Abstract
GABAergic interneurons of the cerebral cortex (cINs) play crucial roles in many aspects of cortical function. The diverse types of cINs are classified into subgroups according to their morphology, intrinsic physiology, neurochemical markers and synaptic targeting. Recent advances in mouse genetics, imaging and electrophysiology techniques have greatly advanced our efforts to understand the role of normal cIN function and its dysfunction in neuropsychiatric disorders. In schizophrenia (SCZ), a wealth of data suggests that cIN function is perturbed, and that interneuron dysfunction may underlie key symptoms of the disease. In this review, we discuss the link between cINs and SCZ, focusing on the evidence for GABAergic signaling deficits from both SCZ patients and mouse models.
Keywords: schizophrenia, interneurons, cortex, inhibition, GABA, NMDA, gamma oscillations, parvalbumin, somatostatin, neuregulin
Introduction
Schizophrenia (SCZ) is a heterogeneous, heritable, neuropsychiatric disorder that affects about 1% of the population worldwide. It is characterized by positive symptoms (hallucinations and delusions), negative symptoms (social withdrawal, avolition and anhedonia) and cognitive symptoms (impairments in working memory, basic sensory processing and higher order cognitive functions). Although the positive and negative symptoms are often the most striking features of the disease, the cognitive defects lie at the core of the disorder and may precede the development of other symptoms (Walker et al., 1994;Green, 2006;Keefe and Fenton, 2007;Reichenberg et al., 2010;Lesh et al., 2011).
One of the most prominent cognitive deficits observed in SCZ involves working memory, an aspect of short-term memory by which information is consciously maintained during the execution of other cognitive tasks. An important aspect of working memory is the sustained firing of dorsolateral prefrontal cortex (DLPFC) pyramidal neurons between cue presentation and behavioral response (Funahashi et al., 1993). During this period, GABAergic transmission is required for working memory, and infusion of GABA antagonists into the DLPFC perturbs working memory by disrupting the spatial tuning of these cells (Sawaguchi et al., 1989;Rao et al., 2000).
Another cognitive deficit in SCZ patients is their inability to filter irrelevant sensory information (Javitt, 2009). A neurophysiological correlate of this deficit is evaluated by a pre-attentive paradigm called paired pulse inhibition (PPI). In PPI, subjects are presented a startling (test) stimulus with or without a preceding non-startling (conditioning) stimulus. In control subjects, the response to the test stimulus is suppressed by the conditioned stimulus, but this suppression is reduced in SCZ subjects (Freedman et al., 1996). Investigation of the neurophysiological correlates of PPI identified a crucial role for GABAergic transmission through presynaptic GABAB receptors on glutamatergic terminals (Freedman et al., 2000). Although deficits in neither PPI nor working memory are unique to SCZ (Daskalakis et al., 2007), a detailed understanding of the physiological basis for these cognitive functions would likely advance efforts to improve therapies for SCZ.
Cognitive functions depend on the precise balance of excitatory and inhibitory (E-I) activity of cortical circuits. Any alteration in the E-I balance could perturb brain function unless there are compensatory mechanisms to counteract this change. Much of the data presented in this review supports the hypothesis that this E-I balance is altered in SCZ. For example, a recent study used optogenetics to manipulate cortical circuits and found that elevated excitation, but not elevated inhibition, in neurons of the prefrontal cortex (PFC), leads to impaired cognition and social behavior (Yizhar et al., 2011). Additionally, increasing inhibition during elevated excitation, which likely restores the normal E-I balance, reduced these behavioral deficits. Although it was not possible to directly test disinhibition in this study, it is likely that deficits in GABAergic transmission tip the E-I balance in the cortex. A gradual increase in the E-I imbalance may occur in SCZ, which could eventually increase the vulnerability of cortical circuits due to insufficiency of compensatory mechanisms. Such a scenario is also consistent with the progressive worsening of SCZ symptoms.
Cortical GABAergic neurotransmission plays a central role in controlling cognitive processes that are disrupted in SCZ. In this review, we discuss the recent evidence that links perturbations in GABAergic cortical interneuron function to SCZ. We will summarize findings detailing the deficits in neurochemical and synaptic markers in cINs from postmortem SCZ tissue. Additionally, we will focus on several models that shed insight into how cIN dysfunction could underlie many of the cognitive symptoms associated with SCZ.
GABAergic interneurons of the cortex
In the cerebral cortex, the majority of GABAergic neurons are local circuit neurons and referred to as cortical interneurons (cINs). cINs are classified into subgroups and subtypes according to their morphology (e.g. chandelier, martinotti, neurogliaform), intrinsic physiology (e.g. fast-spiking, low threshold spiking), neurochemical markers and their targeting of specific subcellular compartments (Ascoli et al., 2008). Different forms of inhibition (tonic, feed-forward, feedback and shunting) by distinct subtypes of cINs modulate cortical excitability and neuronal plasticity (Hensch, 2005;Daskalakis et al., 2007).
Most cortical interneurons can be divided into three distinct subgroups based on several neurochemical markers: parvalbumin- (PV+), somatostatin- (Sst+), or calretinin-expressing (CR+) interneurons, although a small percentage of cINs express both CR and Sst. PV+ cINs are fast-spiking (FS) and comprise two major subtypes: basket and chandelier cells. Basket cells can be divided into several subgroups morphologically while chandelier cells (ChCs) appear to be a homogeneous group (Markram et al., 2004;Ascoli et al., 2008;Woodruff et al., 2011). Basket cells primarily innervate the somata and proximal dendrites of pyramidal neurons, as well as other interneurons, whereas chandelier cells selectively target the axon initial segments of pyramidal neurons (AIS). Thus, both basket and chandelier cells synapse near the site of action potential initiation. Due to their rapid and non-accommodating spiking properties, PV+ cINs are potent regulators of cortical circuits and could play important roles in neurological diseases (Keefe and Fenton, 2007;Uhlhaas et al., 2009;Woodruff et al., 2009;Uhlhaas and Singer, 2010;Woodruff et al., 2011).
The effect of GABAergic transmission depends on the postsynaptic chloride equilibrium potential (ECl−), since GABA receptors are permeable to Cl− and their reversal potential (EGABA) is close to ECl−. If EGABA is more negative than the resting membrane potential (Vrest) then it has a hyperpolarizing (inhibitory) effect. If EGABA is higher than Vrest then it has a depolarizing (excitatory) effect. During development, intracellular Cl− is high due to relatively high levels of NKCC1 and low levels of KCC2, co-transporters that uptake and extrude Cl−, respectively. Relative Cl− concentration and EGABA are also different in distinct subcellular neuronal compartments, with lower Cl− levels in dendrites and higher Cl− levels in AIS (Khirug et al., 2008). Hence, although basket cell synapses are always inhibitory, the cortical axo-axonic synapses of ChCs can be depolarizing (Szabadics et al., 2006;Khirug et al., 2008;Woodruff et al., 2009). On the other hand, examination of ChC influence on pyramidal neuron excitability, under conditions designed to mimic pyramidal neuron activity in vivo, revealed an inhibitory effect (Woodruff et al., 2011). In addition, GABAergic transmission at axo-axonic synapses of the hippocampus was reported to be hyperpolarizing (Glickfeld et al., 2009).
Cortical interneurons and brain oscillations in SCZ
There is extensive literature on the role of GABAergic transmission in synchronization of network oscillations through electrical and synaptic coupling of cINs (Beierlein et al., 2000;Tamas et al., 2000;Szabadics et al., 2001;Uhlhaas and Singer, 2010;Moran and Hong, 2011). This rhythmic inhibition synchronizes cortical circuits by generating a narrow window for effective excitation. For example, Sst+ Martinotti cells were reported to fire rhythmically at theta frequency (Fanselow et al., 2008) and neurogliaform cells were suggested to have the potential to shape cortical oscillations due to their extensive electrical coupling even with different subtypes of cINs (Simon et al., 2005). PV+ cINs can entrain gamma frequency oscillations via the combinatorial action of synaptic and electrical coupling (Tamas et al., 2000;Bartos et al., 2002), and loss of PV+ cells in the PFC correlates with reduced gamma-band response in a mouse model of SCZ (Lodge et al., 2009). Additionally, recent in vivo studies demonstrated that PV+ cIN driven gamma oscillations enhance responsiveness to sensory input (Cardin et al., 2009;Sohal et al., 2009).
Cortical EEG synchronization in response to a visual gestalt task increases dramatically during late adolescence (Uhlhaas et al., 2009), the time period when SCZ symptoms often manifest. Although EEG abnormalities have been reported in SCZ at all frequencies (Sponheim et al., 1994;Spencer et al., 2003;Spencer et al., 2004;Cho et al., 2006;Boutros et al., 2008;Siekmeier and Stufflebeam, 2010;Moran and Hong, 2011), gamma frequency oscillations have attracted the most attention due to their association with cognitive functions that are commonly disrupted in SCZ, such as working memory and attention (Lisman and Idiart, 1995;Jefferys et al., 1996;Steriade et al., 1996;Howard et al., 2003;Tallon-Baudry et al., 2005;Spencer, 2008). These findings, together with the role of PV+ cINs in gamma oscillations and abnormalities observed at PV+ cIN synapses (see below), led to the hypothesis that cognitive dysfunction in SCZ may arise from abnormal cortical gamma oscillations due to deficiencies at PV+ cIN synapses (Uhlhaas and Singer, 2010;Nakazawa et al., 2011;Lewis et al., 2012).
So how does disinhibition affect gamma oscillations and cortical activity? One might expect reduced cIN activity to produce enhanced pyramidal neuron activity. Consistent with this idea, there is a robust enhancement of cerebral blood flow in the ventral hippocampus of SCZ patients, where a reduction of both PV+ and Sst+ cINs may be present (Konradi et al., 2011). This increased blood flow is thought to be secondary to enhanced pyramidal neuron activity (Schobel et al., 2009). Moreover, activity correlates with the degree of psychosis experienced, corroborating the importance of E-I balance for proper brain activity. SCZ has also been associated with ”hypfrontality” during tasks requiring frontal activation (Glahn et al., 2005), albeit in the context of enhanced gamma power at baseline (pre-stimulus) conditions. It is conceivable that reduced activity of cINs could result in some basal increase in pyramidal neuron activity leading to an enhanced gamma power at baseline. However, the cIN deficit becomes functionally relevant when phase synchrony of gamma oscillations fail to occur normally during tasks that strongly engage the frontal cortex, resulting in reduced gamma stimulus-to-baseline ratios in SCZ (Gandal et al., 2012).
Deficits of cIN neurochemical subgroup markers in SCZ
Numerous studies have examined changes in neurochemical cIN markers and overall cIN numbers in postmortem SCZ brains, but there is often conflicting results. For example, despite the consensus that GAD67 mRNA levels are generally decreased in schizophrenic cortex, there is evidence both for (Todtenkopf et al., 2005;Konradi et al., 2011) and against (Akbarian et al., 1995;Selemon et al., 1995;Woo et al., 1997;Thune et al., 2001;Hashimoto et al., 2003) a reduction of cIN numbers. It is likely that methodological differences (i.e. marker used, controls for postmortem status, cortical region evaluated) underlie these conflicting data. Additionally, the tremendous genetic and diagnostic heterogeneity in SCZ minimizes the power to identify real differences in the context of relatively small sample sizes (generally less than 20 brains per group). Critical evaluation of the literature remains key to separating well-designed studies (preferably N=10 or more brains for SCZ and control groups matched for age, sex, and post-mortem interval or brain pH; Table 1) from less complete studies. For example, one study reported an increase in PV+ interneurons in the cingulate cortex of SCZ brains (Kalus et al., 1997), which is in contrast to most of the literature. In this study, the authors analyzed N=5 SCZ and control brains, with a post-mortem interval averaging around 40 and 20 hours for the SCZ individuals and controls, respectively. Such dramatic differences between groups can certainly introduce significant artifacts into the results.
Table 1.
Summary of the molecular deficits in cINs found in SCZ patients and the phenotypes observed. Only postmortem studies that included ≥ 10 SCZ and age-matched control brains are included in the table. All postmortem studies controlled for postmortem interval, brain pH or both between SCZ and control subjects. All measurements of mRNA and/or protein were taken from the PFC of postmortem brains (specifically Brodmann’s areas 9, 10 and 46) unless indicated below.
Konradi et al., 2011 – only examined the hippocampus.
Hashimoto et al., 2008 – also examined anterior cingulated, M1 and V1.
Guidotti et al., 2000 – also examined cerebellum.
Thompson et al., 2009 – examined PFC and other cortical areas, as well as striatum and thalamus. Chandelier cell, ChC; pyramidal cell, PC; basket cell, BC.
In terms of neurochemically-defined interneuron subgroups, Sst mRNA is reduced in the DLPFC of SCZ patients, with some data supporting both a reduction in the total number of Sst+ cINs and Sst levels within individual cINs (Hashimoto et al., 2008a;Hashimoto et al., 2008b;Morris et al., 2008). Concurrently, there is a decrease in the expression of the Sst receptor subtype 2 (SSTR2) in pyramidal cells of SCZ patients (Beneyto et al., 2012). The specific Sst+ subtype that is affected, as well as a functional consequence for this decrease in Sst signaling, remains unclear.
Regarding the PV+ interneurons, there is mixed evidence regarding reductions of PV levels in SCZ patients. While some data supported a decrease in PV+ cells in SCZ patients (Beasley and Reynolds, 1997), others found similar densities of PV+ neurons in the DLPFC of SCZ and control patients (Woo et al., 1997;Beasley et al., 2002). At the same time, PV mRNA levels are diminished in the DLPFC of SCZ patients (Hashimoto et al., 2003;Hashimoto et al., 2008b;Fung et al., 2010). As PV is an activity-regulated gene (Philpot et al., 1997;Patz et al., 2004), an intracellular reduction in PV could indicate a decrease in cIN activity and a potential shift in the E/I balance.
In contrast to the PV+ and Sst+ subgroups, the levels of CR mRNA and the density of CR+ neurons appears to be normal in SCZ tissue (Daviss and Lewis, 1995;Woo et al., 1997;Hashimoto et al., 2003). As most CR+ cINs have a distinct embryonic origin from the Sst+ and PV+ subgroups in rodents (Xu et al., 2004) and probably in humans (Fertuzinhos et al., 2009), the above results suggest that cINs derived from the medial ganglionic eminence may be most likely to be altered in SCZ. Given the evidence that ventral hippocampal overactivation may drive psychotic symptoms in SCZ (Schobel et al., 2009), it would be interesting to see if other groups can replicate the recent finding of a reduction in PV+ and Sst+ cINs in the hippocampus (Konradi et al., 2011).
Synaptic deficits of cINs in SCZ
Evidence for the role of GABAergic neurotransmission in the cognitive functions that are impaired in SCZ led researchers to examine the components of GABA signaling (Table 1). Several studies performed DNA microarray analyses to identify large populations of genes whose expression was altered in the DLPFC of SCZ patients (Mirnics et al., 2000;Vawter et al., 2002;Hashimoto et al., 2008a) whereas other studies were based on candidate gene approaches. Identifying genes and genomic regions that underlie the pathogenesis of SCZ has been a formidable challenge. Initial linkage studies identified numerous loci that were linked with SCZ, but only a few of these regions were successfully replicated (several of which are discussed below). A series of genome-wide association studies failed to identify any clear SCZ susceptibility alleles, while many studies examining copy number variations and de novo mutations have provided little insight (reviewed in (Girard et al., 2012)). There appears to be significant genetic heterogeneity in SCZ, which presents a major challenge for developing genetic models of SCZ and linking these findings with human disease. Here we will focus on several candidate genes and mechanisms that appear to be associated with defects cIN synaptic transmission defects in SCZ.
Deficits at input synapses onto cINs
NMDA receptor hypofunction
N-Methyl-D-asparatic acid (NMDA) receptors (NMDARs) are molecular coincidence detectors that are only active when the synaptic membrane is depolarized and glutamate is bound to the receptor. Activation and opening of NMDARs leads to an influx of Ca2+, which can activate many downstream signaling cascades. One important function of NMDAR activation is the regulation of AMPA receptor trafficking, which plays a major role in synaptic plasticity. NMDARs are important mediators of various forms of synaptic plasticity, such as spike-timing dependent plasticity (STDP), long-term potentiation (LTP) and long-term depression (LTD). NMDAR-dependent plasticity plays a major role in learning and memory (Takahashi et al., 2003) and synapse formation (Inan and Crair, 2007;Lamsa et al., 2010). Synaptic plasticity can be induced at glutamatergic synapses on to cINs as well as at GABAergic synapses, but the form of synaptic plasticity at these synapses is specific to the brain region and cIN subtype (Lamsa et al., 2010;Kullmann and Lamsa, 2011).
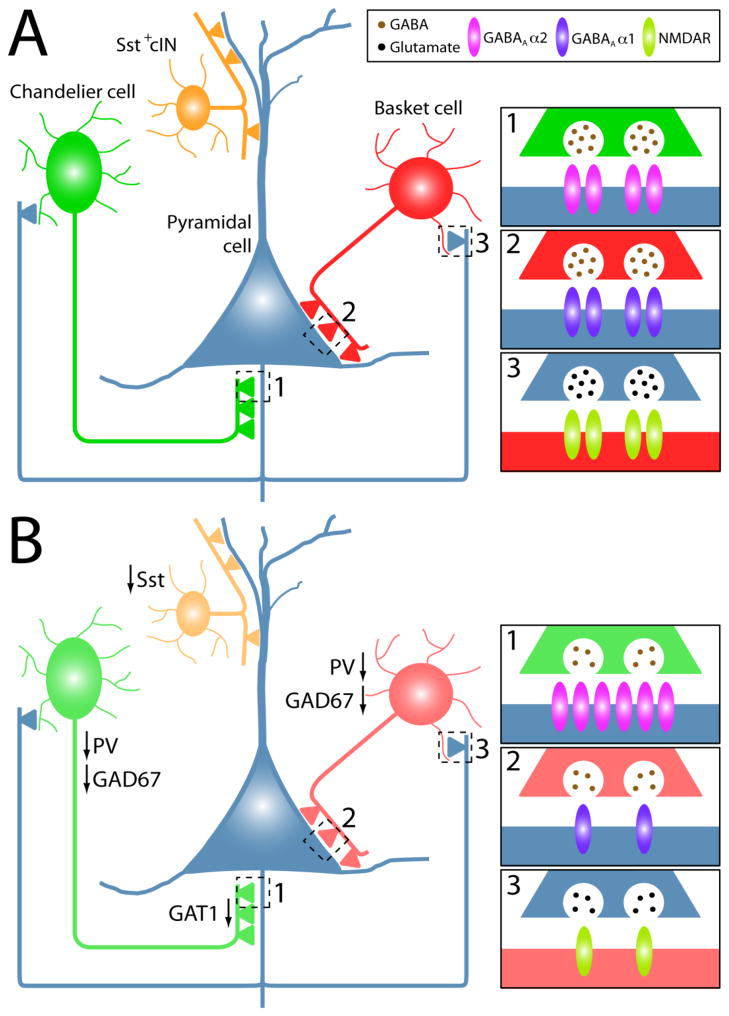
The theory of NMDA receptor (NMDAR) hypofunction as a root cause of SCZ has been a long-standing hypothesis since phencyclidine (PCP), a drug that induces SCZ-like symptoms, was found to be a non-competitive NMDAR antagonist (Lodge and Anis, 1982). Administration of NMDAR antagonists such as PCP, ketamine and MK801 results in cortical excitatory neuron hyperactivation (Krystal et al., 1994;Suzuki et al., 2002;Jackson et al., 2004) and decreased GAD67 and PV expression in cINs (Cochran et al., 2003;Keilhoff et al., 2004;Kinney et al., 2006;Rujescu et al., 2006;Behrens et al., 2007;Gietl et al., 2007;Morrow et al., 2007). This excitatory neuron hyperactivation is possibly due to disinhibition of excitatory neurons via NMDAR hypofunction in cINs (Homayoun and Moghaddam, 2007). Additionally, the density of cINs (specifically PV+ cINs) expressing the NR2A subunit of NMDAR is notably reduced in SCZ brains (Woo et al., 2004;Woo et al., 2008;Bitanihirwe et al., 2009). Furthermore, transplantation of cIN progenitor cells can prevent the induction of PCP-induced cognitive defects (Tanaka et al., 2011). These studies led to the hypothesis that NMDAR hypofunction in GABAergic interneurons contributes to excitatory neuron hyperexcitability in the etiology of SCZ-like symptoms (Figure 1).
Figure 1.
Schematic depicting a simplified cortical circuit (A) and some of the cortical interneuron (cIN)-related changes that are reported in SCZ patients (B). Sst, PV, GAD67 and GAT1 expression are reduced in SCZ patients. Postmortem studies reveal an increase in GABAA 2 receptors at chandelier cell synapses onto pyramidal cells (1), a decrease in GABAA 1 receptors at basket cell synapses onto pyramidal cells (2), and a decrease of NMDA receptors (NR2A subunit, in particular) at excitatory synapses onto PV+ cINs (3). Although only pyramidal synapses onto basket cells are shown, the reduction of NMDA receptors may also occur at synapses onto chandelier cells.
Compelling evidence exists for NMDAR hypofunction in PV+ cINs contributing to hyperactivity in DLPFC excitatory neurons. However, NMDAR-mediated synaptic currents appear to decrease in PV+ cINs with age (Wang and Gao, 2009, 2010;Rotaru et al., 2011). Thus, it is unclear how NMDAR hypofunction in PV+ cINs would manifest into a later-developing disease like SCZ, when NMDAR-mediated currents are already low in these neurons at this stage of development. A recent study may explain this conflict with the finding that prolonged exposure of MK801 leads to a reduction in the amplitude of AMPAR-mediated synaptic currents in FS PV+ cINs but an increase in the amplitude of AMPAR-mediated synaptic currents in pyramidal cells (Wang and Gao, 2012). Hence, early NMDAR hypofunction may lead to a reduction in AMPA-mediated synaptic currents specifically in FS cINs, possibly through an LTD-like mechanism, although an NMDAR-dependent LTD is yet to be shown at these synapses. Reduced activation of FS cINs could lead to disinhibition of pyramidal cells resulting in an increase in AMPAR-mediated synaptic currents during basal synaptic transmission.
On the other hand, MK801 also induced an increase in the frequency of AMPAR-mediated currents in DLPFC pyramidal cells but not in FS PV+ cINs. Furthermore, the addition of new presynaptic NMDARs is observed in axon terminals targeting pyramidal cells but not FS PV+ cINs (Wang and Gao, 2012). Since presynaptic NMDARs can facilitate glutamate release (Corlew et al., 2008), this mechanism likely explains the observation of increased frequency of AMPAR-mediated synaptic currents of pyramidal cells and not that of FS PV+ cINs. Hence, consistent with earlier findings, this study provided a plausible mechanism on how chronic MK801 treatment can have opposing effects on these two cell types: reduction of FS cIN activity via decreased AMPAR-mediated currents while hyperactivation of pyramidal cells by increased glutamate release and reduced inhibition. These findings provide a clearer explanation by which NMDAR hypofunction could induce distinct processes at different synapses to underlie the complex SCZ pathophysiology. Additional support for this mechanism could be obtained by determining if other NMDAR antagonists such as PCP or ketamine can replicate these findings.
In order to better tease a part NMDAR function and its relation to SCZ in different cell types, several groups disrupted NMDAR function specifically in a subset of GABAergic cINs. Removal of the NR1 subunit (the mandatory subunit for NMDAR function) in ~50% of cINs, the majority of which were PV+, during the second postnatal week led to behavioral phenotypes similar to the symptoms observed in SCZ patients such as hyperactivity, increased anxiety, and perturbations in working memory and PPI (Belforte et al., 2010). NR1-null cINs contained reduced levels of GAD67 and PV, and excitatory neurons displayed increased firing rates, which are possibly due to disinhibition. These findings are consistent with activity-dependent PV expression and they suggest that NMDAR-dependent activity is upstream of GABAergic transmission in this model. Furthermore, ablation of NMDAR function in cINs at a later age (~8 weeks old, which corresponds to post-adolescence) did not reveal any SCZ-like behavioral phenotype. These findings suggest a crucial role for intact NMDAR function in cINs during early development.
Two other groups utilized a PV-Cre mouse line to remove NR1 in PV+ cINs and found that these mice have impairments in gamma oscillations and in spatial and working memory tasks (Korotkova et al., 2010;Carlen et al., 2012). In contrast to the previous study, these groups did not observe abnormal social behavior, which may be due to the relatively later knockout of NMDAR function in PV-Cre mice (3rd postnatal week), again emphasizing the developmental role of NMDAR function in these cINs.
These studies addressing the role of NMDAR hypofunction in FS PV+ cINs have several caveats. First, the phenotypes analyzed are not symptoms unique to SCZ, making it difficult to draw clear connections between the observed findings and SCZ. Second, these studies either do not specifically ablate NMDARs only from FS PV+ cINs, or they ablate NMDARs in FS cINs at a relatively late developmental stage. Third, studies using NMDAR antagonists to model SCZ inhibit NMDARs throughout the brain, making it difficult to correlate site of action with phenotype. Future studies are needed to ablate NMDARs specifically in FS PV+ cINs early during development to determine if NMDA hypofunction in PV cells is sufficient to produce SCZ-like symptoms. While this aim will be aided by the recent production of many cIN subtype-specific Cre lines (Taniguchi et al., 2011), an early marker of PV-fated cINs is still unknown. Another beneficial approach would be to analyze whether animal models that better mimic mutations identified in genetic linkage studies, such as 22q11.2 or ErbB4 deletion models (see below), have NMDAR deficits in their FS PV+ cINs.
Perturbation in Neuregulin1/ErbB4 signaling
One of the replicated hits from linkage studies was located on chromosome 8p. Extensive haplotype analysis from several Icelandic SCZ families identified a series of SNPs and microsatellites within the 5′ through the second intron of the Nrg1, making Nrg1 a candidate SCZ susceptibility gene (Stefansson et al., 2002). Additional studies have found genetic associations between Nrg1 and SCZ in multiple affected populations (Harrison and Law, 2006;Mei and Xiong, 2008).
Neuregulins are trophic growth factors that stimulate ErbB receptor tyrosine kinases. There are four different Neuregulin genes, with Neuregulin1 (Nrg1) being the best characterized and most prominent in the developing and adult brain. Although there are multiple ErbB receptors, ErbB4 is the only autonomous Nrg1-specific receptor that becomes catalytically active upon Nrg1 interaction. Numerous studies identified ErbB4 as a candidate susceptibility gene for SCZ (Nicodemus et al., 2006;Norton et al., 2006;Silberberg et al., 2006;Benzel et al., 2007;Walsh et al., 2008). The Nrg1-ErbB4 interaction plays a prominent role in many aspects of neuronal development, including neuronal migration, axon guidance and synapse formation and plasticity (Mei and Xiong, 2008;Rico and Marín, 2011).
Most SNPs for Nrg1 and ErbB4 are found in non-coding regions (introns, 5′ or 3′ sequences), making it unclear how these SNPs regulate gene expression or function. There are conflicting reports for both an increase (Hashimoto et al., 2004;Law et al., 2006;Chong et al., 2008) and decrease (Bertram et al., 2007) of Nrg1 mRNA in the PFC cortex and hippocampus of SCZ postmortem brains. Although the levels of Nrg1 protein in the brain of SCZ patients remain unknown, Nrg1 levels are decreased in the serum of SCZ patients compared to controls (Shibuya et al., 2011). Additionally, some isoforms of the ErbB4 mRNA were also increased in SCZ brains (Silberberg et al., 2006;Law et al., 2007), and there is some evidence for increased expression of Nrg1 and ErbB4 protein in the DLPFC of SCZ patients (Chong et al., 2008). Another study reported that the expression levels of Nrg1 and ErbB4 are similar between SCZ postmortem tissue and controls, but there is an increase in Nrg1-induced ErbB4 activation and increases in ErbB4-PSD95 interactions (Hahn et al., 2006).
Until recently, the cellular localization of ErbB4 in the cortex was controversial, with the predominant view being that Nrg1/ErbB4 signaling functioned at excitatory synapses. However, several recent studies have demonstrated that ErbB4 is largely restricted to PV+ GABAergic interneurons in the murine cortex and hippocampus, with limited (if any) expression in projection neurons or Sst+ interneurons (Woo et al., 2007;Vullhorst et al., 2009;Fazzari et al., 2010;Neddens and Buonanno, 2010). Specifically, ErbB4 was present in the postsynaptic densities of PV+ cINs receiving glutamatergic inputs and the presynaptic axon terminals of both basket cells and chandelier cell cartridges. This Nrg1-ErbB4 interaction regulates synapse formation and GABAergic transmission (Woo et al., 2007). In addition, there is some evidence that this ErbB4 expression pattern is conserved in higher mammals (Neddens et al., 2011).
Nrg1 and ErbB4 knockout mice die during embryogenesis, so the initial data obtained from mouse models came from heterozygous or hypomorphic mice. Nrg1 mutant mice display impairments in behavioral tasks that are relevant to the negative and cognitive symptoms of SCZ, such as decreased PPI, hyperexcitability, and perturbations in social interactions and fear conditioning (Stefansson et al., 2002;Karl et al., 2007;O’Tuathaigh et al., 2007;O’Tuathaigh et al., 2008;Ehrlichman et al., 2009;Duffy et al., 2010;O’Tuathaigh et al., 2010). Spatial and working memory remains intact in these mice, but mice with specific mutations of Type III Nrg1 did show evidence of impaired working memory (Chen et al., 2008b). Surprisingly, overexpression of Nrg1 also increased locomotor activity, reduced PPI and perturbed fear conditioning and social interactions (Deakin et al., 2009;Kato et al., 2010). Restricted Nrg1 overexpression in individual pyramidal neurons greatly enhanced the number of GAD65+ boutons targeting somata and AIS (likely basket and chandelier cells, respectively) (Fazzari et al., 2010).
ErbB4 mutant mice also display increased hyperactivity, impaired working memory and decreased PPI (albeit to a lesser extent compared to Nrg1 mutants) (Stefansson et al., 2002;Golub et al., 2004;Barros et al., 2009). The discovery of ErbB4 restriction to PV+ cINs has allowed researchers to knockout ErbB4 specifically in these cells to better characterize its function in cINs. Early disruption of ErbB4 using GFAP-Cre or Dlx5/6-Cre mice decreased excitatory synapses, overall spine number, the number of chandelier synapses, synaptic transmission between cINs and projection neurons, and impaired PPI (Barros et al., 2009;Fazzari et al., 2010). Elimination of ErbB4 with PV-Cre decreased GABAergic transmission, induced locomotor hyperactivity, and caused impairments in PPI, working memory and fear conditioning (Chen et al., 2010b;Wen et al., 2010).
These studies outline the function Nrg1-ErbB4 in cIN synaptic transmission and demonstrate that perturbations in this signaling system induce cellular and behavioral changes that are consistent with a SCZ phenotype. However, since SCZ-associated Nrg1 and ErbB4 SNPs are generally restricted to non-coding regions (but see (Walss-Bass et al., 2006;Walsh et al., 2008)), it will be interesting to determine whether Nrg1 or ErbB4 mouse models that better mimic SCZ-associated SNPs induce similar effects as the above studies (Chen et al., 2010a).
Deficits at output synapses of cINs
Presynaptic defects
Glutamate is converted to GABA via two isoforms of glutamatic acid decarboxylase (GAD), GAD65 and GAD67. Numerous postmortem studies have found that GAD67 mRNA (Akbarian et al., 1995;Volk et al., 2000) and protein (Guidotti et al., 2000;Curley et al., 2011) are decreased in a subset (~25–35%) of DLPFC interneurons in SCZ patients, whereas GAD65 expression is largely intact in these brains (Benes et al., 2000;Guidotti et al., 2000). Further studies found that the loss of GAD67 mRNA and protein was restricted to PV+ cells, with nearly 50% of PV+ cINs in the PFC of SCZ patients lacking GAD67 expression (Hashimoto et al., 2003;Curley et al., 2011). It appears that reduced GAD67 levels are present in basket cells (Figure 1), since decreased GAD67 protein was observed in non-chandelier PV+ axon terminals (Curley et al., 2011), but more definitive data on the cIN subtype distribution of decreased GAD67 levels in SCZ is needed. GAD67 expression is also decreased in other cortical regions of SCZ patients (Hashimoto et al., 2008b;Thompson et al., 2009), indicating that this defect is not specific to GABAergic DLPFC cINs. Of note, a decrease in GAD67 does not necessarily indicate a decrease in GABA levels, and attempts at measuring GABA levels in SCZ patients has produced conflicting results (Goto et al., 2009;Ongur et al., 2010). However, GAD67 is activity regulated (Benson et al., 1994), indicating that cells with a reduction in GAD67 level may be firing less than normal. These findings suggest that there is a reduction in GABAergic neurotransmission in PV+ cINs of SCZ patients, which could lead to disinhibition of cortical excitatory neurons.
In addition to GAD67, expression of the GABA membrane transporter 1 (GAT1), which is involved in the reuptake of GABA, is reduced in a subset of cINs in the PFC of SCZ patients (Ohnuma et al., 1999;Volk et al., 2001). Several studies utilized GAT1 staining of chandelier cell cartridges to identify a decrease in the density of GAT1+ cartridges in the PFC of SCZ patients (Figure 1) (Woo et al., 1998;Pierri et al., 1999). It remains unclear whether these findings reflect a decrease in GAT1 levels with normal cartridge numbers, or a decrease in the number of cartridges in SCZ patients. Interestingly, the density of non-chandelier GAT1+ terminals was unchanged in SCZ patients. Similar to GAD67, the exact distribution of decreased GAT1 levels between basket and chandelier cells is unknown and requires further study.
Postsynaptic defects
Two prominent GABAA receptor subunits have been examined in SCZ patients: GABAA 1 receptors are postsynaptic to basket cell synapses on pyramidal neuron cell bodies and proximal dendrites, whereas GABAA 2 receptors are primarily postsynaptic to chandelier cells on the AIS of pyramidal neurons. Several studies have identified a decrease in GABAA α1 receptor mRNA in the DLPFC cortex of SCZ patients (Hashimoto et al., 2008a;Hashimoto et al., 2008b;Beneyto et al., 2011), with some evidence that the decrease is specific to pyramidal cells (Figure 1) (Glausier and Lewis, 2011). These findings suggest that inhibition is reduced at basket cell synapses on pyramidal neurons. Consistent with this idea, two kinases that differentially modulate the chloride ion transporters NKCC1 and KCC2 were found to be elevated in the DLPFC of SCZ patients (Arion and Lewis, 2011). Enhanced activation of NKCC1 relative to KCC2 would be expected to increase intracellular chloride levels and thus diminish GABA-mediated inhibition of pyramidal neurons. These molecular deficits are consistent with the neurophysiological defects observed for cortical inhibition in SCZ patients (Daskalakis et al., 2007) as well as the disinhibition hypothesis of excitatory neurons in SCZ. Recently, such a reduction in inhibition at basket cell synapses was proposed to be a homeostatic response to the pyramidal neuron’s receiving decreased excitatory inputs in SCZ (Lewis et al., 2012).
GABAA α2 receptor protein (Volk et al., 2002) and mRNA (Beneyto et al., 2011) expression is increased in the PFC of SCZ patients compared to controls, with a clear enrichment of GABAA α2 receptor protein at the AIS (Figure 1). Both the decrease in GAT1 in chandelier cartridges and the increase in postsynaptic GABAA α2 receptors were suggested to be compensatory mechanisms to enhance GABA transmission at the axo-axonic synapses of SCZ patients (Lewis et al., 2012).
The role of cINs in other SCZ models
In addition the models described above, there are other SCZ-associated genes that have received significant attention. Here we describe several SCZ mouse models in which cIN function is beginning to be examined.
Dysbindin and clathrin-mediated endocytosis
Dysbindin-1 is encoded by dystrobrevin-binding protein 1 gene (DTNBP1) located at chromosome 6p22.3. SNP and haplotype analyses of the DTNBP1 locus of SCZ patients from multiple populations found linkage between DTNBP-1 and SCZ, making dysbindin a potential SCZ susceptibility gene (Riley et al., 2009;Talbot et al., 2009;Zuo et al., 2009;Rethelyi et al., 2010). DTNBP1 gene variations were also shown to influence cognitive functions related to the PFC (Burdick et al., 2006;Fallgatter et al., 2006). DTNBP1 haplotypes with increased protein expression are associated with enhanced working memory performance (Wolf et al., 2011). On the other hand, risk haplotypes of DTNBP1 are associated with reduced spatial memory (Donohoe et al., 2007). Dysbindin-1 is found at both presynaptic and postsynaptic sites of glutamatergic synapses (Talbot et al., 2006), and it is found to be reduced in the hippocampus and PFC of SCZ brains (Talbot et al., 2004;Tang et al., 2009a;Talbot et al., 2011).
Dysbindin-1 regulates biogenesis of lysosome-related organelles and is involved in protein trafficking through the lysosomal pathway. Receptor trafficking is mediated by recycling endosomes or clathrin-mediated endocytosis (CME), which targets proteins to lysosomes for degradation. Alteration in Dysbindin-1 expression was found to affect surface expression of the D2 subunit of dopamine receptors, as well as NR1 and NR2A subunits of NMDAR, all of which are trafficked via CME (Ji et al., 2009;Tang et al., 2009b;Jeans et al., 2011;Karlsgodt et al., 2011;Schubert et al., 2011). Consistent with its role in NMDAR trafficking, Dysbindin-1 knockout mice (Dys−/−) have altered NMDAR-mediated currents and synaptic plasticity. Of note, loss of Dysbindin-1 was found to have differential effects on the NMDAR-mediated currents at hippocampal and PFC glutamatergic synapses, which may be due to differential regulation of distinct NMDAR subunits (Jentsch et al., 2009;Ji et al., 2009;Tang et al., 2009b;Karlsgodt et al., 2011).
Dys−/− mice also display deficits in spatial working memory and PPI, increased locomotion, impaired gamma-band oscillations, and reduced inhibition in the hippocampus with no change in excitation (Jentsch et al., 2009;Carlson et al., 2011;Karlsgodt et al., 2011). One group observed a reduction in the number of PV+ cells in the hippocampus (Carlson et al., 2011). Dys−/− mice also have reduced inhibition of layer V PFC pyramidal neurons, possibly due to decreased excitability of FS cINs (Ji et al., 2009). Deficits in dysbindin-1 mutants may not be restricted to the postsynaptic side. In the Sandy mouse (Sdy), an inbred line carrying a spontaneous mutation in dysbindin-1, larger vesicle size and reduced readily releasable pool of synaptic vesicles were detected (Chen et al., 2008a).
In sum, by regulating multiple neurotransmitter systems on both sides of the synapse, dysbindin-1 function may be relevant for multiple aspects of synaptic activity and SCZ pathophysiology. However, further analysis is necessary to understand the molecular basis of this regulation. Finally, although Dysbindin-1 was detected at glutamatergic synapses of the hippocampus, its presence at GABAergic synapses of the cortex is still unclear. Since GABA receptor subunits are trafficked to the postsynaptic membrane via CME (Tretter and Moss, 2008), it is also possible that Dysbindin-1 regulates the surface expression of GABA receptors.
Disrupted in Schizophrenia-1 (DISC1)
A truncating mutation in the Disrupted in Schizophrenia-1 (DISC1) gene was first identified in a Scottish family with a high incidence of psychiatric disorders including SCZ, depression and anxiety diseases (St Clair et al., 1990;Millar et al., 2000;Blackwood et al., 2001). Although many independent genetic linkage and association studies support a linkage between DISC1 and psychiatric illnesses, there is significant controversy whether a specific linkage between DISC1 and SCZ actually exists (Kvajo et al., 2011b). DISC1 encodes a large protein with relatively little sequence homology to other known genes, hampering progress for identifying a clear function for DISC1. Despite these drawbacks, DISC1 has been shown to play a role in neuronal cell proliferation, maturation, migration, neuronal plasticity, and regulation of numerous intracellular signaling cascades (Singh et al., 2011;Soares et al., 2011). However, these studies used knockdown or overexpression approaches to study DISC1 function in a manner that does not model the susceptibility allele. In fact, many of these migration and maturation defects were not observed in a mouse model with a truncating lesion in the Disc1 gene that mimics the mutation in the Scottish family (Kvajo et al., 2008;Kvajo et al., 2011a), highlighting the importance for creating transgenic mice that best recapitulate the human SCZ-related genetic mutations.
Eight distinct DISC1 mutant mice have been reported, several of which display some SCZ symptoms, such as impairments in working memory and deficits in PPI (Kelly and Brandon, 2011). In several mouse models that overexpress a truncated version DISC1, PV immunoreactivity was decreased in the medial PFC compared to controls (Hikida et al., 2007;Shen et al., 2008;Ayhan et al., 2011). There was no decrease in either calretinin or calbindin in Disc1 mutant mice. Of note, although there was no reduction of PV+ cells in the DLPFC, the laminar organization of PV+ cells was perturbed (Shen et al., 2008). However, no changes in the number of PV+ or calbindin+ cINs were detected in a mouse model that mimics the Disc1 allele from the Scottish family (Kvajo et al., 2008). shRNA-mediated knockdown of Disc1 in pyramidal cells of the prefrontal cortex also led to a reduction in PV+ cells in this area, indicating that the decrease in PV+ interneurons could be non-autonomous (Niwa et al., 2010). Recent evidence indicates that Disc1 may regulate dendritic growth during periods of prolonged GABA-mediated depolarization in hippocampal neurogenesis (Kim et al., 2012). It also appears that DISC1 functions in cIN tangential migration (Steinecke et al., 2012). Future studies are needed to link dysfunction of Disc1-containing risk alleles to the specific pathobiology of SCZ.
22q11.2 deletion syndrome (22qDS)
One known genetic factor underlying the risk of developing SCZ is the microdeletion in the chromosomal region 22q11.2, which occurs in roughly 1 in 3000 births and leads to the 22q deletion syndrome (22qDS, DiGeorge syndrome, velocardiofacial syndrome) (Shprintzen et al., 2005). 22qDS patients display impairments in a variety of cognitive tasks (Karayiorgou et al., 2010), and roughly 30% of carriers with this deletion are diagnosed with SCZ (Pulver et al., 1994;Murphy et al., 1999), such that this mutation constitutes approximately 1–2% of the sporadic cases of SCZ (Bassett et al., 2008;Xu et al., 2008). The size of the microdeletion is either 1.5 Mb or 3 Mb, and both deletions are associated with a higher risk of SCZ (Karayiorgou et al., 1995). The 1.5 Mb region contains 27 functional genes (Edelmann et al., 1999) whose dosage reduction are proposed to have adverse effects on early patterning, neurogenesis and synaptogenesis (Meechan et al., 2011). In mice, orthologues of 26 of these genes are located on chromosome 16, although the gene order is slightly different (Puech et al., 1997;Maynard et al., 2006).
Among several mouse lines with different chromosome 16 deletions, two lines, Df(16A+/−) and Lgdel, carry a deletion in chromosome 16 that is syntenic to the 1.5 Mb microdeletion of the human chromosome 22q11.2 (Meechan et al., 2006;Stark et al., 2008). Both Df(16A+/−) and Lgdel display impaired PPI, but only Df(16A+/−) mice have conditioned fear and spatial working memory deficits (Long et al., 2006;Stark et al., 2008). Analyses of network oscillations during a working memory task, in which Df(16A+/−) mice show impairments, revealed reduced synchrony of hippocampal and PFC oscillations (Sigurdsson et al., 2010). Whether the underlying pathophysiology of hippocampal-cortical oscillation synchrony in Df(16A+/−) mice involves cINs is not clear. However, inhibition of pyramidal neurons in the CA1 region of hippocampus is also reduced in Df(16A+/−) mice (Drew et al., 2011b). Lgdel mice display alterations in PV+ cIN layer distribution and cIN migration deficits, although the total number of PV+ cINs is not changed (Meechan et al., 2009). A recent study found that cIN migration and lamination defects in Lgdel mice might be caused by disruption of C-X-C chemokine receptor type 4 (Cxcr4) signaling (Meechan et al., 2012), a well-characterized guidance mechanism required for proper cIN migration (Wang et al., 2011). Thus, unlike several of the mouse models mentioned above, Df(16A+/−) and Lgdel mice may provide a more accurate model of the human 22q deletion, though the extent to which these mice recapitulate SCZ symptoms is still under investigation (Drew et al., 2011a).
Interneuron dysfunction in SCZ: cause or effect?
The evidence for various input and output-related deficits in cINs from SCZ studies begs the question of whether cIN alterations could be an effect, rather than a cause, of the core SCZ pathology. A wealth of data from imaging studies suggest that SCZ is associated with reduced gray matter thickness that is present early in the illness. Postmortem studies suggest that the volume loss mainly results from a reduction in neuropil and not cells (Selemon et al., 1995;Thune et al., 2001). Several lines of evidence indicate that SCZ is associated with a network-wide reduction of PFC pyramidal neuron activity that could lead to reduced excitatory drive of cINs (Buchsbaum et al., 1984a;Buchsbaum et al., 1984b;Berman et al., 1992). This could result in downregulation of various activity-dependent cIN parameters, such as PV or GAD expression. Coupled with evidence for loss of dendritic spines (Garey et al., 1998;Glantz and Lewis, 2000), the above findings suggest that the onset of SCZ may originate from reduced excitatory neurotransmission in the PFC, resulting in secondary cIN deficits. Interestingly, adolescence is normally associated with extensive pruning of excitatory connectivity within the PFC (Mirnics et al., 2000). Since plasticity of excitatory connectivity during adolescence is influenced by the maturation and function of PV+ cINs (Katagiri et al., 2007), early cIN dysfunction could conceivably contribute to overpruning of excitatory connectivity onto pyramidal neurons early in the illness progression, which would then result in the reduction of cIN activity.
In addition to pyramidal neuron excitation, two other mechanisms associated with SCZ have also been suggested to influence cIN activity. PV+ cINs receive stimulatory dopaminergic inputs (Mrzljak et al., 1996) that increase during adolescence (Tseng et al., 2006;Tseng and O’Donnell, 2007;Tang et al., 2011). In a hippocampal rat lesion model of SCZ, PFC dysfunction involves alteration of cIN responsiveness to dopamine (DA) (Gruber et al., 2010). In a mouse model of SCZ in which striatal D2 receptor activity is genetically enhanced, resulting in decreased DA in the PFC, GABAergic inputs onto cortical pyramidal neurons are also decreased (Li et al., 2011). Therefore, reduced cortical DA can result in dampened interneuron activity, and is thus a potential upstream mediator of cIN-related pathology in SCZ.
Finally, cholinergic inputs provide a critical excitatory drive to cINs (Mok and Kew, 2006;Arnaiz-Cot et al., 2008) and appear to mainly target cINs during the first two weeks of postnatal development (Janiesch et al., 2011). Cholinergic terminals can also enhance GABA release at perisomatic synapses onto hippocampal pyramidal neurons (Tang et al., 2011). In addition, cholinergic antagonists block PV+ cIN-mediated gamma oscillations in the hippocampus, while nicotine administration reduces the methylation status of the GAD67 promoter (Satta et al., 2008). Post-mortem findings on cholinergic deficits are sparse, but some evidence exists for a reduction in SCZ brains (Guan et al., 1999;Holt et al., 1999;Freedman et al., 2000;Hyde and Crook, 2001).
In sum, due to extensive connectivity and crosstalk between different neurotransmitter systems in the cortex, a deficit in any of these systems could induce significant changes within the circuitry. Thus, whether the neuropathology of SCZ originates in excitatory pyramidal neuron connectivity, inhibitory cINs, or subcortical DA or cholinergic systems remains unclear. Regardless of the molecular and genetic causes of SCZ, cIN dysfunction is a consistent symptom and remains a promising therapeutic target.
Future directions
Research over the last decade has significantly advanced our understanding of cIN function. While cINs appear to play a vital role in the etiology of SCZ, there are still many questions that remain unanswered. A significant challenge in studying the genetic basis of SCZ is generating accurate models of the disorder. Single gene knockouts may replicate certain phenotypes associated with SCZ and provide potential tools to understand the molecular and genetic mechanisms underlying these phenotypes. However, transgenic models that better mimic the genetic mutations in humans, such as Lgdel/Df(16+/−) mice, are promising tools to study disease mechanisms in which cIN phenotypes can further be deciphered.
The advent of embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) technology has opened up a new gateway for studying normal neuronal development and disease (Cundiff and Anderson, 2011;Petros et al., 2011). Recently, several groups have succeeded in generating cINs from mouse (Maroof et al., 2010;Danjo et al., 2011) and human (Goulburn et al., 2011) ESCs. The ability to produce an unlimited source of cINs will expand our ability to study hypotheses on interneuron dysfunction in SCZ. In addition, the ability to create patient specific iPSCs permits the study of different genetic predispositions to SCZ. Several groups have successfully created iPSCs from SCZ patients (Brennand et al., 2011;Chiang et al., 2011;Pedrosa et al., 2011), with some evidence that iPSC-derived neurons from SCZ patients have general defects in neuronal connectivity (Brennand et al., 2011). Application of this technology to the study of specific hypotheses on cortical pyramidal and interneuron dysfunction in SCZ promises to open new avenues of research on the cellular and molecular bases, and treatments, of this highly debilitating and treatment-resistant mental illness.
Acknowledgments
We thank members of the Anderson lab for helpful comments on this manuscript, and we apologize to our colleagues whose work we were unable to discuss due to space limitations. This work was supported by R01 MH066912 and K02 MH070031 (Stewart Anderson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melis Inan, Email: inanmeli@gmail.com.
Timothy J. Petros, Email: timtros@gmail.com.
Stewart A. Anderson, Email: anderson3@email.chop.edu.
References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics [see comments] Archives of General Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. discussion 267–278. [DOI] [PubMed] [Google Scholar]
- Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Archives of general psychiatry. 2011;68:21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz-Cot JJ, Gonzalez JC, Sobrado M, Baldelli P, Carbone E, Gandia L, Garcia AG, Hernandez-Guijo JM. Allosteric modulation of alpha 7 nicotinic receptors selectively depolarizes hippocampal interneurons, enhancing spontaneous GABAergic transmission. Eur J Neurosci. 2008;27:1097–1110. doi: 10.1111/j.1460-9568.2008.06077.x. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Molecular psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 2008;17:4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cerebral cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Morris HM, Rovensky KC, Lewis DA. Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology. 2012;62:1598–1605. doi: 10.1016/j.neuropharm.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- Benzel I, Bansal A, Browning BL, Galwey NW, Maycox PR, McGinnis R, Smart D, St Clair D, Yates P, Purvis I. Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behavioral and brain functions : BBF. 2007;3:31. doi: 10.1186/1744-9081-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Torrey EF, Daniel DG, Weinberger DR. Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Arch Gen Psychiatry. 1992;49:927–934. doi: 10.1001/archpsyc.1992.01820120015004. [DOI] [PubMed] [Google Scholar]
- Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, Bogerts B. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–156. doi: 10.1196/annals.1397.080. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BKY, Lim MP, Kelley JF, Kaneko T, Woo TUW. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Cappelletti J, Ball R, Hazlett E, King AC, Johnson J, Wu J, DeLisi LE. Positron emission tomographic image measurement in schizophrenia and affective disorders. Annals of neurology. 1984a;15(Suppl):S157–165. doi: 10.1002/ana.410150730. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, DeLisi LE, Holcomb HH, Cappelletti J, King AC, Johnson J, Hazlett E, Dowling-Zimmerman S, Post RM, Morihisa J, et al. Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch Gen Psychiatry. 1984b;41:1159–1166. doi: 10.1001/archpsyc.1984.01790230045007. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, Kucherlapati R, Malhotra AK. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008a;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hancock ML, Role LW, Talmage DA. Intramembranous valine linked to schizophrenia is required for neuregulin 1 regulation of the morphological development of cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010a;30:9199–9208. doi: 10.1523/JNEUROSCI.0605-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, Wang XM, Lai C, Xiong WC, Mei L, Gao TM. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008b;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CH, Su Y, Wen Z, Yoritomo N, Ross CA, Margolis RL, Song H, Ming GL. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol Psychiatry. 2011;16:358–360. doi: 10.1038/mp.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophrenia research. 2008;100:270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14:609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff PE, Anderson SA. Impact of induced pluripotent stem cells on the study of central nervous system disease. Curr Opin Genet Dev. 2011 doi: 10.1016/j.gde.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. The American journal of psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci. 2011;31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain research reviews. 2007;56:427–442. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Daviss SR, Lewis DA. Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res. 1995;59:81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport. 2009;20:1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, Garavan H, Robertson IH, Gill M, Corvin A. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia. 2007;45:454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2011a;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Stark KL, Fenelon K, Karayiorgou M, Macdermott AB, Gogos JA. Evidence for altered hippocampal function in a mouse model of the human 22q11.2 microdeletion. Mol Cell Neurosci. 2011b;47:293–305. doi: 10.1016/j.mcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64:1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, Kreibich AS, Abel T, Brodkin ES, Hahn CG, Siegel SJ. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain research. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, Deckert J. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective Depletion of Molecularly Defined Cortical Interneurons in Human Holoprosencephaly with Severe Striatal Hypoplasia. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry. 1996;53:1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of neurology, neurosurgery, and psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietl A, Giegling I, Hartmann AM, Schneider B, Schnabel A, Maurer K, Moller HJ, Rujescu D. ABCG1 gene variants in suicidal behavior and aggression-related traits. Eur Neuropsychopharmacol. 2007;17:410–416. doi: 10.1016/j.euroneuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Girard SL, Dion PA, Rouleau GA. Schizophrenia genetics: putting all the pieces together. Current neurology and neuroscience reports. 2012;12:261–266. doi: 10.1007/s11910-012-0266-7. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human brain mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α 1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, Hori H, Ueda N, Korogi Y, Nakamura J. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Human psychopharmacology. 2009;24:639–645. doi: 10.1002/hup.1070. [DOI] [PubMed] [Google Scholar]
- Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, Hatzistavrou T, Bourke J, Watmuff B, Lang RJ, Haynes JM, Pouton CW, Giudice A, Trounson AO, Anderson SA, Stanley EG, Elefanty AG. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of clinical psychiatry. 2006;67(Suppl 9):3–8. discussion 36–42. [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E, DiGiorgi Gerevini V. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of general psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature medicine. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and Schizophrenia: Genetics, Gene Expression, and Neurobiology. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Molecular psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2008a;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. The American journal of psychiatry. 2008b;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andradé M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Herman MM, Hyde TM, Kleinman JE, Sinton CM, German DC, Hersh LB, Graybiel AM, Saper CB. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94:21–31. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Crook JM. Cholinergic systems and schizophrenia: primary pathology or epiphenomena? J Chem Neuroanat. 2001;22:53–63. doi: 10.1016/s0891-0618(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Inan M, Crair MC. Development of cortical maps: perspectives from the barrel cortex. Neuroscientist. 2007;13:49–61. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiesch PC, Kruger HS, Poschel B, Hanganu-Opatz IL. Cholinergic control in developing prefrontal-hippocampal networks. J Neurosci. 2011;31:17955–17970. doi: 10.1523/JNEUROSCI.2644-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeans A, Malins R, Padamsey Z, Reinhart M, Emptage N. Increased expression of dysbindin-1A leads to a selective deficit in NMDA receptor signaling in the hippocampus. Neuropharmacology. 2011;61:1345–1353. doi: 10.1016/j.neuropharm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009;34:2601–2608. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A. 2009;106:19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Senitz D, Beckmann H. Altered distribution of parvalbumin-immunoreactive local circuit neurons in the anterior cingulate cortex of schizophrenic patients. Psychiatry Research. 1997;75:49–59. doi: 10.1016/s0925-4927(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes, brain, and behavior. 2007;6:677–687. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry. 2011;69:28–34. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PloS one. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Brandon NJ. Taking a bird’s eye view on a mouse model review: a comparison of findings from mouse models targeting DISC1or DISC1-interacting proteins. Future Neurology. 2011;6:661–677. [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, Christian K, Callicott JH, Weinberger DR, Song H, Ming GL. Interplay between DISC1 and GABA Signaling Regulates Neurogenesis in Mice and Risk for Schizophrenia. Cell. 2012;148:1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology. 2011;60:712–719. doi: 10.1016/j.neuropharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Drew LJ, Lepagnol-Bestel AM, Xiao L, Levy RJ, Blazeski R, Arguello PA, Lacefield CO, Mason CA, Simonneau M, O’Donnell JM, MacDermott AB, Karayiorgou M, Gogos JA. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proc Natl Acad Sci U S A. 2011a;108:E1349–1358. doi: 10.1073/pnas.1114113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Gogos JA. Avoiding mouse traps in schizophrenia genetics: lessons and promises from current and emerging mouse models. Neuroscience. 2011b doi: 10.1016/j.neuroscience.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Kullmann DM, Woodin MA. Spike-timing dependent plasticity in inhibitory circuits. Frontiers in synaptic neuroscience. 2010;2:8. doi: 10.3389/fnsyn.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Human molecular genetics. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Lodge D, Anis NA. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. doi: 10.1016/0014-2999(82)90022-x. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, LaPorte P, Merscher S, Funke B, Saint-Jore B, Puech A, Kucherlapati R, Morrow BE, Skoultchi AI, Wynshaw-Boris A. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 2006;7:247–257. doi: 10.1007/s10048-006-0054-0. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Brown K, Shi SH, Studer L, Anderson SA. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J Neurosci. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard TM, Meechan DW, Heindel CC, Peters AZ, Hamer RM, Lieberman JA, LaMantia AS. No evidence for parental imprinting of mouse 22q11 gene orthologs. Mamm Genome. 2006;17:822–832. doi: 10.1007/s00335-006-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci. 2011;29:283–294. doi: 10.1016/j.ijdevneu.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Maynard TM, Wu Y, Gopalakrishna D, Lieberman JA, LaMantia AS. Gene dosage in the developing and adult brain in a mouse model of 22q11 deletion syndrome. Mol Cell Neurosci. 2006;33:412–428. doi: 10.1016/j.mcn.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, Lamantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature reviews Neuroscience. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human molecular genetics. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mok MHS, Kew JN. Excitation of rat hippocampal interneurons via modulation of endogenous agonist activity at the alpha7 nicotinic ACh receptor. J Physiol. 2006;574:699–710. doi: 10.1113/jphysiol.2006.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 2011;37:659–663. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 2007;192:283–290. doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2011;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry. 2011;70:636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Molecular psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo KI, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, Huang B, Kohda K, Noda Y, O'Donnell P, Nakajima K, Sawa A, Nabeshima T. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CMP, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Harvey R, Waddington JL. Phenotypic characterization of spatial cognition and social behavior in mice with 'knockout' of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CMP, Harte M, O'Leary C, O'Sullivan GJ, Blau C, Lai D, Harvey RP, Tighe O, Fagan AJ, Kerskens C, Reynolds GP, Waddington JL. Schizophrenia-related endophenotypes in heterozygous neuregulin-1 'knockout' mice. The European journal of neuroscience. 2010;31:349–358. doi: 10.1111/j.1460-9568.2009.07069.x. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CMP, O’Connor AM, O’Sullivan GJ, Lai D, Harvey R, Croke DT, Waddington JL. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous 'knockout' of the schizophrenia risk gene neuregulin-1. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:462–466. doi: 10.1016/j.pnpbp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz S, Grabert J, Gorba T, Wirth MJ, Wahle P. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an Early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- Pedrosa E, Sandler V, Shah A, Carroll R, Chang C, Rockowitz S, Guo X, Zheng D, Lachman HM. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J Neurogenet. 2011;25:88–103. doi: 10.3109/01677063.2011.597908. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Tyson JA, Anderson SA. Pluripotent stem cells for the study of CNS development. Frontiers in molecular neuroscience. 2011;4:30. doi: 10.3389/fnmol.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Lim JH, Brunjes PC. Activity-dependent regulation of calcium-binding proteins in the developing rat olfactory bulb. J Comp Neurol. 1997;387:12–26. [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. The American journal of psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Puech A, Saint-Jore B, Funke B, Gilbert DJ, Sirotkin H, Copeland NG, Jenkins NA, Kucherlapati R, Morrow B, Skoultchi AI. Comparative mapping of the human 22q11 chromosomal region and the orthologous region in mice reveals complex changes in gene organization. Proc Natl Acad Sci U S A. 1997;94:14608–14613. doi: 10.1073/pnas.94.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. The Journal of nervous and mental disease. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi JM, Bakker SC, Polgar P, Czobor P, Strengman E, Pasztor PI, Kahn RS, Bitter I. Association study of NRG1, DTNBP1, RGS4, G72/G30, and PIP5K2A with schizophrenia and symptom severity in a Hungarian sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:792–801. doi: 10.1002/ajmg.b.31049. [DOI] [PubMed] [Google Scholar]
- Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Current opinion in genetics & development. 2011;21:262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Riley B, Kuo PH, Maher BS, Fanous AH, Sun J, Wormley B, O’Neill FA, Walsh D, Zhao Z, Kendler KS. The dystrobrevin binding protein 1 (DTNBP1) gene is associated with schizophrenia in the Irish Case Control Study of Schizophrenia (ICCSS) sample. Schizophr Res. 2009;115:245–253. doi: 10.1016/j.schres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Moller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, Harkavy-Friedman J, Malaspina D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert KO, Focking M, Prehn JH, Cotter DR. Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol Psychiatry. 2011 doi: 10.1038/mp.2011.123. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, Chatzi C, He S, Mackie I, Brandon NJ, Marquis KL, Day M, Hurko O, McCaig CD, Riedel G, St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Komi E, Wang R, Kato T, Watanabe Y, Sakai M, Ozaki M, Someya T, Nawa H. Measurement and comparison of serum neuregulin 1 immunoreactivity in control subjects and patients with schizophrenia: an influence of its genetic polymorphism. J Neural Transm. 2011;117:887–895. doi: 10.1007/s00702-010-0418-3. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Higgins AM, Antshel K, Fremont W, Roizen N, Kates W. Velo-cardio-facial syndrome. Current opinion in pediatrics. 2005;17:725–730. doi: 10.1097/01.mop.0000184465.73833.0b. [DOI] [PubMed] [Google Scholar]
- Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. J Clin Neurophysiol. 2010;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, De Rienzo G, Drane L, Mao Y, Flood Z, Madison J, Ferreira M, Bergen S, King C, Sklar P, Sive H, Tsai LH. Common DISC1 polymorphisms disrupt Wnt/GSK3β signaling and brain development. Neuron. 2011;72:545–558. doi: 10.1016/j.neuron.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DC, Carlyle BC, Bradshaw NJ, Porteous DJ. DISC1: Structure, Function, and Therapeutic Potential for Major Mental Illness. ACS chemical neuroscience. 2011;2:609–632. doi: 10.1021/cn200062k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. Visual gamma oscillations in schizophrenia: implications for understanding neural circuitry abnormalities. Clin EEG Neurosci. 2008;39:65–68. doi: 10.1177/155005940803900208. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. American journal of human genetics. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32:738–745. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience. 2002;114:769–779. doi: 10.1016/s0306-4522(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Lorincz A, Tamas G. Beta and gamma frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. J Neurosci. 2001;21:5824–5831. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, Kamins J, Hahn CG, Blake DJ, Arnold SE. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn CG, Siegel SJ, Trojanowski JQ, Gur RE, Blake DJ, Arnold SE. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Louneva N, Cohen JW, Kazi H, Blake DJ, Arnold SE. Synaptic dysbindin-1 reductions in schizophrenia occur in an isoform-specific manner indicating their subsynaptic location. PLoS One. 2011;6:e16886. doi: 10.1371/journal.pone.0016886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC, Arnold SE. Dysbindin-1 and its protein family with special attention to the potential role of dysbindin-1 in neuronal functions and the pathophysiology of schizophrenia. In: Javitt DC, Kantrowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology: Schizophrenia. Vol. 27. New York: Springer Scientific; 2009. pp. 107–241. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Toriumi K, Kubo KI, Nabeshima T, Nakajima K. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14116–14125. doi: 10.1523/JNEUROSCI.2786-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Karson MA, Nagode DA, McIntosh JM, Uebele VN, Renger JJ, Klugmann M, Milner TA, Alger BE. Nerve terminal nicotinic acetylcholine receptors initiate quantal GABA release from perisomatic interneurons by activating axonal T-type (Cav3) Ca(2) channels and Ca(2) release from stores. J Neurosci. 2011;31:13546–13561. doi: 10.1523/JNEUROSCI.2781-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, Blake DJ, Arnold SE, Talbot K. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009a;18:3851–3863. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, Lu B. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci U S A. 2009b;106:21395–21400. doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. Journal of psychiatric research. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Thune JJ, Uylings HB, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Frontiers in molecular neuroscience. 2008;1:7. doi: 10.3389/neuro.02.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophrenia research. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Walss-Bass C, Liu W, Lew DF, Villegas R, Montero P, Dassori A, Leach RJ, Almasy L, Escamilla M, Raventos H. A novel missense mutation in the transmembrane domain of neuregulin 1 is associated with schizophrenia. Biol Psychiatry. 2006;60:548–553. doi: 10.1016/j.biopsych.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology. 2012;62:1808–1822. doi: 10.1016/j.neuropharm.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Vazdarjanova A, Xiong WC, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Jackson MC, Kissling C, Thome J, Linden DE. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry. 2011;16:145–155. doi: 10.1038/mp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain research. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TUW, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of general psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31:17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Luo X, Kranzler HR, Lu L, Rosenheck RA, Cramer J, van Kammen DP, Erdos J, Charney DS, Krystal J, Gelernter J. Association study of DTNBP1 with schizophrenia in a US sample. Psychiatr Genet. 2009;19:292–304. doi: 10.1097/ypg.0b013e32832a50bc. [DOI] [PMC free article] [PubMed] [Google Scholar]