Abstract
Objective
We monitored the epidemiology and microbiology of oral yeast colonization in patients undergoing hemopoietic progenitor cell transplantation (HPCT) to examine associations between yeast colonization and oral mucositis.
Study Design
One hundred twenty-one consecutive HPCT patients were sampled for oral yeasts prior to fluconazole (FLC) prophylaxis, at transplant, and weekly until discharge. Clinical oral mucositis screenings were performed tri-weekly.
Results
Yeast colonization was evident at 216 of 510 total visits. Candida albicans and C. glabrata were the predominate organisms. Eight patients showed elevated MICs to FLC. One patient developed fungal septicemia. Patients with OMAS mucositis scores <20 had higher colonization rates than those with higher scores.
Conclusions
FLC is very effective in controlling a variety of oral yeasts in HPCT recipients. FLC resistant yeasts do emerge and can be the source of fungal sepsis. A positive association was not shown between yeast colonization and presence or severity of oral mucositis.
INTRODUCTION
High-dose chemotherapy with hematopoietic progenitor cell transplantation (HPCT) is an established therapy for patients with hematologic malignancies and selected solid tumors 1. Oral mucositis and fungal infections remain major complications of HPCT 2, 3. Routine antifungal prophylaxis with fluconazole (FLC) in HPCT has greatly decreased the incidence of serious Candida albicans infections 4–6. However, other Candida species resistant to fluconazole, including C. glabrata and C. krusei, have emerged 3, 4. We have previously reported that oral colonization with C. glabrata 7 or C. krusei 8 can lead to fungal sepsis in hemopoietic progenitor cell recipients. In the era of FLC prophylaxis, there is limited information regarding the oral fungal colonization of transplant recipients before and throughout the transplant course.
We performed a prospective longitudinal surveillance study of HPCT recipients using oral sampling to evaluate the prevalence of oral yeast microbiology before transplantation and the epidemiology of yeast resistance before and during the HPCT process. The study cohort was also evaluated for levels of oral mucositis since this is a common side effect of HPCT conditioning regimens 9–11. Severe mucositis can cause painful dysphagia, limiting the patient’s ability to maintain a normal diet or take oral medications. This debilitating complication can increase the need for opioid analgesics, prolong inpatient stays and increase costs 12, 13. Ulcerative mucositis compromises oral mucosal integrity and can increase risk of bacteremia or fungemia due to systemic invasion of endogenous flora 10, 14. Our purpose was to determine the change in oral yeast colonization in patients undergoing HPCT who received fluconazole prophylaxis. Additionally, we sought to determine whether there was any association between oral Candida colonization and presence of oral mucositis.
MATERIALS and METHODS
Patient population
We conducted a longitudinal, prospective study of 121 consecutive HPCT recipients with hematologic malignancies who underwent high dose chemotherapy (Table 1) followed by transplantation from July 2005 through February 2008. Our patients were referred to the South Texas Veterans Health Care System, Audie L. Murphy Division, San Antonio Texas Bone Marrow Transplant Unit from 28 different referring centers throughout the nation and Commonwealth of Puerto Rico. Each patient received antifungal prophylaxis with oral fluconazole 400 mg daily starting with conditioning regimen and continuing through engraftment. As inpatients, compliance was assured by the daily nursing staff administration and documentation of all medicinal regimens. If the patient was unable to tolerate p.o. medications intravenous routes were utilized. Informed consent was obtained from all participants/patients, and all procedures were in accordance with the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA) and the Research and Development Committee of the South Texas Veterans Health Care System.
Table 1.
Diagnosis and conditioning regimens
| Diagnosis (Number of patients) | Conditioning Regimen |
|---|---|
| Multiple Myeloma (74) | Melphalan |
| Non-Hodgkins Lymphoma (27) | CBV or BEAM |
| Hodgkin Lymphoma (7) | CBV1 or BEAM2 |
| Acute Myelogenous Leukemia (7) | BuCy3 or BuF4 |
| Testicular Germ Cell (3) | CEC6 |
| Chronic Lymphocytic Leukemia (2) | BuF4 or MelF5 |
| Myelodysplactic Syndrome (1) | BuCy3 |
CBV = cyclophosphamide, etoposide, carmustine
BEAM =carmustine, cytarabine, etoposide, melphalan
BuCy = busulfan, cyclophosphamide
BuF = busulfan, fludarabine
MelF = melphalan, fludarabine
CEC = cyclophosphamide, etoposide, carboplatin
Oral Rinse Sample Collection and Microbiological Characterization, Mucositis assessment
Sampling consisted of a 20 second oral swish with 10 ml of sterile water. On three occasions, an oral swab culture was substituted since oropharyngeal candidiasis (OPC) was evident or the patient was unable to swish and expectorate. Samples were taken before the initiation of the conditioning regimen and fluconazole prophylaxis (visit 1), the day of transplant (visit 2) which was 5.5±2.59 days later with weekly sampling until discharge (visits 3 and 4) 12.02±2.77 and 19.04 ±3.38 days later respectfully.
Samples were plated on CHROMagar Candida (DRG International, Mountainside, NJ) media containing chloramphenicol (0.5g/L) with fluconazole (8 and 16 μg/mL) or without fluconazole for presumptive fungal identification and resistance screening 15. These chromogenic medium plates were prepared in 100-mm-diameter petri dishes and stored at 4°C for up to one week prior to use. CHROMagar Candida-specific color patterns were used for presumptive yeast identification. Yeasts were further characterized using germ tube analysis after incubation in human serum at 37°C for 3 h, and by biochemical utilization patterns determined using API 20C (bioMérieux, Marcy-l’Etoile, France). Tri-weekly oral examinations utilizing both the World Health Organization (WHO) 12, 14 and the Oral Mucositis Assessment Scale (OMAS) 16 criteria were utilized for mucositis assessment and were performed by the same two healthcare providers (SDW and JJT). All patients participated in periodic oral fungal surveillance.
Antifungal Susceptibility
Minimal inhibitory concentrations (MICs) of these yeasts were determined using the Clinical and Laboratory Standards Institute (CLSI) methodology 17 by the Fungus Testing Laboratory at UTHSCSA. Yeast isolates with fluconazole MICs of ≥16 μg/mL were considered to be resistant in this study 18.
RESULTS
Our study population was 94% (112 of 119) male with a median age of 58 years (range 19 to 74, mean 55.4±11.5 years). Fifty-three percent (63 of 119) were white, 25 % (30 of 119) hispanic, 20% (24 of 119) black, and 2% (2 of 119) other races. Patients enrolled in this cohort received 106 autologous, 11 allogeneic and 2 syngeneic transplants. Two patients died prior to transplantation.
A variety of yeasts were cultured from the HPCT patients enrolled in this study. Yeast colonization was evident in 216 of the 510 total visits (42%; Table 2). Nine different yeast species were cultured from samples taken at these visits. Candida albicans and Candida glabrata were the predominate organisms in the cultures, and most specimens were susceptible to fluconazole, as shown in Table 2.
Table 2.
Yeast distribution with fluconazole MIC data in 121 HPCT recipients
| Yeast | Colonized Visits (216) | Total Visits (510) | MIC range (median) |
|---|---|---|---|
| C. albicans | 96 (44%) | 96 (19%) | 0.125 – 1.0 (0.125) |
| C. glabrata | 76 (35%) | 76 (15%) | 2–64 (4) |
| C. tropicalis | 24 (11%) | 24 (4.7%) | 0.125–32 (.25) |
| C. dubliniensis | 23 (11%) | 23 (4.5%) | 0.125–32 (0.125) |
| C. krusei | 6 (3%) | 6 (1%) | 8–16 (8) |
| S. cerevisiae | 5 (2%) | 5 (1%) | 0.5–1 (0.5) |
| C. parapsilosis | 4 (2%) | 4 (.8%) | 0.125–0.25 (0.25) |
| C. magnoliae | 1 (0%) | 1 (0%) | 0.5 (0.5) |
| C. lusitaniae | 1 (0%) | 1 (0%) | 0.5 (0.5) |
Three patients with OPC and five other patients who were only colonized with yeasts showed elevated MICs to FLC. In our patient population, fluconazole resistance, defined as MIC ≥16 μg/mL, was seen in C. glabrata, C. krusei, C. dubliniensis and C. tropicalis, as shown in Table 3 In each of these patients the fluconazole. MICs increased over time (data not shown) with the exception of patient 34, in whom the C. glabrata isolate rapidly regained susceptibility once prophylaxis with FLC was discontinued and echinocandin therapy was initiated 19.
Table 3.
Patients carrying yeasts with decreased fluconazole susceptibility
| Pt # | Yeast | Elevated MIC |
|---|---|---|
| 26 | C. glabrata | 16 |
| 28 | C. krusei | 16/64 |
| 34 | C. glabrata | 32 |
| 34 | C. dubliniensis | 32 |
| 34 | C. tropicalis | 32 |
| 41 | C. glabrata | 32 |
| 50 | C. glabrata | 64 |
| 56 | C. glabrata | 16 (48hr) |
| 57 | C. glabrata | 64 |
| 102 | C. glabrata | 32 |
Colonization by single species was common throughout the duration of the study with C. albicans and C. glabrata being the predominating organisms. However, the rate of colonization by multiple organisms was relatively low, being noted in only 27 of 216 (13%) total colonized visits. Candida glabrata was present in 25 of 27 (93%) of the visits where multiple colonization was noted. In addition, C. glabrata was present in 6 of 8 (75%) of patients colonized with multiple organisms, while C. krusei, C. dubliniensis and C. tropicalis were each present in only one patient. Patient 34 was colonized concurrently with C. glabrata and C. dubliniensis in the first visit, C. glabrata and C. tropicalis on the second visit, and only with C. glabrata on subsequent visits. Rates of colonization decreased by visit 4 when engraftment had occurred and antifungal therapy had been discontinued as shown in Figure 1. Interestingly, despite the large number of colonized visits, only three episodes of clinical OPC were seen during the study. OPC manifested as a pseudomembranous form with soft friable, creamy colored plaques on the oral mucosa. One patient had C. albicans OPC on his initial visit, while a second patient (patient 28) developed OPC on his third and fourth visits with C. krusei. This patient also developed Candida fungemia secondary to oral carriage and has been described in a previous publication 8. As a result of their pretransplant conditioning regimens (Table 1) most of our patients developed various degrees of oral mucositis starting 5–7 days after their conditioning therapy and continuing through neutrophil engraftment. None of our patients received Total Body Irradiation (TBI) as part of their treatment protocol. Ten of the patients showed no evidence of oral mucositis throughout their course of treatment while 25 had WHO mucositis scores of 3 or above. Interestingly, 21 of our patients who developed significant oral ulcerative mucositis with OMAS scores >20 at a single visit showed lower rates of yeast colonization or infection than those who did not develop significant mucositis. Yeast colonization during one or more of the first four visits was seen in 9 of these 21 (43%) patients with severe mucositis (Table 4). In contrast, those patients with mucositis scores <20 had higher colonization rates (66 of 98; 67%; p < 0.05 per Fischer’s exact test). WHO mucositis scores showed a similar pattern with patients receiving the highest scores (>3) having the lowest rate of yeast colonization. Interestingly, the WHO and OMAS mucositis grading systems associated very nicely when the categories of pain (p<0.01), hospitalization days, and febrile days in this cohort of patients were compared. Both WHO and OMAS scores increased as neutropenia days increased except at a WHO score of 4, and OMAS score of >35 where we noted a slight, nonstatistical decrease (Table 5).
Figure 1.
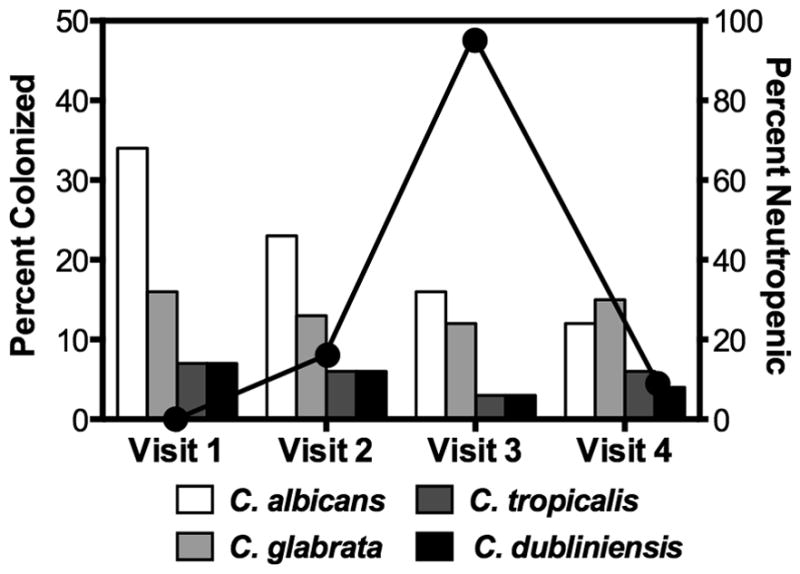
Bar graphs represent the incidence of predominant Candida species found in the total patient population for each visit. Percentages do not equal 100% due to mixed cultures. Line graph represents the percentage of patients who were neutropenic (< 1000 cells/mL) at each visit.
Table 4.
OMAS mucositis scores >20 and yeast colonization by visit
| ID | Total OMAS | WHO | Yeast V-1 | Yeast V-2 | Yeast V-3 | Yeast V-4 |
|---|---|---|---|---|---|---|
| 7 | 23 | 2 | CA1 | CA | CA | CA |
| 9 | 22 | 2 | NG2 | NG | NG | NG |
| 17 | 31 | 3 | NG | NG | NG | NG |
| 22 | 34 | 4 | NG | NG | NG | NG |
| 28 | 32 | 4 | NG | NG | CK3 | CK |
| 37 | 25 | 2 | NG | NG | NG | NG |
| 44 | 20 | 2 | NG | NG | NG | NG |
| 46 | 32 | 3 | CA | CA | CA | CA |
| 50 | 39 | 4 | CA | CA | NG | NG |
| 51 | 37 | 4 | NG | NG | NG | NG |
| 59 | 27 | 2 | NG | SC4 | SC | SC |
| 67 | 42 | 3 | CA | CA | CA | CA |
| 69 | 34 | 3 | NG | NG | NG | NG |
| 78 | 35 | 4 | NG | NG | NG | NG |
| 82 | 38 | 4 | NG | NG | NG | NG |
| 90 | 41 | 3 | CT5/CG6 | CT/CG | CT/CG | CT/CG |
| 105 | 25 | 2 | CG | NG | NG | NG |
| 107 | 34 | 3 | CT | CT | CT | CT |
| 112 | 26 | 3 | NG | NG | NG | NG |
| 113 | 37 | 3 | CG | CG | CG | CG |
| 123 | 26 | 3 | NG | NG | NG | NG |
CA: Candida albicans
NG: No growth
CK: Candida krusei
SC: Saccharomyces cerevisiae
CT: Candida tropicalis
CG: Candida glabrata
Table 5.
WHO/OMAS Scores versus therapy sequelae
| WHO | OMAS | Pain* | Diarrhea Days | Fever Days | Hospital Days | Neutropenic Days |
|---|---|---|---|---|---|---|
| 0 | 0.25 | 0 | 7 | 1.66 | 15.8 | 6.91 |
| 1 | 3 | 1.13 | 6.13 | 1.06 | 16.8 | 7.86 |
| 2 | 11.23 | 3.29 | 6.37 | 2.11 | 19.34 | 8.25 |
| 3 | 23.05 | 6.58 | 8.95 | 3.42 | 24.21 | 8.84 |
| 4 | 35.83 | 8.83 | 8.33 | 9 | 53.33 | 7.66 |
P<0.01 vs WHO and OMAS
DISCUSSION
Most institutions utilize antifungal prophylaxis for patients undergoing HPCT 4. We utilize fluconazole 400 mg p.o. daily from pretransplant conditioning though neutrophil engraftment. Despite this antifungal prophylaxis 45% (53 of 119) of our patients were still colonized at visit 2 (day of transplant). Our patients’ conditioning regimen of high dose chemotherapy without TBI in the mainly autologous transplant population resulted in only 18% (21 of 119) of our patients developing significant ulcerative mucositis. In agreement with a similar oral candidiasis study of mainly allogeneic transplant recipients, a positive association was not determined between Candida colonization and degree or presence of oral mucositis in our transplant cohort 20. Our reported lower oral colonization in patients with the highest OMAS and WHO scores may have resulted from less vigorous oral swishing by HPCT recipients as mucositis symptoms increased, which may have captured fewer yeasts. The patients were also encouraged to use an oncology triple mix (lidocaine, kaopectate and diphenhydramine) for symptomatic relief; these additional rinses may have lowered our yeast counts. During the course of this study, both WHO and OMAS mucositis scoring systems were determined and found to have similar utility.
Colonization by C. albicans was most prevalent in these patients, however, relatively high numbers of yeasts with a propensity for fluconazole resistance, notably C. glabrata and C. dubliniensis, were recovered. Also, C. glabrata was commonly found in mixed colonization. Despite their presence, most yeast isolates were highly susceptible to fluconazole. In addition, fluconazole prophylaxis appears highly effective as only one patient developed a documented fungemia, and rates of oral yeast colonization deceased by the third visit despite maximum neutropenia at this time. This was especially evident for those with C. albicans as the rates of colonization with this species significantly decreased between visit 1 and visit 3 (34% vs. 16%; p = 0.006). This is important, as major risk factors for Candida fungemia include neutropenia, mucositis and the presence of a central venous catheter, which were present in many of those in this cohort 4. Fortunately, fungal sepsis with its origin from oral colonization is rare in these patients. However, patient 28 demonstrates that under conditions of severe neutropenia and ulcerative oral mucositis, while receiving fluconazole prophylaxis, oral colonization with C. krusei can lead to candidemia with the same colonizing organism. We compared the third and fourth oral swab cultures of C. krusei to subsequent blood and urine isolates using molecular techniques to determine that the initial oral colonization was the likely source of systemic infection. To our knowledge, this was the first report of the relationship documenting C. krusei fungemia with oral carriage in an autologous HPCT recipient 8. We have also previously described, in another population of patients, one other case of Candida fungemia from an oral source in an allogeneic HPCT recipient. In this case the offending organism was C. glabrata 7. These two cases, with an oral source, have shown elements common to patients with Candida fungemia including severe neutropenia and severe oral mucositis. However they also included colonization with an organism with elevated MICs to fluconazole, such as C. glabrata and C. krusei 7, 8. When these organisms are found in oral colonization in HPCT recipients, modification of antifungal prophylaxis should be considered.
CONCLUSIONS
Measurements for both the WHO and OMAS mucositis grading systems are comparable and closely follow other patient complications such as fever days, hospital inpatient days and pain scores. A positive association was not shown between yeast colonization and the presence or severity of oral mucositis. Fungal organisms that are resistant to FLC can lead to fungal sepsis. When FLC resistant organisms are detected in HPCT recipients, modifications of antifungal prophylaxis should be considered.
Acknowledgments
FINANCIAL SUPPORT
This work was supported in part by Public Health Service Grant DE-18096 from the National Institute of Dental and Craniofacial Research.
The authors acknowledge Marcos Olivo for his technical assistance with yeast identification and isolation. We are grateful to the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio for their assistance in determining the MICs of the yeast isolates.
Footnotes
CONFLICT OF INTEREST
TFP: Research Support/Consultant: Astellas Pharma US, Merck & Co., Viamet Pharmaceuticals, Inc., Toyama Chemical Company, and Pfizer Inc. SWR: Research Support: Pfizer Inc., Merck & Co., Astellas Pharma US Inc. NPW: Research Support/Consultant: Astellas Pharma US, Merck & Co., CyDex, Viamet Pharmaceuticals, Inc., Toyama Chemical Company, and Pfizer Inc. SDW, WRK, JJT, COF: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JB, Raber-Durlacher JE, Wilkins A, Chavarria MG, Myint H. Advances in hematologic stem cell transplant: an update for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:301–12. doi: 10.1016/j.tripleo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 4.Marr KA, Bow E, Chiller T, Maschmeyer G, Ribaud P, Segal B, et al. Fungal infection prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44:483–7. doi: 10.1038/bmt.2009.259. [DOI] [PubMed] [Google Scholar]
- 5.Wirk B, Wingard JR. Current approaches in antifungal prophylaxis in high risk hematologic malignancy and hematopoietic stem cell transplant patients. Mycopathologia. 2009;168:299–311. doi: 10.1007/s11046-009-9188-6. [DOI] [PubMed] [Google Scholar]
- 6.Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–51. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 7.Redding SW, Marr KA, Kirkpatrick WR, Coco BJ, Patterson TF. Candida glabrata sepsis secondary to oral colonization in bone marrow transplantation. Med Mycol. 2004;42:479–81. doi: 10.1080/13693780410001731574. [DOI] [PubMed] [Google Scholar]
- 8.Westbrook SD, Kirkpatrick WR, Freytes CO, Toro JJ, Bernardo S, Patterson TF, et al. Candida krusei sepsis secondary to oral colonization in a hemopoietic stem cell transplant recipient. Med Mycol. 2007;45:187–90. doi: 10.1080/13693780601164306. [DOI] [PubMed] [Google Scholar]
- 9.Blijlevens N, Schwenkglenks M, Bacon P, D’Addio A, Einsele H, Maertens J, et al. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy--European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26:1519–25. doi: 10.1200/JCO.2007.13.6028. [DOI] [PubMed] [Google Scholar]
- 10.Laheij AM, de Soet JJ, von dem Borne PA, Kuijper EJ, Kraneveld EA, van Loveren C, et al. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support Care Cancer. 2012 Apr 26; doi: 10.1007/s00520-012-1463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–31. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 12.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 13.Jones JA, Qazilbash MH, Shih YC, Cantor SB, Cooksley CD, Elting LS. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112:1096–105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979. [Google Scholar]
- 15.Patterson TF, Revankar SG, Kirkpatrick WR, Dib O, Fothergill AW, Redding SW, et al. Simple method for detecting fluconazole-resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–7. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH, Jr, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group Cancer. 1999;85:2103–13. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard. 3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. (M27-A3) [Google Scholar]
- 18.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24:235–47. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 19.Westbrook SD, Wiederhold NP, Vallor AC, Kotara S, Bernardo S, Lee SA, et al. Loss of in vitro resistance in Candida glabrata following discontinuation of fluconazole prophylaxis in a hematopoietic stem cell transplantation patient. Med Mycol. 2010;48:557–60. doi: 10.3109/13693780903213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein JB, Hancock PJ, Nantel S. Oral candidiasis in hematopoietic cell transplantation patients: an outcome-based analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:154–63. doi: 10.1016/s1079-2104(03)00296-8. [DOI] [PubMed] [Google Scholar]


