Abstract
Recent studies have suggested a link between exposure to ambient particulate matter <2.5μm in diameter (PM2.5) and adverse cardiovascular outcomes. The objective of this study was to examine the effects of differing community-level exposure to PM2.5 on daily measures of blood pressure (BP) among an adult population. During the period May 2002 through April 2003, BP was examined at two time points for 347 adults residing in three distinct communities of Detroit, MI. Exposure to PM2.5 was assessed in each community during this period, along with multivariate associations between PM2.5 and BP. In models combining all three communities, PM2.5 was significantly associated with systolic pressure (SP); a 10 μg/m3 increase in daily PM2.5 was associated with a 3.2 mm Hg increase in SP (p=0.05). However, in models that added a location interaction, larger effects were observed for SP within the community with highest PM2.5 levels; a 10 μg/m3 increase in daily PM2.5 was associated with a 8.6 mm Hg increase in SP (p=0.01). We also found young age (<55 years) and not taking BP medications to be significant predictors of increased BP effects. Among those taking BP medications, the PM2.5 effect on BP appeared to be mitigated, partially explaining the age effect, as those participants less than 55 years were less likely to take BP medications. Short-term increases in exposure to ambient PM2.5 are associated with acute increases in BP in adults, especially within communities with elevated levels of exposure.
Keywords: air pollution, particulate matter, blood pressure, urban, cardiovascular outcomes
Introduction
Several observational studies have demonstrated that short-term exposure to fine particulate matter < 2.5 μm in diameter (PM2.5) can acutely raise blood pressure (BP).1-5 However, not all studies have been positive.6-9 Discrepancies between previous studies may result from variations in: characteristics or susceptibility of study participants, PM exposure mischaracterizations, varying chemical composition of the PM, protective medication effects taken by some participants, possible lack of adjustments for other confounders, and inaccurate determinations of BP.10 Importantly, no previous study that has linked PM2.5 exposure and BP has reported the effect of varying pollutant exposure types within a metropolitan area in order to identify potentially sensitive sub-populations and/or particularly toxic local PM environments. This is important because the pro-hypertensive actions of PM2.5 may be limited to a specific subset of at-risk individuals and/or may be mediated only by PM of certain chemical composition.
Thus, in the current study, we examined the effect of daily exposure to PM2.5 on BP among an adult population characteristic of the general population across three distinct Detroit, Michigan communities with differing levels of exposure to ambient PM2.5. Since the communities vary in their socioeconomic and racial-ethnic compositions, with high concentrations of socioeconomically and racially-ethnically disadvantaged persons, the study also contributes to understanding the potential role of differential exposure to air pollution in health disparities of socioeconomic and racial-ethnic classes.
Methods
Data for this study was collected as part of the Detroit Healthy Environments Partnership (HEP),11 an affiliated project of the Detroit Community-Academic Urban Research Center (DCA-URC).12 The goals of HEP include gathering and analyzing biological indicators of cardiovascular disease risk, and the contributions of social and physical environments to those risk factors, in eastside, northwest, and southwest Detroit. These three communities differ in racial, ethnic, and socioeconomic composition.11 As a community-based participatory research effort,13 HEP engages researchers based in academic institutions, and representatives from health service organizations and community-based organizations in a collaborative effort to address these questions. Representatives of partner organizations comprise the HEP Steering Committee, which is involved in all aspects of the research process. The HEP study was approved in January 2001 by the University of Michigan (UM) Institutional Review Board for Protection of Human Subjects.
Blood Pressure Measures and Covariates
A stratified probability sample of 919 residents of the 3 Detroit study communities (northwest, southwest, and eastside) participated in the HEP study, with 347 of those participants completing both a stratified face-to-face survey and a biomarker component of the study.11 All BP measures and other relevant covariates were collected during the period May 2002 through April 2003 (see Table 1). These measures were made at 2 different time points for each study participant (mean of four weeks between each measurement time point). The measures included systolic and diastolic BP collected using a portable cuff device (Omron model #HEM 711AC) that passed Association for the Advancement of Medical Instrumentation (AAMI) standards.14 Self-reports of age, sex, race-ethnicity, household income, education, body mass index, smoking behavior, doctor diagnosed diabetes, and medication use for hypertension, along with measures of total cholesterol. In brief, of the variables listed in Table 1, only 2 were found to be significantly different between biomarker participants and non-participants. A slightly higher percentage of biomarker participants had an annual household income of less than $10,000 (32% vs. 26%, p=0.01), and fewer biomarker participants were characterized as “never smoked” (34% vs. 45%, p=0.02).
Table 1.
Baseline Demographics of HEP Biomarker Study Participants.
| Full Biomarker Sample | Eastside Detroit | Northwest Detroit | Southwest Detroit | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | % | Mean | SD | N | % | Mean | SD | N | % | Mean | SD | N | % | Mean | SD | |
| Systolic BP | 347 | 129.2 | 20.8 | 116 | 130.1 | 20.5 | 96 | 128.9 | 21.8 | 135 | 128.7 | 20.5 | |||||
| Diastolic BP | 347 | 79.4 | 12.7 | 116 | 80.2 | 11.9 | 96 | 80.7 | 13.8 | 135 | 77.9 | 12.4 | |||||
| Pulse BP | 347 | 49.8 | 14.7 | 116 | 49.9 | 15.1 | 96 | 48.2 | 12.9 | 135 | 50.8 | 15.6 | |||||
| BMI | 347 | 30.9 | 7.9 | 116 | 31.0 | 7.2 | 96 | 31.1 | 8.3 | 135 | 30.8 | 8.3 | |||||
| Age | 347 | 46.2 | 13.7 | 116 | 47.7 | 14.1 | 96 | 45.4 | 13.8 | 135 | 45.7 | 13.3 | |||||
| < 55 years | 236 | 75.4 | 86 | 73.4 | 69 | 72.1 | 107 | 79.3 | |||||||||
| 55 years or more | 85 | 24.6 | 31 | 26.6 | 27 | 27.9 | 28 | 20.7 | |||||||||
| Sex | Males | 99 | 44.5 | 21 | 40 | 21 | 33.6 | 53 | 55.8 | ||||||||
| Location | East | 116 | 31.8 | ||||||||||||||
| South | 135 | 40.0 | |||||||||||||||
| North | 96 | 28.2 | |||||||||||||||
| Race-Ethnicity | Hispanic | 55 | 18.0 | 1 | 1 | 53 | 43.3 | ||||||||||
| White | 70 | 20.1 | 1 | 25 | 22.2 | 44 | 33.6 | ||||||||||
| Black | 212 | 58.4 | 112 | 94.3 | 67 | 72.1 | 33 | 20.1 | |||||||||
| Household Income | <$10,000 | 124 | 35.0 | 43 | 34.9 | 34 | 37.1 | 47 | 33.5 | ||||||||
| $10,000-19,999 | 93 | 28.0 | 31 | 31.8 | 21 | 21.8 | 41 | 29.4 | |||||||||
| $20,000-34,999 | 81 | 22.2 | 30 | 21.6 | 22 | 20.0 | 29 | 24.2 | |||||||||
| $35,000+ | 49 | 14.8 | 12 | 11.7 | 19 | 21.1 | 18 | 12.9 | |||||||||
| Education | Less than 8th grade | 37 | 11.3 | 9 | 7.8 | 3 | 2.4 | 25 | 20.4 | ||||||||
| Some High School | 78 | 22.4 | 24 | 19.7 | 19 | 20.4 | 35 | 25.8 | |||||||||
| High School Graduate | 97 | 29.6 | 36 | 33.7 | 23 | 27.0 | 38 | 28.1 | |||||||||
| Some College | 79 | 20.8 | 32 | 25.8 | 27 | 26.2 | 20 | 13.0 | |||||||||
| College or More | 50 | 13.9 | 13 | 11.0 | 21 | 19.3 | 16 | 12.3 | |||||||||
| Hypertension Medication Use | 259 | 77.9 | 74 | 66.3 | 71 | 78.2 | 114 | 86.9 | |||||||||
BP was measured following the methodology utilized by the NHANES study,15 in a seated position using the right arm, with a large cuff used in instances where arm circumference was greater than 15 inches. Three consecutive measures of systolic and diastolic pressure, separated by about one minute, were taken at each of the two time points, with the mean of the 2nd and 3rd measures used for all data analysis. Pulse pressure was calculated as systolic minus diastolic BP.
Community-Level Characterization of PM2.5
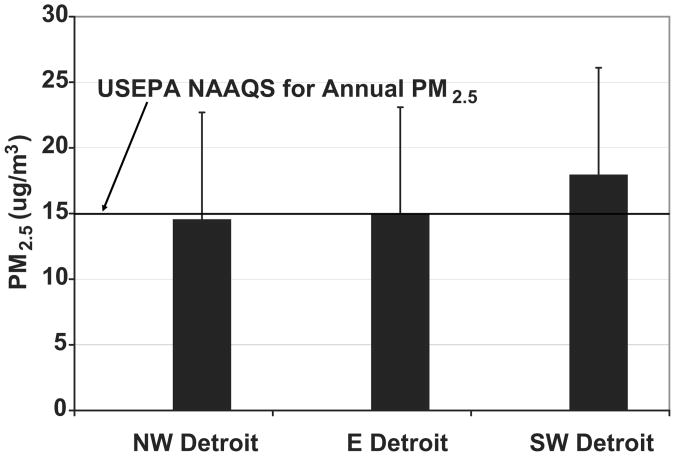
Levels of ambient PM2.5 were characterized in the 3 Detroit communities during the years 2000 to 2003 using tapered element oscillating microbalances (TEOM Model 1400a, Rupprecht and Patashnick, Inc).11,16 Two of the three monitoring sites were established for the sole purpose of conducting this study, the northwest site was previously established by the state of Michigan. Each monitoring site was located within a 5 km radius of all study participants in each respective community, allowing for a considerable increase in the geographic representativeness of community-level assessment of exposure to ambient PM2.5 over many previous studies. For days in which PM2.5 was not available from the northwest site, data was interpolated using regression with data from the eastside site, with justification for this being that daily comparative exposure data for both sites was available for 79% of the study days. Three full years of data collection found levels of PM2.5 at these two sites to be nearly identical (Figure 1), allowing the eastside site to serve as a reliable surrogate estimator of exposure for the northwest site on days when northwest data was missing. Standard meteorological variables including temperature, atmospheric pressure, relative humidity, wind speed, and wind direction were also recorded at each site.
Figure 1.
Mean PM2.5 measured in each HEP study community, 2000-2003 (error bars represent standard deviation).
Statistical Analysis
Multivariate associations between ambient PM2.5 and BP outcomes were assessed using the PROC SURVEYREG procedure of SAS for WINDOWS 9.13. These procedures are specially designed for the analysis of complex sample survey data. PROC SURVEYREG incorporates the complex sample weights (final weights, strata, and psu) for the standard error estimates, and was determined most appropriate for complex sampling designs like that of our study. Models investigated lagged exposure in two ways: 1) Individual 24-hour spans: exposure measured 1 day prior to health outcome (Lag1), 2 days prior (Lag2), up to 4 days prior (Lag4), and 2) Large spans: 48-hour average prior (2 days average), 72-hour average prior (3 days average) up to 120-hour average prior (5 days average). Covariates adjusted for in all models included: age, sex, race-ethnicity, household income, education, body mass index, smoking behavior, doctor diagnosed diabetes, total cholesterol, and medication use for hypertension. We also estimated models that controlled for meteorological variables. However, due to the previously known high level of covariance between ambient PM2.5 and temperature (correlation coefficients as high as 0.7 for our study), we were not able to include temperature in the final models since this resulted in non-convergence of the model.
Results
The mean (SD) level of PM2.5 measured across all three community-level monitoring sites for the period 2000-2003 was 15.0 (8.2) μg/m3 (mean levels at each individual site are shown in Figure 1). Concentrations observed at the southwest Detroit site were significantly elevated (by roughly 20%) over those measured at the northwest and eastside monitoring locations. These levels are above the USEPA-National Ambient Air Quality Standard (NAAQS) of 15 μg/m3 for annual PM2.5.
Multivariate associations between BP and community-level exposure to PM2.5 were examined at varying lag levels (1-5 days), and included analyses to assess the modification of the relationship by community location, age, baseline BP, and medication use. Overall, regression equations demonstrated positive associations between exposure to PM2.5 and increased systolic pressure and pulse pressure. In particular, significant effect modification of these associations were observed for community location, age, and medication use (data presented below), while no significant effects were found for baseline BP (data not presented).
Effects of Community Location
Table 2a presents analysis results for individual day lag effects. As is shown, PM2.5 was significantly associated with systolic pressure (as well as pulse pressure) for Lag2 (p=0.05), as a 10 μg/m3 increase in daily PM2.5 was associated with a 3.2 mm Hg increase in systolic pressure. However, the inclusion of a community location interaction term in the model found the observed effects to be greatly enhanced in the southwest Detroit community relative to the other two communities. For example, as is seen in Table 2b, a significant increase in systolic pressure (as well as pulse pressure) was observed for Lags 2, 3, & 4. The effects of PM2.5 were not only more consistent across lags for the location interaction model, but the magnitude of the effect was also greater [ex: a 10 μg/m3 increase in daily PM2.5 was associated with a 8.6 mm Hg increase in systolic pressure for Lag4 (p=0.01)]. Models were also assessed for effects of multi-day averaged exposure to PM2.5 on BP outcomes. Similar to the analysis of individual day lag effects, analysis of multi-day averaged exposures found significant effects on systolic pressure (5 days) without a location interaction included in the model (Table 3a). However, inclusion of the location interaction found the observed effects on systolic pressure (as well as pulse pressure) to be enhanced in the southwest Detroit community relative to the other two communities (Table 3b).
Table 2.
(a and b) Individual day lag effects of PM2.5 on BP outcomes (per 10 μg/m3 increase in 2.5) assessing community location interaction.
| a.) Total Sample - Lags | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Blood Pressure | Exposure | Lag 1 | Lag 2 | Lag 3 | Lag 4 | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | ||
| Systolic | PM2.5 | -0.33 | 0.83 | 3.24 | 0.05 | 1.37 | 0.32 | 3.75 | 0.11 |
| Diastolic | PM2.5 | -1.42 | 0.14 | -0.92 | 0.41 | -0.13 | 0.91 | 1.54 | 0.34 |
| Pulse | PM2.5 | 1.10 | 0.29 | 4.16 | 0.01 | 1.53 | 0.10 | 2.36 | 0.11 |
| b.) Location Interaction (Southwest Detroit) - Lags | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Blood Pressure | Exposure | Lag 1 | Lag 2 | Lag 3 | Lag 4 | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | ||
| Systolic | PM2.5 | -2.71 | 0.23 | 4.66 | 0.01 | 3.47 | 0.02 | 8.58 | 0.01 |
| Diastolic | PM2.5 | -1.95 | 0.16 | -1.16 | 0.42 | 0.63 | 0.71 | 2.44 | 0.41 |
| Pulse | PM2.5 | -0.73 | 0.74 | 5.93 | 0.01 | 3.00 | 0.02 | 6.40 | 0.01 |
Note: The following variables are controlled/included in all equations: age, sex, race-ethnicity, household income, education, body mass index, doctor diagnosed diabetes, smoking behavior, total cholesterol, and medication use for hypertension.
Table 3.
(a and b) Combined day lag effects of PM2.5 on BP outcomes (per 10 μg/m3 increase in PM2.5) assessing community location interaction.
| a.) Total Sample - Averages | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Blood Pressure | Exposure | 2 Days | 3 Days | 4 Days | 5 Days | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | ||
| Systolic | PM2.5 | 1.19 | 0.56 | 2.17 | 0.26 | 3.87 | 0.08 | 4.73 | 0.05 |
| Diastolic | PM2.5 | -1.89 | 0.15 | -1.27 | 0.38 | -0.59 | 0.75 | 0.89 | 0.64 |
| Pulse | PM2.5 | 3.15 | 0.04 | 3.56 | 0.01 | 4.62 | 0.01 | 4.04 | 0.02 |
| b.) Location Interaction (Southwest Detroit) - Averages | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Blood Pressure | Exposure | 2 Days | 3 Days | 4 Days | 5 Days | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | ||
| Systolic | PM2.5 | 0.07 | 0.98 | 3.27 | 0.08 | 5.65 | 0.01 | 5.93 | 0.01 |
| Diastolic | PM2.5 | -2.09 | 0.16 | 0.09 | 0.96 | 1.57 | 0.51 | 2.02 | 0.36 |
| Pulse | PM2.5 | 2.49 | 0.39 | 3.55 | 0.04 | 4.50 | 0.02 | 4.24 | 0.02 |
Note: The following variables are controlled/included in all equations: age, sex, race-ethnicity, household income, education, body mass index, doctor diagnosed diabetes, smoking behavior, total cholesterol, and medication use for hypertension.
Effects of Age and Medication Use
Table 4 presents analysis results for effect of age on individual day lag relationships. Contrary to expected outcomes based on previous literature, we found young age (those <55 years) to be a significant predictor of increased BP effects (both systolic and pulse pressure for Lag2 and Lag4). Since our data showed increased medication use among older study participants, we then analyzed for effect modification by prevalence of BP medication use. These results (Table 5a) clearly showed that not taking BP medication was a strong predictor of increased BP effects for both systolic and pulse pressure. When we then added the community location interaction to the model, we saw further increases in BP specific to residing in the SW Detroit community (Table 5b). For example, a 10 μg/m3 increase in daily PM2.5 was associated with a 10.3 mm Hg increase in systolic pressure for Lag4 (p=0.01). Among those taking BP medications, the PM2.5 effect on BP appeared to be mitigated, partially explaining the age effect, as those participants less than 55 years were less likely to use BP medications.
Table 4.
Individual day lag effects of PM2.5 on BP outcomes (per 10 μg/m3 increase in PM2.5) assessing effect modification by age.
| Total Sample - Lags | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Blood Pressure | Exposure | Effect Modification | Lag 1 | Lag 2 | Lag 3 | Lag 4 | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | |||
| Systolic | PM2.5 | 55+ | 3.33 | 0.30 | 1.23 | 0.78 | 0.55 | 0.87 | -1.25 | 0.73 |
| 25-54 years | -1.23 | 0.44 | 4.24 | 0.02 | 1.50 | 0.26 | 6.28 | 0.02 | ||
| Diastolic | PM2.5 | 55+ | -1.70 | 0.20 | -3.76 | 0.06 | -1.67 | 0.40 | -0.93 | 0.64 |
| 25-54 years | -1.36 | 0.20 | 0.25 | 0.84 | 0.44 | 0.71 | 2.74 | 0.17 | ||
| Pulse | PM2.5 | 55+ | 5.07 | 0.08 | 4.78 | 0.17 | 2.20 | 0.38 | -0.29 | 0.92 |
| 25-54 years | 0.08 | 0.94 | 4.02 | 0.02 | 1.11 | 0.17 | 3.61 | 0.01 | ||
Note: The following variables are controlled/included in all equations: sex, race-ethnicity, household income, education, body mass index, doctor diagnosed diabetes, smoking behavior, total cholesterol, and medication use for hypertension.
Table 5.
(a and b) Individual day lag effects of PM2.5 on BP outcomes (per 10 μg/m3 increase in PM2.5) assessing effect modification by prevalence of BP medication use and including community location interaction.
| a.) Total Sample - Lags | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Blood Pressure | Exposure | Effect Modification | Lag 1 | Lag 2 | Lag 3 | Lag 4 | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | |||
| Systolic | PM2.5 | Taking BP Med. | 3.75 | 0.41 | 5.76 | 0.32 | 1.45 | 0.68 | 0.67 | 0.89 |
| Not Taking BP Med. | -1.30 | 0.32 | 2.93 | 0.07 | 1.88 | 0.17 | 6.01 | 0.01 | ||
| Diastolic | PM2.5 | Taking BP Med. | 1.42 | 0.58 | -0.70 | 0.84 | 0.04 | 0.99 | -1.58 | 0.59 |
| Not Taking BP Med. | -2.27 | 0.01 | -0.93 | 0.39 | 0.06 | 0.96 | 3.42 | 0.06 | ||
| Pulse | PM2.5 | Taking BP Med. | 2.35 | 0.40 | 5.85 | 0.18 | 1.31 | 0.53 | 2.39 | 0.42 |
| Not Taking BP Med. | 0.92 | 0.41 | 3.94 | 0.01 | 1.87 | 0.08 | 2.72 | 0.04 | ||
| b.) Location Interaction (Southwest Detroit) - Lags | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Blood Pressure | Exposure | Effect Modification | Lag 1 | Lag 2 | Lag 3 | Lag 4 | ||||
| Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | Δ mm Hg | p-value | |||
| Systolic | PM2.5 | Taking BP Med. | 2.11 | 0.67 | 7.64 | 0.20 | 4.02 | 0.29 | 4.55 | 0.31 |
| Not Taking BP Med. | -2.84 | 0.23 | 4.71 | 0.01 | 3.18 | 0.04 | 10.25 | 0.01 | ||
| Diastolic | PM2.5 | Taking BP Med. | 1.22 | 0.67 | -1.36 | 0.70 | 1.31 | 0.63 | -0.71 | 0.84 |
| Not Taking BP Med. | -2.30 | 0.07 | -1.09 | 0.45 | 0.55 | 0.74 | 4.00 | 0.16 | ||
| Pulse | PM2.5 | Taking BP Med. | 0.96 | 0.78 | 8.65 | 0.07 | 2.94 | 0.30 | 5.55 | 0.02 |
| Not Taking BP Med. | -0.54 | 0.81 | 5.98 | 0.01 | 2.85 | 0.04 | 6.54 | 0.01 | ||
Note: The following variables are controlled/included in all equations: age, sex, race-ethnicity, household income, education, body mass index, doctor diagnosed diabetes, smoking behavior, and total cholesterol.
Discussion
In this study of 347 adults in three Detroit communities, short-term increases in exposure to PM2.5 levels less than the current daily USEPA NAAQS (65 μg/m3) were significantly associated with an increase in systolic and pulse pressure. These results confirm and extend previous epidemiological studies to a broad population of adults by demonstrating these effects in a multi-ethnic community sample. Moreover, not only was PM2.5 related to alterations in BP, but the effect of air pollution varied by community location, age, and BP medication use. This provides critically important insight of the cardiovascular risk conveyed by air pollutants by strongly supporting that PM2.5 from differing sources and/or chemical composition have a differential impact on BP, and therefore likely cardiovascular risk as well.
Even relatively small increases in systolic and/or pulse pressure of similar magnitudes found in this study are well-established to substantially increase the long-term risk for both coronary and cerebrovascular events.17,18 However, these associations are presumably related to sustained BP elevations. It is not clear whether the differences in BP due to PM exposures found in this study are maintained in a chronic fashion and thereby contribute to a long-term elevated cardiovascular risk. This is hypothetically possible and requires further investigation. Nonetheless, this hemodynamic pro-hypertensive change has been consistently implicated as one of the major triggers of cardiovascular events in vulnerable individuals.19 It is conceivable that in susceptible people, a rapid pro-hypertensive response (or the underlying mediating hemodynamics responsible such as arterial vasoconstriction and increased vascular resistance) over a few days could trigger atherosclerotic plaque disruption and thus promote an acute myocardial infarction or stroke. In vulnerable coronary heart disease patients, the BP increase could also instigate myocardial ischemia due to increases in cardiac afterload and oxygen demand. Moreover, the relation between BP increase and PM2.5 was shown to be linear. The actual increase in BP therefore could be substantially larger on days with extreme elevations in air pollution. For example, the 5th and 95th percentile PM2.5 pollution days for the SW Detroit community for our study period were 4.9 and 35.1 μg/m3, respectively. Based on results in Table 5a, an individual residing in SW Detroit and not taking BP medications would have a theoretical increase in systolic pressure of 31 mm Hg (based on the 10.3 mm Hg increase in systolic pressure per 10 μg/m3 increase in daily PM2.5, Lag4) from PM2.5 exposure on a 5th percentile pollution day to a 95th percentile pollution day. Finally, there is a wide range in the magnitude of BP elevation within subjects, and certain susceptible individuals may actually respond with much larger degrees of BP increase than the population mean. Therefore, our findings may provide an important explanation of a key mechanism whereby air pollutants are capable of increasing the risk both for acute coronary and cerebrovascular events over a few-day period.
Community Location Effect
Elevated levels of PM2.5 have been reported for southwest Detroit16 and attributed to the density of traffic and industrial facilities present in this community relative to other areas of the city.20 Results of the community location analysis in this study suggest that increased levels of PM2.5, and possibly differences in chemical composition of the PM emitted from nearby emission sources may be responsible for the adverse effect observed on BP outcomes. Two specific studies of PM using animal models have previously been conducted in southwest Detroit and have observed impacts of nearby emission sources. One study assessed levels of plasma asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthase, in rats following three days of exposure to concentrated ambient PM2.5,21 and found a significant increase of ADMA in rats exposed to PM compared to a control group exposed to filtered air. The measured meteorological conditions and the elemental tracers observed in the PM2.5 suggested that emissions from a nearby industrial complex (including coal combustion, oil refineries and coke ovens) may have considerably contributed to the overall mass of PM2.5 in this study. Another animal-based study conducted in southwest Detroit found that the chemical composition of PM, rather than the PM2.5 mass concentration, was most indicative of adverse effects.22 These analyses determined that increased pulmonary retention of specific chemical components of PM2.5 were associated with the enhancement of airway inflammation, specifically in rodents with increased eosinophilic infiltrates in lungs of allergic rats. Further, the analysis determined the likely source of the retained chemical components in the lung tissue to be from the nearby industrial source complex located within southwest Detroit and upwind of the study site during the exposure period.
Most research to date has focused on ambient PM2.5 mass and has not involved extensive exposure characterization; therefore, little is known regarding the effects of specific PM2.5 sources and components on human health. Our findings provide evidence that exposure to PM2.5 from different communities within the same city (differing sources and chemical composition) can have a differential impact on human health outcomes, in this case BP. This corroborates two recent studies, where long-term exposure to PM2.5 was associated with widely different cardiovascular outcomes across different communities within the same urban area.23,24 However, further studies are required in order to help determine the most toxic and responsible PM constituents.
Effects of Age and BP Medication Use
Contrary to what might have been expected based on previous literature on susceptibility to PM, we found that young age (those <55 years) modified the relationship between BP and individual day lag exposures to PM2.5. Since there was higher medication use among older study participants, we then analyzed for effect modification by prevalence of medication use for hypertension. These results clearly showed that not taking medication was a strong predictor of increased BP effects (both systolic and pulse pressure). Among those taking BP medications, the PM2.5 effect on BP appeared to be mitigated, partially explaining the age effect, as participants <55 years were less likely to take BP medications.
Blood pressure medications appeared to be protective in our study against the effects of PM exposure. While we were not able to assess if different classes of BP medications were more or less protective, it is likely that there would be differences, and further investigation of this finding is needed in future studies. Beta blockers may be most protective by blocking SNS responses, or perhaps that ACEI and ARBs may be most protective due to their anti-oxidant and anti-inflammatory responses. Controlled studies with hypertensive versus normotensive participants not on BP meds (looking at beta blockers vs. ace inhibitors or angiotensin receptor blockers vs. calcium blockers vs. diuretics in responses - each separately) could assess if there are differences in responses following PM exposure.
Potential Mechanisms
Several biological mechanisms could be responsible for affecting cardiovascular hemodynamics in response to PM2.5.25 While the actual etiology must remain speculative, plausible pathways have been described in human and animal studies, and theories to explain these findings include the release of pro-inflammatory/oxidative mediators from pulmonary cells and/or trans-located PM constituents effecting the function of the system arterial circulation.25 A third hypothesis is that PM within the lung may promote arterial vasoconstriction via altering CV autonomic nervous system balance. The inhalation of PM has been shown to induce changes in autonomic balance favoring sympathetic activity, mediate systemic oxidative stress and inflammation, and promote vascular dysfunction leading to arterial vasoconstriction.25-28 The pulmonary tree is widely innervated by vagal afferents.29 Stimulation of many of the nervous receptor subtypes can instigate reflex autonomic responses and alter CV sympathetic/parasympathetic balance.29 Several studies have shown that PM rapidly effects CV autonomic tone.30-34 Overlapping and different mechanisms may be responsible for alterations in BP at varying time points. Nevertheless, these pathways are each individually, or in sum, hypothetically capable of promoting physiological BP elevations.35
Limitations
Significant relationships were observed after controlling for several potential confounders; however, residual confounding remains possible and other important variables may not have been considered. Furthermore, this study was conducted over a relatively short time duration, and in a limited adult sample with a low median income. Because PM exposure and hypertension are associated with socioeconomic status, the finding of significant effects within this sample with limited income may be conservative. The results and conclusions reported here need to be confirmed with larger samples with a broader range of socioeconomic characteristics. The lack of detailed medication information was also a limitation, and this study did not determine PM chemical components and source impacts on a daily basis. Future studies will be required to clarify the relevant biologic mechanisms and to identify the specific PM constituents responsible for mediating the observed adverse BP effects.
Perspectives
Despite these limitations, we found that exposure to levels of PM2.5 that do not exceed the current daily USEPA-NAAQS was associated with potentially clinically meaningful increases in systolic and pulse pressure. We found young age (<55 years) to be a significant predictor of increased BP effects, partially explained by an apparent mitigating effect of taking BP medication, with older participants more likely to be using medication. Our findings corroborate and extend previous much smaller studies and demonstrate that PM2.5 within individual communities of an urban area may have varying effects on BP. There is substantial evidence that low-income communities of color are more likely to be exposed to sources of air pollutants. Given that the differentials in exposure to and BP impact of PM2.5 are associated with variations in the racial-ethnic and socioeconomic composition of community populations, future research should further explore not only the pollution emission sources contributing to and mechanisms producing these effects, but also their implications for understanding and potentially alleviating racial-ethnic and socioeconomic disparities in health.
Acknowledgments
HEP is a project of the DCA-URC. We thank members of the HEP Steering Committee11 for their contributions to the work presented here. We also thank members of the UM Air Quality Laboratory for assistance with PM data collection, and the UM Nutritional Biomarkers Laboratory for assistance with BP measures.
Sources of Funding: This research was supported by the NIEHS, #RO1-ES10936-0. Work by the Michigan DEQ and funding from the MCECH (U.S.EPA-#R826710-01, NIEHS-#P01-ES09589-01 and #R01-ES10688-03) helped support air quality data analyzed as part of HEP.
Footnotes
Disclosures: None.
Contributor Information
J. Timothy Dvonch, Email: dvonch@umich.edu, The University of Michigan, Department of Environmental Health Sciences Ann Arbor, MI 48109.
Srimathi Kannan, Email: skannan@nutrition.umass.edu, University of Massachusetts, Department of Nutrition Amherst, MA 01003.
Amy J. Schulz, Email: ajschulz@umich.edu, The University of Michigan, Department of Health Behavior and Health Education Ann Arbor, MI 48109.
Gerald J. Keeler, Email: jkeeler@umich.edu, The University of Michigan, Department of Environmental Health Sciences Ann Arbor, MI 48109.
Graciela Mentz, Email: gmentz@umich.edu, The University of Michigan, Department of Health Behavior and Health Education Ann Arbor, MI 48109.
James House, Email: jimhouse@umich.edu, The University of Michigan, Department of Epidemiology Ann Arbor, MI 48109.
Alison Benjamin, Email: alison_swdev@flash.net, Southwest Detroit Environmental Vision Detroit, MI 48209.
Paul Max, Email: maxp@health.ci.detroit.mi.us, Detroit Department of Health and Wellness Promotion Detroit, MI 48202.
Robert L. Bard, Email: bbard@umich.edu, The University of Michigan, Department of Internal Medicine Ann Arbor, MI 48109.
Robert D. Brook, Email: robdbrok@umich.edu, The University of Michigan, Department of Internal Medicine Ann Arbor, MI 48109.
References
- 1.Linn WS, Gong H, Clark KW, Anderson KR. Day-to-day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc. 1999(49):PM108–115. doi: 10.1080/10473289.1999.10463890. [DOI] [PubMed] [Google Scholar]
- 2.Ibald-Mulli A, Stieber J, Wichmann H-E, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Pub Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Xu QS, Park SY, Kim JH, Hwang SS, Lee KH, Lee HJ, Hong YC. Seasonal variation of effect of air pollution on blood pressure. J Epidemiol Community Health. 2007;61:314–318. doi: 10.1136/jech.2006.049205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibald-Mulli A, Timonen KL, Peters A, Heinrich J, Wölke G, Lanki T, Buzorius G, Kreyling WG, de Hartog J, Hoek G, ten Brink HM, Pekkanen J. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect. 2004;112:369–377. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipfert FW, Perry HM, Jr, Miller JP, Baty JD, Wyzga RE, Carmody SE. Air pollution, blood pressure, and their long-term associations with mortality. Inhal Toxicol. 2003;15:493–512. doi: 10.1080/08958370304463. [DOI] [PubMed] [Google Scholar]
- 8.Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO) Eur J Epidemiol. 2006;21:485–491. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 9.Harrabia I, Rondeaub V, Dartiguesc J-F, Tessiera J-F, Filleuld R. Effects of particulate air pollution on systolic blood pressure: A population-based approach. Environ Res. 2006;101:89–93. doi: 10.1016/j.envres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Brook RD. You are what you breathe: evidence linking air pollution and blood pressure. Curr Hypertens Rep. 2005;7:427–434. doi: 10.1007/s11906-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 11.Schulz AJ, Kannan S, Dvonch JT, Israel BA, Allen A, 3rd, James SA, House JS, Lepkowski J. Social and physical environments and disparities in risk for cardiovascular disease: the Healthy Environments Partnership conceptual model. Environ Health Perspect. 2005;113:1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel BA, Lichtenstein R, Lantz P, McGranaghan R, Allen A, Guzman JR, Softley D, Maciak B. The Detroit Community–Academic Urban Research Center: development, implementation and evaluation. J Public Health Manag Pract. 2001;7:1–19. doi: 10.1097/00124784-200107050-00003. [DOI] [PubMed] [Google Scholar]
- 13.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 14.Yarows SA, Brook RD. Measurement variation among 12 electronic home blood pressure monitors. Am J Hypertens. 2000;13:276–282. doi: 10.1016/s0895-7061(99)00182-x. [DOI] [PubMed] [Google Scholar]
- 15.National Health and Nutrition Examination Staff Procedures Manual for the NHANES Survey 1971-73. part 15a Washington, DC: National Center for Health Statistics; 1972. [Google Scholar]
- 16.Keeler GJ, Dvonch JT, Yip FY. Assessment of personal and community-level exposures to particulate matter among children with asthma in Detroit, Michigan, as part of Community Action Against Asthma (CAAA) Environ Health Perspect. 2002;110:173–181. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 18.Panagiotakos DB, Kromhout D, Menotti A, Chrysohoou C, Dontas A, Pitsavos C, Adachi H, Blackburn H, Nedeljkovic S, Nissinen A. The relation between pulse pressure and cardiovascular mortality in 12,763 middle-aged men from various parts of the world. Arch Intern Med. 2005;165:2142–2147. doi: 10.1001/archinte.165.18.2142. [DOI] [PubMed] [Google Scholar]
- 19.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 20.Morishita M, Keeler GJ, Wagner JG, Harkema JR. Source identification of ambient PM2.5 during summer inhalation exposure studies in Detroit, MI. Atmos Environ. 2006;40:3835–3844. [Google Scholar]
- 21.Dvonch JT, Brook RD, Keeler GJ, Rajagopalan S, D'Alecy LG, Marsik FJ, Morishita M, Yip FY, Brook JR, Timm EJ, Wagner JG, Harkema JR. Effects of concentrated fine ambient particles on rat plasma levels of asymmetric dimethylarginine. Inhal Toxicol. 2004;16:473–480. doi: 10.1080/08958370490439678. [DOI] [PubMed] [Google Scholar]
- 22.Morishita M, Keeler GJ, Wagner JG, Marsik FJ, Timm EJ, Dvonch JT, Harkema JR. Pulmonary retention of particulate matter is associated with airway inflammation in allergic rats exposed to air pollution in urban Detroit. Inhal Toxicol. 2004;16:663–674. doi: 10.1080/08958370490476550. [DOI] [PubMed] [Google Scholar]
- 23.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 24.Jerrett M, Burnett RT, Ma R, Pope CA, 3rd, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE, Thun MJ. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 25.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004(109):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 26.Brook RD, Brook JR, Rajagopalan S. Air pollution: the “heart” of the problem. Curr Hypertens Rep. 2003;5:32–39. doi: 10.1007/s11906-003-0008-y. [DOI] [PubMed] [Google Scholar]
- 27.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 28.Pope CA., III Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108:713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109:579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope CA, 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:33–45. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 32.Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J. 2003;21:76S–80S. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 33.Zareba W, Nomura A, Couderc JP. Cardiovascular effects of air pollution: What to measure in ECG? Environ Health Perspect. 2001;109:533–538. doi: 10.1289/ehp.01109s4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SK, O'Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: The VA normative aging study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oparil S, Zaman A, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–776. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]