Abstract
Glaucoma, a major cause of blindness worldwide, is a neurodegenerative optic neuropathy in which vision loss is caused by loss of retinal ganglion cells (RGCs). To better define the pathways mediating RGC death and identify targets for the development of neuroprotective drugs, we developed a high-throughput RNA interference screen with primary RGCs and used it to screen the full mouse kinome. The screen identified dual leucine zipper kinase (DLK) as a key neuroprotective target in RGCs. In cultured RGCs, DLK signaling is both necessary and sufficient for cell death. DLK undergoes robust posttranscriptional up-regulation in response to axonal injury in vitro and in vivo. Using a conditional knockout approach, we confirmed that DLK is required for RGC JNK activation and cell death in a rodent model of optic neuropathy. In addition, tozasertib, a small molecule protein kinase inhibitor with activity against DLK, protects RGCs from cell death in rodent glaucoma and traumatic optic neuropathy models. Together, our results establish a previously undescribed drug/drug target combination in glaucoma, identify an early marker of RGC injury, and provide a starting point for the development of more specific neuroprotective DLK inhibitors for the treatment of glaucoma, nonglaucomatous forms of optic neuropathy, and perhaps other CNS neurodegenerations.
Keywords: neuroprotection, MAP3K12, drug discovery
Glaucoma is the leading cause of irreversible blindness worldwide (1). It is a neurodegenerative disease in which vision loss is caused by the axonal injury and death of retinal ganglion cells (RGCs) (2), the projection neurons that process and transmit vision from the retina to the brain. Current therapies (i.e., surgery, laser, and eye drops) all act by lowering intraocular pressure (IOP). However, pressure reduction can be difficult to achieve, and even with significant pressure lowering, RGC loss can continue. Efforts have therefore been made to develop neuroprotective agents that would complement IOP-lowering therapies by directly inhibiting the RGC cell death process (3, 4). However, no neuroprotective agent has yet been approved for clinical use.
Protein kinases provide attractive targets for the development of neuroprotective agents. A number of kinases, including cyclin-dependent kinases, death-associated protein kinases, JNK1–3, MAPKs, and glycogen synthase kinase-3β, are involved in neuronal cell death (5–12). An additional attraction is that protein kinases are readily druggable. The pharmacology and medicinal chemistry of kinase inhibitors are well-developed, with kinases now being the most important class of drug targets after G protein–coupled receptors (13). Although the primary clinical use of kinase inhibitors continues to be as antineoplastic agents, increasing attention is being paid to their use in other areas (14, 15).
To identify, in a comprehensive and unbiased manner, kinases that could serve as targets for neuroprotective glaucoma therapy, we screened the entire mouse kinome for kinases whose inhibition promotes RGC survival. For this screen, we developed a high-throughout method for transfecting primary RGCs with small interfering RNA oligonucleotides (siRNAs) and coupled it with a quantitative assay of RGC survival. Here we present the results of the screen, which identified the dual leucine zipper kinase (DLK; MAP3K12) as being important in JNK activation and RGC cell death after injury. Moreover we demonstrate, using a conditional knockout approach, that inhibition of DLK signaling can promote RGC survival in vivo and identify a small molecule kinase inhibitor that protects RGC somata and axons in a rodent model of glaucoma. Our findings, which implicate DLK as being an important player in pathologic neuronal cell death, fit in well with the growing literature demonstrating DLK’s role as a key signaling molecule in synapse formation, neuronal development, axonal injury and regeneration, and developmental neuronal degeneration (16–23).
Results
RNAi-Based Screen Identifies DLK.
To develop a biologically relevant RGC survival assay, we chose to use primary RGCs immunopanned from perinatal mice (24). Despite the inherent challenges in the use of primary neurons, they are more likely than established cell lines to be predictive of in vivo efficacy (25). Individually inhibiting the function of each kinase in the genome required an efficient method for siRNA delivery to the primary RGCs. Because traditional transfection procedures were either toxic or minimally effective with RGCs, we adapted a magnetic nanoparticle-based reagent (NeuroMag, Oz Biosciences) for high-efficiency, high-throughput siRNA delivery (Fig. 1A). NeuroMag-based transfection resulted in consistent and efficient suppression of target gene expression in unselected RGC populations (Fig. S1).
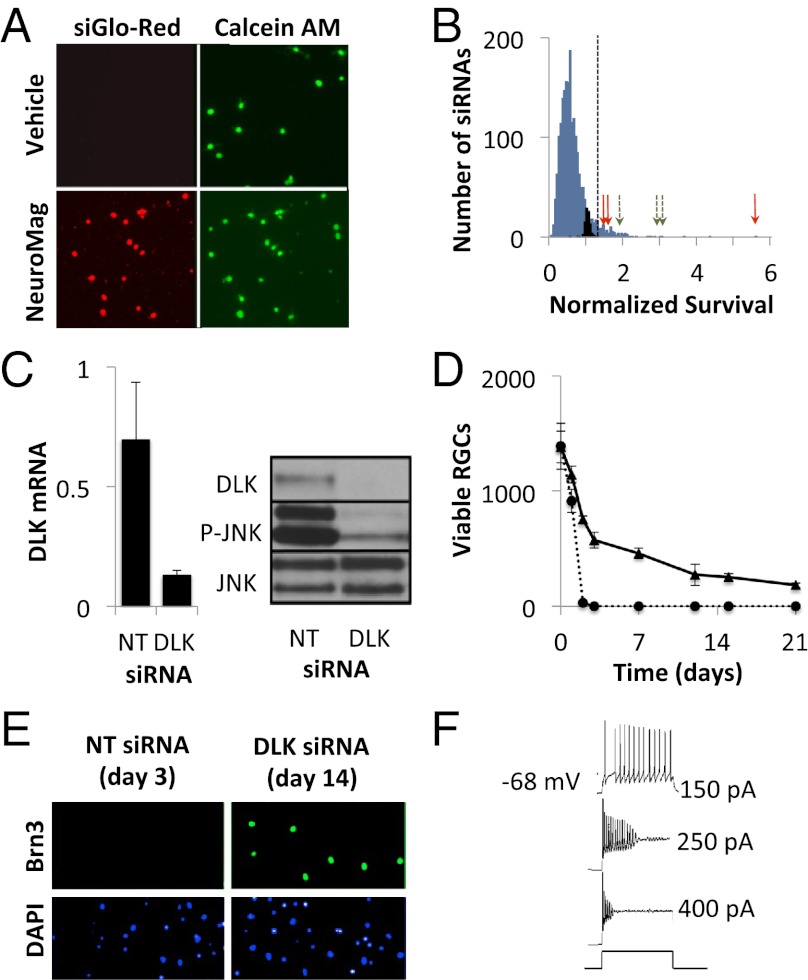
Fig. 1.
Identification of DLK as a mediator of cell death in RGCs. (A) RGCs were transfected at the time of immunopanning with a fluorescently labeled siRNA (siGLO-Red, Dharmacon) in the presence or absence of the magnetic nanoparticle, NeuroMag. After 24 h, RGCs were imaged for viability (calcein-AM staining) and nuclear accumulation of siRNA. (B) Histogram showing the normalized survival for control (black bars), kinome library (blue bars), DLK (red arrows), and MKK7 (green arrows) siRNAs. Oligonucleotides conferring survival more than 3 SD from the nontargeting siRNAs (dashed line) were considered neuroprotective (106 siRNA, 5.4%). (C) RGCs were transfected with DLK or a nontargeting control (NT) siRNA. mRNA (Left) and protein (Right) levels were quantified at 24 h using RT-PCR and immunoblotting, respectively. (D) Survival of immunopanned RGCs transfected with nontargeting (dashed) or DLK siRNA (solid). (E) Primary RGCs were transfected with DLK or NT siRNA. After the indicated period (before cell death), cells were fixed and stained for Brn3 expression. (F) Patch-clamp recordings from RGCs maintained with DLK siRNA in response to depolarizing current.
Using this approach, we screened an arrayed library of 1,869 siRNAs against 623 kinases, providing threefold coverage of the mouse kinome, for the ability to promote the survival of RGCs grown in neurotrophin-deficient media (Fig. 1B). To minimize the number of false-positive leads resulting from off-target silencing, we took the conservative approach of focusing only on kinases for which all three siRNAs were protective. Indeed, only two kinases met this criterion: DLK and its only known substrate, mitogen-activated protein kinase kinase 7 (MKK7) (26). Secondary testing, using an independent set of siRNA with distinct targeting sequences, confirmed that both kinases were the relevant targets (Fig. S2A). Supporting the biological relevance of this finding, MKK7 and its homolog, MKK4, are the canonical activators of JNK1–3 (27), key regulators of RGC cell death (9–12). As an additional validation that our siRNA finding was specifically a result of DLK pathway inhibition, RGCs were isolated from mice containing a floxed allele of Dlk (22) or wild-type controls and then transduced with adenovirus expressing the P1 bacteriophage Cre recombinase or a GFP control. Similar to the results with RNA interference, genetic deletion of DLK led to increased RGC survival (Fig. S2B).
DLK Down-Regulation Promotes Long-Term Survival and Function of RGCs in Vitro.
We next studied the kinetics of RGC cell death after DLK knockdown. Immunopanned RGCs were transfected with Dlk siRNA or a nontargeting control and followed over time. By 24 h, Dlk siRNA efficiently reduced DLK expression at both the mRNA and protein levels. Consistent with DLK being a major activator of JNK in injured RGCs, DLK knockdown inhibited JNK phosphorylation, indicating attenuation of downstream JNK signaling (Fig. 1C). By 48 h there was a clear survival effect (Fig. 1D). Although there were very few live control cells, RGCs transfected with Dlk siRNA had greater than 50% viability. The prosurvival effect of DLK inhibition persisted for at least 3 wk. We found that Dlk mRNA levels stayed low throughout this period, which is consistent with reports that siRNA knockdown in postmitotic neurons can be long-lived (28, 29).
Axonal injury typically reduces the expression of many RGC-specific markers secondary to the down-regulation of the Brn3 family of transcription factors (30). However, in RGCs with DLK knockdown, Brn3 continued to be expressed (Fig. 1E). This suggested that DLK may be a relatively upstream injury signal and that injured RGCs, in the absence of DLK signaling, maintain characteristics of uninjured RGCs. At the functional level, patch-clamp recordings showed that RGCs kept alive for 2 wk with Dlk siRNA continue to generate action potentials in response to depolarizing current (Fig. 1F).
DLK Inhibition Promotes RGC Survival in Vivo After Optic Nerve Injury.
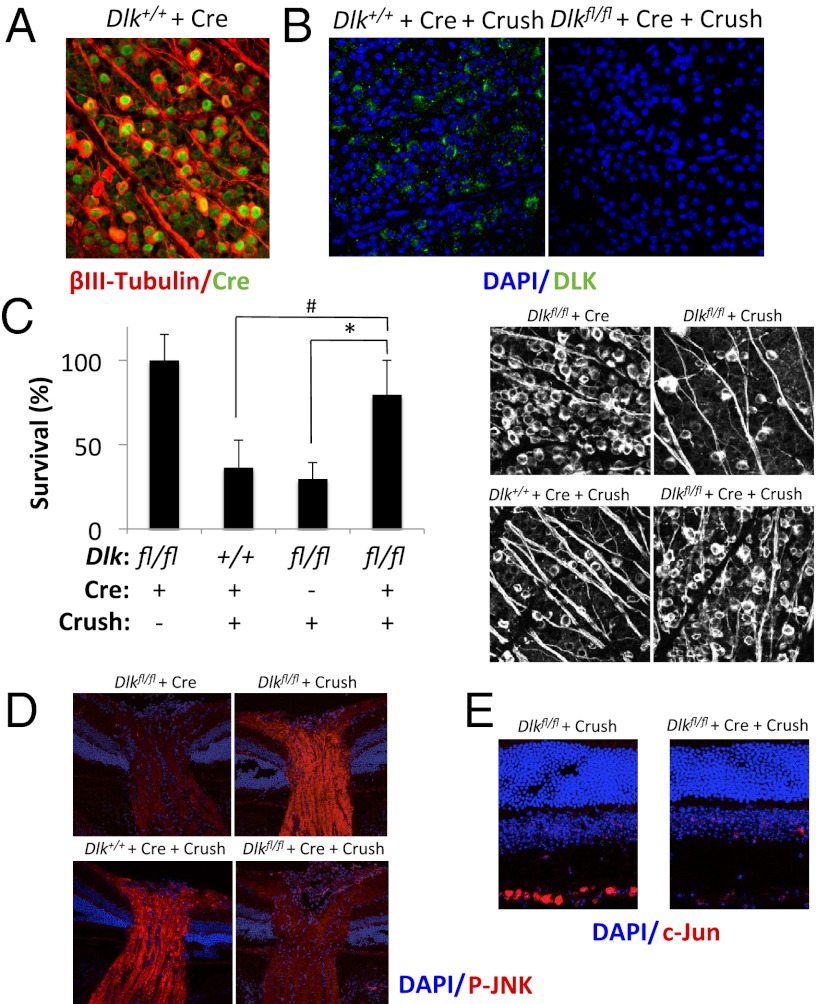
To test the role of DLK on RGC survival in vivo, we turned to the mouse optic nerve crush model. In response to axonal injury, 50%–75% of RGCs die by 2 wks (31). Mice carrying a floxed allele of Dlk (Dlkfl/fl) were injected intravitreally with a self-complementary, capsid-modified adeno-associated virus 2 (AAV2) (32, 33) expressing Cre. Injection of the AAV2-Cre resulted in Cre expression in nearly 100% of RGCs (Fig. 2A). One week after injection, to allow sufficient time for Cre-mediated deletion of Dlk (Fig. 2B), optic nerve crush was performed. Ten days later, retinal flatmounts were prepared and stained for the RGC-specific marker βIII-tubulin and the number of surviving RGCs was determined. Compared with control animals (Dlk+/+ mice injected with AAV2-Cre or Dlkfl/fl mice in the absence of Cre), Dlkfl/fl mice injected with AAV2-Cre showed a 75% reduction in RGC loss (Fig. 2C). This increase in RGC survival was associated with decreased JNK phosphorylation (Fig. 2D) and c-Jun expression (Fig. 2E), which are markers of JNK signaling (34, 35). These results suggest that DLK may be the primary kinase responsible for JNK pathway activation after axonal injury.
Fig. 2.
Genetic deletion of DLK protects RGCs from axonal injury-induced cell death in vivo. (A) Dlk+/+ mice were intravitreally injected with adeno-associated virus 2 (AAV2)-Cre. Seven days after infection, retinal flatmounts were stained for βIII-tubulin and Cre. (B) Three-month-old Dlk+/+ or Dlkfl/fl mice were intravitreally injected with AAV2-Cre. Seven days later, eyes were subjected to optic nerve crush. Four days after injury, retinal flatmounts were prepared and stained for DLK. (C) Survival of RGCs 10 d after optic nerve crush in Dlkfl/fl mice (n = 7), Dlkfl/fl mice injected with AAV2-Cre (n = 8), or Dlk+/+ mice injected with AAV2-Cre (n = 9), normalized to uninjured control mice (n = 6). *P < 0.05; #P < 0.005; error bars, SD. (Right) Representative images. Immunofluorescent staining of optic nerves (D) and retinas (E) 24 h after nerve crush in the mice described in (C).
Axonal Injury Up-Regulates DLK Expression Through a Posttranscriptional Mechanism.
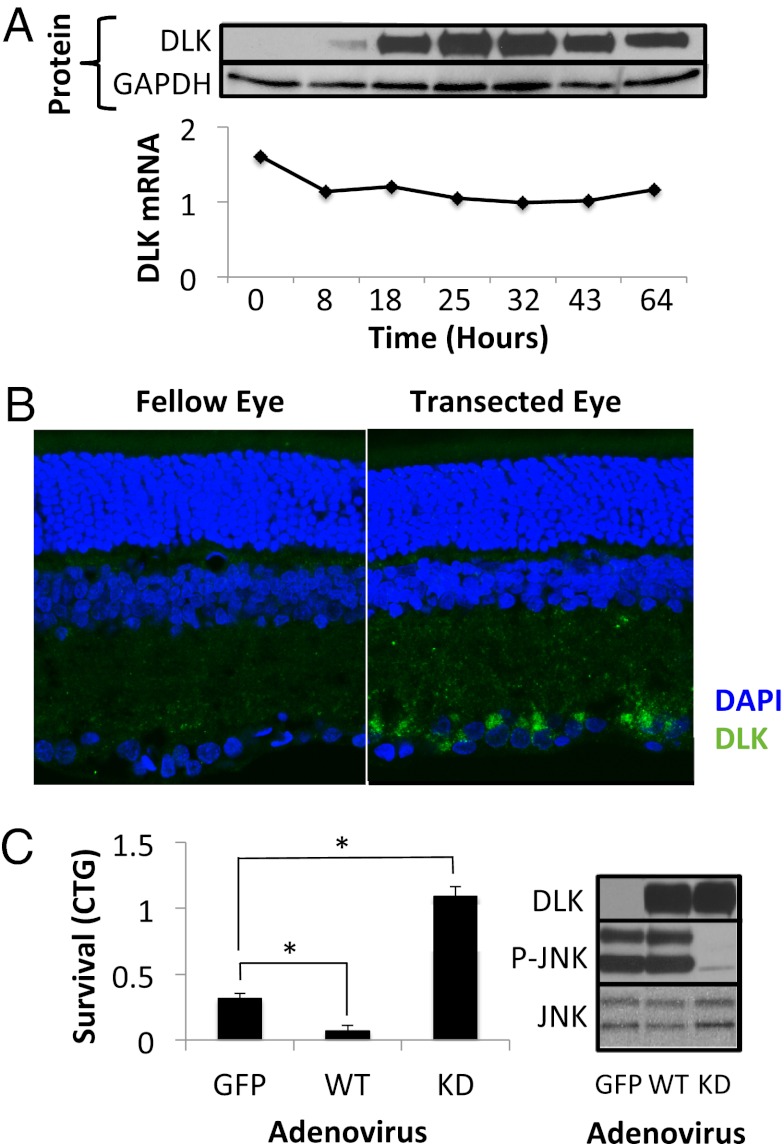
We next examined the mechanism of DLK regulation. Surprisingly, and unlike other members of the JNK cascade, DLK protein is undetectable in uninjured RGCs both in vitro and in vivo (Fig. 3 A and B, Left). However, culturing RGCs in vitro (which necessarily involves axotomy and cell injury) and transection in vivo both lead to robust up-regulation of DLK protein (Fig. 3 A and B, Right). In vitro, DLK protein levels increased more than 10-fold within 18 h from the initiation of cell culture. In contrast, Dlk transcript levels remained relatively constant during this period (Fig. 3A), indicating that increased translation and/or decreased protein turnover must underlie the mechanism of DLK up-regulation. In Drosophila melanogaster, Wallenda/DLK is posttranslationally regulated by the E3 ubiquitin ligase Highwire (36, 37). However, mice with a brain-specific conditional knockout of Phr1 (Pam/Highwire/RPM-1 1, the vertebrate Highwire homolog) show no difference in the overall brain levels of DLK protein (38). Furthermore, knockdown of PHR1 in our RGC cultures did not affect DLK levels, suggesting that either PHR1 regulates DLK levels only in certain settings/neuronal subtypes or that DLK levels in vertebrates are regulated by another as-yet-unidentified protein.
Fig. 3.
DLK protein is up-regulated in RGCs in response to injury. (A) Levels of DLK protein (Upper) and mRNA (Lower), normalized to GAPDH, after various times in culture. (B) DLK immunofluorescence of retinal sections 72 h after optic nerve transection in rats. (C) Survival, measured by CellTiter-Glo (CTG; Promega) luminescence, of immunopanned RGCs 48 h after transduction with adenovirus expressing wild-type (WT) or kinase-dead DLK. Western blot showing the up-regulation of DLK protein and corresponding response of the JNK pathway. *P < 0.05; error bars, SD.
Because DLK down-regulation promotes RGC survival, we wanted to test the complementary hypothesis that increased DLK expression can trigger RGC cell death. We used adenovirus to overexpress GFP, DLK, or a kinase-dead version of DLK (K185R) (39). Primary RGCs were transduced and survival measured 48 h later. Consistent with our model, wild-type DLK overexpression hastened cell death, whereas overexpression of K185R DLK, which functioned as a dominant-negative mutant as assessed by JNK phosphorylation, actually promoted RGC survival (Fig. 3C).
Small Molecule Kinase Inhibitor Tozasertib Binds DLK and Promotes RGC Survival.
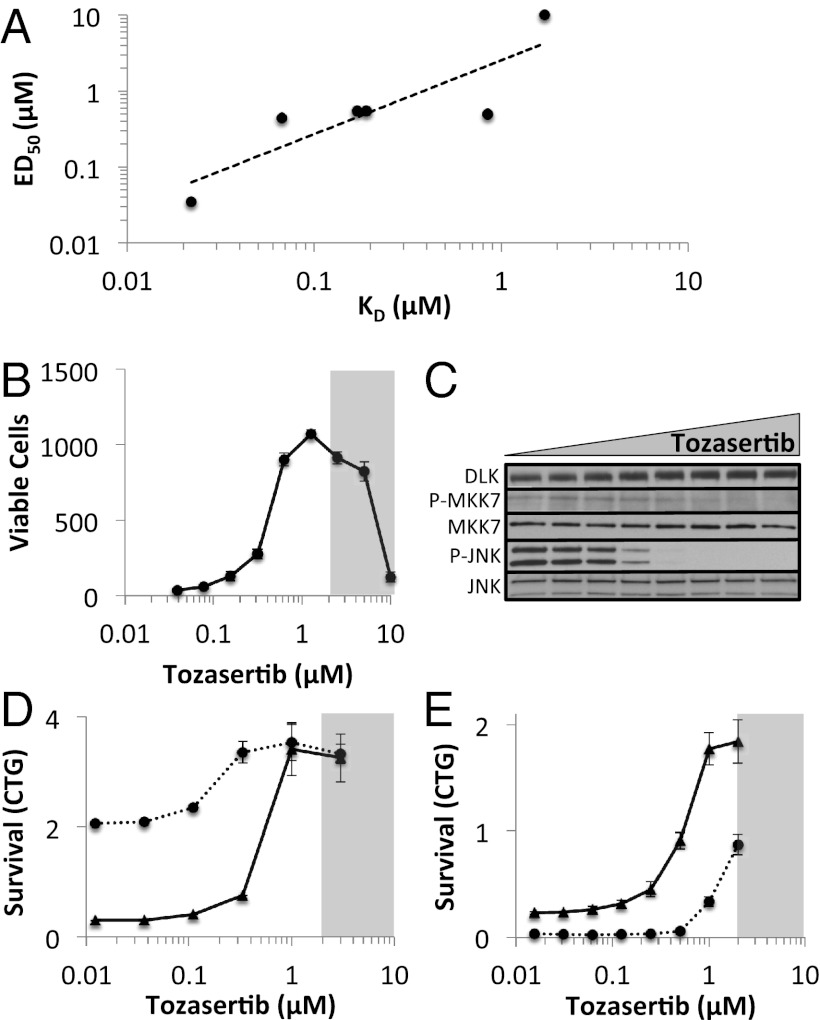
Our siRNA screening and conditional knockout results implicated DLK as a key mediator of RGC cell death. Thus, as a proof of principle that our approach could lead to the identification of small molecules for glaucoma therapy, we explored whether pharmacologic inhibition of DLK would be neuroprotective. First, we obtained a series of kinase inhibitors with known spectra (40, 41), including some that bind DLK at sub- or low-micromolar doses (tozasertib, crizotinib, foretinib, KW-2449, axitinib, and lestaurtinib) and others that do not bind purified DLK (vatalanib, vandetanib, imatinib, tandutinib, and sorafenib). We then tested them for their ability to promote survival of RGCs in vitro and found that only those with anti-DLK activity were neuroprotective (Figs. S3 and S4). Moreover, of the inhibitors with neuroprotective activity, there was a correlation between the survival-promoting ED50 and the DLK dissociation constant (R2 = 0.75; Fig. 4A). From this group of neuroprotective inhibitors, we chose tozasertib, a small molecule originally developed as an aurora kinase inhibitor for cancer treatment (42), for further in vitro and in vivo analysis. Tozasertib is commercially available, amenable to packaging with poly(lactic-co-glycolic acid) (PLGA) microspheres, and showed significant neuroprotective activity, demonstrating increased RGC survival in a dose-dependent manner (Fig. 4B). Furthermore, the same concentrations that increased RGC survival caused a decrease in the phosphorylation of targets downstream of DLK, including MKK7 and JNK (Fig. 4C). This effect is unlikely to be a result of direct inhibition of MKK7 and/or JNK, as published kinase-inhibitor profiling data indicate that tozasertib does not have significant affinity for either kinase (40, 41). Given tozasertib’s ability to inhibit multiple kinases, we wanted to more directly test whether its neuroprotective activity at least partially involved DLK inhibition. We postulated that if DLK were a key biologically relevant target of tozasertib, then reducing the amount of DLK with siRNA should sensitize the cells to lower doses of tozasertib (Fig. 4D). Indeed, RGCs transfected with DLK siRNA had a left-shift of the tozasertib-survival dose–response curve compared with RGCs transfected with a control siRNA. However, another prediction of our model is that increased levels of DLK necessitate higher levels of tozasertib to achieve the same amount of survival. As expected, RGCs transduced with adenovirus overexpressing DLK had a right-shift of the tozasertib-survival dose–response curve compared with control adenovirus-transduced cells (Fig. 4E).
Fig. 4.
Tozasertib inhibits DLK signaling in RGCs. (A) Correlation between the DLK dissociation constant (KD) and the neuroprotective ED50 for each of the protein kinase inhibitors tested. (B) Survival of immunopanned RGCs, treated with increasing doses of tozasertib, after 72 h in culture. The shaded area indicates the toxic range for tozasertib. (C) Western blot of the DLK pathway members in RGCs 4 h after immunopanning in the presence of 0, 0.03, 0.06, 0.125, 0.25, 0.5, 1, or 2 μM tozasertib. (D) Survival of cultured RGCs 72 h after transfection with DLK (dashed) or nontargeting siRNA (solid) in the presence of increasing doses of tozasertib. (E) Survival of cultured RGCs 48 h after transduction with DLK-expressing (dashed) or control-expressing (solid) adenovirus in the presence of increasing doses of tozasertib. Error bars, SD.
Tozasertib Promotes RGC Survival in Vivo.
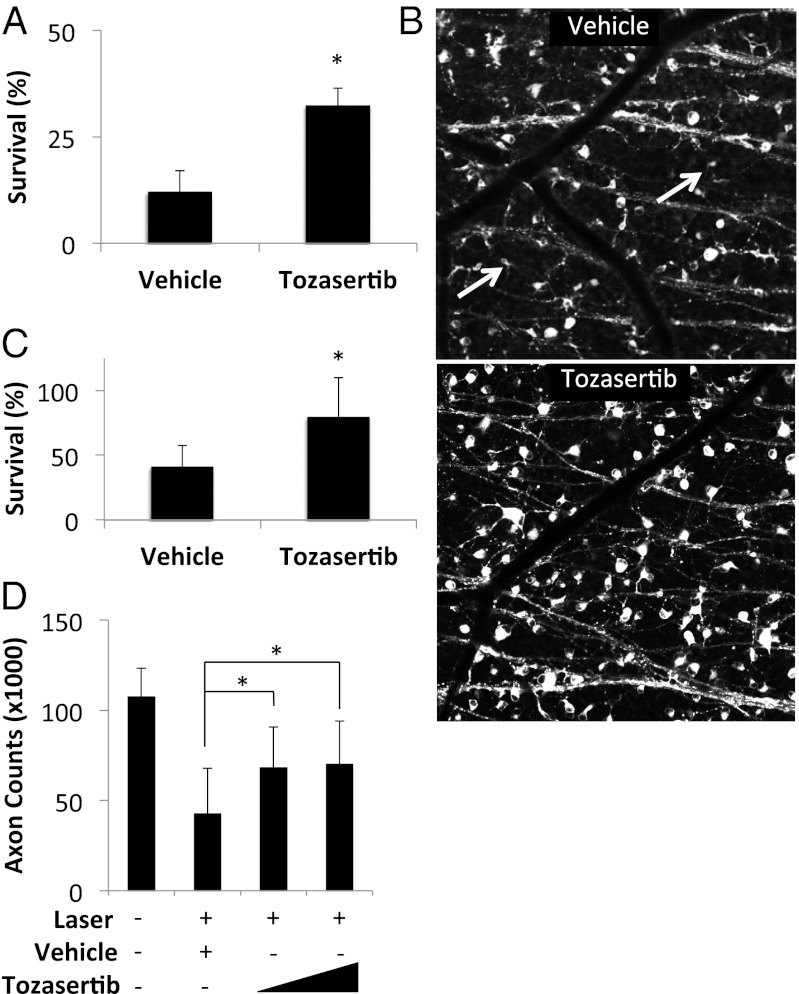
We tested the ability of tozasertib to promote RGC survival after optic nerve transection. To provide sustained, local ocular drug delivery, tozasertib-containing, PLGA-based, slow-eluting microspheres were generated (43). The microspheres demonstrated drug release in vitro for more than 1 mo (Fig. S5). For these experiments, rats were chosen over mice because their larger eye size is more amenable to microsphere injection. Wistar rats were pretreated with intravitreal microspheres containing tozasertib or vehicle. Seven days later, optic nerves were transected and RGCs were retrogradely labeled by applying the lipophilic tracer 4-Di-10-ASP to the proximal nerve stump (44). Two weeks after transection, retinal flatmounts were examined by confocal microscopy to identify and quantify the number of surviving RGCs. Vehicle-treated eyes showed an average of 12.0% surviving RGCs compared with 32.3% surviving cells in the tozasertib-treated eyes (Fig. 5 A and B). Given the complex pharmacokinetics of intravitreal tozasertib and the possibility of enhanced off-target effects with drug accumulation, it is not surprising that the magnitude of the neuroprotective effect is smaller than with genetic deletion of Dlk. Nonetheless, these results validate that our primary RGC screen of druggable targets (i.e., kinases) was able to lead to the identification of a small molecule kinase inhibitor that protects RGCs in vivo.
Fig. 5.
Tozasertib promotes RGC survival in vivo. (A) Survival of RGCs after optic nerve transection in rats pretreated with intravitreal drug-eluting microspheres containing vehicle (n = 10) or 275 ng tozasertib (n = 5). (B) Representative images shown to the right. (C) Survival of RGCs after laser-induced ocular hypertension in rats pretreated with intravitreal drug-eluting microspheres containing vehicle (n = 14) or 275 ng tozasertib (n = 14). (D) Optic nerve axon counts after laser-induced ocular hypertension in rats pretreated with intravitreal drug-eluting microspheres containing vehicle (n = 14) or 82 ng (n = 22) or 275 ng (n = 21) tozasertib. Fellow eyes (n = 57) shown for comparison. *P < 0.05; error bars, SD.
To evaluate tozasertib’s neuroprotective activity in a glaucoma model, we pretreated rats with intravitreal tozasertib- or vehicle-eluting microspheres and then used diode laser treatment of the trabecular meshwork to increase IOP (Fig. S6A) (45). A pretreatment paradigm is appropriate given that glaucoma in humans is thought to result from chronic, repeated injury, and thus drug administration at any point in the disease is likely to affect future degeneration. The drug- and vehicle-treated eyes showed similar degrees of IOP elevation (Fig. S6B). In eyes injected with control microspheres, there was a 60% reduction in RGC cell bodies and optic nerve axons at 1 mo. However, in eyes treated with tozasertib-eluting microspheres, soma loss was decreased to 21% (Fig. 5C) and axon loss was decreased to 34% (Fig. 5D and Fig. S6C). Taken together, our data suggest that DLK inhibition is a key part of the mechanism by which tozasertib promotes RGC survival in glaucoma and optic nerve crush models. However, as tozasertib is a broad-spectrum protein kinase inhibitor, it is certainly possible that inhibition of kinases other than DLK may also contribute to its neuroprotective activity. Future experiments such as a comparison of the relative efficacy of tozasertib treatment and Cre-mediated DLK knockout in mouse models of glaucoma will be needed to resolve this issue.
Discussion
Large-scale RNAi-based phenotypic screens in lower organisms have successfully identified genes involved in the rescue of neuronal degenerations (46–48). Parallel screens using primary vertebrate neurons, however, have been more difficult because of the challenges in working with and transfecting primary neuronal cell cultures. Using a magnetic nanoparticle-based method that is easily compatible with automation, we have overcome these challenges and performed a kinome-wide survival screen using a disease-relevant primary neuron. This global and unbiased approach led to the identification of DLK signaling as a key cell death pathway in RGC degeneration. Moreover, it establishes the proof of principle for a whole-genome scan in primary RGCs to identify additional potential neuroprotective pathways and drug targets.
Several previous studies have implicated the JNK pathway in both traumatic and glaucomatous models of optic neuropathy (9–12). However, the mechanism by which axonal injury leads to JNK activation in RGC cell bodies has been unclear. Our results, as well as those by Watkins et al. (49), suggest that DLK may be the as-yet-unidentified trigger for JNK activation and cell death in injured RGCs. Such a role for DLK integrates well with accumulating data about the involvement of DLK in axonal injury and neuronal apoptosis. DLK has been shown to mediate developmental apoptosis in peripheral motor and sensory neurons (17, 18). In adult peripheral neurons, it has been implicated as an important mediator of distal axonal degeneration (22) and proximal axonal regeneration (16, 19, 20) after axonal injury. It is required for the retrograde transmission of injury mediators such as the JNK scaffold protein JNK interacting protein 3 (JIP3) and phosphorylated STAT3 and plays a role in the induction of expression of proregenerative genes (21).
The work by Watkins et al. suggests that DLK is also involved in the regeneration of optic nerve axons after optic nerve crush (49). It remains to be determined whether DLK is involved in glaucoma-related axonal degeneration. If axonal degeneration persists even in the setting of DLK inhibition-mediated soma preservation, then associated inhibition of axonal regeneration might a priori seem like an undesirable adverse effect. However, as there is little evidence that regeneration occurs as part of the natural history of glaucoma, such a strategy is unlikely to be harmful At the very least, preservation of RGC cell bodies would provide an important component of a comprehensive neuroprotective therapy. Furthermore, definition of what determines whether activation of DLK signaling leads to primarily axonal regeneration and/or neuronal cell death may make possible the separation of these signals and the development of treatment strategies that could inhibit cell death without interfering with regeneration.
An advantage of promoting RGC survival through DLK-mediated inhibition of JNK signaling, rather than directly inhibiting JNK, is that JNK signaling has a number of important physiologic roles, such as tumor suppression (50). Because multiple independent pathways feed into JNK activation (51), the finding that the DLK branch is the major pathway leading to proapoptotic JNK activation after RGC injury makes possible a more fine-tuned and specific approach. Our demonstration that tozasertib is neuroprotective in both glaucoma and traumatic optic neuropathy models can be seen as a proof of principle indicating that such a strategy can succeed. It also supports the possibility that pharmacologic inhibition of DLK could provide a therapeutic approach for other forms of CNS neurodegeneration.
Materials and Methods
Statistical Analysis.
All statistical analyses were performed with the unpaired Mann-Whitney-Wilcoxon test.
Rat Optic Nerve Transection.
The optic nerve was exposed by a partial peritomy and intraorbital dissection of the extraocular muscles and then transected with a 25-gauge needle; 4-Di-10-ASP was then applied to the proximal nerve stump. Care was taken to avoid vascular injury during the transection, and retinal perfusion was examined after nerve transection. Two weeks after transection, rats were killed and enucleated. Retinas were flatmounted, imaged with a Zeiss LSM 510 META confocal microscope with a Zeiss Plan-Apochromat 20×/0.75 numerical aperture (NA) objective. Images were taken from four fields of 230 × 230 mm squares located 2 mm superior, inferior, temporal, and nasal to the optic disk. The number of 4-Di-10-ASP-labeled cells with RGC morphology was quantified. Imaging and quantification of RGC survival were performed in a masked fashion.
Rat Laser-Induced Ocular Hypertension.
IOP was unilaterally elevated by laser treatment of the trabecular meshwork as previously described (45). Briefly, 6-wk-old Wistar male rats were anesthetized with ketamine/xylazine. On two consecutive weeks, 40–50 532-nm diode laser spots were applied to the prelimbal region (50 μm diameter, 600 mW power, and 0.6 s duration). Under anesthesia, the IOP of laser-treated and fellow eyes was measured with TonoLab (Icare) 1 and 3 d after laser treatment. Four weeks after laser treatment, toluidine blue–stained optic nerves were imaged and the axons were counted. The laser treatment and acquisition of optic nerve images were performed in a masked fashion. RGC somata were measured by Brn3 staining of retina sections. The number of Brn3-positive cells was normalized by the number of DAPI-stained cells in GCL on the same sections.
Mouse Intravitreal Injection and Optic Nerve Crush.
Three-month-old male C57BL/6 and Dlk floxed mice (BL/6 background) were anesthetized with ketamine/xylazine and intravitreally injected with 1010 DNA-containing particles of capsid-mutant (Y444, 500, 730F) AAV2 expressing Cre recombinase from the chicken β-actin promoter. Seven days later, optic nerve was surgically exposed and crushed with Dumont N7 self-closing forceps 1 mm behind the globe for 3 s. Ten days after nerve crush, eyes were enucleated and fixed and surviving RGC was immunostained for βIII-tubulin and Brn3. The retinas were then imaged with a Nikon Eclipse TE2000-5 fluorescence microscope and Plan-fluor 40×/0.6 objective. Images were acquired from the four fields in the superior, inferior, temporal, and nasal quadrants 1 mm from the optic disk. RGCs were counted manually from each image. In a separate cohort of animals, optic nerves and retinas were sectioned and stained for phospho-JNK and c-Jun, respectively, 24 h after optic nerve crush. Intravitreal injection, optic nerve crush, immunofluorescence, and RGC counting were performed in a masked fashion.
Reagents, RGC Culture, Electrophysiology, AAV Vectors, and Microspheres.
See SI Materials and Methods for more details.
Supplementary Material
Acknowledgments
We thank Noriko Esumi, Akrit Sodhi, Ted Dawson, and Valina Dawson for reviewing the manuscript and/or scientific advice; Mary Ellen Pease and Matthew Steinhart for help with the rat glaucoma experiment; and Jiangxia Wang and John McGready for assistance with the statistics. This study was supported, in part, by grants from the National Eye Institute; NIH; the American Health Assistance Foundation; Glaucoma Research Foundation; Research to Prevent Blindness, Inc.; the Macula Vision Research Foundation; the Eldon Family Foundation; Vision for Children; and generous gifts from the Guerrieri Family Foundation and from Mr. and Mrs. Robert and Clarice Smith.
Footnotes
Conflict of interest statement: D.S.W., Z.Y., S.E.M., H.Q., C.A.B., and D.J.Z. are inventors on patents related to the work described; these patents are being managed by The Johns Hopkins University School of Medicine. W.W.H. and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211284110/-/DCSupplemental.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179(7):1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EE, Goldberg JL. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danesh-Meyer HV. Neuroprotection in glaucoma: Recent and future directions. Curr Opin Ophthalmol. 2011;22(2):78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 5.Tu W, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140(2):222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277(1):22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang JT, Medress ZA, Barres BA. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196(1):7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisanaga S-I, Endo R. Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J Neurochem. 2010;115(6):1309–1321. doi: 10.1111/j.1471-4159.2010.07050.x. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, et al. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Mol Vis. 2011;17:864–875. [PMC free article] [PubMed] [Google Scholar]
- 10.Ribas VT, Arruda-Carvalho M, Linden R, Chiarini LB. Early c-Jun N-terminal kinase-dependent phosphorylation of activating transcription factor-2 is associated with degeneration of retinal ganglion cells. Neuroscience. 2011;180:64–74. doi: 10.1016/j.neuroscience.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 11.Bessero A-C, Chiodini F, Rungger-Brändle E, Bonny C, Clarke PGH. Role of the c-Jun N-terminal kinase pathway in retinal excitotoxicity, and neuroprotection by its inhibition. J Neurochem. 2010;113(5):1307–1318. doi: 10.1111/j.1471-4159.2010.06705.x. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes KA, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis. 2012;46(2):393–401. doi: 10.1016/j.nbd.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P. Protein kinases—the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1(4):309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 14.Scott DL. Role of spleen tyrosine kinase inhibitors in the management of rheumatoid arthritis. Drugs. 2011;71(9):1121–1132. doi: 10.2165/11591480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: Important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301(2):H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 16.Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun. 2009;383(2):258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Itoh A, et al. ZPK/DLK, a mitogen-activated protein kinase kinase kinase, is a critical mediator of programmed cell death of motoneurons. J Neurosci. 2011;31(20):7223–7228. doi: 10.1523/JNEUROSCI.5947-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh AS, et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol. 2011;194(5):751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138(5):1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74(6):1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12(4):387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, et al. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci. 2008;28(3):672–680. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1(9):791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Ando DM, Daub A, Kaye JA, Finkbeiner S. High-throughput screening in primary neurons. Methods Enzymol. 2012;506:331–360. doi: 10.1016/B978-0-12-391856-7.00041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merritt SE, et al. The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate. J Biol Chem. 1999;274(15):10195–10202. doi: 10.1074/jbc.274.15.10195. [DOI] [PubMed] [Google Scholar]
- 27.Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94(14):7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Asaoka M, Yanagawa Y, Hirashima N. Long-term gene-silencing effects of siRNA introduced by single-cell electroporation into postmitotic CNS neurons. Neurochem Res. 2011;36(8):1482–1489. doi: 10.1007/s11064-011-0474-6. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Yanagawa Y, Hirashima N. Transfer of small interfering RNA by single-cell electroporation in cerebellar cell cultures. J Neurosci Methods. 2009;178(1):80–86. doi: 10.1016/j.jneumeth.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, et al. Changes in gene expression in experimental glaucoma and optic nerve transection: The equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48(12):5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40(5):1004–1008. [PubMed] [Google Scholar]
- 32.Petrs-Silva H, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrs-Silva H, et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19(2):293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dérijard B, et al. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 35.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 36.Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51(1):57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191(1):211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloom AJ, Miller BR, Sanes JR, DiAntonio A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev. 2007;21(20):2593–2606. doi: 10.1101/gad.1592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robitaille K, et al. Tissue transglutaminase triggers oligomerization and activation of dual leucine zipper-bearing kinase in calphostin C-treated cells to facilitate apoptosis. Cell Death Differ. 2004;11(5):542–549. doi: 10.1038/sj.cdd.4401392. [DOI] [PubMed] [Google Scholar]
- 40.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 41.Davis MI, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29(11):1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 42.Harrington EA, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 43.Edwards DA, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 44.Pavlidis M, Fischer D, Thanos S. Photoreceptor degeneration in the RCS rat attenuates dendritic transport and axonal regeneration of ganglion cells. Invest Ophthalmol Vis Sci. 2000;41(8):2318–2328. [PubMed] [Google Scholar]
- 45.Levkovitch-Verbin H, et al. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43(2):402–410. [PubMed] [Google Scholar]
- 46.Bhattacharya MRC, et al. A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci. 2012;32(15):5054–5061. doi: 10.1523/JNEUROSCI.4951-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimitriadi M, et al. Conserved genes act as modifiers of invertebrate SMN loss of function defects. PLoS Genet. 2010;6(10):e1001172. doi: 10.1371/journal.pgen.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulte J, Sepp KJ, Wu C, Hong P, Littleton JT. High-content chemical and RNAi screens for suppressors of neurotoxicity in a Huntington’s disease model. PLoS ONE. 2011;6(8):e23841. doi: 10.1371/journal.pone.0023841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins TA, et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci USA. 2013;110:4039–4044. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies C, Tournier C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem Soc Trans. 2012;40(1):85–89. doi: 10.1042/BST20110641. [DOI] [PubMed] [Google Scholar]
- 51.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.