is characterized by significant changes in the physiology, structure, and mechanical function of the heart. Presently, therapy for patients with diabetes focuses largely on glucose control, and attention to the heart commences with the onset of symptoms. When the latter develops, standard therapy for heart failure is applied. However, recent studies highlight that specific elements of the pathogenesis of diabetic heart disease are unique, raising the prospect of diabetes-specific therapeutic intervention. Here, we review recently unveiled insights into the pathogenesis of diabetic cardiomyopathy and associated metabolic remodeling with an eye toward identifying novel targets with therapeutic potential.
Keywords: diabetic cardiomyopathy, insulin resistance, heart, metabolism, FoxO, mTOR
Introduction
The incidence and prevalence of diabetes mellitus (DM) are each rising rapidly (Roger et al., 2012). DM affects 350 million people around the world, and the WHO has projected that diabetes deaths will double between 2005 and 2030 (http://www.who.int/diabetes/en/). Within this rapidly expanding public health epidemic of worldwide proportions, type 2 DM (T2DM) accounts for 90–95% of all diagnosed diabetes in adults ((Roger, Go, 2012). Patients with diabetes are at increased risk for developing coronary artery disease (CAD), hypertension, and heart failure (HF), and the majority of these patients succumb ultimately to heart disease. However, despite the importance of heart disease-promoting comorbidities, ventricular dysfunction and a clinical syndrome of HF can develop independent of underlying CAD, a condition termed “diabetic cardiomyopathy” (Rubler et al., 1972). Indeed, epidemiological studies demonstrate that obesity and diabetes are critical risk factors for cardiovascular disease, independent of blood pressure and coronary atherosclerosis (Grundy, 2012)). Diabetic cardiomyopathy and the other diabetes-associated cardiovascular risks are the leading cause of morbidity and mortality in these individuals.
T2DM is typified by hyperglycemia, hyperinsulinemia and obesity, and insulin resistance is a cardinal feature (Witteles and Fowler, 2008). At later stages of disease, some patients manifest insufficient insulin action. Together, these events, acting through a variety of mediators such as altered intracellular calcium, increased reactive oxygen species (ROS), ceramides, hexosamines, advanced glycation end products, and more, contribute to the pathogenesis of the disorder (Battiprolu et al., 2010). Additionally, the interplay between dysregulated function of endothelial cells and fibroblasts contributes, highlighting the multifactorial etiology of diabetic cardiomyopathy. Now, metabolic derangements within the cardiomyocyte itself are emerging as important elements in the pathogenesis of the disorder.
Cardiomyocytes are capable of metabolizing a spectrum of substrates. These “metabolic omnivores” normally rely on fatty acids and glucose, and to a lesser extent lactate and ketone bodies, to produce ATP (Hue and Taegtmeyer, 2009). These substrates, however, are unable to enter the cardiomyocyte by simple diffusion and must be taken up by facilitated transport. Fatty acid uptake is mediated by FAT (fatty acid translocase, also known as cluster of differentiation 36, CD36), and glucose intake is accomplished by GLUT4 (glucose transporter type 4). In response to availability of nutrients or increased cardiac work, plasma insulin concentrations rise (Schwenk et al., 2008). This, in turn, provokes GLUT4 as well as FAT/CD36 translocation to the myocyte sarcolemma. To date, many studies have implicated signaling pathways that regulate GLUT4 translocation with those involved in transport of FAT/CD36 to the sarcolemma of (Schwenk, Luiken, 2008, Steinbusch et al., 2011). However, during the development of insulin resistance and T2DM, FAT/CD36 becomes preferentially sarcolemma-localized, whereas GLUT4 is internalized. This reciprocal positioning of GLUT4 and FAT/CD36 is central to aberrant substrate uptake in the diabetic heart, where fatty acid metabolism is chronically increased at the expense of glucose (Schwenk, Luiken, 2008, Steinbusch, Schwenk, 2011). In addition, the interplay of preferential substrate utilization is impacted by a variety of other mediators, as previously reviewed (Battiprolu, Gillette, 2010).
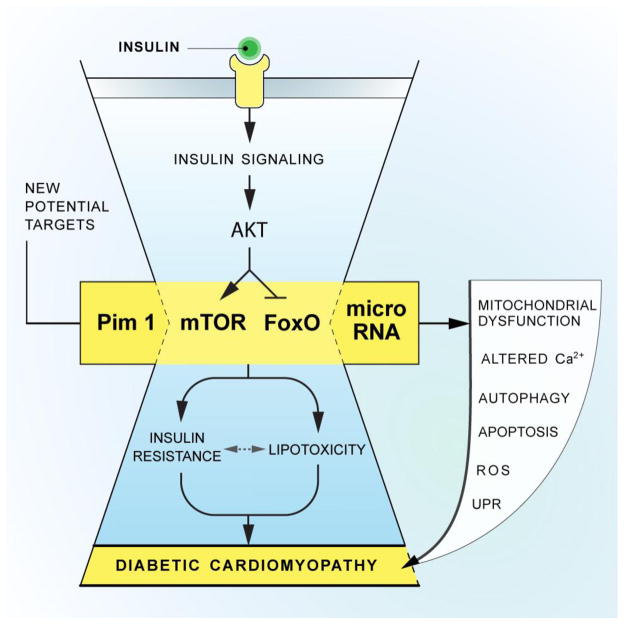
Dissection of the pathophysiology of diabetic cardiomyopathy and disease-related metabolic remodeling in the heart has progressed considerably in recent years. As a result, several novel mechanisms have emerged. Here, we highlight several of these molecular targets (graphical abstract, Figure 1), acknowledging that space limitations and the scope of this review do not allow us to discuss them all.
Figure 1. New molecular targets and their role in diabetic cardiomyopathy.
Schematic depiction of important elements of disease pathogenesis in diabetic cardiomyopathy, highlighting recently discovered molecular pathways.
Forkhead transcription factors
FoxO (Forkhead box-containing protein, O subfamily) proteins are emerging as important targets of insulin and other growth factor action in the myocardium (Ferdous et al., 2010, Ronnebaum and Patterson, 2010). Abundant evidence demonstrates that three members of the FoxO subfamily (FoxO1, −3, −4) are critical to maintenance of cardiac function and stress responsiveness (Ferdous, Battiprolu, 2010, Ronnebaum and Patterson, 2010). FoxO transcription factors regulate cardiac growth and govern insulin signaling and glucose metabolism in heart (Ni et al., 2006, Ni et al., 2007). Further, recent work has implicated chronic activation of FoxOs in the pathogenesis of diabetic cardiomyopathy (Battiprolu et al., 2012). Specifically, cardiomyocyte-specific inactivation of FoxO1 (FoxO1 KO) rescued high fat diet (HFD)-induced myocyte hypertrophy and associated declines in cardiac function while preserving cardiomyocyte insulin responsiveness (Battiprolu, Hojayev, 2012). FoxO1-depleted cardiomyocytes displayed a shift in their metabolic substrate usage from free fatty acids to glucose, and accumulation of myocardial lipids was reduced (Battiprolu, Hojayev, 2012). Furthermore, a direct causal link was demonstrated, where FoxO1-dependent down-regulation of insulin receptor substrate 1 (IRS1) resulted in blunted Akt signaling and insulin resistance. While these findings suggest that activation of FoxO1 is a significant mechanism underlying diabetic cardiomyopathy, in-depth understanding of specific molecular targets and the transcriptional interplay between FoxO1 and IRS1 will be required in order to move this biology toward the clinic.
On the other hand, excessive cardiac insulin signaling can also be detrimental to heart function. Shimizu et al studied cardiac insulin signaling in mice subjected to pressure overload. Both streptozotocin treatment and cardiac-specific IRS-1 knockdown were beneficial, suggesting that over-activation of cardiac insulin signaling can convey maladaptive actions (Shimizu et al., 2010).
FoxO has emerged recently as a major mechanism governing insulin signaling and glucose metabolism in a variety of tissues, including the liver (Cheng and White, 2012, Lu et al., 2012). Chronic activation of hepatic FoxO1 triggers dysregulated expression of a wide array of gluconeogenic genes, a process which contributes to systemic insulin resistance (Cheng and White, 2012, Lu, Wan, 2012). Interestingly, concomitant liver-specific deletion of both Akt1/2 and FoxO1 in mice restored appropriate adaptation to the fed and fasted states, as well as normal insulin action to suppress hepatic glucose production (Cheng and White, 2012, Lu, Wan, 2012). Accordingly, a major role of Akt is to inhibit FoxO1 activity which appears to be dispensable for other insulin actions. Moreover, silencing of hepatic FoxO1 largely normalized gluconeogenesis gene expression, lowered the concentration of circulating glucose, and diminished the basal rate of glucose production in insulin resistant, diabetic mice (Cheng and White, 2012, Dong et al., 2008, Lu, Wan, 2012). Thus, inhibition of FoxO1 activity might emerge as a promising strategy to ameliorate features of the metabolic syndrome, such as hyperglycemia, hyperinsulinemia, and insulin resistance (Cheng and White, 2012). However, the role of FoxO1 in hypertriglyceridemia and hepatic steatosis, which typically accompany insulin resistance and hyperglycemia, warrants further investigation (Cheng and White, 2012, Lu, Wan, 2012). It has also been reported that Notch1 signaling can act in a coordinated manner with FoxO1 to regulate hepatic glucose production, and pharmacological inhibition of the Notch1 cascade enhanced insulin sensitivity in diet-induced obese mice (Pajvani et al., 2011).
Mammalian target of rapamycin
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth and metabolism and is dysregulated in cancer and DM (Laplante and Sabatini, 2009, Zoncu et al., 2011). mTOR comprises two multiprotein complexes: mTORC1, which regulates pathways involved in mRNA translation and autophagy, and mTORC2, which regulates insulin signaling and other cellular processes. Insulin and insulin-like growth factors (IGFs) are major mTOR activators that signal through phosphoinositide-3-kinase (PI3K) and Akt (Hay and Sonenberg, 2004). Also, AMPK (adenosine monophosphate-activated protein kinase), which is activated upon energy depletion, calorie restriction, or genotoxic damage has been implicated in stress-responsive inhibition of mTOR (Hay and Sonenberg, 2004, Towler and Hardie, 2007). mTOR stimulates cell growth and anabolism by increasing protein and lipid synthesis through activation of S6K (p70 ribosomal protein S6 kinase), 4E-BP (eukaryotic translation initiation factor 4E-binding protein), and SREBP (sterol response element binding protein) (Hay and Sonenberg, 2004, Porstmann et al., 2008, Wullschleger et al., 2006) and by decreasing autophagic catabolism through inhibition of ATG1 (Wullschleger, Loewith, 2006). Persistent activation of mTOR has been implicated in diverse disorders, including cancer and obesity-related metabolic pathologies (Wullschleger, Loewith, 2006). Sestrins, another group of conserved stress-responsive proteins are increased by ROS accumulation, leading to activation of JNKs (c-Jun N-terminal kinase) and FoxOs (Lee et al., 2010). In contrast, loss of sestrins resulted in triglyceride accumulation, mitochondrial dysfunction, muscle degeneration and cardiac dysfunction, suggesting that these proteins function as negative feedback regulators of mTOR (Lee, Budanov, 2010).
mTOR, along with other kinases such as JNK, phosphorylate IRS-1 on serine residues, leading ultimately to IRS-1 degradation (Hiratani et al., 2005). Indeed, deletion of mTOR substrates such as S6K1 is sufficient to improve insulin sensitivity and to extend lifespan in mice (Selman et al., 2009). This suggests that rapamycin, an established inhibitor of mTORC1, might act in a similar manner. Conversely, recent work suggests that chronic treatment with rapamycin impaired, rather than improved, glucose homeostasis (Lamming et al. 2012). This effect was shown to be mediated by mTORC2 inhibition, provoking insulin resistance and impaired glucose homeostasis potentially by blocking insulin-responsive Akt (Hughes and Kennedy, 2012, Lamming et al., 2012). Thus, modulators of either mTOR (e.g. rapamycin) or the distinct branches of the mTOR signaling cascade and their downstream molecular targets (e.g. GRB10, growth factor receptor-bound protein 10) (Hsu et al., 2011, Yu et al., 2011), are of interest as potential points of therapeutic attack in diabetic heart disease.
MicroRNAs
MicroRNAs (miRNAs or miRs) are naturally occurring, small noncoding single-strand RNAs that regulate gene expression usually by targeting mRNAs for degradation or by repressing protein translation. In some cases, miRNAs up-regulate translation of some mRNAs, especially during cell cycle arrest or in terminally differentiated cells (Vasudevan et al., 2007). miRNAs have been identified as important molecular regulators participating in many biological functions. However, their actions are complex and nuanced, as they target a wide range of transcripts, often in a cluster of processes involved in given biological event (e.g. fibrosis, cell death). Further, miRNAs merely fine-tune, as opposed to frankly suppress, the actions of their target mRNAs.
Numerous miRNAs are altered in diabetes (Greco et al., 2012, Lu et al., 2010, Shantikumar et al., 2012, Shen et al., 2011). For example, miRNAs 103 and 107 (miR-103/107) are negative regulators of hepatic insulin sensitivity (Trajkovski et al., 2011), and both are up-regulated in obesity. Silencing miR-103/107 rescues insulin sensitivity in ob/ob and diet-induced obese mice by affecting adipocyte differentiation (Trajkovski, Hausser, 2011). One of targets for miR-103/107 is the gene encoding caveolin-1, the major protein of caveolae, the distinctive lipid- and cholesterol-enriched invaginations of the plasma membrane. Caveolin-1 stabilizes caveolae and their associated insulin receptors, promoting insulin signaling. By reducing caveolin-1 levels, miR-103/107 alters insulin receptor stability and activation (Trajkovski, Hausser, 2011). However, whereas both miRNAs are strongly expressed in cardiac and skeletal muscles (Finnerty et al., 2010), their potential role in insulin resistance in the heart remains unknown.
miR-223 is another miRNA which is consistently up-regulated in diabetes, including in cardiac tissue (Lu, Buchan, 2010). miR-223 expression increases basal glucose uptake in cardiomyocytes, and exposure to insulin does not lead to further increases (Lu, Buchan, 2010). This enhanced glucose uptake is caused by elevated expression and preferential plasma membrane translocation of GLUT4 (Lu, Buchan, 2010). Because plasma membrane-localized GLUT4 is markedly down-regulated in diabetic hearts (Cook et al., 2010), the increase in miR-223 expression in diabetic patients could be an adaptive response to restore glucose uptake.
A recent report demonstrated that the cardiac-specific miR-208a regulates systemic energy homeostasis by targeting MED13, a subunit of the mediator complex which controls transcription by nuclear hormone receptors, including the thyroid hormone receptor (Grueter et al., 2012). Pharmacological inhibition of miR-208a or cardiac over-expression of MED13 enhances metabolic rate, confers resistance to obesity, improves glucose homeostasis and lowers plasma lipid levels in mice (Grueter, van Rooij, 2012). Further research remains to be done to elucidate mechanisms whereby MED13 alters systemic metabolic rate.
Alterations in intracellular calcium handling and impaired SERCA2a (sarcoendoplasmic reticulum Ca2+-ATPase 2) activity are cardinal features of the failing heart. Indeed, SERCA2a gene therapy in failing hearts improves cardiac function and reduces arrhythmias in vivo (Lyon et al., 2011, Miyamoto et al., 2000). Interestingly, elevated cytoplasmic calcium concentrations in failing cardiomyocytes promote CaMKK (calcium/calmodulin-dependent protein kinase kinase)-dependent activation of Akt, which in turn inhibits FoxO3a activity, leading to down-regulation of miR-1, a FoxO3a target (Kumarswamy et al., 2012). NCX-1 (sodium-calcium exchanger 1) mRNA is one of the main targets of miR-1, and increases in NCX-1 levels may contribute to calcium mishandling in HF. SERCA2a gene therapy restored calcium levels in cardiomyocytes from failing hearts, normalizing Akt and FoxO3a activity and miR-1 and NCX-1 levels (Kumarswamy, Lyon, 2012).
Pim-1
In addition to altered calcium homeostasis, down-regulation of prosurvival signaling factors has also been implicated in diabetic cardiomyopathy (Katare et al., 2011). Pim-1 (proviral integration site for Moloney murine leukemia virus-1) is a serine/threonine protein kinase that modulates SERCA and promotes cardiomyocyte survival and function (Katare, Caporali, 2011, Muraski et al., 2007). Pim-1 is upregulated in failing hearts, potentially as an inefficient, last-ditch attempt to preserve cardiac function (Muraski et al., 2008). Interestingly, Pim-1 is down-regulated in the initial phase of diabetic cardiomyopathy and continues to decline as contractile dysfunction and HF progress (Katare, Caporali, 2011). Furthermore, Pim-1 is positively regulated by STAT3 (signal transducer and activator of transcritption 3) and Akt (Muraski, Rota, 2007), both of which are down-regulated in diabetic cardiomyopathy (Katare et al., 2010). Both STAT3 and Akt act as modulators of insulin and nutritional status in the heart (Shiojima et al., 2002). On the other hand, Pim-1 is inactivated by PP2A (protein phosphatase 2A) (Ma et al., 2007) and is a target of miR-1 (Nasser et al., 2008). It has been proposed that the increased levels of intracellular ceramide in diabteic myocardium may in part explain the upregulation of PP2A (Ma, Arnold, 2007) and hence its fate over Pim-1. Pim-1 is also implicated in promotion of cardiomyocyte survival via activation of Bcl2 (B-cell lymphoma-2), BAD (phosphorylation/inhibition of BCL-2 associated death promoter), and in the maintenance of mitochondrial integrity (Borillo et al., 2010, Muraski, Rota, 2007). Furthermore, Pim-1 increases the proliferative activity of cardiac progenitor cells by inducing c-Myc, nucleostemin, cyclin E expression and p21 phosphorylation ((Cottage et al., 2010, Tjwa and Dimmeler, 2008). Therefore, it is tempting to speculate that the accrual of alterations in upstream Pim-1 activators (Katare, Caporali, 2010) and the confounding up-regulation of Pim-1 inhibitors, such as PP2A and miR-1, contributes to the unique features observed in hearts of diabetic mice compared with other ischemic and pressure-overload models. Additionally, as noted above, some work suggests that cardiac-specific Pim1 gene therapy attenuates the progression of diabetic cardiomyopathy (Katare, Caporali, 2011), raising yet further the prospects of targeting this interesting molecule.
It is important to point out that studies of Pim-1 have been performed in streptozotocin-treated animals, a model used to mimic the later stage of T2DM characterized by insufficient insulin action. Studies to investigate the relevance of Pim-1 in earlier stages of T2DM pathogenesis, as well as metabolic syndrome, are warranted
Mitochondrial dysfunction
Mitochondrial dysfunction contributes to progression of diabetes and diabetic cardiomyopathy (Duncan, 2011). The transcription factor p53 is induced by ischemia, chronic pressure overload, or metabolic disturbances. A recent report suggests that p53 contributes to cardiac dysfunction in diabetes by promoting mitochondrial oxygen consumption, ROS production, and lipid accumulation (Nakamura et al., 2012). The SCO2 (synthesis of cytochrome c oxidase 2) gene is a transcriptional target of p53, and its protein product plays a key role in the assembly of mitochondrial respiration complex IV, the center of oxygen consumption. Nakamura et al reported a marked increase in cardiac SCO2 expression in diabetic mice that contributed to increases in mitochondrial respiration rate. This elevated mitochondrial activity triggers enhanced lipid uptake which exceeds mitochondrial oxidation capacity, leading to lipid accumulation and increased mitochondrial ROS production, together culminating in cardiac dysfunction.
Unfolded protein response
Accumulating evidence points to disruption of endoplasmic reticulum (ER) homeostasis in diabetic cardiomyopathy (Ceylan-Isik et al., 2011, Li et al., 2010, Miki et al., 2009, Wu et al., 2011). The ER is the central organelle for secretory/transmembrane protein folding, calcium storage, and lipid synthesis. Elevated demand for synthesis of new proteins and lipids poses a special burden on the ER. When the throughput of proteins being processed in the ER exceeds folding capacity, “ER stress” ensues and the so-called unfolded protein response (UPR) is activated (Back and Kaufman, 2012, Walter and Ron, 2011). To ameliorate ER stress, the UPR activates three pathways that antagonize the cellular stress (Harding et al., 2002). PERK, a transmembrane protein kinase in the ER phosphorylates eIF2α, which in turn promotes chaperone synthesis and transiently suppresses other protein synthesis. ATF6 undergoes proteolytic cleavage triggered by the UPR. The processed ATF6 functions as a transcription factor for a host of ER chaperone proteins to enhance ER protein folding capacity. IRE1 manifests nuclease activity when phosphorylated during ER stress, and splices a downstream target mRNA, Xbp1. The resulting Xbp1s drives expression of ER chaperones and molecules involved in the ER-associated protein degradation pathway. Thus, all three branches of the UPR (PERK, ATF6, IRE1-Xbp1) act coordinately to relieve ER stress and restore protein processing homeostasis. When ER stress is persistent, however, excessive and unremitting activation of the UPR becomes maladaptive and may activate apoptotic pathways for cell destruction (Tabas and Ron, 2011).
Mounting evidence suggests that the UPR plays critical roles in the heart during diabetic cardiomyopathy. Using a genetic model of T2DM, Miki et al found markers of ER stress were induced in OLETF rats compared to control LETO rats (Miki, Miura, 2009). Similarly, it has been reported that the UPR is triggered in hearts of ob/ob mice, including up-regulation of BIP (binding immunoglobulin protein, also known as GRP-78, 78 kDa glucose-regulated protein), and eIF2α and PERK phosphorylation (Ceylan-Isik, Sreejayan, 2011). Moreover, induction of the UPR is evident in the myocardium in streptozotocin-induced type 1 DM (Li, Zhu, 2010). Consistently, ER stress is seen in cardiomyocytes maintained in culture medium containing high glucose (28 mM) (Younce et al., 2010).
Whereas induction of the UPR has been observed both in vitro and in vivo, the association between ER stress and diabetic cardiomyopathy remains correlative, and clear mechanistic links are lacking. Moreover, although the three branches of the UPR share similar targets, their temporal induction patterns differ and their specific functions are distinct, conferring additional complexity to the putative role(s) of ER stress in diabetic cardiomyopathy (Lin et al., 2007). During ER stress, PERK is activated to terminate protein synthesis and reduce the burden of ER cargo, providing a window for rejuvenated protein folding. Subsequently, IRE1-Xbp1 and ATF6 are stimulated to increase ER chaperone production and enhance folding capacity. Some evidence points to the IRE1-Xbp1 and ATF6 pathways as pro-survival and adaptive in diabetes, cancer, and cardiomyopathy (Doroudgar et al., 2009, Lin, Li, 2007, Thuerauf et al., 2006).
Several groups have tested drugs and chemical chaperones to manipulate ER stress in diabetic cardiomyopathy. Studies using valsartan (Wu, Dong, 2011), tauroursodeoxycholic acid (Ceylan-Isik, Sreejayan, 2011, Miki, Miura, 2009), and apocynin (Li, Zhu, 2010) all reported improved heart function in the setting of diabetic stress. Consistently, activation of ER stress markers was decreased, assessed mainly as GRP78 levels and PERK phosphorylation. These studies, although well designed and carefully conducted, only demonstrate correlation between ER stress and diabetic cardiomyopathy and fall short of providing a clear picture of the degree and extent of cellular ER stress activation. To address these questions, gain- and loss-of function of individual ER stress elements, ideally at selected temporal windows of disease progression, are required.
Adipokines
Adipose tissue expansion is, of course, a hallmark of obesity. Over the past 20 years, our view of adipose tissue has been revolutionized from previously being viewed as an inert energy storage tissue now to clear recognition of its role as the largest endocrine organ in the body (Scherer, 2006). Beside fatty acids, adipocytes synthesize and secrete a host of proteins, including adiponectin, leptin, resistin, tumor necrosis factor alpha TNFα, interleukin-6 (IL-6), and many more, collectively named adipokines (Deng and Scherer, 2010). In a wide array of circumstances, adipose tissue communicates with multiple organs and tissues throughout the body through these adipokines.
Adiponectin, the only adipokine manifesting reverse correlation with adipose tissue mass, has attracted tremendous attention during the past two decades. Adiponectin is specifically synthesized in adipocytes, folded in the ER, and secreted as a range of complexes, including a trimer, hexamer, and high molecular weight form (18-mer). Circulating levels of adiponectin as well as the distribution of its various protein complexes, are altered in obesity and diabetes (Wang and Scherer, 2008). Low levels of adiponectin are a marker of cardiovascular disease, and some evidence suggests that genetic and pharmacological approaches to increase adiponectin levels may have efficacy in modulating obesity-associated cardiac disease (Hui et al., 2012).
Adiponectin antagonizes diabetic cardiomyopathy by various mechanisms. For one, adiponectin manifests potent anti-hypertrophic activity in cardiomyocytes. For example, Shibata et al found that adiponectin inhibits hypertrophic growth of the heart in the setting of pressure overload, events which are mediated by AMPK activation (Shibata et al., 2004). Adiponectin can reverse endothelial dysfunction by increasing endothelial nitric oxide synthase and nitric oxide production, leading to improved blood pressure control (Wang and Scherer, 2008). Moreover, adiponectin blunts cardiac cell death via apoptosis by inhibiting ceramide production and inflammation (Holland et al., 2011). Administration of adiponectin to adult cardiomyocytes isolated from db/db animals rescues over-activation of IRS1 and c-Jun in association with increased intracellular calcium and enhanced calcium decay (Dong and Ren, 2009). In summary, circulating adiponectin manifests cardioprotective effects by blunting cardiac and endothelial cell death, inhibiting inflammation, and suppressing hypertrophy.
In contrast, resistin, another adipokine synthesized by and secreted from adipose tissue (Rajala et al., 2002), may participate in declines in cardiac function in diabetic cardiomyopathy. Similar to adiponectin, resistin circulates as multiple protein complexes. Clinical studies have shown that serum levels of resistin are positively correlated with insulin resistance, obesity, T2DM and cardiovascular disease (DeClercq et al., 2008, Nogueiras et al., 2010).
Recent studies suggest resistin directly affects heart function in rodents. Resistin is expressed in heart at both mRNA and protein levels. Cardiomyocyte resistin levels are higher in the setting of diabetes (Kim et al., 2008). Cardiomyocyte over-expression of resistin by means of adeno-associated virus serotype 9-mediated gene delivery is associated with pronounced cardiac remodeling, including increased fibrosis and hypertrophy, reduced cardiac performance, and elevated markers of inflammation (Chemaly et al., 2011). The same group reported that resistin over-expression in cultured cardiomyocytes in vitro significantly increased cellular hypertrophy, activated JNK, and provoked serine phosphorylation (and desensitization) of IRS1, which together correlated with insulin resistance under high resistin conditions (Kang et al., 2011, Kim, Oh, 2008).
Metabolic role of autophagy
Autophagy is an evolutionarily ancient process of intracellular protein and organelle recycling (Yang and Klionsky, 2010). Faced with nutrient deprivation, most cells manifest a complex autophagic response that initiates with the formation of an intracellular membrane organelle that engulfs cytoplasmic material forming an autophagosome. After fusion with a lysosome, the intra-autophagosomal cargo is digested and the resulting degradation products are released to provide nutrients and cellular building blocks for sustenance of energy, cellular integrity and function. Autophagy is also critical for clearing defective organelles and degrading long-lived proteins. Autophagy has been implicated in numerous biological processes and diseases, including development, starvation, obesity, diabetes, infectious disease, cancer and cardiovascular disease (Mizushima et al., 2008, Wang et al., 2010). Some evidence suggests that autophagy is altered in diabetic cardiomyopathy. H9c2 cells maintained in high glucose-containing culture medium (28 mM) manifest increases in autophagy (Younce, Wang, 2010). Mice fed a high fructose diet manifest insulin resistance, cardiac remodeling, and elevated levels of cardiomyocyte autophagy (Mellor et al., 2011). However, another report noted reduced autophagy in diabetic heart of OVE26 mice (Xie et al., 2011). Importantly, in all these studies, autophagy was assessed as steady state levels of LC3 measured by immunoblot. Autophagy, however, is a highly dynamic process of flux, with constant generation and processing of autophagosomes (Wang, Rothermel, 2010), and a snapshot in time of LC3 abundance is insufficient to distinguish between enhanced autophagy initiation versus defective processing downstream in the autophagic cascade. This important point may explain some of the discrepancies reported in the literature.
Due to the complexity of autophagy, its role in diabetic cardiomyopathy has yet to be elucidated. It has been reported that metformin-induced AMPK activation in diabetic mice is associated with increased autophagy and preservation of cardiac function (Xie, Lau, 2011). Without more detailed analysis, however, this correlation cannot be used to attribute the apparent cardioprotective effects of metformin solely to autophagic changes. Likewise, correlations between autophagic activation and cardiac impairment with fructose feeding in rodents fall short of demonstrating a compelling mechanistic link (Mellor, Bell, 2011). In conclusion, cardiomyocyte autophagic responses are altered in diabetic cardiomyopathy, yet further work is required to ferret out potential causal roles.
Conclusions and perspective
HF has remained the leading cause of death in industrialized nations for some years. Numerous events contribute to the rise in HF, but the increasing prevalence of DM is an important contributor. Derangements in insulin signaling have widespread and devastating effects in numerous tissues, including the cardiovascular system. The multiple, interlacing events occurring in patients with diabetes culminate in an environment which, coupled with insulin resistance, leads to diabetic cardiomyopathy. In recent years, novel insights into mechanisms that increase vulnerability of the diabetic heart to failure have emerged. Despite these recent efforts, our understanding of diabetic cardiomyopathy – a disease which is at once intricate and clinically significant – remains rudimentary.
Constant and unremitting metabolic stress on the heart leads over time to progressive deterioration of myocardial structure and function. This suggests that therapeutic interventions early in the disease, targeting specific metabolic and structural derangements, may be required. This is especially relevant as rigid control of hyperglycemia, however central to treatment, has not fulfilled hopes of meaningful morbidity and mortality benefit (Gerstein et al., 2011). Recent and ongoing research into mechanisms of metabolic control, insulin resistance, and diabetes-associated derangements portend novel therapies designed to benefit the rapidly expanding cohort of patients with diabetes. Continued efforts to identify effective preventive strategies and treatments are essential. At the same time, there remains a growing need to identify therapies that slow, arrest, or even reverse disease progression, and ongoing research efforts suggest that such may emerge with time.
Acknowledgments
This work was supported by grants from the NIH (HL-075173, JAH; HL-080144, JAH; HL-090842, JAH), AHA (0640084N, JAH; 12POST9030041, PKB), ADA mentor-based postdoctoral fellowship (7-08-MN-21-ADA, JAH and PKB), the AHA-Jon Holden DeHaan Foundation (0970518N, JAH), and the Fondo Nacional de Desarrollo Cientifico y Tecnologico: FONDECYT 1120212 and FONDAP 15010006 (SL). C.L.C is a recipient of a CONICYT fellowship, Chile.
Footnotes
Conflict of interest
The authors have declared that no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Kaufman RJ. Endoplasmic Reticulum Stress and Type 2 Diabetes. Annu Rev Biochem. 2012 doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic Cardiomyopathy: Mechanisms and Therapeutic Targets. Drug discovery today Disease mechanisms. 2010;7:e135–e43. doi: 10.1016/j.ddmec.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. The Journal of clinical investigation. 2012;122:1109–18. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borillo GA, Mason M, Quijada P, Volkers M, Cottage C, McGregor M, et al. Pim-1 kinase protects mitochondrial integrity in cardiomyocytes. Circ Res. 2010;106:1265–74. doi: 10.1161/CIRCRESAHA.109.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol. 2011;50:107–16. doi: 10.1016/j.yjmcc.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chemaly ER, Hadri L, Zhang S, Kim M, Kohlbrenner E, Sheng J, et al. Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J Mol Cell Cardiol. 2011;51:144–55. doi: 10.1016/j.yjmcc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, White MF. The AKTion in non-canonical insulin signaling. Nat Med. 2012;18:351–3. doi: 10.1038/nm.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClercq V, Taylor C, Zahradka P. Adipose tissue: the link between obesity and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets. 2008;8:228–37. doi: 10.2174/187152908785849080. [DOI] [PubMed] [Google Scholar]
- Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Ren J. Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 2009;17:262–8. doi: 10.1038/oby.2008.545. [DOI] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–45. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta. 2011;1813:1351–9. doi: 10.1016/j.bbamcr.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous A, Battiprolu PK, Ni YG, Rothermel BA, Hill JA. FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res. 2010;3:355–64. doi: 10.1007/s12265-010-9200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. Journal of molecular biology. 2010;402:491–509. doi: 10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. The New England journal of medicine. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S, Fasanaro P, Castelvecchio S, D’Alessandra Y, Arcelli D, Di Donato M, et al. MicroRNA Dysregulation in Diabetic Ischemic Heart Failure Patients. Diabetes. 2012;61:1633–41. doi: 10.2337/db11-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter CE, van Rooij E, Johnson BA, Deleon SM, Sutherland LB, Qi X, et al. A Cardiac MicroRNA Governs Systemic Energy Homeostasis by Regulation of MED13. Cell. 2012;149:671–83. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–99. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, Kobayashi M. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun. 2005;335:836–42. doi: 10.1016/j.bbrc.2005.07.152. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–22. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–91. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KJ, Kennedy BK. Cell biology. Rapamycin paradox resolved. Science. 2012;335:1578–9. doi: 10.1126/science.1221365. [DOI] [PubMed] [Google Scholar]
- Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–90. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011;286:18465–73. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare R, Caporali A, Zentilin L, Avolio E, Sala-Newby G, Oikawa A, et al. Intravenous gene therapy with PIM-1 via a cardiotropic viral vector halts the progression of diabetic cardiomyopathy through promotion of prosurvival signaling. Circ Res. 2011;108:1238–51. doi: 10.1161/CIRCRESAHA.110.239111. [DOI] [PubMed] [Google Scholar]
- Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ Heart Fail. 2010;3:294–305. doi: 10.1161/CIRCHEARTFAILURE.109.903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–80. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Lyon AR, Volkmann I, Mills AM, Bretthauer J, Pahuja A, et al. SERCA2a gene therapy restores microRNA-1 expression in heart failure via an Akt/FoxO3A-dependent pathway. Eur Heart J. 2012;33:1067–75. doi: 10.1093/eurheartj/ehs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–8. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of Rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress and reduces myocardial remodeling in type-I diabetic mice. Diabetes. 2010 doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res. 2010;86:410–20. doi: 10.1093/cvr/cvq010. [DOI] [PubMed] [Google Scholar]
- Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–95. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–72. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Arnold HK, Lilly MB, Sears RC, Kraft AS. Negative regulation of Pim-1 protein kinase levels by the B56beta subunit of PP2A. Oncogene. 2007;26:5145–53. doi: 10.1038/sj.onc.1210323. [DOI] [PubMed] [Google Scholar]
- Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol. 2011;50:1035–43. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, et al. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes. 2009;58:2863–72. doi: 10.2337/db09-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–8. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, et al. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci U S A. 2008;105:13889–94. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–75. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Matoba S, Iwai-Kanai E, Kimata M, Hoshino A, Nakaoka M, et al. p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail. 2012;5:106–15. doi: 10.1161/CIRCHEARTFAILURE.111.961565. [DOI] [PubMed] [Google Scholar]
- Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, et al. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem. 2008;283:33394–405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–68. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20517–22. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Novelle MG, Vazquez MJ, Lopez M, Dieguez C. Resistin: regulation of food intake, glucose homeostasis and lipid metabolism. Endocr Dev. 2010;17:175–84. doi: 10.1159/000262538. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011;17:961–7. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008;8:224–36. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala MW, Lin Y, Ranalletta M, Yang XM, Qian H, Gingerich R, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16:1920–30. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annual review of physiology. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–45. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–58. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–93. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E, Diao X, Wang X, Chen R, Hu B. MicroRNAs involved in the mitogen-activated protein kinase cascades pathway during glucose-induced cardiomyocyte hypertrophy. Am J Pathol. 2011;179:639–50. doi: 10.1016/j.ajpath.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. The Journal of clinical investigation. 2010;120:1506–14. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–7. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- Steinbusch LK, Schwenk RW, Ouwens DM, Diamant M, Glatz JF, Luiken JJ. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol Life Sci. 2011;68:2525–38. doi: 10.1007/s00018-011-0690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–82. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- Tjwa M, Dimmeler S. A nucleolar weapon in our fight for regenerating adult hearts: nucleostemin and cardiac stem cells. Circ Res. 2008;103:4–6. doi: 10.1161/CIRCRESAHA.108.179994. [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–53. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–4. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. The Journal of biological chemistry. 2010;285:8509–14. doi: 10.1074/jbc.R109.025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Wu T, Dong Z, Geng J, Sun Y, Liu G, Kang W, et al. Valsartan protects against ER stress-induced myocardial apoptosis via CHOP/Puma signaling pathway in streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2011;42:496–502. doi: 10.1016/j.ejps.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–8. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nature cell biology. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87:665–74. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–6. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]