Abstract
Decades of research have examined the effects of cannabis on neurocognition. Recent advances in this field provide us with a better understanding of how cannabis use influences neurocognition both acutely (during intoxication) and non-acutely (after acute effects subside). Evidence of problems with episodic memory is one of the most consistent findings reported; however, several other neurocognitive domains appear to be adversely affected by cannabis use under various conditions. There is significant variability in findings across studies, thus a discussion of potential moderators is increasingly relevant. The purpose of this review was to 1) provide an update on research of cannabis’ acute and non-acute effects on neurocognition, with a focus on findings since 2007 and 2) suggest and discuss how neurodevelopmental issues and sex differences may influence cannabis effects on neurocognition. Finally we discuss how future investigations may lead to better understanding of the complex interplay among cannabis, stages of neurodevelopment, and sex on neurocognitive functioning.
Keywords: cannabis, cognition, marijuana, neuropsychology, sex differences, THC
1. Introduction
In the last five years, significant advances have been made in understanding the endogenous cannabinoid system and how cannabis may affect its functioning, particularly as it pertains to the brain. The abundance of cannabinoid receptors in the hippocampus, amygdala, basal ganglia, and prefrontal cortex (Mackie 2005; Piomelli 2003) suggests that disruption of the cannabinergic system by administration of exogenous cannabinoids, like delta-9-tetrahydrocannbinol (THC; the main psychoactive compound in cannabis), may have important implications for various neurobehavioral processes, including mood and anxiety regulation (Crippa et al. 2009; Crippa et al. 2011), learning, memory, motivation, motor control, reward processing, and executive functions (Crean et al. 2011; R. Gonzalez 2007; Solowij and Pesa 2010). Indeed, it seems frontal-limbic neurocircuitry is most prominently affected by cannabis use (for review see Martin-Santos et al. 2010), while other structures in the brain, including the brain stem, occipital lobe, and parietal lobe may be less affected. Not surprisingly, the neurobehavioral effects of cannabis use have been intensely studied over several decades and summarized in numerous published reviews (Crean et al. 2011; Ferraro 1980; R. Gonzalez et al. 2002; R. Gonzalez 2007; I. Grant et al. 2003; Iversen 2003; Jager and Ramsey 2008; Pope et al. 1995; Ranganathan and D’Souza 2006; Schweinsburg et al. 2008; Solowij and Battisti 2008; Solowij and Pesa 2010; Wert and Raulin 1986). However, many of these reviews conclude that the findings for several neurocognitive domains are equivocal, speaking to the importance of further research in this field.
Research on the neurocognitive effects of cannabis continues to grow rapidly. This is an especially pertinent area of research because cannabis continues to be the most widely used illicit substance worldwide (UNODC 2011). In the U.S., cannabis use has recently risen and now surpasses that of cigarette use among adolescents (Johnston 2012). These statistics are particularly concerning, given that cannabis use is associated with negative health outcomes (Kalant 2004), psychosocial and cognitive impairments (Kalant 2004; Solowij and Pesa 2010), and other neurobehavioral consequences such as an increased risk of an automobile crash when driving while intoxicated (Asbridge et al. 2012; Drummer et al. 2003; Li et al. 2012; Mura et al. 2003; Ramaekers et al. 2004). Furthermore, some have argued that the potency of cannabis, as indexed by THC content, has increased in recent years (Burgdorf et al. 2011) and may account for increases in cannabis use disorder diagnoses (Compton et al. 2004), as well as cannabis-related psychosis (Malone et al. 2010). On the other hand, societal acceptance of cannabis use for medicinal applications continues to increase (Cohen 2010; Fairfield et al. 1998; O’Connell and Bou-Matar 2007) and there is a growing body of literature suggesting medical benefits from cannabis use (Elikkottil et al. 2009; Ellis et al. 2009; Ware et al. 2005; Watson et al. 2000). All of these factors underscore the continued importance of investigating the acute and non-acute neurocognitive effects of cannabis use.
Given the outstanding reviews currently available on acute and non-acute effects of cannabis use on neurocognitive functioning and the rapid growth of new research in this area, we aimed to provide an update of research principally conducted during the past five years (since 2007) in the first half of this review. To orient the reader and provide a consistent organizational framework, we begin sections on acute and non-acute effects by first briefly summarizing the general findings from recent reviews of the field and then describing detailed findings from studies conducted since 2007. Studies that examine the impact of cannabis on measures generally thought to assess the same neurocognitive domain are discussed together when feasible. However, it is important to keep in mind that most neurocognitive measures are multidimensional in nature and some of these groupings, although not arbitrary, are not absolute. We also decided to present studies from samples with adolescents separately from those that employed adult samples in the non-acute section. In the second half of the review, we focus on two emerging influential factors, neurodevelopmental influences and sex differences, by reviewing critical animal and human studies and examining how these factors may interact with cannabis use to affect neurocognition.
2. Acute Effects of Cannabis on Neurocognitive Functioning
Studies on the acute effects of cannabis allow us to understand the immediate and direct effects that the psychoactive constituents of cannabis have on neurocognitive functioning during intoxication. These studies often rely on oral or intravenous administration of THC, but some have participants smoke actual cannabis joints under standardized conditions. Several review articles have been written on acute cannabis intoxication and neuropsychological functioning (Crean et al. 2011; R. Gonzalez 2007; Ferraro 1980; Ranganathan and D’Souza 2006; Solowij and Pesa 2010), with equivocal findings for most neurocognitive domains other than aspects of episodic memory - the autobiographical memory of specific events, situations, and experiences (Tulving 2001). For example, some of the first acute administration studies reported equivocal findings on several measures of executive functions, but found intoxicated individuals performed more poorly in the domain of episodic memory, especially recall of newly acquired information (Ferraro 1980). More recent studies also suggest that acute THC intoxication seems to impair retrieval-based memory of new material, but evidence regarding the effects of THC on other neuropsychological domains remained mixed (R. Gonzalez 2007; Ranganathan and D’Souza 2006). For instance, a recent review of cannabis’ effects on executive functioning suggests that THC administration adversely affects inhibition, impulsivity, and working memory, but not verbal fluency, and findings are mixed for decision-making, risk-taking, and aspects of attention (Crean et al. 2011).
2.1. Acute Effects of Cannabis: Update from Research Published in the Last Five Years
2.1.1. Learning and Memory
Recent research on acute effects of cannabis has benefited from using placebo-controlled designs and administration of different doses of THC in the laboratory. Data from the past five years is consistent with previous studies, generally showing acute administration of THC to impair episodic memory, including immediate (D’Souza et al. 2008a; D’Souza et al. 2008b; Dumont et al. 2011; Morrison et al. 2009) and delayed recall (D’Souza et al. 2008a; D’Souza et al. 2008b); procedural memory (Dumont et al. 2011); and associative learning and memory (Ballard et al. 2012) among occasional and regular cannabis users as well as non-users. Evidence indicates there may be a dose-dependent relationship between amount of intravenous THC and impairments in total free and delayed verbal recall, but not delayed cued or recognition recall (D’Souza et al. 2008b). D’Souza and colleagues (2008b) also found that, compared to non-using controls, frequent cannabis users showed blunted THC-related impairments in verbal episodic memory, suggesting potential tolerance effects to cannabis’ negative influence on memory. In studies of visuospatial memory, THC impaired accuracy in regular cannabis users (Weinstein et al. 2008a; Weinstein et al. 2008b). On the other hand, others report no effect from THC on visuospatial memory (Anderson et al. 2010), associative memory (Bossong et al. 2012a), or verbal episodic memory (Sugarman et al. 2011). Hart and colleagues (2010) also found that THC administration did not affect overall performance on a task of verbal episodic memory in regular cannabis users, but participants performed more poorly on a recognition task suggesting a deleterious impact of cannabis on retrieval-based memory.
2.1.2. Attention, Concentration, and Working Memory
Most studies document impairments in attention and concentration following administration of small (i.e., 2.5mg) and large (i.e., 0.5 mg/kg) doses of THC in cannabis users and non-users compared to placebo administration (Anderson et al. 2010; D’Souza et al. 2008a; D’Souza et al. 2008b; Hunault et al. 2009; Ramaekers et al. 2009; Ramaekers et al. 2011; Theunissen et al. 2011), with some evidence for a dose-dependent relationship between amount of THC smoked and degree of impairment (Hunault et al. 2009). However, two studies found no differences in performance on measures of auditory selective attention and concentration in regular cannabis users after smoking cannabis standardized to 20mg THC (O’Leary et al. 2007) or on a measure of sustained attention in occasional cannabis users after ingesting 15mg THC (Sugarman et al. 2011) compared to placebo. Impairments in working memory have been observed following acute administration of vaporized THC (Bossong et al. 2012b), oral THC (D’Souza et al. 2008a; Dumont et al. 2011), smoked cannabis containing different doses of THC (Hart et al. 2010), and intravenous THC (Morrison et al. 2009).
2.1.3. Abstract Reasoning, Decision-Making, and Risk-Taking
Research on the effects of cannabis on abstract reasoning and problem-solving have been conducted for many years; however, a recent trend in cannabis research has been the study of decision-making and risk-taking - often defined as processes of evaluating a variety of response options and choosing the option considered to be optimal in the present moment (Clark et al. 2004). THC has been found to impair healthy young adults’ performance on a reasoning task (but not a verbal fluency task; Morrison et al. 2009) and significantly increased total errors and nonpreservative errors in regular cannabis users on a task of abstract reasoning (Weinstein et al. 2008a; Weinstein et al. 2008b). Additionally, problems with cognitive flexibility have been reported after smoking cannabis standardized to different doses of THC compared to a placebo condition (Anderson et al. 2010). In contrast, most current evidence suggests that acute intoxication does not negatively impact decision-making or risk-taking in occasional, regular, or heavy cannabis users (D’Souza et al. 2008a; Ramaekers et al. 2009; Ramaekers et al. 2011; Vadhan et al. 2007; Weinstein et al. 2008a; Weinstein et al. 2008b), but it may slow decision-making (Vadhan et al. 2007). Some contradictory evidence does exist. Whereas a previous study demonstrated increasing levels of cannabis intoxication were associated with more risky responses in occasional cannabis users (Lane et al. 2005), a recent study found THC may actually reduce risk-taking behaviors in healthy young adults (Rogers et al. 2007).
2.1.4. Inhibitory Control, Motor Impulsivity, and Psychomotor Control
Similar to the findings for decision-making and risk taking, studies examining inhibition, motor impulsivity, and psychomotor control (these domains are invariably linked and generally defined as behavior involving rapid reactions to internal and external stimuli; Moeller et al. 2001) are mixed. THC was found to negatively influence inhibitory control, as evidenced by increased stop reaction time and decreased accuracy of responses in occasional and heavy cannabis users during a stop signal task in two studies (Ramaekers et al. 2009; Theunissen et al. 2011), but THC administration, three hours after placebo alcohol administration, did not have an effect on inhibition or motor impulsivity in heavy cannabis users on the same task in another study (Ramaekers et al. 2011). Given the motor components inherent in the aforementioned tasks, it is worth considering that some studies find THC to impair psychomotor performance (D’Souza et al. 2008a; Hunault et al. 2009; Ramaekers et al. 2009), but several others do not (Dumont et al. 2011; O’Leary et al. 2007; Ramaekers et al. 2011). Interestingly, Ramaekers and colleagues (2009) found THC administration impaired psychomotor control in occasional cannabis users, but not in heavy cannabis users, suggesting a potential tolerance effect. Hunault and colleagues (2009) found THC to significantly decrease response time and increase errors in a dose-dependent manner in heavy cannabis users on a motor control task.
2.1.5. Recent Innovations in Acute Administration Studies: The Role of Cannabidiol
It is important to consider that the Cannabis sativa plant contains hundreds of known compounds and at least 70 cannabinoids (Elsohly and Slade 2005), but only THC has been extensively studied. Yet cannabidiol (CBD), another major cannabinoid found in cannabis, has opposing effects on CB1 and CB2 receptors compared to THC, mediates the effects of THC (Pertwee 2008), and has neuroprotective properties (Hampson et al. 1998; Lastres-Becker et al. 2005; Mechoulam et al. 2002; Mechoulam et al. 2007). Ratios of THC to CBD in street cannabis can vary considerably (Burgdorf et al. 2011), which may have implications for neurocognitive sequelae from use; however, only recently have the combined effects of THC and CBD administration been studied.
To better understand the potential differential impact of THC and CBD on neurocognition, Borgwardt and colleagues (2008) administered 10mg THC, 600 mg CBD, or placebo capsules to 15 men with minimal cannabis exposure (<15 times in life) on three separate occasions in a double-blind, within-subject design. In this study, and across several other investigations using the same sample, participants’ performance was unaffected by THC or CBD on measures of inhibition, verbal memory, accuracy of gender discrimination, and visual and auditory processing (Bhattacharyya et al. 2009; Bhattacharyya et al. 2010; Borgwardt et al. 2008; Fusar-Poli et al. 2009; Winton-Brown et al. 2011). In contrast, Roser and colleagues (2009) found combined THC and CBD, but not THC alone, impaired psychomotor control in 24 occasional cannabis users who were administered a combination of 10mg THC and 5.4mg CBD, 10mg THC alone, or a placebo pill on three separate visits in a double-blind, cross-over design. Recently, Morgan and colleagues (2010) found 22 cannabis users with low cumulative levels of CBD (as evidenced from hair analysis) demonstrated poorer performance on immediate and delayed episodic memory (but not verbal and category fluency) compared to 22 users with higher cumulative CBD exposure when both groups were intoxicated. The same group showed that daily cannabis users who had higher cumulative THC exposure demonstrated poorer performance on measures of immediate and delayed episodic memory, as well as source memory, compared to daily cannabis users with lower cumulative THC exposure when participants were intoxicated (Morgan et al. 2011). However, there were no differences in episodic memory and source memory performance between the 54 occasional cannabis users regardless of the cumulative THC exposure. On the other hand, both daily and occasional cannabis users with CBD present in hair analysis displayed better recognition memory than users without CBD present when all individuals were intoxicated (Morgan et al. 2011).
Recent neuroimaging studies suggest differential neurophysiological effects of THC and CBD. A number of functional neuroimaging studies demonstrated that THC and CBD can exert opposite actions on the neurofunctional correlates of cognitive and emotional processes in humans (Bhattacharyya et al. 2009; Bhattacharyya et al. 2010; Bhattacharyya et al. 2012; Borgwardt et al. 2008; Fusar-Poli et al. 2009; Fusar-Poli et al. 2010a). Specifically, although no neurocognitive differences were found, THC and CBD had opposite effects of brain activation patterns in the striatum during verbal recall, in the hippocampus during inhibition, in the superior temporal cortex during auditory processing, in the occipital cortex during visual processing, and in the amygdala while subjects viewed fearful faces when compared to placebo (Bhattacharyya et al. 2010). These complex effects of cannabinoids on underlying neural networks may help to explain some of aforementioned mixed findings in the field and warrant further investigation.
2.1.6. Summary of Recent Advances on the Acute Effects of Cannabis
In summary, the recent literature suggests acute THC administration impairs specific aspects of learning and memory, in line with the previous literature, as well as attention, concentration, and working memory. A notable contribution to the literature during the past five years has been examining how acute cannabis intoxication affects neuropsychological measures of abstract reasoning, decision-making, risk-taking, inhibition, motor impulsivity, and psychomotor control. Studies indicate abstract reasoning may be impaired after acute THC administration, while decision-making and risk-taking largely remain intact, despite some evidence for THC potentially slowing decision-making. However, future research in this area may benefit from more detailed analyses on the various components of decision-making and risk-taking (Busemeyer and Stout 2002) and examining different types of risk (i.e., ambiguous versus explicit risk; Weller et al. 2010) in affecting performance. The mixed findings for measures of inhibition, motor impulsivity, and psychomotor control, suggest more work is needed to understand the role of motor slowing and dose-response effects. Moreover, findings of differential performance following THC administration in heavy cannabis users, occasional cannabis users, and healthy controls, as well as the potential role of different types of cannabinoids present in cannabis, highlight the importance considering these issues in future research. Finally, examining neurocognitive effects of other naturally-occurring cannabinoids in combination with THC is a promising line of research. The literature on acute administration (although nascent) suggests little behavioral differences from THC alone compared to THC/CBD combinations. Conversely, associations between higher cumulative CBD exposure and better neuropsychological functioning indicate CBD may buffer against the negative effect of THC, but these neurocognitive differences may only emerge after prolonged administration.
3. Non-Acute Effects of Cannabis on Neurocognitive Functioning
Studies on the non-acute effects of cannabis focus on the period after the intoxicating effects of cannabis have subsided. As such, they often examine participants from after about 8 hours to a month since their last cannabis use. These studies are best apt to inform the chronicity of cannabis-associated neuropsychological deficits that may linger after acute intoxication has ceased. As with acute-administration studies, various review articles have broached this topic over the years. Some suggest residual effects (i.e., after several hours to several days of abstinence) and long-term effects (i.e., after several weeks of abstinence and beyond), while others find no clear evidence for residual or long-term neuropsychological deficits among cannabis users across several domains (Crean et al. 2011; R. Gonzalez et al. 2002; R. Gonzalez 2007; I. Grant et al. 2003; Pope et al. 1995; Schweinsburg et al. 2008; Solowij and Battisti 2008; Solowij and Pesa 2010; Wert and Raulin 1986). As found in the acute intoxication studies, the evidence is mixed for most domains other than episodic memory, which remains adversely impacted for up to 28 days following abstinence (R. Gonzalez 2007; I. Grant et al. 2003; Solowij and Battisti 2008). Novel contributions from recent studies include increased attention to adolescent samples and assessing measures of decision-making, risk-taking, and other aspects of impulsive behavior.
3.1 Non-Acute Effects of Cannabis: Update from Research Published in the Past 5 Years
3.1.1. Learning and Memory
As found in previous reviews, aspects of memory are often impaired among recently abstinent cannabis users, particularly verbal episodic memory. Regular adolescent cannabis users who have been abstinent between 12 hours and 21 days demonstrate poorer immediate (Hanson et al. 2010; Harvey et al. 2007; Solowij et al. 2011) and delayed recall (Harvey et al. 2007; Solowij et al. 2011), as well as impaired recognition (Solowij et al. 2011). However, one of these studies found no difference in recognition between regular and occasional cannabis users (Harvey et al. 2007). After longer periods of abstinence (28 days), Tapert and colleagues (2007) found adolescent cannabis users made more recall intrusions than controls, while other studies found no differences between groups on any measure of episodic memory (Jacobsen et al. 2007; Mahmood et al. 2010; Medina et al. 2007a). Recently abstinent adult cannabis users also demonstrate poorer immediate (Battisti et al. 2010b; R. Gonzalez et al. In press; Hadjiefthyvoulou et al. 2011; Korver et al. 2010; Nestor et al. 2008; Wagner et al. 2010; Yucel et al. 2008) and delayed recall (R. Gonzalez et al. In press; Wadsworth et al. 2006; Yucel et al. 2008), but have intact recognition (R. Gonzalez et al. In press; Nestor et al. 2008; Wadsworth et al. 2006). Although this is not invariably the case, as some studies report intact immediate (Chang et al. 2006; Gruber et al. 2011; Wadsworth et al. 2006) and delayed recall (Chang et al. 2006; Gruber et al. 2011) among cannabis users. Other evidence suggests that recall performance is negatively associated with amount of past year (Jager et al. 2007) and lifetime cannabis use (Indlekofer et al. 2009; Jager et al. 2007; P. Murphy et al. 2011; Solowij et al. 2011), duration of cannabis use (Solowij et al. 2011; Wadsworth et al. 2006), frequency of cannabis use, and age of first cannabis use (Solowij et al. 2011). In a longitudinal study of young adults, trajectories of performance on measures of episodic memory in non-users, former users, and current users were different, such that non-users and former users immediate and delayed recall improved over a period of eight years, while performance on these measures worsened over time in light and heavy current users (Tait et al. 2011).
Deficits in other aspects of learning and memory among cannabis users not acutely intoxicated are less commonly reported than those for verbal episodic memory. Adolescent cannabis users who were abstinent between 12 to 24 hours demonstrated intact associative learning (Harvey et al. 2007; Jager et al. 2010). After longer periods of abstinence (28 days), adolescent cannabis users exhibited deficits on a story memory task (Medina et al. 2007a), but had intact visuospatial memory (Mahmood et al. 2010; Medina et al. 2007a; Schweinsburg et al. 2010). In recently abstinent adult cannabis users, some studies report visuospatial memory deficits (Hermann et al. 2007; McHale and Hunt 2008), but others do not (Chang et al. 2006; Gruber et al. 2011). Amount of lifetime cannabis use did not predict visuospatial memory in a community sample of recently abstinent polydrug users (Indlekofer et al. 2009). Recently abstinent adult cannabis users have demonstrated intact associative learning (Fisk and Montgomery 2008) and semantic memory (Wadsworth et al. 2006). Evidence for prospective memory deficits is split with one study reporting deficits (Montgomery et al. 2012) and another no deficits (Hadjiefthyvoulou et al. 2011).
3.1.2. Attention and Concentration
Attention and concentration continue to be well-studied among adolescent and adult samples. Several studies cite impairments in attention and concentration in recently abstinent adolescent cannabis users (Abdullaev et al. 2010; Hanson et al. 2010; Harvey et al. 2007; Lane et al. 2007). After at least 21 days of abstinence, complex attention continues to be impaired in adolescent cannabis users (Hanson et al. 2010; Jacobsen et al. 2004; Medina et al. 2007a; Tapert et al. 2007), with evidence of a dose-dependent relationship with amount of lifetime use (Medina et al. 2007a). Conversely, others reported that after 45 days of abstinence, selective and divided attention was intact in adolescent cannabis users (Jacobsen et al. 2004). Recently abstinent adult cannabis users also demonstrated impairments in attention and concentration in several studies (Hermann et al. 2007; Indlekofer et al. 2009; Scholes and Martin-Iverson 2009; Scholes-Balog and Martin-Iverson 2011; Wadsworth et al. 2006), which persisted for several hours to one week of abstinence. However, a few studies report no impairments among adult cannabis users when the time of last cannabis use was either unknown (J. Grant et al. 2011; Korver et al. 2010) or after at least 4 hours to approximately 38 months of abstinence (Chang et al. 2006).
3.1.3. Working Memory
In studies examining working memory, the evidence for cannabis-associated deficits is mixed. Adolescent regular cannabis users who are abstinent demonstrated poorer working memory compared to controls after 3 and 13 days, during a challenging task that required active manipulation of items (Hanson et al. 2010), but not after 8 days, during a more simple matching task (Jager et al. 2010). Thus, it is possible that impairments in working memory are only detected among adolescent cannabis users with more complex tasks that place a greater load on working memory systems, as seen in Jacobsen et al. (2007). On the other hand, working memory deficits may be related to amount of cannabis use, as Harvey and colleagues (2007) found regular adolescent cannabis users performed more poorly on spatial working memory than occasional cannabis users and amount of cannabis use was one of the strongest predictors of poorer spatial working memory. The evidence is also mixed regarding the persistence of working memory deficits among adolescents after abstinence, with some studies reporting deficits after 28 days (Jacobsen et al. 2004; Jacobsen et al. 2007), whereas others do not (Hanson et al. 2010; Padula et al. 2007; Schweinsburg et al. 2008; Schweinsburg et al. 2010). In contrast to studies with adolescent samples, most studies report intact working memory among recently abstinent adult cannabis users (Becker et al. 2010; Chang et al. 2006; Fisk and Montgomery 2008; J. Grant et al. 2011; Gruber et al. 2011; Jager et al. 2006; Looby and Earleywine 2010; Piechatzek et al. 2009; Scholes and Martin-Iverson 2010). Additionally, in a longitudinal study, working memory performance remained intact among recently abstinent current cannabis users, former cannabis users, and controls over a period of eight years (Tait et al. 2011). On the other hand, frequency of cannabis use was negatively correlated with working memory performance among abstinent cannabis users (Wadsworth et al. 2006) and among polysubstance users (Fernandez-Serrano et al. 2010).
3.1.4. Verbal Fluency
Evidence for deficits in verbal fluency among abstinent cannabis users is also mixed. Only one recent study examined verbal fluency in a sample of adolescent cannabis users and found it to be impaired after 28 days of abstinence (Tapert et al. 2007). Studies of recently abstinent adult cannabis users report deficits in verbal fluency after 8-24 hours since last use (Korver et al. 2010; Mason et al. 2012; McHale and Hunt 2008) and after 15 days of abstinence (Fernandez-Serrano et al. 2010). However, others report intact verbal fluency after a few hours to a week since last cannabis use (Chang et al. 2006; Fisk and Montgomery 2008; Gruber et al. 2011; Piechatzek et al. 2009). Of note, recently abstinent adult cannabis users have also been reported to demonstrate deficits in text comprehension (Huestegge et al. 2010).
3.1.5. Abstract Reasoning
Poorer cognitive flexibility has been reported among adolescent cannabis users abstinent for approximately 8 hours compared to controls (Lane et al. 2007), but not between recently abstinent occasional and regular adolescent cannabis users (Harvey et al. 2007). After at least 23 days of abstinence, adolescent cannabis users appear to evidence intact cognitive flexibility (Medina et al. 2007a). As with adolescent cannabis users, abstinent adult cannabis users also demonstrated impairments in cognitive flexibility (Fontes et al. 2011a, 2011b; J. Grant et al. 2011; Mason et al. 2012). Similarly, planning and reasoning were poorer among adult cannabis users who were abstinent for at least 5 days compared to controls (Montgomery et al. 2012). Furthermore, amount of lifetime cannabis use predicted worse performance on a reasoning task for adult polysubstance users who were abstinent for at least 15 days (Fernandez-Serrano et al. 2010).
3.1.6. Decision-Making and Risk-Taking
Compared to findings from acute investigations, recently abstinent adult cannabis users show more consistent deficits in decision-making and risk-taking (J. Grant et al. 2011; Vaidya et al. 2012; Wesley et al. 2011), even after 15 days of abstinence (Fernandez-Serrano et al. 2010). Fridberg and colleagues (2010) also reported impairments in decision-making among cannabis users, and through using computational modeling they found these impairments were likely due to cannabis users’ decreased sensitivity to losses and increased sensitivity to immediate gains. However, others have not observed such deficits (Boggio et al. 2010; Hermann et al. 2009; Martin-Soelch et al. 2009). In our own studies, cannabis users and non-users, who were matched on various potential comorbidities, performed equivalently on measures of decision-making and risk-taking (R. Gonzalez et al. In press). However, we found that decision-making performance (but not performance on measures of episodic memory or other measures associated with impulsive behavior) is related to more DSM-IV cannabis use dependence symptoms among cannabis users (R. Gonzalez et al. In press). Similarly, we found that amount of cannabis use is related to more problems experienced from cannabis use among individuals with poor decision-making performance, but not among those with intact decision-making (R. Gonzalez 2012). Further, poorer decision-making and more risk-taking among cannabis users predicted more risky sexual behaviors in our sample (Schuster et al. 2012). Thus, despite a lack of differences between cannabis users and non-users on measures of decision-making and risk-taking in our sample, performance on some neurocognitive measures influenced important functional outcomes.
3.1.7. Inhibitory Control, Motor Impulsivity, and Psychomotor Control
The evidence seems to be mixed as to whether deficits in inhibition, motor impulsivity, or psychomotor control are evident in abstinent cannabis users. Among recently abstinent adolescent cannabis users, regular and non-regular users showed no difference in inhibition or psychomotor control (Harvey et al. 2007). After 28 days of abstinence, adolescent cannabis users demonstrated intact inhibition and motor impulsivity (Tapert et al. 2007). Yet, after at least 23 days of abstinence, adolescent cannabis users had impairments in psychomotor performance compared to controls, and there was a negative, dose-dependent association between performance and lifetime cannabis use episodes (Medina et al. 2007a). In recently abstinent adult cannabis users, some studies report impairments in inhibition and motor impulsivity (Battisti et al. 2010a; Clark et al. 2009; Cunha et al. 2010; Fontes et al. 2011a, 2011b; Scholes and Martin-Iverson 2010), with some evidence for a dose-dependent relationship between amount of past 30 day use and performance (Cunha et al. 2010; Piechatzek et al. 2009). However, many other studies report no differences in performance between recently abstinent adult cannabis users and controls (Aharonovich et al. 2008; Becker et al. 2010; Cane et al. 2009; Chang et al. 2006; Fernandez-Serrano et al. 2010; Fontes et al. 2011b; R. Gonzalez et al. In press; J. Grant et al. 2011; Gruber et al. 2011; Hermann et al. 2007; Hester et al. 2009; Roberts and Garavan 2010; Scholes and Martin-Iverson 2010). Interestingly, Mason and colleagues (2012) found recently abstinent adult cannabis users were able to successfully inhibit prepotent responses on a simple task of inhibition, but their performance became significantly more impaired compared to normative data as the task became more complex. Performance on measures of psychomotor control are also mixed, with one investigation finding deficits in psychomotor control across several measures among adult cannabis users abstinent for 12 hours (King et al. 2011), but others suggesting intact psychomotor control in recently abstinent cannabis users (Chang et al. 2006; Korver et al. 2010; Wadsworth et al. 2006) and after 28 days of abstinence (Pillay et al. 2008). Relatedly, Gonzalez and colleagues (2011) found poorer complex psychomotor performance (but no procedural learning deficits) among polydrug-using individuals with a history of cannabis dependence compared to those without a history of cannabis dependence and this deficit was exacerbated in the context of HIV.
3.1.8. Summary of Recent Advances on Non-Acute Effects of Cannabis
In conclusion, recent studies of episodic memory continue to suggest non-acute impairments among cannabis users both with adult and adolescent samples. On the other hand, the results for deficits in other types of memory seem to be more nuanced when considering length of abstinence, age at which cannabis users discontinue their use, amount and duration of cannabis use, whether prospective study designs were used, and whether samples consisted of adolescents or adults, as emerging evidence suggests memory deficits among younger samples may be more pronounced than what has been previously observed in adults (Pope et al. 2003; Solowij et al. 2011). Attention and concentration seem to be consistently impaired among adolescent and adult cannabis users, while evidence is mixed regarding the enduring effects of cannabis use on working memory and verbal fluency. Abstract reasoning seems to be impaired after short periods of abstinence, but these deficits may resolve after longer periods of abstinence, at least in adolescent users. Similarly, findings suggest abstinent cannabis users often demonstrate poorer decision-making and risk-taking, which have been reported alongside functional brain alterations, especially in the orbitofrontal cortex, and may persist even after 28 days of abstinence in heavy cannabis users (Bolla et al. 2005). Further, although there are equivocal findings in working memory, inhibitory control, motor impulsivity, and psychomotor control, preliminary evidence suggests cognitive load may be an important factor to consider in understanding cannabis’ effect on some of these domains. It is also important to consider that the same neurocognitive domain may be assessed with different tasks that may also vary in complexity and sensitivity. Less complex tasks may be less sensitive to more subtle brain abnormalities among cannabis users, but as the difficulty of the task increases, impairments in performance may emerge, as observed with some measures of inhibition (Mason et al. 2012) and working memory (Jacobsen et al. 2007). Moreover, as mentioned previously, some evidence suggests THC and CBD may exert opposing effects on neurocognition after prolonged exposure; however, to our knowledge this has not been examined in studies of non-acute use. Thus, it is possible that regional and individual differences in the THC/CBD ratios of the cannabis consumed by cannabis users may also contribute to discrepant findings. There are many other factors may contribute to the disparate findings and identifying these sources of variability is crucial in helping us to understand the non-acute effects of cannabis on cognition. One factor not discussed in this review, but that deserves mention, includes interactions of cannabis with other substances. Some evidence suggests differential neurocognitive effects of cannabis that depend on whether the individual also uses other substances. For example, there is some evidence indicating cannabis may exert neuroprotective effects among heavy alcohol using adolescents (Jacobus et al. 2009; Mahmood et al. 2010; Medina et al. 2007b; Schweinsburg et al. 2011), alcohol-dependent adults (Nixon et al. 1998), and methamphetamine-dependent adults (R. Gonzalez et al. 2004). On the other hand, nicotine may mask cannabis-related neurocognitive impairments, and these deficits may only be evident during abstinence from nicotine (Jacobsen et al. 2007). Given that cannabis users often use other substances as well, this is an important factor that needs further examination, especially considering the overlap in the neurocognitive domains affected by cannabis and other drugs of abuse. These domains include learning and memory, attention and concentration, working memory, abstract reasoning, decision-making and risk-taking, inhibitory control, and psychomotor control; however, the presence and magnitude of such deficits may vary by substance. For example, Gonzalez et al. (2007) reported deficits in decision-making both among abstinent individuals with recent history of alcohol dependence and among those with recent methamphetamine dependence; however, only those with methamphetamine dependence also demonstrated deficits in working memory. Comparisons of the neurocognitive impact of various substances of abuse have been reviewed by others (Bava and Tapert 2010; Fernandez-Serrano et al. 2011; R. Gonzalez 2009).
4. Neurodevelopment and Sex Differences as Emerging Influential Factors
It is evident that disparate findings are common across studies examining acute and non-acute effects of cannabis on neurocognition. Heterogeneity in methods, participant samples, dosing, and cannabis use history are all likely contributors. However, in recent years there has been a more systematic search for potential neurobiological factors that may influence the impact of cannabis use on neurocognition. Two areas receiving substantial attention include neurodevelopmental factors and the potential for cannabis to affect males and females differently (i.e., sex differences). Although many of the issues discussed below have received more attention recently, unlike the previously cited studies, our review on these factors was not limited to the last five years.
4.1. Cannabis Use and Neurodevelopmental Processes
Initiation of cannabis use often occurs during adolescence, an important period for neurodevelopment; thus, it is important to consider this time when examining the impact of cannabis use on neurocognitive functioning. Although 90% of the brain’s total volume has developed by approximately age 6 (Giedd 2004), global cortical development follows an inverted U-shaped trajectory, peaking around 12 to 14 years of age then decreasing in volume and thickness over adolescence (Giedd et al. 1999; Gogtay et al. 2004). This synaptic pruning of gray-matter density occurs first in more primary sensorimotor areas and last in higher-order association areas like the prefrontal cortex (Giedd 2004; Gogtay et al. 2004). In contrast, white matter density increases during adolescence and into adulthood, reflecting significant increases in projectional, associational, and commissural myelination (Durston et al. 2001; Giedd 2004). Given the extensive and complex development going on at this time, it is conceivable that the brain may be more vulnerable to insults and disturbances from exogenous compounds that may alter normal brain functioning. Moreover, the endocannabinoid system plays an important role in neuromaturation and synaptic pruning (Viveros et al. 2012). Thus, it is possible that use of cannabis during this time period may be disruptive to normal neuromaturation (Bava and Tapert 2010). Further, cannabis use during this neurodevelopmental period may trigger a psychotic disorder in individuals vulnerable for psychosis (Fusar-Poli et al. 2012; Fusar-Poli et al. In press). The neural networks showing functional (Fusar-Poli et al. 2010b; Fusar-Poli et al. 2011b; Fusar-Poli et al. 2011d), structural (Fusar-Poli et al. 2011a; Fusar-Poli et al. 2011c) and neurochemical alterations (Fusar-Poli and Meyer-Lindenberg 2012a, 2012b) during the pre-psychotic phases are similar to those affected by cannabinoids, supporting the neurodevelopmental hypothesis of psychosis.
To better understand how cannabis use may interfere with neurodevelopmental processes, animal studies have compared how administration of botanical or synthetic cannabinoids during different developmental time periods affect neurocognitive processes in adolescent and adult rats. Many of these studies have focused on working memory. For example, rats exposed to synthetic cannabinoids or THC during adolescence experience impaired working memory during adulthood (O’Shea et al. 2004; O’Shea et al. 2006; Rubino et al. 2009a; Rubino et al. 2009b). These impairments have been correlated with less active synapses in the prefrontal cortex (Rubino et al. 2009a), as well as shorter dendrites and reduced spine densities in the hippocampus, suggesting enduring neurobiological consequences of early cannabis exposure (Rubino et al. 2009b). The heightened vulnerability of younger brains is highlighted by the fact that the same amount of THC exposure that led to decreased working memory performance in adolescent rats had no effect in adult rats (Quinn et al. 2008). However, some have suggested that heavy THC administration during adolescence may be needed to produce significant and persistent impairments in working memory (Realini et al. 2009). With regards to learning, impairments with synthetic cannabinoid administration during late adolescence were reported, but these resolved after a prolonged period of abstinence (Abush and Akirav 2012). Conversely, rats exposed to chronic doses of THC during adolescence, but not during late adolescence, evidenced deficits in learning during adulthood (Harte and Dow-Edwards 2010). There may be a critical time during adolescence when cannabis use may have the most negative effects. When taken together, these studies suggest that cannabis use during adolescence may have a more significant and lasting impact on neurocognition than if cannabis use is initiated during adulthood.
In line with the animal literature, accumulating evidence from studies with human subjects suggests initiation of cannabis use during early adolescence may be more detrimental to some aspects of neurocognition compared to later initiated use. Indeed, earlier age of cannabis use onset is negatively associated with neurocognitive functioning in several studies (see Table 1). For example, those who initiate use before 15 to 17 years of age demonstrate more pronounced deficits in visual attention (Ehrenreich et al. 1999), verbal fluency (Gruber et al. 2011; Pope et al. 2003), inhibition (Fontes et al. 2011a; Gruber et al. 2011; Pope et al. 2003), and other aspects of executive functioning (Fontes et al. 2011a) as compared to those who initiate use later on. Further, episodic memory was poorer in those with an earlier onset of cannabis use in two studies (Pope et al. 2003; Solowij et al. 2011); however, another study failed to find this effect (Gruber et al. 2011). Poorer performance on measures of inhibition (Battisti et al. 2010a; Gruber et al. 2011) and impulsivity (Solowij et al. 2012) have also been associated with earlier age of onset. Moreover, Gruber and colleagues (2012) reported that early-onset users made more errors and showed greater disruptions in brain activation patterns than late-onset users during an inhibition task. On the other hand, others have reported no differences between early and late-onset cannabis users (and healthy controls) on measures of working memory and attention (Ehrenreich et al. 1999; Gruber et al. 2011; Pope et al. 2003), as well as on a task of visuospatial memory (Gruber et al. 2011). The current evidence suggests that earlier onset of cannabis use is associated with poorer neurocognitive outcomes, but more research is needed to better determine how additional parameters of cannabis use (e.g., age of daily use, age of meeting cannabis use disorder), other substance use, periods of rapid neurodevelopment, and sex (as discussed below) influence findings.
Table 1. Studies Examining Age of Onset of Cannabis Use on Neurocognitive Functioning.
| Study | Age | Age of Onset | Healthy Controls |
Length of Abstinence |
Neurocognitive Domains Poorer in Early-Onset Cannabis Users |
Neurocognitive Domains Intact in Early-Onset Cannabis Users |
|
|---|---|---|---|---|---|---|---|
| M (SD) | Early (n) | Late (n) | n | minimum | |||
| Battisti et al. 2010a | 36.10 (11.11) | Mean age = 15.0 (25) | 19 | 12 hours | Earlier onset associated with worse inhibition |
-- | |
| Ehrenreich et al. 1999 | 23.25 (unk) | <16 (48) | ≥16 (51) | 49 | 2 hours | Visual attention | Divided attention, flexibility in attention, working memory |
| Fontes et al. 2011a | 30.21(unk) | <15 (49) | ≥15 (55) | 44 | Unknown | Executive functioning, inhibition | None |
| Gruber et al. 2011 | 22.80 (6.57) | <16 (19) | ≥16 (15) | 28 | 12 hours | Verbal fluency, inhibition | Attention, working memory, visuospatial & episodic memory |
| Gruber et al. 2012 | 22.75 (2.82) | <16 (9) | ≥16 (14) | 16 | 12 hours | None | Inhibition (compared to the late-onset users) |
| Pope et al. 2003 | 39.48 (unk) | <17 (69) | ≥17 (53) | 87 | 28 days | Verbal fluency, inhibition, episodic & visuospatial memory |
Attention, working memory |
| Solowij et al. 2011 | 18.30 (0.63) | Mean age = 16.5 (52) | 62 | 12 hours | Earlier onset associated with worse impulsivity |
-- | |
| Solowij et al. 2012 | 18.30 (0.63) | Mean age = 16.0 (48) | 62 | 12 hours | Earlier onset associated with worse episodic memory |
-- | |
Note: All values are means and standard deviations unless otherwise noted; unk, unknown; --, no other domains evaluated.
4.2. Sex Differences in Cannabis’ Influence on Neurobehavioral Functioning
4.2.1. Neurodevelopmental Considerations
Accumulating evidence indicates sex-specific effects of cannabinoids. Neurodevelopmental trajectories vary by sex, such that female brains appear to undergo earlier maturation than male brains. Females’ total brain size peaks between 10 and 11 years of age, while males’ total brain size peaks at about 14 to 15 years of age (Lenroot et al. 2007). Similarly, females’ prefrontal cortex gray matter volume peaks 1 to 2 years earlier than in males (Giedd et al. 1999). Therefore, if cannabis use is initiated in early to mid-adolescence, it is possible that males may be more vulnerable to neurobehavioral disturbances, especially functional and/or structural disruptions in the prefrontal cortex, compared to females.
In addition to differential rates of neuromaturation, CB1 density may also vary by sex, with animal studies citing greater CB1 receptor density among males across several brain regions (Burston et al. 2010; Mateos et al. 2011; Reich et al. 2009; Rubino et al. 2008). Whereas males either maintain or lose some CB1 binding sites in later adulthood (i.e., 45 to 70 years of age), adult female brains show increases in CB1 receptor density across the lifespan, eventually surpassing that of males (Van Laere et al. 2008). Furthermore, adolescent females evidence greater CB1 desensitization after exposure to THC in the prefrontal cortex, hippocampus, striatum, amygdala, and midbrain (Burston et al. 2010; Rubino et al. 2008). Given that endocannabinoid signaling plays a crucial role in establishing normal sex differences in the brain (Viveros et al. 2012), it is not surprising that disruption of this system may produce sex-specific differences in neurocognitive outcomes.
The interaction between sex and age of cannabis use onset has been examined in several animal studies, with somewhat mixed results, but generally suggesting the importance of considering these factors together in determining neurocognitive outcomes. Acute THC administration disrupted learning in adolescent female, adolescent male, and adult female rats, but did not disrupt learning in adult male rats (Cha et al. 2007). Chronic doses of THC administration did not affect learning in either adolescent or adult male and female rats (Cha et al. 2007), but others found that male rats evidenced worse novel object recognition than female rats (Mateos et al. 2011). In contrast, female rats administered chronic THC during adolescence perform worse on an object location task than female controls, while no differences emerged among male rats (Mateos et al. 2011). Although both male and female rats demonstrated deficits in spatial working memory after exposure to THC in adolescence (O’Shea et al. 2004; O’Shea et al. 2006; Rubino et al. 2009a; Rubino et al. 2009b), this exposure in adulthood had long-term impairments on memory in male rats, but not in female rats (O’Shea et al. 2004; O’Shea et al. 2006). Thus, cannabis use during adolescence may produce sex-specific alterations in neurodevelopment that may lead to enduring cognitive impairments.
4.2.2. Sex Hormones and Cannabinoids
Many recent studies have focused on the endocannabinoid system’s bi-directional interactions with gonadal hormones (Lopez 2010). Cannabinoids affect the endocrine system, specifically the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes (which interact in their functioning), while gonadal hormones also modulate endocannabinoid activity and function (Lopez 2010). More specifically, evidence from animal studies suggest exogenous cannabinoids, like THC, have the ability to significantly alter pituitary function (L. Murphy et al. 1991a; L. Murphy et al. 1991b; Wenger et al. 1999) and may inhibit the HPG axis (L. Murphy et al. 1990; L. Murphy et al. 1994; L. Murphy et al. 1999; Steger et al. 1980; Steger et al. 1983), thereby dysregulating key motivational systems involved in drug-seeking behavior. Additionally, the endocannabinoid system plays an important role in regulating the neuroendocrine and behavioral response to stress, and evidence supports an association between disrupted endocannbinoid signaling and a blunted stress response (Gorzalka et al. 2008). However, hormones significantly influence the impact of cannabinoids on the HPG axis, as estrogen reduces the inhibitory effect of cannabinoids on HPG function (Scorticati et al. 2003; Scorticati et al. 2004). Furthermore, hormones play a key role in structural and functional changes in the endocannabinoid system, evidenced by fluctuations in endocannabinoid receptor (especially CB1) density across the estrus cycle (Bradshaw et al. 2006; S. Gonzalez et al. 2000; Rodriguez de Fonseca et al. 1994). Moreover, human studies have demonstrated that, during the follicular phase of the menstrual cycle (when estradiol is high and progesterone is low), females are more responsive to the effects of stimulants, such as cocaine (Sofuoglu et al. 1999) and nicotine (Newman 2009; Perkins et al. 2000); however, this relationship has not yet been explored with cannabis. It is presently unclear how these factors may differentially affect the neuropsychological functioning of male and female cannabis users; however, the potential detrimental downstream effects of cannabinoids on the HPA and HPG axes and how these alterations may differentially affect neuropsychological functioning in males and females warrant further investigation.
4.2.3. Behavioral, Tolerance, and Metabolic Considerations
Factors outside of the central nervous system may also play an important role in how cannabis use differently affects neurocognition in males and females. Males are more likely to use cannabis than females (rates in 2010 were 11.2% vs. 6.8%, respectively; SAMSHA 2011) and initiate use at an earlier age (Gfroerer and Epstein 1999; Pope et al. 2003). However, similar to patterns observed in alcohol dependence, a telescoping effect (i.e., accelerated progression of a substance-use disorder) seems to occur. In this sense, females enter treatment for marijuana use disorders significantly sooner (i.e., after fewer years of use) and after less cumulative cannabis use compared to males (Hernandez-Avila et al. 2004). This may be due to various differences in the pharmacologic effects of THC in males and females. Preclinical studies have demonstrated that female rats preferentially metabolize THC to its most highly active metabolite, while male rats metabolize THC to multiple compounds (Narimatsu et al. 1991). Moreover, physiological data shows that when smoking a second cigarette, females have less tachycardia than males (Cocchetto et al. 1981), suggesting females may develop tolerance to cannabis more quickly than males, perhaps accelerating their escalation of use. Furthermore, some evidence indicates that females may feel greater hedonic reinforcement from cannabis compared to males, and that ovarian hormones (such as estrogen and progesterone) may facilitate stronger learned associations between drug effects and drug-related stimuli (Fattore et al. 2007). Taken together, these data suggest the potential for different behavioral, tolerance, and metabolic effects that influence patterns of use and abuse between males and females. Such differences may also indirectly influence the impact of cannabis use on the neurocognitive functioning of males and females. For example, it is possible that females may be more vulnerable to the negative effects of cannabis on frontal-limbic neurocircuitry, thus leading to an accelerated progression to cannabis use disorders. However, it remains unclear how factors like age of initiated use and amount of cannabis use may contribute to sex-specific neurocognitive outcomes among cannabis users.
5. Neurocognitive Sex Differences in Cannabis Users
When considering sex differences in cannabis effects on neurocognition, it is first important to account for the sex differences in neurocognitive performance often reported among healthy human subjects. Males often perform better than females on measures of decision-making (Bolla et al. 2004; Overman et al. 2004; Reavis and Overman 2001), spatial processing, and psychomotor performance (Gur et al. 2012); whereas females perform better on measures of verbal memory (Kramer et al. 1988), visual recognition, attention, and reasoning abilities (Gur et al. 2012). In light of this and the aforementioned sex differences in neurodevelopment, endocannabinoid distribution and functioning, metabolism of THC, tolerance to THC, patterns of cannabis use, and cannabis’ effects on hormonal systems, it seems reasonable to expect differences in how cannabis use may influence neuropsychological functioning in males and females. Nonetheless, this has been examined by only a few studies (see Table 2), but has promise to be a more significant component of the literature in coming years.
Table 2. Studies Examining Sex Differences in Cannabis Users on Neurocognitive Functioning.
| Study | Age | Female Users | Female Controls | Male Users | Male Controls | Length of Abstinence | Findings Pertaining to Sex Differences |
|---|---|---|---|---|---|---|---|
| M (SD) | n | n | n | n | minimum | ||
| Acute Neurocognitive Studies | |||||||
| Anderson et al. 2010 | 20.50 (2.70) |
35 | -- | 35 | -- | After smoking THC (2.9%) |
No difference on visuospatial processing, time estimation, and cognitive flexibility |
| Makela et al. 2006 | 21.80 (0.93) |
-- | 7 | -- | 12 | After ingesting 8.5mg THC |
Spatial working memory was enhanced in females, but not males |
| McDonald et al. 2003 | 23.00 (4.48) |
19 | -- | 18 | -- | After ingesting 7.5 or 15mg THC |
No difference on impulsivity |
| Roser et al. 2009 | 27.90 (2.90) |
-- | 12 | -- | 12 | After ingesting 10mg THC or THC w/5.4mg CBD |
Males had faster left-hand tapping than females in both conditions |
| Non-Acute Neurocognitive Studies | |||||||
| Clark et al. 2009 | 23.25 (unk) |
10 | 7 | 5 | 12 | Unk | Male users psychomotor performance < male controls; no difference among females |
| King et al. 2011 | 22.70 (unk) |
14 | 14 | 16 | 16 | 12 hours | Male users psychomotor performance and visuospatial organization < male controls; no difference among females |
| Pope et al. 1997 | Unk | 24 | -- | 31 | -- | 19 hours | Heavy female visuospatial performance < light female; no difference among the males; or in attention & inhibition |
| Solowij et al. 2011 | 18.30 (0.63) |
21 | 44 | 31 | 18 | 12 hours | No interaction of group or sex on episodic memory |
| Tait et al. 2011 | 22.65 (unk) |
586 | 239 | 493 | 181 | (baseline
measures of 8 year study) |
No interaction of group or sex on episodic and working memory |
| Neuroimaging Studies | |||||||
| McQueeny et al. 2011 | 17.81 (unk) |
8 | 11 | 27 | 36 | 28 days | Female users right amygdalar volume > female controls, no difference among males |
| Medina et al. 2009 | 18.06 (unk) |
4 | 6 | 12 | 10 | 28 days | Female users prefrontal cortex > female controls; male users prefrontal cortex < male controls |
Note: All values are means and standard deviations unless otherwise noted; unk, unknown; THC, tetrahydrocannabinol; CBD, cannabidiol
In one of the first studies to examine sex differences after THC administration, McDonald and colleagues (2003) found no differences in the effects of THC (0mg, 7.5mg, or 15mg capsules) on measures of impulsivity between 18 male and 19 female recreational cannabis users. Similarly, 35 male and 35 female cannabis users did not demonstrate differential effects of smoked THC (joints with either 0% THC or 2.9% THC) in performance on measures of visuospatial processing, time estimation, and cognitive flexibility (Anderson et al. 2010). However, it is important to note that despite no differences found in subjective “high” scores in the aforementioned study, females discontinued smoking before males, but performed similarly. Rogers and colleagues (2007) found no sex differences in risk-taking performance between 7 male and 8 female cannabis users after administration of either 0mg or 5mg THC. Surprisingly, spatial working memory was enhanced in females (n= 7), but not in males (n=12) after 0mg or 8.5mg of sublingual THC (Makela et al. 2006). Additionally, in all five sessions of a finger tapping task, males (n= 12) had faster left-hand tapping than females (n= 12) after 10mg THC administration and for two sessions after THC and CBD combined administration (10mg THC and 5.4mg CBD), despite no differences in baseline performance (Roser et al. 2009). In summary, preliminary evidence suggests there may be little or no sex-specific effects of acute THC administration on neurocognition; however, studies are few and have relied on very small sample sizes.
In the few studies examining potential sex differences in the non-acute effects of cannabis on neuropsychological functioning, consistent evidence for sex-specific relationships are emerging. One of the first studies on this topic found that females who used cannabis heavily (i.e., median frequency of use was 29 out of the past 30 days) performed worse than light users (i.e., median frequency of use was 1 out of the past 30 days) on a visuospatial task - no differences were found between heavy and light male cannabis users, nor were any sex differences found on task of attention and inhibition (Pope et al. 1997). More recently, male cannabis users evidenced impairments on psychomotor performance (Clark et al. 2009; King et al. 2011) and visuospatial organization (King et al. 2011) compared to male healthy controls, but no differences were observed between cannabis-using females and their non-using counterparts (Clark et al. 2009; King et al. 2011). Preliminary evidence from our laboratory suggests a sex-specific dissociation in how amount of cumulative lifetime cannabis use relates to decision-making, but not episodic memory, among male (n= 44) and female (n = 25) young adult cannabis users (unpublished). Specifically, more lifetime cannabis use was associated with poorer decision-making performance for male cannabis users, but not female cannabis users. In contrast, more lifetime cannabis use was negatively associated with episodic memory performance in both male and female cannabis users. Other studies report no interaction effects of sex and cannabis use on episodic memory (Solowij et al. 2011; Tait et al. 2011) or working memory (Tait et al. 2011).
A few neuroimaging studies provide further preliminary evidence for sex-specific differences in the residual influence of cannabis use. After 28 days of abstinence, female adolescent chronic cannabis users had larger right amygdala volumes compared to female adolescent healthy controls, while there was no difference in amygdala volumes among adolescent male chronic cannabis users and controls (McQueeny et al. 2011). Female adolescent chronic cannabis users also evidenced larger prefrontal cortex volumes than female controls, whereas males showed the opposite pattern relative to a male control group (Medina et al. 2009). In this sample, smaller prefrontal cortex volume was associated with better executive functioning among the cannabis users, but larger prefrontal cortex volume was associated with better executive functioning among the controls.
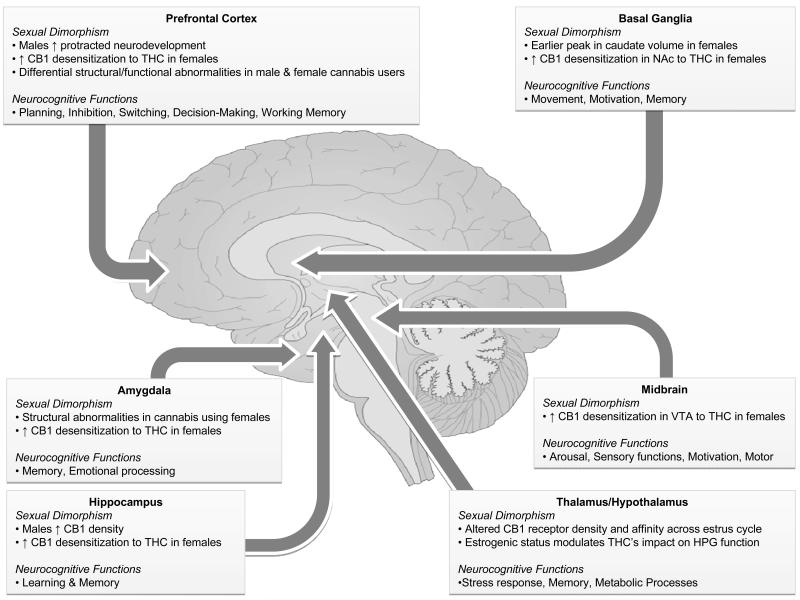
Although still nascent, the current evidence points to sex differences in the impact of cannabis on neurocognitive functioning, but more so for non-acute than acute effects. However, the sex-dependent influence of cannabis appears to be domain specific (see Figure 1), which may reflect some of the aforementioned differences in neurodevelopment, CB1 receptor density, hormones, and both the pharmacological and subjective effects of cannabis. Other factors not covered in this review may also contribute to possible sex differences. For example, one study found that stereotype threat (i.e., individuals’ belief that they belong in a group that is seen as inferior) differentially affected neurocognitive measures among male and female cannabis users (Looby and Earleywine 2010). Although the emerging neuroimaging data suggest that some of the neurobehavioral differences may be driven by structural differences, the overall picture remains largely unstudied and likely involves a complex contribution of many factors.
Figure 1.
Sexual dimorphism and neurocognitive functions of brain regions with high CB1 receptor density. This figure simplifies and summarizes some sexual dimorphisms relevant to the effects of cannabis on brain functioning in regions with high CB1 receptor density and some of the neurocognitive functions commonly ascribed to these regions. Arrows indicate the general location of the noted structures, although some lie outside of the shown midsaggital plane.
6. Concluding Remarks
Accumulating research in the past five years has contributed considerable advances regarding cannabis’ acute and non-acute effects on human cognition. These studies continue to demonstrate the negative effect of cannabis on learning and memory, in addition to deficits in attention, concentration, and abstract reasoning in both acute and non-acute studies. Given the acute and non-acute deficits in attention and concentration, it is possible that the cannabis-related impairments in episodic memory may be more influenced by encoding (through disruptions in the prefrontal cortex), rather than through temporal-lobe mediated storage and retrieval - or it may be a combination of both. However, the authors of a recent review on the acute effects of cannabis on memory function reported that deficits in learning and recall could not be attributed to cannabis’ disruption of attentional processes; instead, acute cannabis intoxication may disrupt retrieval and not encoding (Ranganathan and D’Souza 2006). On the other hand, results from a recent study by our group found that poorer performances in delayed recall by cannabis users on an auditory episodic memory task did not persist after controlling for performance on immediate memory (R. Gonzalez et al. In press). Cannabis-related effects in other neurocognitive domains are often less consistent. Working memory is impaired during acute intoxication, but remains largely intact among recently abstinent adult cannabis users, while the data is mixed for recently abstinent adolescent cannabis users. The findings for measures of inhibition, motor impulsivity, and psychomotor control are mixed, whereas measures of decision-making and risk-taking remain intact during acute intoxication, but are impaired among cannabis users. Taken together with cannabinoid receptor distribution and evidence from neuroimaging data, it may be that cannabis’ effects on prefrontal, striatal, and hippocampal structures may drive the observed neurocognitive profiles of cannabis users.
These new results have implications for clinical practice. For example, problems with decision-making, inhibition and/or risk-taking may serve as risk factors for the development of substance use disorders, as found in research on other drugs of abuse (de Wit 2009; Goldstein and Volkow 2002; Schepis et al. 2008), making it more difficult to resist the urge to continue using the drug even when its use is likely to be harmful (Bechara 2005). It is also possible that cannabis negatively impacts these neurocognitive domains, especially if use is initiated during adolescence, when the prefrontal cortex is still developing. However, it is important to keep in mind the many pitfalls to interpreting and generalizing findings from studies presented in a systematic review. Many of these can be addressed with meta-analysis; however, despite the rapid growth in research, the last meta-analysis examining non-acute effects of cannabis on neurocognition was published almost a decade ago (I. Grant et al. 2003).
Importantly, many recent studies have also examined specific variables that may contribute to some of the discrepant findings pertaining to the neuropsychological profile of cannabis use. Here we suggest that neurodevelopmental factors and sex differences are important to consider when examining neurocognitive effects of longer term cannabis use. For example, onset of use during adolescence, as compared to later lifetime epochs, may be associated with disruption of normal neuromaturation and result in neurocognitive deficits. There is accumulating evidence that neurodevelopmental trajectories, regional CB1 densities, as well as endocrinological and behavioral contributions are not uniform across sex, resulting in a sex-dependent impact of cannabis use on neurocognitive functioning.
Together, data reviewed in the current manuscript indicates that cannabis use may have a negative impact on some aspects of neurocognition, especially those mediated principally by frontal-limbic systems. However, there is a high degree of inconsistency related to the exact nature and chronicity or reversibility of these deficits. Future investigations that continue to employ longitudinal methodologies and capitalize on large sample sizes across the full range of cannabis use experience are critical to disentangle the temporal ordering of cognitive deficits and cannabis use. Additionally, more nuanced examinations that take into account individual difference variables are crucial in order to develop a more stable understanding of how cannabis interferes with various domains of neuropsychological functioning. We suggest that a specific focus on pre-existing neurobehavioral factors and sex-differences, as well as their complex interactions, will be fruitful in understanding the subjective liability to the neurocognitive effects of cannabis.
Acknowledgements
This review was made possible by grants from the National Institute on Drug Abuse (K23 DA023560, R01 DA031176, and R01 DA033156, PI: Gonzalez) and (F31 DA032244-01, PI: Schuster). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or the National Institutes of Health.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behavioural Brain Research. 2010;215(1):45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Abush H, Akirav I. Short- and long-term cognitive effects of chronic cannabinoids administration in late-adolescence rats. PLoS One. 2012;7(2):e31731. doi: 10.1371/journal.pone.0031731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Brooks AC, Nunes EV, Hasin DS. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug and Alcohol Dependence. 2008;95(3):279–283. doi: 10.1016/j.drugalcdep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS. Sex, drugs, and cognition: effects of marijuana. Journal of Psychoactive Drugs. 2010;42(4):413–424. doi: 10.1080/02791072.2010.10400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, Gallo DA, de Wit H. Psychoactive drugs and false memory: comparison of dextroamphetamine and delta-9-tetrahydrocannabinol on false recognition. Psychopharmacology (Berl) 2012;219(1):15–24. doi: 10.1007/s00213-011-2374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology (Berl) 2010a;212(4):613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstone SJ, Respondek C, Hermens DF, Solowij N. Chronic use of cannabis and poor neural efficiency in verbal memory ability. Psychopharmacology (Berl) 2010b;209(4):319–330. doi: 10.1007/s00213-010-1800-4. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20(4):398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(6):837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Archives of General Psychiatry. 2012;69(1):27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Archives of General Psychiatry. 2009;66(4):442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC) Drug and Alcohol Dependence. 2010;112(3):220–225. doi: 10.1016/j.drugalcdep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biological Psychiatry. 2008;64(11):966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Jager G, van Hell HH, Zuurman L, Jansma JM, Mehta MA, et al. Effects of Delta9-tetrahydrocannabinol administration on human encoding and recall memory function: a pharmacological FMRI study. Journal of Cognitive Neuroscience. 2012a;24(3):588–599. doi: 10.1162/jocn_a_00156. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Jansma JM, van Hell HH, Jager G, Oudman E, Saliasi E, et al. Effects of Delta9-Tetrahydrocannabinol on Human Working Memory Function. Biological Psychiatry. 2012b doi: 10.1016/j.biopsych.2012.01.008. doi:S0006-3223(12)00046-7 [pii]10.1016/j.biopsych.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2006;291(2):R349–358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug and Alcohol Dependence. 2011;117(1):59–61. doi: 10.1016/j.drugalcdep.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. British Journal of Pharmacology. 2010;161(1):103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment:decomposing performance on the Bechara gambling task. Psychological Assessment. 2002;14(3):253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Cane JE, Sharma D, Albery IP. The addiction Stroop task: examining the fast and slow effects of smoking and marijuana-related cues. Journal of Psychopharmacology. 2009;23(5):510–519. doi: 10.1177/0269881108091253. [DOI] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behavioural Pharmacology. 2007;18(5-6):563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(Pt 5):1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and Cognition. 2004;55(1):41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Robbins TW, Sahakian BJ. Disrupted ‘reflection’ impulsivity in cannabis users but not current or former ecstasy users. Journal of Psychopharmacology. 2009;23(1):14–22. doi: 10.1177/0269881108089587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchetto DM, Owens SM, Perez-Reyes M, DiGuiseppi S, Miller LL. Relationship between plasma delta-9-tetrahydrocannabinol concentration and pharmacologic effects in man. Psychopharmacology (Berl) 1981;75(2):158–164. doi: 10.1007/BF00432179. [DOI] [PubMed] [Google Scholar]
- Cohen PJ. Medical marijuana 2010: it’s time to fix the regulatory vacuum. Journal of Law, Medicine & Ethics. 2010;38(3):654–666. doi: 10.1111/j.1748-720X.2010.00519.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291(17):2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An Evidence Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions. Journal of Addiction Medicine. 2011;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of Psychopharmacology. 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, et al. Cannabis and anxiety: a critical review of the evidence. Human Psychopharmacology. 2009;24(7):515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: Abstract reasoning, motor programming, and cognitive flexibility. Addictive Behaviors. 2010;35(10):875–881. doi: 10.1016/j.addbeh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008a;198(4):587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008b;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn JR, Robertson MD, et al. The incidence of drugs in drivers killed in Australian road traffic crashes. Forensic Science International. 2003;134(2-3):154–162. doi: 10.1016/s0379-0738(03)00134-8. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, van Hasselt JG, de Kam M, van Gerven JM, Touw DJ, Buitelaar JK, et al. Acute psychomotor, memory and subjective effects of MDMA and THC co-administration over time in healthy volunteers. Journal of Psychopharmacology. 2011;25(4):478–489. doi: 10.1177/0269881110376687. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Elikkottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. Journal of Opioid Management. 2009;5(6):341–357. [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sciences. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Archives of Internal Medicine. 1998;158(20):2257–2264. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. British Journal of Pharmacology. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. Journal of Psychopharmacology. 2010;24(9):1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience and Biobehavior Reviews. 2011;35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ferraro DP. Acute effects of marijuana on human memory and cognition. NIDA Research Monographs. 1980;31:98–119. [PubMed] [Google Scholar]
- Fisk JE, Montgomery C. Real-world memory and executive processes in cannabis users and non-users. Journal of Psychopharmacology. 2008;22(7):727–736. doi: 10.1177/0269881107084000. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. British Journal of Psychiatry. 2011a;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Frontal Assessment Battery (FAB) is a simple tool for detecting executive deficits in chronic cannabis users. Journal of Clinical and Experimental Neuropsychology. 2011b;33(5):523–531. doi: 10.1080/13803395.2010.535505. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, et al. Cognitive Mechanisms Underlying Risky Decision-Making in Chronic Cannabis Users. Journal of Mathematical Psychology. 2010;54(1):28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. International Journal of Neuropsychopharmacology. 2010a;13(4):421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, et al. At Risk for Schizophrenic or Affective Psychoses? A Meta-Analysis of DSM/ICD Diagnostic Outcomes in Individuals at High Clinical Risk. Schizophrenia Bulletin. 2012 doi: 10.1093/schbul/sbs060. doi:10.1093/schbul/sbs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high risk state: a comprehensive state of the art review. Archives of General Psychiatry. doi: 10.1001/jamapsychiatry.2013.269. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews. 2011a;35(5):1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Archives of General Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Archives of General Psychiatry. 2010b;67(7):683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Molecular Psychiatry. 2011b;16(1):67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal Presynaptic Dopamine in Schizophrenia, Part I: Meta-analysis of Dopamine Active Transporter (DAT) Density. Schizophrenia Bulletin. 2012a doi: 10.1093/schbul/sbr111. doi:10.1093/schbul/sbr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. Striatal Presynaptic Dopamine in Schizophrenia, Part II: Meta-Analysis of [18F/11C]-DOPA PET Studies. Schizophrenia Bulletin. 2012b doi: 10.1093/schbul/sbr180. doi:10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical Maps of Psychosis Onset: Voxel-wise Meta-Analysis of Antipsychotic-Naive VBM Studies. Schizophrenia Bulletin. 2011c doi: 10.1093/schbul/sbr134. doi:10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Stone JM, Broome MR, Valli I, Mechelli A, McLean MA, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Archives of General Psychiatry. 2011d;68(9):881–890. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Epstein JF. Marijuana initiates and their impact on future drug abuse treatment need. Drug and Alcohol Dependence. 1999;54(3):229–237. doi: 10.1016/s0376-8716(98)00167-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychology Review. 2007;17(3):347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Carey C, Grant I. Nonacute (residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. Journal of Clinical Pharmacology. 2002;42(11 Suppl):48S–57S. doi: 10.1002/j.1552-4604.2002.tb06003.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, et al. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76(2):181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Crane NA, Martin EM, Vassileva J. Decision-Making Performance Influences the Relationship between Amount of Cannabis use and its Negative Consequences; Annual Meeting of the International Neuropsychological Society; Montreal, Canada. 2012; published abstract. [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationships to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803395.2012.703642. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Vassileva J, Martin EM. Impact of HIV and a history of marijuana dependence on procedural learning among individuals with a history of substance dependence. Journal of Clinical and Experimental Neuropsychology. 2011;33(7):735–752. doi: 10.1080/13803395.2011.553584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Scott JC. Neuropsychological Consequences of Drug Abuse. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. 3rd Edition New York; Oxford University Press; 2009. pp. 455–479. [Google Scholar]
- Gonzalez S, Mauriello-Romanazzi G, Berrendero F, Ramos JA, Franzoni MF, Fernandez-Ruiz J. Decreased cannabinoid CB1 receptor mRNA levels and immunoreactivity in pituitary hyperplasia induced by prolonged exposure to estrogens. Pituitary. 2000;3(4):221–226. doi: 10.1023/a:1012874029689. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neuroscience and Biobehavioral Reviews. 2008;32(6):1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychology Society. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and Alcohol Dependence. 2011 doi: 10.1016/j.drugalcdep.2011.08.015. doi:10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neuroscience Letters. 2012;511(2):89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2011 doi: 10.1037/a0026269. doi:10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiefthyvoulou F, Fisk JE, Montgomery C, Bridges N. Prospective memory functioning among ecstasy/polydrug users: evidence from the Cambridge Prospective Memory Test (CAMPROMPT) Psychopharmacology (Berl) 2011;215(4):761–774. doi: 10.1007/s00213-011-2174-y. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, et al. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacology, Biochemistry, and Behavior. 2010;96(3):333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicology and Teratology. 2010;32(5):515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. European Addiction Research. 2009;15(2):94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biological Psychiatry. 2007;61(11):1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and Alcohol Dependence. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34(11):2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestegge L, Kunert HJ, Radach R. Long-term effects of cannabis on eye movement control in reading. Psychopharmacology (Berl) 2010;209(1):77–84. doi: 10.1007/s00213-009-1769-z. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Bocker KB, Schipper CM, Kruidenier M, Leenders ME, et al. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC) Psychopharmacology (Berl) 2009;204(1):85–94. doi: 10.1007/s00213-008-1440-0. [DOI] [PubMed] [Google Scholar]
- Indlekofer F, Piechatzek M, Daamen M, Glasmacher C, Lieb R, Pfister H, et al. Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. Journal of Psychopharmacology. 2009;23(5):495–509. doi: 10.1177/0269881108091076. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126(6):1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of the New York Academy of Sciences. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(6):561–572. doi: 10.1016/j.jaac.2010.02.001. 572 e561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 2006;185(3):358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: an overview of animal and human research. Current Drug Abuse Reviews. 2008;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. European Neuropsychopharmacology. 2007;17(4):289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings. Vol. 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. p. 78. [Google Scholar]
- Kalant H. Adverse effects of cannabis on health: an update of the literature since 1996. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28(5):849–863. doi: 10.1016/j.pnpbp.2004.05.027. [DOI] [PubMed] [Google Scholar]
- King GR, Ernst T, Deng W, Stenger A, Gonzales RM, Nakama H, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. Journal of Neuroscience. 2011;31(49):17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver N, Nieman DH, Becker HE, van de Fliert JR, Dingemans PH, de Haan L, et al. Symptomatology and neuropsychological functioning in cannabis using subjects at ultra-high risk for developing psychosis and healthy controls. Australian and New Zealand Journal of Psychiatry. 2010;44(3):230–236. doi: 10.3109/00048670903487118. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex-Differences in Verbal-Learning. Journal of Clinical Psychology. 1988;44(6):907–915. [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30(4):800–809. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behaviors. 2007;32(5):977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiology of Disease. 2005;19(1-2):96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MC, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G. Marijuana use and motor vehicle crashes. Epidemiologic Reviews. 2012;34(1):65–72. doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looby A, Earleywine M. Gender moderates the impact of stereotype threat on cognitive function in cannabis users. Addictive Behaviors. 2010;35(9):834–839. doi: 10.1016/j.addbeh.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Lopez HH. Cannabinoid-hormone interactions in the regulation of motivational processes. Hormones and Behavior. 2010;58(1):100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handbook of Experimental Pharmacology. 2005;(168):299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. Journal of Studies on Alcohol and Drugs. 2010;71(6):885–894. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31(2):462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. British Journal of Pharmacology. 2010;160(3):511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use: a systematic review of the literature. Psychological Medicine. 2010;40(3):383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Kobel M, Stoecklin M, Michael T, Weber S, Krebs B, et al. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. 2009;60(2):94–103. doi: 10.1159/000239685. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, et al. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. Journal of Psychopharmacology. 2011;25(12):1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Human Psychopharmacology. 2008;23(5):409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol--recent advances. Chemistry & Biodiversity. 2007;4(8):1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Science Signaling. 2002;2002(129):re5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007a;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addiction Biology. 2009;14(4):457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007b;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Seddon AL, Fisk JE, Murphy PN, Jansari A. Cannabis-related deficits in real-world memory. Human Psychopharmacology. 2012;27(2):217–225. doi: 10.1002/hup.1273. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychological Medicine. 2011:1–10. doi: 10.1017/S0033291711001322. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected] British Journal of Psychiatry. 2010;197(4):285–290. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychological Medicine. 2009;39(10):1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- Mura P, Kintz P, Ludes B, Gaulier JM, Marquet P, Martin-Dupont S, et al. Comparison of the prevalence of alcohol, cannabis and other drugs between 900 injured drivers and 900 control subjects: results of a French collaborative study. Forensic Science International. 2003;133(1-2):79–85. doi: 10.1016/s0379-0738(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Adrian BA, Kohli M. Inhibition of luteinizing hormone secretion by delta9-tetrahydrocannabinol in the ovariectomized rat: effect of pretreatment with neurotransmitter or neuropeptide receptor antagonists. Steroids. 1999;64(9):664–671. doi: 10.1016/s0039-128x(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Gher J, Steger RW, Bartke A. Effects of delta 9-tetrahydrocannabinol on copulatory behavior and neuroendocrine responses of male rats to female conspecifics. Pharmacology Biochemistry and Behavior. 1994;48(4):1011–1017. doi: 10.1016/0091-3057(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Newton SC, Dhali J, Chavez D. Evidence for a direct anterior pituitary site of delta-9-tetrahydrocannabinol action. Pharmacology Biochemistry and Behavior. 1991a;40(3):603–607. doi: 10.1016/0091-3057(91)90370-h. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Rodriguez de Fonseca F, Steger RW. delta 9-Tetrahydrocannabinol antagonism of the anterior pituitary response to estradiol in immature female rats. Steroids. 1991b;56(2):97–102. doi: 10.1016/0039-128x(91)90131-e. [DOI] [PubMed] [Google Scholar]
- Murphy LL, Steger RW, Smith MS, Bartke A. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rat. Neuroendocrinology. 1990;52(4):316–321. doi: 10.1159/000125604. [DOI] [PubMed] [Google Scholar]
- Murphy PN, Erwin PG, Maciver L, Fisk JE, Larkin D, Wareing M, et al. The relationships of ‘ecstasy’ (MDMA) and cannabis use to impaired executive inhibition and access to semantic long-term memory. Human Psychopharmacology. 2011;26(7):460–469. doi: 10.1002/hup.1228. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochemical Pharmacology. 1991;41(8):1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40(3):1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Newman JL, Mello NK. Neuroactive gonadal steroid hormones and drug addicton in women. In: Brady KT, Back SE, Greenfield SF, editors. Women and Addiction: A Comprehensive Textbook. Guildford Press; New York, NY: 2009. pp. 35–64. [Google Scholar]
- Nixon SJ, Paul R, Phillips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcoholism, Clinical and Experimental Research. 1998;22(7):1414–1420. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- O’Connell TJ, Bou-Matar CB. Long term marijuana users seeking medical cannabis in California (2001-2007): demographics, social characteristics, patterns of cannabis and other drug use of 4117 applicants. Harm Reduction Journal. 2007;4:16. doi: 10.1186/1477-7517-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, Ponto LB, et al. Effects of smoking marijuana on focal attention and brain blood flow. Human Psychopharmacology. 2007;22(3):135–148. doi: 10.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;20(5):611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology. 2004;18(4):502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42(13):1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21(4):478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, et al. Tobacco withdrawal in women and menstrual cycle phase. Journal of Consulting and Clinical Psychology. 2000;68(1):176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addiction Biology. 2008;13(2):147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Piechatzek M, Indlekofer F, Daamen M, Glasmacher C, Lieb R, Pfister H, et al. Is moderate substance use associated with altered executive functioning in a population-based sample of young adults? Human Psychopharmacology. 2009;24(8):650–665. doi: 10.1002/hup.1069. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Gruber S, Simpson N, Pope HG, et al. Cannabis and motor function: fMRI changes following 28 days of discontinuation. Experimental and Clinical Psychopharmacology. 2008;16(1):22–32. doi: 10.1037/1064-1297.16.1.22. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: the current status of research. Drug and Alcohol Dependence. 1995;38(1):25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychotherapy and Psychosomatics. 1997;66(4):179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33(5):1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug and Alcohol Dependence. 2004;73(2):109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. Journal of Psychopharmacology. 2009;23(3):266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Theunissen EL, de Brouwer M, Toennes SW, Moeller MR, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214(2):391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188(4):425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research. 2009;60(2):132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115(1):196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behavioural Brain Research. 2009;203(2):264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GM, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage. 2010;52(2):429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sciences. 1994;54(3):159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Wakeley J, Robson PJ, Bhagwagar Z, Makela P. The effects of low doses of delta-9 tetrahydrocannabinol on reinforcement processing in the risky decision-making of young healthy adults. Neuropsychopharmacology. 2007;32(2):417–428. doi: 10.1038/sj.npp.1301175. [DOI] [PubMed] [Google Scholar]
- Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM. Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. European Archives of Psychiatry and Clinical Neuroscience. 2009;259(5):284–292. doi: 10.1007/s00406-009-0868-5. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Alberio T, Capurro V, Vigano D, et al. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotoxicity Research. 2009a;15(4):291–302. doi: 10.1007/s12640-009-9031-3. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009b;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- SAMSHA . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- Schepis TS, Desai RA, Smith AE, Cavallo DA, Liss TB, McFetridge A, et al. Impulsive sensation seeking, parental history of alcohol problems, and current alcohol and tobacco use in adolescents. Journal of Addiction Medicine. 2008;2(4):185–193. doi: 10.1097/adm.0b013e31818d8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes KE, Martin-Iverson MT. Alterations to pre-pulse inhibition (PPI) in chronic cannabis users are secondary to sustained attention deficits. Psychopharmacology (Berl) 2009;207(3):469–484. doi: 10.1007/s00213-009-1679-0. [DOI] [PubMed] [Google Scholar]
- Scholes KE, Martin-Iverson MT. Cannabis use and neuropsychological performance in healthy individuals and patients with schizophrenia. Psychological Medicine. 2010;40(10):1635–1646. doi: 10.1017/S0033291709992078. [DOI] [PubMed] [Google Scholar]
- Scholes-Balog KE, Martin-Iverson MT. Cannabis use and sensorimotor gating in patients with schizophrenia and healthy controls. Human Psychopharmacology. 2011 doi: 10.1002/hup.1217. doi:10.1002/hup.1217. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, Gonzalez R. The Influence of Inhibitory Control and Episodic Memory on the Risky Sexual Behavior of Young Adult Cannabis Users. Journal of the International Neuropsychological Society. 2012;18(5):827–833. doi: 10.1017/S1355617712000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42(3):401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorticati C, Fernandez-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, et al. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorticati C, Mohn C, De Laurentiis A, Vissio P, Fernandez Solari J, Seilicovich A, et al. The effect of anandamide on prolactin secretion is modulated by estrogen. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2134–2139. doi: 10.1073/pnas.0437924100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and Clinical Psychopharmacology. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews. 2008;1(1):81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216(1):131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl) 2012;219(2):575–586. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- Solowij N, Pesa N. Cognitive abnormalities and cannabis use. Revista Brasileira de Psiquiatria. 2010;32(Suppl 1) [PubMed] [Google Scholar]
- Steger RW, DePaolo L, Asch RH, Silverman AY. Interactions of delta 9-tetrahydrocannabinol (THC) with hypothalamic neurotransmitters controlling luteinizing hormone and prolactin release. Neuroendocrinology. 1983;37(5):361–370. doi: 10.1159/000123576. [DOI] [PubMed] [Google Scholar]
- Steger RW, Silverman AY, Siler-Khodr TM, Asch RH. The effects of delta 9-tetrahydrocannabinol on the positive and negative feedback control of luteinizing hormone release. Life Sciences. 1980;27(20):1911–1916. doi: 10.1016/0024-3205(80)90438-5. [DOI] [PubMed] [Google Scholar]
- Sugarman DE, Poling J, Sofuoglu M. The safety of modafinil in combination with oral 9-tetrahydrocannabinol in humans. Pharmacology, Biochemistry, and Behavior. 2011;98(1):94–100. doi: 10.1016/j.pbb.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106(12):2195–2203. doi: 10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen EL, Kauert GF, Toennes SW, Moeller MR, Sambeth A, Blanchard MM, et al. Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology (Berl) 2011;220(2):341–350. doi: 10.1007/s00213-011-2479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and common sense: how far apart? Philosophical Transactions of the Royal Society of London. 2001;356(1413):1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crimes . World Drug Report. United Nations Publications; 2011. [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Block RI, O’Leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37(3):618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, et al. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage. 2008;39(4):1533–1541. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, Rodriguez de Fonseca F. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. Journal of Psychopharmacology. 2012;26(1):164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Wadsworth EJ, Moss SC, Simpson SA, Smith AP. Cannabis use, cognitive performance and mood in a sample of workers. Journal of Psychopharmacology. 2006;20(1):14–23. doi: 10.1177/0269881105056644. [DOI] [PubMed] [Google Scholar]
- Wagner D, Becker B, Gouzoulis-Mayfrank E, Daumann J. Interactions between specific parameters of cannabis use and verbal memory. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(6):871–876. doi: 10.1016/j.pnpbp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. International Journal of Clinical Practice. 2005;59(3):291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Benson JA, Jr., Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Archives of General Psychiatry. 2000;57(6):547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, et al. Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl) 2008a;196(1):119–131. doi: 10.1007/s00213-007-0940-7. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, et al. A study investigating the acute dose-response effects of 13 mg and 17 mg Delta 9-tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. Journal of Psychopharmacology. 2008b;22(4):441–451. doi: 10.1177/0269881108088194. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Bechara A. Do individual differences in Iowa Gambling Task performance predict adaptive decision making for risky gains and losses? Journal of Clinical and Experimental Neuropsychology. 2010;32(2):141–150. doi: 10.1080/13803390902881926. [DOI] [PubMed] [Google Scholar]
- Wenger T, Fernandez-Ruiz JJ, Ramos JA. Immunocytochemical demonstration of CB1 cannabinoid receptors in the anterior lobe of the pituitary gland. Journal of Neuroendocrinology. 1999;11(11):873–878. doi: 10.1046/j.1365-2826.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- Wert RC, Raulin ML. The chronic cerebral effects of cannabis use. II. Psychological findings and conclusions. The International Journal of the Addictions. 1986;21(6):629–642. doi: 10.3109/10826088609027382. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Research. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology. 2011;36(7):1340–1348. doi: 10.1038/npp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]