The mammalian brain is arguably the most complex organ that evolution has brought forward. In the human brain, for example, about 1011 neurons are connected via some 1015 synapses to form multiple specifically connected neural networks, the interplay of which controls all body functions. Synapses, the key signaling units in the brain, represent yet another level of substantial complexity; they are intricate molecular machines composed of thousands of different proteins. The questions as to how the specificity of synaptic connections in the face of huge neuron and synapse numbers is achieved during brain development, and how the molecular composition of synapses is regulated, have been in the focus of neuroscientists for decades. In PNAS, Yim et al. (1) report that different cell-adhesion proteins of the Slitrk (Slit- and Trk-like) family interact with different cell-surface–exposed protein tyrosine phosphatases (PTPs), and thereby allow neurons to recognize each other during synapse formation and establish different synapse types.
Synapses operate by chemical signaling. The sending part of the synapse, the presynaptic bouton, is specialized for the regulated exocytosis of neurotransmitters, which is triggered by membrane depolarization and Ca2+ influx. Released neurotransmitters bind to receptor proteins on the surface of the receiving part of the synapse, the postsynaptic density, and thereby elicit a membrane potential change in the receiving cell. Most synapses in the mammalian forebrain use the neurotransmitter glutamate, which binds to receptors that act as ligand-gated cation channels and cause membrane depolarization and excitation of the receiving cell. Glutamatergic excitatory synaptic transmission in the forebrain is counterbalanced by the activity of inhibitory synapses. Most of these synapses use the neurotransmitter GABA, whose GABAA receptors are ligand-gated chloride channels that hyperpolarize and thus inhibit the receiving cell. The excitation-inhibition balance in the brain is maintained within narrow boundaries. Correspondingly, persistent alterations in neuronal and network activity trigger homeostatic changes in the function and number of excitatory and inhibitory synapses to guarantee an appropriate excitation-inhibition balance (2), and pathological changes of this balance cause brain diseases, such as epilepsy (3) or autism (4).
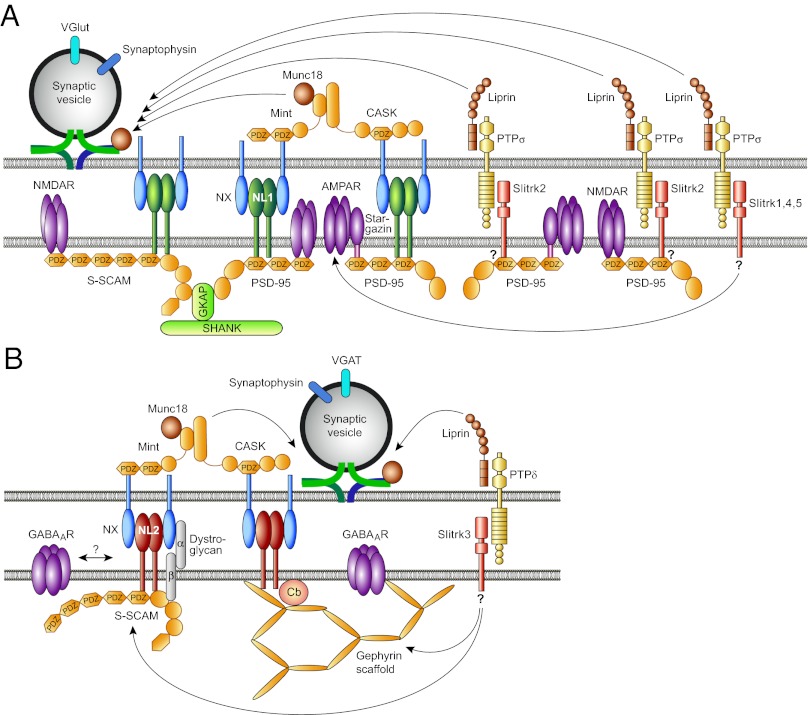
Synapses are thought to form in a two-step process, where an initial cell-type–specific contact between two neurons is formed that subsequently matures into a fully functional synapse. Both, the cell-type–specific formation of a nascent synaptic contact and its subsequent maturation, which involves the recruitment of the presynaptic and postsynaptic protein machineries required for proper synaptic signaling, are mediated by synaptic adhesion proteins (5). Because the corresponding postsynaptic receptors and scaffolding proteins are strikingly different in glutamatergic and GABAergic synapses (Fig. 1), the protein-recruitment processes involved in the formation and maturation of the two synapse types must be fundamentally different. A substantial number of different synaptic cell-adhesion systems have been shown to control the formation of glutamatergic excitatory synapses, and most of them operate by binding PDZ-domain–containing scaffold proteins such as PSD-95, which then—directly or indirectly—recruit glutamate receptors to the maturing synapse (6). In contrast, GABAergic postsynapses contain very few PDZ-domain proteins and instead require the scaffold protein gephyrin and, in many cases, its recruitment factor collybistin to accumulate GABAA receptors. As regards the corresponding cell-adhesion proteins involved, only the α/β-dystroglycan complex and two members of the neuroligin family, neuroligin-2 and neuroligin-4, have been shown to operate in the formation and maturation of inhibitory postsynaptic signaling complexes (7).
Fig. 1.
Comparison of the neurexin-neuroligin and Slitrk-PTP adhesion systems at excitatory (A) and inhibitory (B) synapses. See text for details. AMPAR, AMPA receptor; CASK, calcium/calmodulin-dependent serine protein kinase; Cb, Collybistin; GABAAR, GABAA receptor; GKAP, guanylate kinase associated protein; MINT, Munc18 interacting protein; NL, Neuroligin; NMDAR, NMDA receptor; NX, Neurexin; PDZ, PSD-95/disk-large/zona-occludens-1 domain; PSD-95, postsynaptic density protein of 95 kDa; PTP, protein tyrosine phosphatase; SHANK, SH3 and ankyrin repeat containing protein; S-SCAM, synaptic scaffolding molecule; Slitrk, Slit- and Trk-like; VGAT, vesicular GABA/glycine transporter; VGlut, vesicular glutamate transporter.
The α/β-dystroglycan complex binds presynaptic adhesion proteins of the neurexin family and the intracellular scaffold protein S-SCAM, but how this leads to GABAA receptor recruitment to synapses is unknown. Neuroligin-2 and neuroligin-4 also bind presynaptic neurexins, but in contrast to the α/β-dystroglycan complex, their intracellular C termini interact directly with gephyrin and bind and activate collybistin, which leads to the postsynaptic recruitment of the two scaffold proteins and subsequent postsynaptic assembly of GABAA receptors (7). Strikingly, however, only a subset of inhibitory synapses is dependent upon the presence of neuroligin-2, neuroligin-4, or collybistin, whereas gephyrin is obligatory for most inhibitory synapses (7). Thus, to explain the omnipresence and diversity of inhibitory synapses in the brain, there is a dire need for alternative synaptogenic adhesion proteins that can operate instead of neuroligin-2 or neuroligin-4 and their presynaptic interaction partners and that can trigger GABAA receptor recruitment in a partly collybistin-independent manner.
It is this need that the PNAS article by Yim et al. (1) addresses. Extending a previous study by others (8), the authors show that different members of the Slitrk family of postsynaptic cell-adhesion proteins interact with different presynaptic receptor PTPs to trigger the formation and maturation of excitatory glutamatergic or inhibitory GABAergic synapses (Fig. 1). Specifically, Slitrk3 interacts with PTPδ to trigger the genesis of inhibitory GABAergic synapses, whereas Slitrk1, Slitrk2, Slitrk4, and Slitrk5 act at excitatory glutamatergic synapses by interacting with PTPσ. The action of Slitrk3
The fascination of the recent findings and the resulting conclusions is particularly evident with regard to the role of Slitrk3 at inhibitory synapses.
at inhibitory synapses is phenomenologically related to that of neuroligin-2 and neuroligin-4 (7), whereas the effects of the other Slitrk family members are reminiscent of most synaptogenic proteins at excitatory synapses, including neuroligin-1 (6).
The molecular mechanisms by which PTPδ, PTPσ, and the different Slitrks trigger synapse formation and maturation are probably different from the ones seen with well-established presynaptically or postsynaptically localized synaptogenic adhesion proteins, such as neurexins or neuroligins. Whereas neurexins seem to trigger presynapse differentiation by binding to calcium/calmodulin-dependent serine protein kinase (9), PTPδ and PTPσ are likely to operate by directly interacting with Liprins (10). On the postsynaptic side, neuroligin-1 and most other postsynaptic adhesion proteins of excitatory synapses operate by interaction with PDZ-domain–containing scaffold proteins (6), and neuroligin-2 and neuroligin-4 interact with collybistin and gephyrin to initiate the differentiation of inhibitory postsynapses (7). In contrast, none of the Slitrks except Slitrk2 has a potential PDZ-domain binding motif that would allow interactions with the most prevalent scaffold proteins of glutamatergic synapses, and Slitrk3, which may be one of the enigmatic cronies of neuroligin-2 and neuroligin-4 in inhibitory synapse formation, lacks recognizable binding sites for collybistin or gephyrin.
The considerations above lead to the fascinating conclusion that Slitrks might exert their synaptogenic effects by unique mechanisms, the elucidation of which represents the most pressing open task left by Yim et al.’s report (1). The fascination of the recent findings and the resulting conclusions are particularly evident with regard to the role of Slitrk3 at inhibitory synapses. The formation and maturation of many GABAergic synapses in the mammalian forebrain appear to be independent of neuroligin-2, neuroligin-4, or collybistin (7). Therefore, homologs or analogs of neuroligin-2, neuroligin-4, and collybistin must exist that operate by similar mechanisms to recruit gephyrin and GABAA receptors to maturing inhibitory synapses. Beyond neuroligin-3, which is present at a subset of inhibitory synapses but does not bind collybistin (7), Slitrk3 is a very interesting candidate substitute for neuroligin-2 and neuroligin-4. It will be interesting to see if the synaptogenic effects of Slitrk3 involve the recruitment of the well-established scaffold proteins of inhibitory synapses or whether it operates by a different mechanism.
Footnotes
The author declares no conflict of interest.
See companion article on page 4057.
References
- 1.Yim YS, et al. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrigiano GG. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel J., Jr Excitation and inhibition in epilepsy. Can J Neurol Sci. 1996;23(3):167–174. doi: 10.1017/s0317167100038464. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15(5):621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21(1):132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22(3):412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, et al. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat Neurosci. 2012;15(3):389–398, S1–S2. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo J, Kwon S-K, Kim E. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol Cell Neurosci. 2009;42(1):1–10. doi: 10.1016/j.mcn.2009.05.008. [DOI] [PubMed] [Google Scholar]