Abstract
Spina bifida (SB) patients afflicted with myelomeningocele typically possess a neurogenic urinary bladder and exhibit varying degrees of bladder dysfunction. Although surgical intervention in the form of enterocystoplasty is the current standard of care in which to remedy the neurogenic bladder, it is still a stop-gap measure and is associated with many complications due to the use of bowel as a source of replacement tissue. Contemporary bladder tissue engineering strategies lack the ability to reform bladder smooth muscle, vasculature, and promote peripheral nerve tissue growth when using autologous populations of cells. Within the context of this study, we demonstrate the role of two specific populations of bone marrow (BM) stem/progenitor cells used in combination with a synthetic elastomeric scaffold that provides a unique and alternative means to current bladder regeneration approaches. In vitro differentiation, gene expression, and proliferation are similar among donor mesenchymal stem cells (MSCs), whereas poly(1,8-octanediol-cocitrate) scaffolds seeded with SB BM MSCs perform analogously to control counterparts with regard to bladder smooth muscle wall formation in vivo. SB CD34+ hematopoietic stem/progenitor cells cotransplanted with donor-matched MSCs cause a dramatic increase in tissue vascularization as well as an induction of peripheral nerve growth in grafted areas compared with samples not seeded with hematopoietic stem/progenitor cells. Finally, MSC/CD34+ grafts provided the impetus for rapid urothelium regeneration. Data suggest that autologous BM stem/progenitor cells may be used as alternate, nonpathogenic cell sources for SB patient-specific bladder tissue regeneration in lieu of current enterocystoplasty procedures and have implications for other bladder regenerative therapies.
Keywords: angiogenesis, cell-seeded matrix, biocompatible polymer, multipotent, organ repair
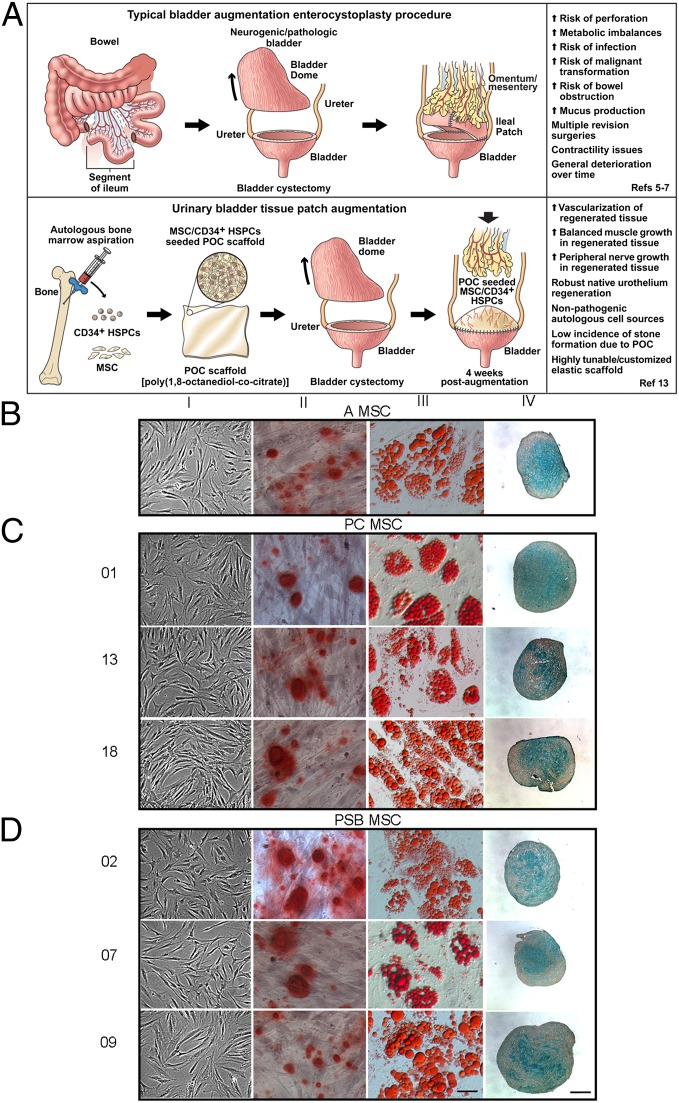
The most common neural tube defect compatible with life presents itself as spina bifida (SB) and consists of three disparate subtypes, of which myelomeningocele is the most common and the most debilitating (1, 2). SB patients affected by myelomeningocele nonexclusively suffer from bowel and urinary bladder abnormalities as a consequence of developmental error (3). The resulting neurogenic bladder predisposes a poor clinical outcome in which bladder function is below acceptable levels, leading to renal insufficiencies and ultimately to renal failure (4). To alleviate pressure from the upper urinary tract, patients undergo surgical intervention in the form of bladder augmentation enterocystoplasty in which a segment of bowel is used as a “patch” to enhance physiological bladder parameters to protect kidney function (Fig. 1A Upper). However, this procedure is associated with many side effects, including electrolyte imbalance, perforation, infection, stone formation, incontinence, and the potential for malignant transformation (5–7). Attempts to recreate functional bladder tissue ex vivo have been met with numerous obstacles that encompass materials design issues and cellular incompatibilities. Thus, alternate strategies using autologous, nonpathogenic, and physiologically compatible cells combined with an elastomeric scaffold that mimics bladder mechanical characteristics would be ideal (Fig. 1A Lower).
Fig. 1.
MSC characterization. (A) Schematic representation of standard bladder augmentation procedure (Upper) along with the alternative urinary bladder tissue patch procedure (Lower). (B–D) Donor MSCs demonstrated expected osteogenic (column II), adipogenic (column III), and chondrogenic (column IV) differentiation via lineage-specific staining along with undifferentiated controls (column I). In B–D, magnification is 400× (scale bar, 50μm); magnification for chondrocytes is 100× (scale bar, 200 μm).
Primitive multipotent CD34+ hematopoietic stem/progenitor cells (HSPCs or CD34+) have demonstrated the ability to differentiate into various cellular constituents of blood under appropriate environmental stimuli (8). CD34+ HSPCs also have the unique ability to either induce angiogenesis or vasculogenesis by mechanisms that have not been fully delineated as demonstrated by neovascularization and physiological enhancement of ischemic myocardium (9). Hence, CD34+ HSPCs could provide a means for tissue revascularization in vivo. Multipotent mesenchymal stem cells (MSCs) have been highly studied over the last several decades and maintain a level of self-renewal with attributes that allow for the terminal differentiation into what are now widely characterized cell types (10). Functional studies in multiple settings further demonstrate that MSCs also possess the unique ability to provide compensatory factors to damaged tissue by aiding in tissue repair exhibited by increased localized angiogenesis (11). In this study, we set forth to establish that SB bone marrow (BM) MSCs are not affected by pathologies associated with SB and can perform analogously to control MSC counterparts within a nude rat bladder augmentation model. Second, we demonstrate that donor-matched SB MSCs with CD34+ HSPCs lead to superior blood vessel and peripheral nerve formation and robust urothelium regeneration in vivo. Hence, SB BM stem/progenitor cells provide distinct advantages over native bladder cells from SB patients as MSC/CD34+ poly(1,8-octanediol-cocitrate) (POC) composites and may provide an alternative solution to current bladder augmentation enterocystoplasty strategies.
Results
MSC Characterization.
BM MSCs isolated from adult (A), pediatric control (PC; donors 01, 13, and 18), and pediatric SB (PSB; donors 02, 07, and 09) samples demonstrated typical fibroblast morphology under MSC maintenance culture conditions visualized by light microscopy (Fig. 1 B–D, column I) (10). MSCs were induced to undergo trilineage differentiation into osteoblasts, adipocytes, and chondrocytes. Osteoblasts were Alizarin Red S+ (column II), whereas adipocytes were Oil Red O+ (column III) and chondrocytes were Alcian blue+ (column IV). Quantitative analyses of cellular differentiation demonstrated similar trends across groups (Table S1). Flow cytometric-based immunophenotyping of MSCs demonstrated cellular expression of MSC surface markers CD29, CD44, CD73, CD90, CD105, and CD166 with the exclusion of hematopoietic cell lineage markers CD14, CD34, CD45, CD117, and CD133 (Fig. S1A). Whole-genome microarray analyses revealed differential expression of 194 genes between PSB and PC samples. Eighty-six probe sets were up-regulated in PSB and 108 were down-regulated in PSB samples (Fig. S1B). Because certain probe sets belonged to the same genes, 129 unique genes were identified consisting of several gene ontological groups (Table S2). Finally, in vitro proliferation assays demonstrated no statistically significant differences between groups at any time point (Fig. S1C). Pathologies associated with SB did not affect MSC multipotentiality or cell surface marker expression. Microarray data further demonstrate that PC and PSB MSCs have remarkably similar molecular signatures. Adult and PC samples were used as control populations throughout this study.
Histological and Morphometric Analyses of Grafts.
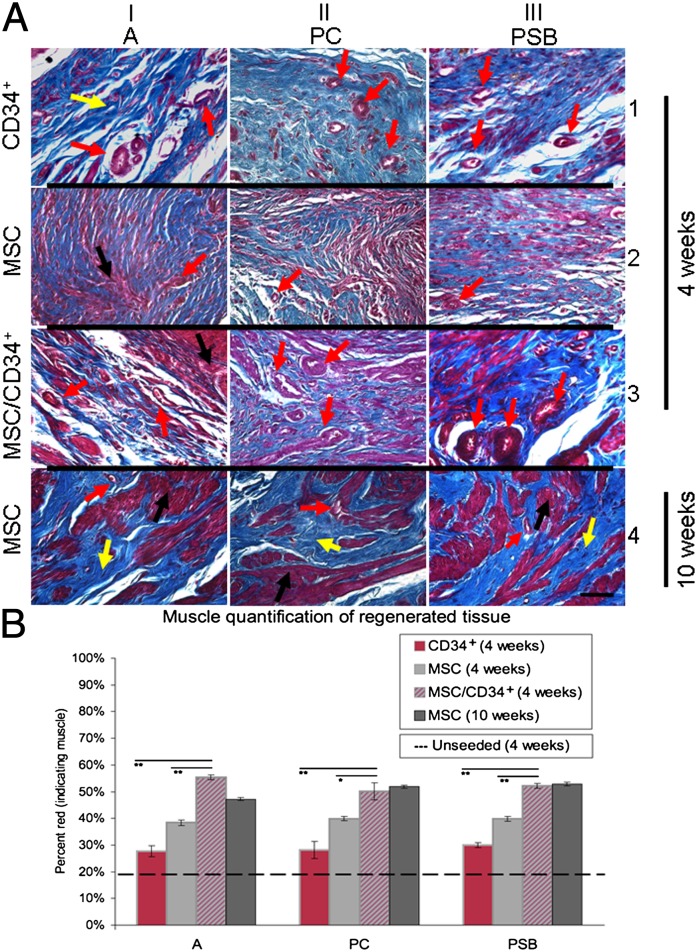
Masson’s trichrome staining of MSC seeded scaffolds 10 wk postaugmentation (Fig. S1D shows a surgical representation of the bladder augmentation procedure) revealed highly defined, well-organized muscle fascicles (red) interspersed with suitable levels of collagen (blue in Fig. 2A, row 4) and is consistent with normal human bladder tissue (Fig. S2A) (12). Varying degrees of tissue organization were observed for all groups 4 wk postaugmentation where MSC and CD34+ groups were highly disorganized (Fig. 2A, rows 1 and 2), whereas MSC/CD34+ groups displayed a greater propensity for tissue organization, including vessel distribution (Fig. 2A, row 3). Evidence of obstruction, stricture, dilation, stone formation, calcification, or diverticulum was not detected upon gross examination of the upper urinary tracts (whole bladders, ureters, and kidneys). Evidence of any necrotic bladder tissue was also lacking. A minimum number of three BM donors were used for SB and PSB groups and one donor was used for the A group for in vivo studies.
Fig. 2.
Effects of graft cell type and in vivo duration on regenerating musculature. (A) Regenerated tissue from MSC groups at 10 wk postaugmentation showed well-organized muscle fascicles (red) with homogenous collagen (blue) distribution (row 4). MSC/CD34+ groups demonstrated better muscle organization than MSC and CD34+ groups 4 wk postaugmentation (rows 1–3). Blood vessel distribution was also found to be greatest in MSC/CD34+ groups, followed by CD34+ then MSC groups 4 wk postaugmentation. Images are representative of multiple samples/animals within a group. Arrows: black, muscle; yellow, collagen; and red, blood vessel. Magnification is 400× ( scale bar, 50 μm). (B) Significantly higher mean muscle content was observed in MSC/CD34+ grafts compared with MSC and CD34+ grafts 4 wk postaugmentation. Mean percent muscle values for MSC/CD34+ groups at 4 wk were similar to those of MSC groups at 10 wk. CD34+ groups demonstrated the lowest muscle content among seeded groups. The dotted line reflects mean muscle content for an unseeded POC graft group (19.3 ± 1.9%). Data presented as means ± SE; *P < 0.01,**P < 0.0001 for MSC/CD34+ vs. MSC and CD34+ groups (4 wk).
Muscle/collagen ratio is indicative of the level of regeneration in areas engrafted with cell-seeded scaffolds (13). Previous data from analogous augmentations demonstrated that unseeded POC grafts contained ∼20% muscle 4 and 10 wk postaugmentation (13). Within this study, grafts from the MSC/CD34+ groups exhibited significantly higher mean muscle content than grafts from MSC and CD34+ groups 4 wk postaugmentation (2.6–2.9 times higher than unseeded scaffolds, vs. 2.0–2.1 times greater and 1.4–1.6 times greater, respectively). Although CD34+ grafts did not attain muscle levels demonstrated by MSC/CD34+ or MSC grafts, the addition of CD34+ HSPCs to MSC grafts increased 4-wk MSC/CD34+ graft muscle content to the levels of MSC groups at 10 wk. No differences were observed with respect to donor (Fig. 2B). The distribution of blood vessels was also found to be greatest in MSC/CD34+ groups, followed by CD34+ then MSC groups at the 4-wk time point.
At 4 wk postaugmentation, significantly higher mean muscle content was observed in MSC/CD34+ grafts compared with MSC and CD34+ grafts (A-CD34+ 27.7 ± 2.1%, PC-CD34+ 28.2 ± 3.2%, PSB-CD34+ 30.0 ± 1.0%; A-MSC 38.4 ± 1.0%, PC-MSC 39.9 ± 0.8%, PSB-MSC 39.9 ± 0.9%; A-MSC/CD34+ 55.3 ± 0.9%, PC-MSC/CD34+ 50.1 ± 3.2%, and PSB-MSC/CD34+ 52.2 ± 1.0%); mean percent muscle values for MSC/CD34+ groups at 4 wk were similar to those of MSC groups at 10 wk (A-MSC 47.2 ± 0.6%, PC-MSC 51.9 ± 0.6%, and PSB-MSC 52.8 ± 0.7%). CD34+ groups demonstrated the lowest muscle content among seeded groups. The dotted line in Fig. 2B reflects mean muscle content for the unseeded graft group (19.3 ± 1.9%), as previously reported (13). All grafts used POC scaffolds.
Blood Vessel Regeneration with MSC/CD34+ Grafts.
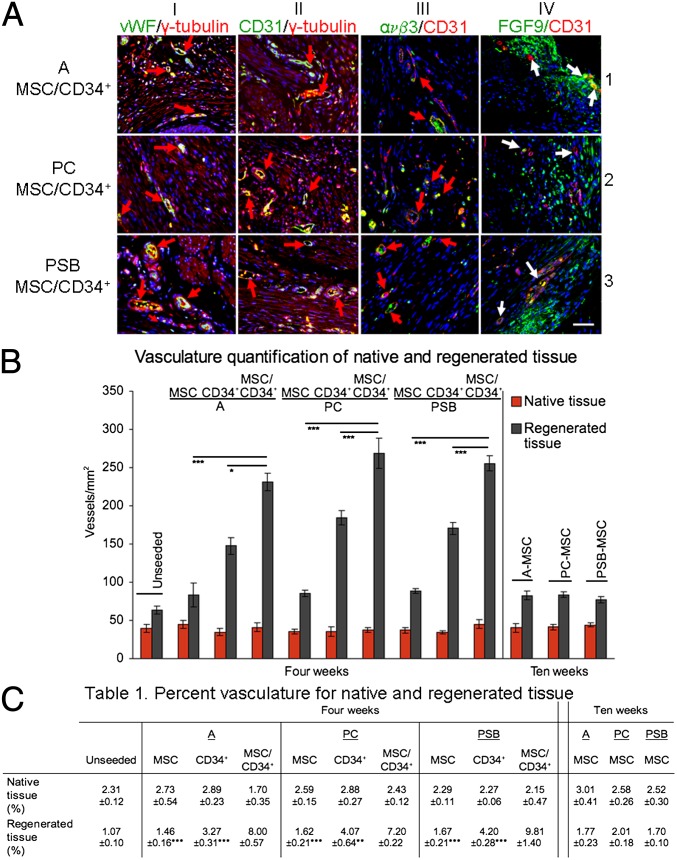
MSC grafts demonstrated a sparse number of blood vessels 4 wk postaugmentation, whereas vessels appeared at greater frequencies with homogenous distribution throughout the grafted tissue of MSC/CD34+ and CD34+ grafts. Vessel diameter in these grafts was also greater compared with MSC grafts (Fig. 3A, rows 1–3; red arrows indicate blood vessels). Vessel identity was confirmed by antibody staining with vascular endothelial cell markers vWF and CD31. MSC/CD34+ groups demonstrated robust vWF+ and CD31+ blood vessels in areas of regeneration (Fig. 3A, columns I and II). Costaining with γ-tubulin [a human-reactive antibody found to be nonreactive with rat bladder tissue (13); Fig. S2 B–D] demonstrated γ-tubulin+/vWF+ and γ-tubulin+/CD31+ cells within the vessel perimeter (red arrows), indicating the presence of human cells within vessels. Quantification of γ-tubulin+/CD31+ vessels in regenerated tissue were similar across cell types and donor groups (Table S3). The identification of putative angiogenic vessels by subsequent costaining with ανβ3/CD31 and FGF9/CD31 (Fig. 3A, columns III and IV) revealed new vessels (red and white arrows, respectively). Prominent FGF9 staining in PSB MSC/CD34+ grafts surrounds a newly formed MSC-derived muscle bundle (column IV, row 3) (MSC and CD34+ grafted groups shown in Fig. S3).
Fig. 3.
Vascular regeneration in MSC/CD34+ grafts. (A) γ-Tubulin+/vWF+ (column I) and γ-tubulin+/CD31+ (column II) cells were detected in regenerated tissue. Presence of ανβ3+/CD31+ and FGF9+/CD31+ (columns III and IV, respectively) cells provide evidence of angiogenesis. Blue, DAPI. Arrows: red, orange colocalization of markers; white, new vessel growth. (Scale bar, 50 µm.) (B) Regenerated tissue in MSC/CD34+ grafts demonstrated higher numbers of vessels and percent vasculature (C, Table 1) than MSC and CD34+ graft tissue 4 wk postaugmentation. Data shown as means ± SE; *P < 0.01,**P < 0.001, ***P ≤ 0.0001 for MSC/CD34+ vs. MSC and CD34+ groups (4 wk).
Analysis of native tissue adjacent to grafts showed a similar number of vessels and total vasculature across all 4- and 10-wk groups (Table S4). No significant differences in vascularization of native tissue with respect to time (4 vs. 10 wk), donor (A vs. PC vs. PSB), or cell type (MSC vs. CD34+ vs. MSC/CD34+) (native vessel number and percent vasculature comparisons were all nonsignificant at P > 0.05) were observed (Fig. 3B). Vascularization of regenerated tissue in MSC-group grafts showed no indication of time or donor effects. Groups showed similar numbers of vessels, a similar level of total vasculature in areas of regenerated tissue, and a similar relationship between native and regenerated tissue. Mean vessel numbers for regenerated tissue were ∼1.7–2.4 times greater than mean vessel numbers for native tissue, with increases of 1.9–2.4 times at 4 wk and 1.7–2.1 times at 10 wk. In comparison, CD34+ group grafts showed enhanced vascularization in regenerated tissue, with mean vessel numbers ∼4.2–5.2 times greater than mean vessel numbers for native tissue. Mean percent vasculature levels were also greater than those seen with MSC grafts, showing a slight increase over native tissue (Fig. 3 B and C). MSC/CD34+ populations resulted in graft vascularization markedly different from all MSC groups, and with a significant increase above CD34+ group levels. Regenerated tissue in grafts from MSC/CD34+ groups exhibited significantly higher numbers of vessels per square millimeter and higher percent vasculature. The relationship between native and regenerated tissue was also altered. A higher degree of increase was seen for vessel number, with mean vessel numbers for regenerated tissue ∼5.6–7.2 times the mean number of vessels in native tissue. Additionally, the regenerated areas of MSC/CD34+ grafts demonstrated levels of percent vasculature significantly higher than the levels in native tissue. This native/regenerated percent vasculature relationship is the converse of that seen in the MSC groups, each of which showed mean percent vasculature levels to be lower in regenerated tissue than in native tissue. No significant differences were detected between the adult and pediatric MSC/CD34+ groups in any aspect of the vascular quantification (Fig. 3 B and C).
In Vivo Graft Evaluation.
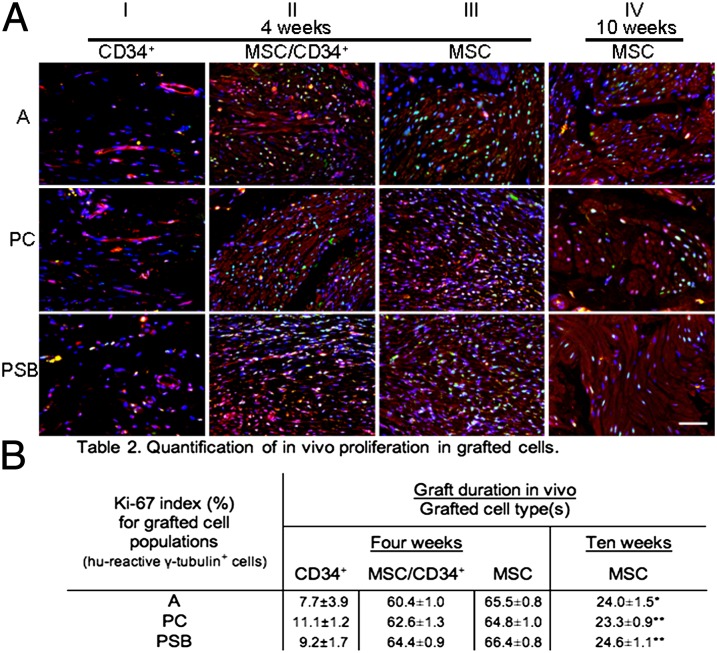
Bladder muscle is composed of specialized smooth muscle cells (SMCs) expressing several key proteins required for repetitive contraction/expansion cycles. MSC seeded grafts demonstrated the morphological appearance of smooth muscle fascicles and grafted MSCs exhibited expression of these smooth muscle proteins 10 wk postaugmentation (Fig. S4) as well as at 4 and 10 wk in vitro (Fig. S5A). In vivo cell proliferation was assessed by Ki-67 expression (green) in grafted cell populations along with γ-tubulin expression (red) in regenerating tissue among all donor groups (Fig. 4A). At 4 wk postaugmentation, comparably fewer grafted cells were detected in CD34+ groups (column I), and fewer of the identified grafted cells expressed Ki-67. MSC/CD34+ and MSC groups (columns II and III) retained a substantive population of grafted cells, with similar levels of Ki-67 expression for both cell types. MSC groups at 10 wk (column IV) showed unchanged retention of grafted cells, but with decreased levels of proliferation. Grafted cells from all CD34+ groups subjectively demonstrated lower levels of Ki-67 expression compared with MSC and MSC/CD34+ groups, which was corroborated by quantitative analysis (Fig. 4B). Proliferation levels showed cell-type and time-point effects, with lower levels in CD34+ groups at 4 wk (A-CD34+ 7.7 ± 3.9%, PC-CD34+ 11.1 ± 1.2%, and PSB-CD34+ 9.2 ± 1.7%) and increased levels in MSC-containing groups (A-MSC/CD34+ 60.4 ± 1.0%, PC-MSC/CD34+ 62.6± 1.3%, PSB-MSC/CD34+ 64.4 ± 0.9%, A-MSC 65.5 ± 0.8%, PC-MSC 64.8 ± 1.0%, and PSB-MSC 66.4 ± 0.8%). MSC groups examined at 4 and 10 wk further demonstrated a 2.7–2.8 times decrease in mean Ki-67 expression (A-MSC 24.0 ± 1.5%, PC-MSC 23.3 ± 0.9%, and PSB-MSC 24.6 ± 1.1%). In contrast to graft muscle content, grafted cell proliferation levels for the combination MSC/CD34+ groups at 4 wk were consistent with MSC group levels at the earlier rather than the later time point (Fig. 4B).
Fig. 4.
Proliferation index of grafted cells in regenerating tissue. (A) Ki-67 expression (green) and γ-tubulin expression (red) was examined in regenerating tissue among all groups. Fewer grafted cells were detected in CD34+ groups (column I), and fewer of the identified grafted cells expressed Ki-67. MSC/CD34+ and MSC groups (columns II and III) retained a substantive population of grafted cells with similar Ki-67 expression. MSC groups at 10 wk showed unchanged retention of grafted cells, but with decreased levels of proliferation. Images are representative of multiple samples/animals within a group. Blue, DAPI. Magnification is 400×. (Scale bar, 50 μm.) (B) Ki-67+ cells shown as percentage of total grafted cells (means ± SE). Proliferation levels showed cell-type and time-point effects, with lower levels in CD34+ groups at 4 wk and a decrease in levels from 4 to 10 wk for the MSC groups (*P < 0.01, **P < 0.0001 for MSC groups 4 vs. 10 wk).
Peripheral Nerve Regeneration with MSC/CD34+ Grafts.
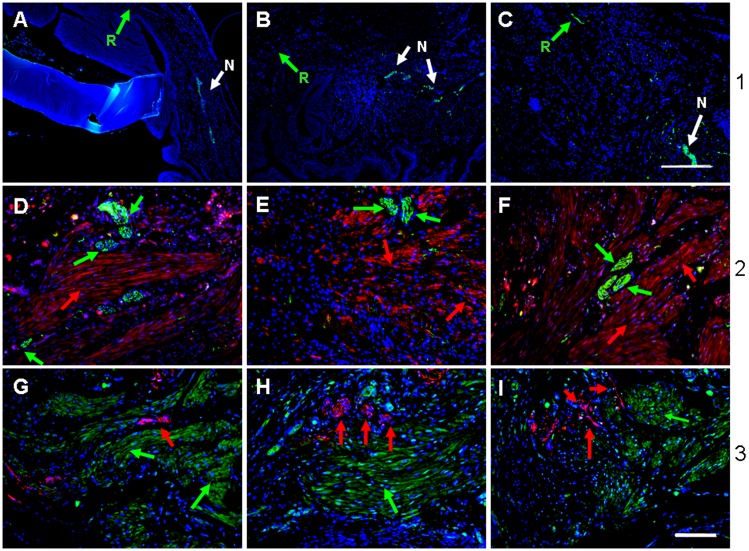
To determine the effect of MSC/CD34+ seeded POC grafts on bladder peripheral nerve regeneration, explanted bladder tissue consisting of adjoining native and regenerated tissue areas was stained with neuronal-specific antibodies βIII tubulin and synaptophysin. Adult MSC seeded scaffolds revealed expression of βIII tubulin (green) in native tissue at all time points, although its expression was absent in regenerated tissue at 4 and 10 wk (Fig. 5 A and B) with nominal expression at 20 wk postaugmentation (Fig. 5C; white and green arrows depict native and regenerated areas, respectively). However, 4 wk MSC/CD34+ grafts exhibited an abundance of peripheral nerve growth emanating from native tissue and traversing regenerated tissue, as confirmed independently by βIII tubulin (green in Fig. 5 D–F) and synaptophysin staining (red in Fig. 5 G–I). Costaining with γ-tubulin revealed that putative nerve bundles (NBs) were of rodent origin. γ-Tubulin+ tissue adjacent to NBs consisted of muscle fascicles composed of grafted human cells (red in Fig. 5 D–F and green in Fig. 5 G–I, respectively). NBs in regenerating tissue populated by grafted cells were detected in all MSC/CD34+ samples (in Fig. 5, A-D, G; PC-E, H; and PSB-F, I). In the absence of MSCs, CD34+ grafts showed minimal neuronal marker expression in regenerated areas at 4 wk (Fig. S5B). The MSC/CD34+ > CD34+ > MSC trend observed with peripheral nerve regeneration is analogous to that of vasculature in regenerating tissue.
Fig. 5.
Bladder peripheral nerve regeneration. βIII tubulin (green) neuronal expression was absent in regenerated tissue of A MSC grafts at 4 and 10 wk and minimal at 20 wk (A–C). Arrows: green, regenerated tissue; white, native tissue. In contrast, regenerated tissue from MSC/CD34+ grafts at 4 wk shows expression of βIII tubulin (D–F, green) and neuronal marker synaptophysin (G–I, red) in areas populated by grafted γ-tubulin+ cells, red or green). D and G, adult samples; E and H, PC samples; F and I , PSB samples. Blue, DAPI. (Scale bar in C, 250 µm; scale bars in remainder of image, 100 µm.)
Discussion
Within the context of this study, we have demonstrated several findings that describe the therapeutic potential of autologous sources of BM cells that could aid in bladder regeneration. Although the focus of this study was directed at the SB neurogenic bladder, this procedure also may be used as an alternative to bladder augmentation cystoplasty for patients who maintain a normal BM microenvironment, such as those suffering from bladder trauma or localized bladder cancer. SB-derived MSCs function analogously to both age-matched counterparts as well as adult MSCs under in vitro and in vivo conditions. Specifically, SB-derived MSCs can function as a surrogate cell source for the musculature of the bladder wall. The addition of donor-matched CD34+ HSPCs to MSC seeded constructs contributes to two facets of bladder tissue engineering that have been sorely lacking to date. The striking level of putative blood vessel formation and peripheral nerve growth in grafted areas demonstrated by MSC/CD34+ seeded grafts, including those of SB origin, commenced at a very early time point in the regenerative process. Data derived from this study warrant further consideration for the utility of these combinations of scaffold/cell populations as a potential alternative therapy to current bladder augmentation enterocystoplasty procedures. However, the examination of overall bladder organization/function as it specifically relates to smooth muscle, peripheral nerve, and blood vessel function must still be further evaluated.
Proper anatomical configuration of the bladder smooth muscle microstructure is imperative for overall bladder function because this feature in part governs repetitive contractile/relaxatory cycles that allow for proper micturition. The transplantation of SB-derived BM MSCs to act as a surrogate cell source for bladder smooth muscle cells in form and function seemed to provide the necessary cellular mimicry required to recapitulate the smooth muscle layer in a bladder augmentation model. Because MSCs are known to express a variety of functional nicotinic and muscarinic receptors and maintain physiological responses to agonist treatment indistinguishable from those of bladder SMCs, physiological responses from environmental cues could aid in the facilitation of proper bladder function (14, 15). The distinct formation of organized muscle tissue was evidenced by the presence of numerous well-developed muscle fascicles oriented in a longitudinal manner, as typically seen in normal bladder anatomy (16). This structural integrity of muscle fascicles was substantiated at the cellular level by the affirmation of key contractile bladder smooth muscle proteins in the grafted areas with no qualitative difference in expression detected between the three different donor populations. The establishment of the MSC-derived muscle layer provided the subsequent foundation for the full formation of the bladder trilayer architecture consisting of muscle, serosa, and urothelium with histological appearance approaching that of normal human bladder tissue (12, 17). Although MSCs provide a potential alternative to bladder SMCs, further testing to determine muscle function is required to evaluate the complete utility of these cells in a bladder regenerative setting. It is imperative that the urothelium of transplanted cell/scaffold composites be quickly established to prevent urine leakage and the development of peritonitis. MSC/CD34+ constructs showed robust urothelium growth several cells in thickness within grafted areas, whereas analogous CD34+ grafts demonstrated a moderate level of urothelial growth. MSC grafts were by far the worst and demonstrated a thin and fragile-appearing urothelium along with disorganized muscle content (Fig. S6A). The combination of MSC/CD34+ HSPCs may have provided specific cues to coax quiescent urothelial stem cells to exit the Go phase of the cell cycle and further potentiate regeneration of the bladder urothelium through heightened up-regulation of Wnt10a and members of the Wnt family of proteins (Fig. S6B). Sonic hedgehog and Wnt signaling pathways have been implicated in urothelium regeneration and in hematopoiesis of CD34+ HSPCs (18, 19).
Adequate vascularization of grafted tissue has been a significant obstacle for many organ systems (20, 21). Developing tissue at the center of implanted cell/scaffold composites is highly susceptible to necrosis because nutrient and gas exchange is poor or nonexistent. The application of CD34+ HSPCs provided a unique solution to overcome this dilemma by supplying an initial foundation for increased levels of potentially functional vasculature. Data from recent studies demonstrate the utility of CD34+ HSPCs: intramyocardial injections of these cells relieve symptoms of angina and promote neovascularization in preclinical models (22, 23). The increase in the number of blood vessels and percent vasculature seen at 4 wk postaugmentation in MSC/CD34+-seeded scaffolds was quite striking. The number of vessels per square millimeter within regenerated areas of these grafts was similar among the three donor groups and was at least fivefold higher than in native areas, which showed vasculature levels similar to those of MSC grafts. This effect was further corroborated via the quantification of percent vasculature in regenerated tissue, which was greatest for grafts that contained both MSCs and CD34+ HSPCs. The similarities with regard to vessel number and percent vasculature between SB and non-SB groups cannot be overstated, because this has implications not only for SB patients in need of bladder replacement but also potentially for bladder cancer patients who are in similar predicaments.
The composition of angiogenic vessels in our study consisted in part of donor MSCs and CD34+ HSPCs. Data demonstrate that these cell populations were indeed incorporated within the walls of blood vessels and were of human origin through antibody staining (Fig. S3). These findings may in part be attributed to one of several physiological phenomena. These include the direct incorporation of MSCs or CD34+ HSPCs into the vessel wall at the onset of the neovascularization program or MSCs’ traversal of tissue into nearby vessels as pericyte cells providing the structural support to endothelial cells that line the walls of blood vessels. Bexell et al. (24) demonstrated the ability of grafted BM MSCs to integrate into tumor vessel walls and express markers affiliated with pericytes. Other studies would indicate that neovascularization is a strong possibility because CD34+ HSPCs have demonstrated the ability to promote neovascularization (25, 26). Additional evidence supporting the possibility that vessels found within the regenerated areas of MSC/CD34+ truly underwent angiogenesis is the coexpression of ανβ3 and CD31 in newly sprouted blood vessels. It has been established that the integrin ανβ3 is expressed on cells within vessels undergoing angiogenesis and is the target of many anticancer therapeutics aimed at targeting ανβ3-based angiogenesis (27). Finally, the expression of FGF9 in areas of regeneration and angiogenesis has great significance as to whether the formation of endothelial sprouts is subsequently followed by their incorporation into vasoactive blood vessels. The expression of FGF9 has been shown to be highly up-regulated as human smooth muscle cells assemble themselves into layered cords that are indicative of their functional capabilities. This expression of FGF9 is also evident in PSB grafts and encompasses MSC-derived muscle bundles. Based on studies by Frontini et al. (28), it is suggestive that the MSC/CD34+ grafts contain microvessels that formed in the presence of FGF9 and are vasoactive. However, bladder perfusion studies evaluating newly formed vessel networks must be conducted to determine the existence and degree of perfusion.
The difficult task of accomplishing bladder nerve regeneration following augmentation cystoplasty has been a major obstacle within the field of bladder tissue engineering. Recent studies have described the controversial use of specific surgical approaches to create artificial somatic–autonomic reflex pathways involving the bladders of patients with spinal cord injuries (29). Within this study, we have coaxed the ingrowth of peripheral nerves as early as 4 wk postaugmentation from native tissue into areas of regenerated tissue seeded with MSC/CD34+ HSPCs, as demonstrated solely with neuronal-specific antibody staining. CD34+ grafts demonstrate the commencement of early nerve growth, whereas grafts lacking the CD34+ HSPC component do not display peripheral nerve growth at 4 or 10 wk. Regenerated peripheral nerves were located throughout the graft, including the core, and appear as clusters that are interspersed among newly formed muscle bundles composed of MSCs (Fig. 5), although the degree of regenerated peripheral nerve functionality requires further evaluation. We postulate that the increased number of blood vessels in MSC/CD34+ grafts (assumingly allowing for greater blood flow to grafted areas) expedited the growth and development of peripheral nerves by providing key nerve-promoting factors. CD34+ HSPCs have also been shown to express a variety of neural proteins with an overlap of hematopoietic and neuropoietic molecular signatures including nerve growth factor, its receptor TrkA, and dopamine receptors DR3 and DR5 and also possess the ability to transdifferentiate into cells of neural lineage (30–33). Dopamine has been determined to be a key molecule in neurite outgrowth, and its interaction with CD34+ HSPCs may have contributed to the peripheral nerve regeneration at the early time point of these experiments (34). Although these findings do not remedy the large neurologic deficits associated with SB patients, it is foreseeable in the future that SB patients or others who lack complete bladder function may use currently available electrostimulatory devices to aid in bladder contraction in conjunction with the aforementioned augmentation procedures. Finally, animals used in this study maintained normal bladder innervation whereas SB patients with neurogenic bladder demonstrate bladder dysfunctionality owing to aberrant bladder innervation. The degree of functional bladder innervation is dependent on the type and severity of SB, which in part is defined by the anatomic locale of the neurological lesion. Patient studies using sacral neuromodulation and intravesical electrostimulation have demonstrated improved functional bladder capacity, compliance, and muscle contraction (35, 36). Hence, some patients with SB do retain some level of functional bladder innervation and may benefit from our procedure with the regeneration of newly functional peripheral nerves, although the degree of functionality needs to be fully evaluated.
We have demonstrated that SB MSCs and CD34+ HSPCs respond similarly to normal adult and pediatric control counterparts under in vitro and bladder regenerative conditions. The addition of the CD34+ HSPCs to grafts containing donor-matched SB MSCs in the case of PC and PSB samples demonstrated remarkable vessel and urothelium growth in areas of regeneration. Finally, the combination of MSCs and CD34+ HSPCs provided a proneural growth environment that allowed for the ingrowth of peripheral nerves into areas of bladder regeneration at only 4 wk postaugmentation. Based on these data, autologous sources of SB-derived BM MSCs in conjunction with CD34+ HSPCs may be successfully used as autologous surrogate cell sources to regenerate various aspects of the urinary bladder.
Materials and Methods
SI Materials and Methods gives a detailed description of materials and methods used.
Cell Isolation from Pediatric Patients.
BM samples were obtained from SB (n = 4) or normal pediatric (nondiseased, n = 4) patients and CD34+ HSPCs and MSCs were isolated. This study was approved by the Institutional Review Board at the Lurie Children’s Hospital of Chicago.
Statistical Analysis.
Differences were determined using t test or ANOVA with Tukey–Kramer adjustment (SAS 9.2). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported in part by an Excellence in Academic Medicine grant funded through the Illinois Department of Healthcare and Family Services (to A.K.S.). Purchase of the Zeiss PALM Microdissection System was supported by National Center for Research Resources Grant 1S10RR025624-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220764110/-/DCSupplemental.
References
- 1.Greene ND, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18(R2):R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebbington MW, Danzer E, Johnson MP, Adzick NS. Open fetal surgery for myelomeningocele. Prenat Diagn. 2011;31(7):689–694. doi: 10.1002/pd.2805. [DOI] [PubMed] [Google Scholar]
- 3.Clayton DB, Brock JW, 3rd, Joseph DB. Urologic management of spina bifida. Dev Disabil Res Rev. 2010;16(1):88–95. doi: 10.1002/ddrr.92. [DOI] [PubMed] [Google Scholar]
- 4.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 5.Guven A, Onal B, Kogan BA. Spontaneous bladder perforations following augmentation cystoplasty in children. Nat Clin Pract Urol. 2006;3(11):584–585. doi: 10.1038/ncpuro0608. [DOI] [PubMed] [Google Scholar]
- 6.Flood HD, et al. Long-term results and complications using augmentation cystoplasty in reconstructive urology. Neurourol Urodyn. 1995;14(4):297–309. doi: 10.1002/nau.1930140402. [DOI] [PubMed] [Google Scholar]
- 7.Austin JC. Long-term risks of bladder augmentation in pediatric patients. Curr Opin Urol. 2008;18(4):408–412. doi: 10.1097/MOU.0b013e328300587c. [DOI] [PubMed] [Google Scholar]
- 8.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89(7):2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losordo DW, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Ishikane S, et al. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26(10):2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich M, Oberbach A, Schlichting N, Stolzenburg J-U, Neuhaus J. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS ONE. 2011;6(6):e20792. doi: 10.1371/journal.pone.0020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma AK, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31(24):6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 14.Hoogduijn MJ, Cheng A, Genever PG. Functional nicotinic and muscarinic receptors on mesenchymal stem cells. Stem Cells Dev. 2009;18(1):103–112. doi: 10.1089/scd.2008.0032. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AK, et al. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol. 2009;182(4) Suppl:1898–1905. doi: 10.1016/j.juro.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Nagatomi J, Toosi KK, Grashow JS, Chancellor MB, Sacks MS. Quantification of bladder smooth muscle orientation in normal and spinal cord injured rats. Ann Biomed Eng. 2005;33(8):1078–1089. doi: 10.1007/s10439-005-5776-x. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, et al. Normal bladder wall morphology in Gd-DTPA-enhanced clinical MR imaging using an endorectal surface coil and histological assessment of submucosal linear enhancement using [14C]Gd-DOTA autoradiography in an animal model. Eur J Radiol. 1998;26(3):290–296. doi: 10.1016/s0720-048x(97)01178-9. [DOI] [PubMed] [Google Scholar]
- 18.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92(9):3189–3202. [PubMed] [Google Scholar]
- 20.Cathey L, Lee KY, Holder W. D, Mooney DJ, Halberstadt CR. In: Lanza R, Langer R, Vacanti J, editors. Principles of Tissue Engineering. 3rd Ed. New York: Elsevier; 2007. pp. 528–530. [Google Scholar]
- 21.Stegemann JP, Kaszuba SN, Rowe SL. Review: Advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13(11):2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahoo S, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109(7):724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Losordo DW, et al. ACT34-CMI Investigators Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bexell D, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheubel RJ, et al. Paracrine effects of CD34 progenitor cells on angiogenic endothelial sprouting. Int J Cardiol. 2010;139(2):134–141. doi: 10.1016/j.ijcard.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Tei K, et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26(3):819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- 27.Murphy EA, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA. 2008;105(27):9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frontini MJ, et al. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol. 2011;29(5):421–427. doi: 10.1038/nbt.1845. [DOI] [PubMed] [Google Scholar]
- 29.Xiao CG, et al. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: preliminary results in 15 patients. J Urol. 2003;170(4 Pt 1):1237–1241. doi: 10.1097/01.ju.0000080710.32964.d0. [DOI] [PubMed] [Google Scholar]
- 30.Steidl U, et al. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 2004;104(1):81–88. doi: 10.1182/blood-2004-01-0373. [DOI] [PubMed] [Google Scholar]
- 31.Bracci-Laudiero L, et al. CD34-positive cells in human umbilical cord blood express nerve growth factor and its specific receptor TrkA. J Neuroimmunol. 2003;136(1-2):130–139. doi: 10.1016/s0165-5728(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 32.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8(10):1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, et al. A neuroinductive biomaterial based on dopamine. Proc Natl Acad Sci USA. 2006;103(45):16681–16686. doi: 10.1073/pnas.0606237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guys JM, et al. Sacral neuromodulation for neurogenic bladder dysfunction in children. J Urol. 2004;172(4 Pt 2):1673–1676. doi: 10.1097/01.ju.0000138527.98969.b0. [DOI] [PubMed] [Google Scholar]
- 36.Choi E, et al. 2012. Effects of intravesical electrical stimulation therapy on urodynamic patterns for children with spina bifida: A 10-year experience. J Pediatr Urol S1477– 5131(12)00257-4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.