Abstract
Recognition of the methyl-7-guanosine (m7G) cap structure on mRNA is an essential feature of mRNA metabolism and thus gene expression. Eukaryotic translation initiation factor 4E (eIF4E) promotes translation, mRNA export, proliferation, and oncogenic transformation dependent on this cap-binding activity. eIF4E–cap recognition is mediated via complementary charge interactions of the positively charged m7G cap between the negative π-electron clouds from two aromatic residues. Here, we demonstrate that a variant subfamily, eIF4E3, specifically binds the m7G cap in the absence of an aromatic sandwich, using instead a different spatial arrangement of residues to provide the necessary electrostatic and van der Waals contacts. Contacts are much more extensive between eIF4E3–cap than other family members. Structural analyses of other cap-binding proteins indicate this recognition mode is atypical. We demonstrate that eIF4E3 relies on this cap-binding activity to act as a tumor suppressor, competing with the growth-promoting functions of eIF4E. In fact, reduced eIF4E3 in high eIF4E cancers suggests that eIF4E3 underlies a clinically relevant inhibitory mechanism that is lost in some malignancies. Taken together, there is more structural plasticity in cap recognition than previously thought, and this is physiologically relevant.
Metabolism of mRNA is a complex and highly regulated process dependent on the association of the methyl-7-guanosine (m7G) cap structure on the 5′ end of transcripts with appropriate proteins (1–3). In mammalian cells, the two major cap-binding proteins are the nuclear cap-binding complex (CBC) (4) and eukaryotic translation initiation factor 4E (eIF4E) (3). Specific cap recognition is key for the fate of transcripts and impacts processes such as mRNA processing and bulk mRNA export via the CBC or the nuclear export of specific transcripts as well as bulk translation by eIF4E (5). NMR and crystallographic studies reveal that m7G cap is specifically recognized by both the CBC and eIF4E via intercalation of the m7G cap moiety with the side chains of two aromatic residues, i.e., an aromatic sandwich (4, 6–9). This is an electrostatically driven process relying on the partial positive charge of the m7G cap and the negative π-electron clouds of the aromatic residues. The aromatic residues are completely conserved in these cap-binding proteins. This recognition motif is used almost exclusively by proteins that specifically bind the m7G cap including eIF4E, CBC, and vaccinia virus protein VP39 (4, 10).
Dysregulation of cap-binding proteins can have striking physiological consequences. For instance through its cap-binding activity and subsequent effects on gene expression, eIF4E plays an important role in proliferation and survival (1, 3). Indeed, eIF4E is overexpressed in about 30% of human cancers and its overexpression is oncogenic in cell culture and animal models (2, 11). Mutation of the cap-binding site of eIF4E impairs its activities in translation, mRNA export, and oncogenic transformation (12). Targeting the eIF4E–cap complex with a competitive m7G cap inhibitor is a promising strategy to generate anticancer agents (13, 14). One such inhibitor, ribavirin, has reached clinical trials where it was used in poor-prognosis leukemia patients. Here, it inhibited eIF4E activity in patients which correlated with clinical responses, including some remissions (13) (http://ClinicalTrials.gov; NCT01056523). Thus, understanding principles of cap recognition and possible variants thereof is clinically relevant.
There has been virtually no investigation into the third member of the eIF4E family, eIF4E3 (15, 16). Other eIF4E family members, eIF4E2 (or 4E-HP) and eIF4E1 (the most commonly studied and referred to here as eIF4E) use the same cap recognition mechanism, i.e., two conserved aromatic residues to sandwich the m7G cap (Fig. 1 A and B and Fig. S1) (3, 17). However, the eIF4E3 subfamily is highly unusual whereby in vertebrates it has only one conserved aromatic (Trp98 in mouse eIF4E3, equivalent to Trp102 in human eIF4E1). The second aromatic site, equivalent to Trp56 in human eIF4E1, is replaced with a cysteine residue (Cys52 in mouse eIF4E3; see Fig. 1A and Fig. S1 for numbering). Here, we demonstrate that, in the absence of the traditional cap-binding residues, eIF4E3 specifically binds m7G cap in vitro and competes for cap in cells. We report structures of eIF4E3 in the apo- and cap-bound forms. Our studies indicate eIF4E3 uses an unusual strategy for m7G cap recognition. No studies into the function of eIF4E3 in cells have been reported previously. Here, we demonstrate that eIF4E3 is an inhibitor, rather than a promoter, of both target transcript expression and oncogenic transformation. This function is dependent on its atypical cap-binding activity. Finally, our data suggest that eIF4E3 is clinically relevant in specific subtypes of leukemia.
Fig. 1.
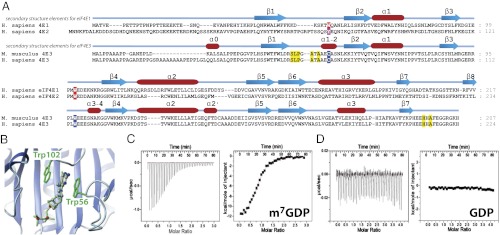
(A) Sequence alignment of the three eIF4E subfamilies. See Fig. S1 for a more comprehensive list. Secondary structures are shown. Aromatic residues important for cap recognition in eIF4E1 and eIF4E2 are highlighted in red, and corresponding residues in eIF4E3 are in blue. Other residues important for cap recognition in eIF4E3 are highlighted in yellow. (B) eIF4E1 structure (PDB ID code 3AM7) highlighting the stacking of the two aromatic residues against the m7G cap. (C and D) ITC data of wild-type eIF4E3 with m7GDP (C) and GDP (D) (Left, raw data; Right, binding isotherm).
Results
eIF4E3 Is Highly Conserved and Specifically Binds the m7G Cap in Vitro.
Nomenclature for eIF4E family members has not evolved without confusion. In this study, eIF4E3 refers to the third subfamily of eIF4E proteins (15, 16). Recent studies have appeared on Drosophila eIF4E3 (Dm4E3) (18) and Ascaris suum eIF4E3 (As4E3) (19); however, these proteins are not eIF4E3 subfamily members but actually belong to the eIF4E1 subfamily (third isoform of eIF4E1 in those species).
eIF4E3 members share ∼25% identity and ∼50% similarity with the two other eIF4E families, and the eIF4E3 family itself is highly conserved with ∼75% identity and ∼85% similarity (Fig. S1). In vertebrates, the conserved pair of aromatic residues critical for cap recognition is replaced by an unusual Cys–Trp pair in eIF4E3 (Fig. 1A). We therefore set out to determine whether eIF4E3 binds the m7G cap and whether it can discriminate between the cap and guanosine. Using purified recombinant mouse eIF4E3 protein and isothermal titration calorimetry (ITC), we observe that eIF4E3 bound m7GDP and m7GTP relatively tightly, with Kd values of 7.7 and 1.8 μM, respectively. The binding affinity is 10- to 40-fold lower than for eIF4E1 (Table 1). Similarly to eIF4E1, a eightfold reduction in affinity was observed for the dinucleotide m7GpppG compared with m7GTP (20). Although ITC did not detect eIF4E3 binding to GTP and GDP (Fig. 1 C and D), an NMR 1H-15N heteronuclear single-quantum correlation (HSQC) titration did detect a very weak association of eIF4E3 with GTP (Kd ∼ 1 mM) indicating ∼1,000-fold increase in affinity of m7G cap relative to guanosine. Thus, our data show eIF4E3 specifically binds the m7G cap and in this way is positioned to associate with mRNAs in the cell.
Table 1.
Thermodynamic parameters measured by ITC for cap binding to wild-type and mutants eIF4E3 and eIF4E1
| eIF4E variant | Cap analog | Kd, μM |
| eIF4E3 WT | m7GDP/m7GTP | 7.7 ± 0.3/1.8 ± 0.1 |
| eIF4E3 WT | m7GpppG | 14.5 ± 1.1 |
| eIF4E3 WT | GDP/GTP | NB*/925 ± 80† |
| eIF4E3 S43G | m7GTP | 15.5 ± 2.0 |
| eIF4E3 A47G | m7GTP | 13.2 ± 1.2 |
| eIF4E3 T48A | m7GDP/m7GTP | 128.9 ± 12.9/31.4 ± 1.5 |
| eIF4E3 A49G | m7GTP | 16.6 ± 1.5 |
| eIF4E3 C52A | m7GDP/m7GTP | NB*/123.9 ± 10.9 |
| eIF4E3 C52W | m7GDP/m7GTP | 24.9 ± 6.5/10.0 ± 0.6 |
| eIF4E3 W98A | m7GDP/m7GTP | NB*/NB* |
| eIF4E3 H197A | m7GDP | 64.9 ± 3.2 |
| eIF4E3 F200A | m7GDP/m7GTP | 324 ± 46†/NB* |
| eIF4E3 Δ205–207 | m7GDP/m7GTP | 12.9 ± 0.8/7.1 ± 0.5 |
| eIF4E3 Δ199–207 | m7GDP/m7GTP | NB*/NB* |
| eIF4E1 WT | m7GDP/m7GTP | 0.17 ± 0.03/0.14 ± 0.02 |
| eIF4E1 W56A | m7GTP | 40.63 ± 4.16 |
| eIF4E1 W102A | m7GTP | 16.81 ± 3.50 |
*No binding observed.
†Kd values determined by NMR spectroscopy.
We then characterized the relative importance of the conserved Cys52 and Trp98 residues for cap recognition. The Trp98Ala and Cys52Ala mutants exhibited severely impaired m7G cap binding by ITC and NMR (Table 1). Importantly NMR studies confirmed that these mutants were folded so that loss of binding was not related to misfolding (SI Materials and Methods). We assessed whether we could increase eIF4E3 affinity for the cap so it would be in the same range as eIF4E1 by engineering a Cys52Trp mutant, but this led to threefold to fivefold reduction in binding relative to wild-type eIF4E3. For comparison, mutation of Trp56Ala severely impairs cap binding in eIF4E1 (21). Thus, both Cys52 and Trp98 play important roles in eIF4E3 cap recognition.
Structure Determination of apo- and m7GDP-Bound eIF4E3.
To understand the molecular basis of eIF4E3 m7G cap recognition, we determined the structures of eIF4E3 in the apo- (Fig. 2A) and m7GDP-bound (Fig. 3A) forms. Structures were determined using NMR distance and dihedral restraints with the autoassign module in CYANA 2.1 (22) and refined with X-PLOR-NIH (23). Structural statistics are given in Table S1. For the eIF4E3–m7GDP complex, 36 intermolecular distance constraints (Fig. S2) were derived from a 3D 13C-edited nuclear Overhauser effect spectroscopy (NOESY) spectrum on an eIF4E3 sample with 13C/15N-labeled m7GTP, and confirmed with a 13C/15N “half-filtered” NOESY experiment (24) on a 13C/15N eIF4E3 sample with unlabeled m7GDP.
Fig. 2.

NMR structure of the apo–eIF4E3. (A) Superposition of the 10 lowest-energy NMR structures of apo–eIF4E3. The side chain of Trp98 is shown. (B) Overlay of the lowest conformation structures of apo–eIF4E3 (orange) and apo–eIF4E1 (green; PDB ID code 2GPQ) (8). (C) Overlay of the apo–eIF4E3 (orange) and m7GDP-bound eIF4E3 (blue) NMR structures.
Fig. 3.

NMR solution structure of the m7GDP–eIF4E3. (A) Superposition of the 10 lowest-energy NMR structures. Secondary structure elements are shown. (B) Overlay of m7G cap structures of eIF4E3 (yellow) and eIF4E1 (blue). Mobile residues and cap-binding regions in eIF4E3 were excluded from the superposition (residues 1–35, 42–56, 92–105, and 195–207). Regions involved in cap binding are highlighted orange for eIF4E3 and dark blue for eIF4E1. (C and D) The m7G cap-binding pockets for eIF4E3 (C) and eIF4E1 (D), highlighting additional m7G cap contacts in eIF4E3. (E and F) Surface electrostatic potential of eIF4E3 in the m7G cap complex. Strength of the potential at the surface is indicated by the color saturation (blue for positive; red for negative), m7GDP is shown as yellow sticks. An enlarged view of the cap-binding pocket is shown in F.
The apo- and m7GDP-bound eIF4E3 structures are well defined (Table S1). Comparisons with the eIF4E1 structures (Figs. 2B and 3B) show the eIF4E3 structures retain the eIF4E fold of a central curved β-sheet consisting of seven antiparallel β-strands flanked by three α helices at its convex surface. The backbone root-mean-square deviation (rmsd) for regular secondary structure elements is 1.86 Å for apo–eIF4E3 versus apo–eIF4E1 (8) and 1.60 Å for m7GDP–eIF4E3 versus m7GTP–eIF4E1 (25). An extra single turn α helix (denoted α0, residues 16–20) is seen in both eIF4E3 structures at the disordered N terminus (residues 1–30) that is not seen in any other eIF4E structure. We detected nuclear Overhauser effects (NOEs) between this helix and the α1 helix for the apo form, but not the m7GDP-bound form. NMR steady-state heteronuclear {1H}-15N NOE (hNOE) data (Fig. S3C) indicates the N terminus is mobile in both forms, suggesting this interaction in apo–eIF4E3 might be transient. The C terminus of apo–eIF4E3 (residues 196–207) is also seen to be disordered from the structure and the hNOE data, but becomes much more structured upon cap binding.
The conserved Cys52 in eIF4E3, which replaces Trp56 in eIF4E1, forms part of a helix in the S1–S2 loop (designated α1–2) in both the apo and m7GDP forms of eIF4E3. Interestingly, in eIF4E1 and eIF4E2, this helix is also observed, but only for cap-bound structures and not the apo form. This preformed helix may play a key role in cap recognition (see below).
Overlay of the apo– and m7GDP–eIF4E3 structures are shown in Fig. 2C. The rmsd for regions that are not mobile or involved in cap binding is 1.2 Å: thus, similar to eIF4E1 only local changes, not global changes, are induced upon m7G–cap binding (8, 17). Regions of eIF4E3 important for m7G–cap binding (Fig. 3 B and C) are highlighted by the per-residue rmsd between apo- and m7GDP-bound eIF4E3 (Fig. S3B). These include similar regions recruited by eIF4E1, i.e., Trp98 (Trp102 in eIF4E1), the α1–2 helix including Cys52 (Trp56 in eIF4E1), and the preceding S1–S2 loop. Unlike eIF4E1, we did not observe a traditional aromatic cap sandwich. Instead a unique C-terminal site (mobile in the apo form), defined by intermolecular NOEs from the side chain of Ala199 and Hδ2 of His197 to m7GDP, forms contacts with the ribose ring. Given the proximity of Phe200 to this region and no aromatic sandwich, we searched exhaustively for NOEs from the cap to Phe200. No NOEs to the aromatic ring of Phe200 were detected even with a fully 14N-, 12C-, 2H-labeled (except for 15N-, 13C-, 1H-labeled Phe, Tyr, and His) eIF4E3 sample. Indeed, the position of the Phe200 is on the same cap-binding face as Trp98, so an aromatic residue from this region would compete with Trp98 rather than complete an aromatic sandwich type binding site. We do note that defining the precise orientation for the m7G moiety is difficult due to low proton density (Fig. S2B), e.g., only six 1H NMR signals are observed for m7GDP (which is comprised of 44 atoms). In the phosphate-binding region, the charged residues Arg152, Lys192, and Arg84 are sufficiently close to the phosphates in three or more of our ensemble of structures to make favorable contacts. The backbone amide resonances of these residues were among the most affected when comparing the 1H-15N HSQC spectra of m7G- and m7GDP-bound eIF4E3, and m7GDP- and m7GTP-bound eIF4E3.
Consistent with our eIF4E3–m7GDP complex structure, significant chemical shift perturbations were observed upon addition of m7GDP for regions incorporating Trp98, Cys52, and the C terminus (Fig. S3A). Relaxation hNOE data (Fig. S3C) showed that the three sites proposed to interact with m7GDP exhibit decreased motions upon association with m7GDP. Especially striking are the data for the S1–S2 loop (residues 43–53) and residues toward the C terminus (196–201). These regions are relatively mobile in apo–eIF4E3 (hNOE values of <0.6), and disordered at the C terminus but become ordered upon cap binding. ITC studies further substantiate our observations. Mutation of His197 or Phe200 and the C-terminal truncation from residue 199 severely impaired cap binding, whereas deletion from residue 205 only had modest effects (Table 1). Notably, the sequence after His194 is highly conserved in the eIF4E3 family, but not in other eIF4E families underlying its importance to cap binding here.
Molecular Insights into m7G Cap Recognition by eIF4E3.
With this structural information in hand, we analyzed the features that underlie cap binding in eIF4E3 (Fig. 3). As expected from sequence alignment with eIF4E1 and the detection of multiple intermolecular NOEs from its aromatic side chain, Trp98 forms extensive contacts with m7GDP in a similar manner to the equivalent residue, Trp102 in eIF4E1, i.e., in an almost parallel arrangement. Cys52, which replaces the second aromatic residue in other eIF4E subfamilies (e.g., Trp56 in eIF4E1), makes contacts with the purine ring and the methyl group of m7GDP, although clearly less extensively than observed for Trp56 in eIF4E1. Instead, eIF4E3 recruits residues on the preceding S1–S2 loop to contribute to cap binding: specifically, the side chain of Ser43, the backbone of Leu44 and Pro45, which contact the sugar, whereas backbone and side chain contacts are observed for Ala47 and Ala49 with the purine ring. Additional contacts are provided by the C terminus, which is disordered in apo–eIF4E3, from residues His197 and Ala199. In contrast, the structure of eIF4E1 shows very few contacts outside the Trp sandwich (Fig. 3D): in fact, both the S1–S2 loop and the C-terminal region point away from the m7G cap in eIF4E1, making no obvious contacts with the m7G cap. Indeed, the equivalent C-terminal residues in eIF4E1 (Fig. 1A) form a loop connecting the β7 and β8 strands, the latter of which cannot be formed in eIF4E3 because these residues are missing. It is possible that the lack of conformational restraints for these residues in eIF4E3, by not forming the β8 strand, allows it to adopt conformations to bind the cap moiety. ITC on eIF4E3 mutants (e.g., Ser43Gly, Ala47Gly, Ala49Gly, His197Ala) confirms that these regions are important to the eIF4E3–m7G cap interaction (Table 1). Additionally, these residues are highly conserved in the eIF4E3 family but not in eIF4E1 or eIF4E2 families (in yellow, Fig. 1A). Thus, eIF4E3 recruits additional contacts to compensate for the loss in binding energy in the absence of the second aromatic residue and associated π-packing.
The electrostatic potential map of the eIF4E3 complex (Fig. 3 E and F) shows the purine pocket is very negatively charged (with contributions from the π cloud of Trp98 and backbone carbonyl oxygens from the S1–S2 loop and the C-terminal arm). This is likely important for forming favorable interactions with the partial positive charge on the m7G base, and therefore a determinant for differentiating m7G versus unmethylated guanine binding. We also note an extensive area of positive charge at the phosphate binding region, which will contribute to complex stabilization.
Thr48 acts as an Ncap for the small α1−2 helix that includes Cys52, stabilizing the helix by formation of a hydrogen bond from its side chain with the backbone of Cys52 and/or Glu51 (both are possible in our ensemble of structures). Although no direct contacts with m7GDP are observed with the side chain of Thr48, a Thr48Ala mutant reduces affinity for m7G by ∼20-fold, similar to the Cys52Ala mutation (Table 1). It is likely that this mutant, where alanine cannot act as an Ncap, destabilizes helix formation, which in turn is important for positioning Cys52 and the S1–S2 loop for favorable interactions with m7GDP. All cap-bound structures of eIF4E1 show that Trp56 (equivalent to Cys52) forms a small helix, but this helix is not present in the apo structure (8). Preforming of this helix in apo–eIF4E3 likely makes the cap interaction more energetically favorable. Interacting residues in the eIF4E3 cap binding S1–S2 loop, including the Ncap Thr48, are highly conserved in the eIF4E3 family, underscoring its particular importance in eIF4E3 cap recognition (Fig. 1A and Fig. S1).
Comparison with Other Cap Complexes.
Other non-eIF4E cap-binding proteins, e.g., CBC (4) and VP39 (26), bind the cap via cation-π or π–π stacking arrangements using two aromatic residues similarly to other eIF4E family members. Indeed, there are only two examples of specific cap-binding proteins that chelate cap via a strategy different to the aromatic sandwich. In reovirus polymerase λ3, the m7G is sandwiched between two largely aliphatic side chains, yet specificity is retained for the m7G cap relative to guanosine (27). In the decapping scavenger enzyme DcpS, the m7G base is stacked between Tyr175 and Leu206 (28). Interestingly, the Leu206Ala mutation does not severely affect DcpS activity (28), adding additional complexity to understanding the basis of this interaction. Clearly, eIF4E3 provides a unique example of cap recognition. Here, a combination of features underlies specific cap recognition; these include electrostatic and van der Waals contributions. For instance, the highly conserved electrostatic features of the protein include negative-charged residues for m7G cap recognition, e.g., the Trp98 π-electron cloud and the carbonyls of the α1–2 helix with Cys52 and the C-terminal arm; as well as positive charges from Arg152, Lys192, and Arg84 for phosphate recognition. Thus, the basic charge features for specific interaction are provided by eIF4E3, but the manner by which these charges are distributed to the appropriate spatial position on the protein surface is different. Interestingly, eIF4E3 contacts are more extensive with the m7G cap than contacts made by other specific cap-binding proteins.
eIF4E3 Uses Its Cap-Binding Activity to Impair Oncogenic Transformation and Target Expression.
Given there were no previous reports on any aspect of the cellular function of eIF4E3, we sought to understand the biological implications for this cap recognition motif. First, we determined that eIF4E3 was endogenously expressed in cells and in primary human specimens. Expression of endogenous eIF4E3 was fairly restricted, being absent in many cell lines such as fibroblasts and U2OS cells, but present in hematopoietic cells such as in the acute myeloid leukemia (AML) cell lines THP-1 and KG1a as well as in primary normal human peripheral mononuclear cells, AML patient specimens, and other lineages (Fig. 4 and Fig. S4A). Further analyses of eIF4E1 and eIF4E3 suggested that levels of the two proteins were very similar in KG1a and THP-1 cells (Fig. S4 C and D). Significantly, cap chromatography experiments demonstrated that endogenous eIF4E3 bound the cap in these cells, where endogenous eIF4E1 is also present (Fig. 4A). In KG1a and THP-1 cells, nearly all of the eIF4E3 (90+%), and at the same time eIF4E1 (90+%), was bound to the cap column (Fig. 4B and Fig. S4D). Thus, eIF4E3 potentially competes with eIF4E1 for mRNAs in these cells. Finally, we examined the subcellular distribution of eIF4E3, as this would impact on its potential functions. We find that, like eIF4E1, eIF4E3 is in the nucleus and cytoplasm and thus could support roles in both mRNA export and translation (Fig. 4F and Fig. S4F).
Fig. 4.
Functional analysis of eIF4E3 activity. (A) Cap column chromatography with THP-1 and KG1a lysates. (B) NIH 3T3 cells stably overexpressing Myc-tagged eIF4E3 wild type, Trp98Ala (W98A) mutant, eIF4E1, or vector controls were used for cap column chromatography. Cap-bound and -unbound samples were analyzed by Western blot (WB), confirming that Trp98 is essential for cap recognition. (C) NIH 3T3 cells were analyzed by WB with indicated antibodies to assess effect of eIF4E3 expression on eIF4E1 targets. (D and E) Foci assays in NIH 3T3 cells stably transfected as indicated. Representative data from three independent experiments, all performed in triplicate, are shown in E. Error bars denote ±SD. (F) U2OS cells overexpressing Xpress-tagged eIF4E3 and appropriate vector control were analyzed by immunofluorescence in conjunction with confocal microscopy with indicated antibodies to assess eIF4E3 subcellular localization. (Magnification: 100×.)
eIF4E1 is a potent stimulator of cellular proliferation and oncogenically transforms cells in culture (1–3). Thus, we determined whether eIF4E3 had similar functions and whether these were dependent on its cap-binding activity. Cap chromatography on wild-type and Trp98Ala eIF4E3 confirmed our in vitro results that the Trp98Ala mutation abolishes cap binding (Fig. 4B and Fig. S4E), and thus this mutant was used to assess the relevance of cap activity to eIF4E3 function. A plethora of previous studies indicate that eIF4E1 specifically affects the expression of some mRNAs but not others (e.g., VEGF is an eIF4E1 target, whereas GAPDH is not) (29). This is thought to arise because of complex highly structured 5′-UTRs sensitizing these transcripts to eIF4E1 levels to better enable multiple rounds of translation initiation, and/or structured 3′-UTR elements involved in recruiting mRNA export machinery in the nucleus enabling transcripts to be eIF4E1 mRNA export targets (5, 29). Given there were no previously identified RNA targets for eIF4E3, we examined its ability to affect the expression of established eIF4E1 targets. Surprisingly, eIF4E3 overexpression decreased expression of all targets examined including VEGF, c-Myc, Cyclin D1, and NBS1 in U2OS and NIH 3T3 cells (Fig. 4C and Fig. S4G). In contrast, the Trp98Ala mutant had no or little effect on target protein levels, giving results similar to vector controls. eIF4E1 overexpression led to an increase in protein levels of these targets, as expected. At the same time, GAPDH, β-Actin, α-Tubulin, and Hsp90 were not altered by either eIF4E3 or eIF4E1, suggesting a similar specificity for these proteins (Fig. 4C and Fig. S4G and E).
Given this unexpected but striking finding that eIF4E3 repressed the expression of a pool of target mRNAs common with eIF4E1, we examined the oncogenic potential of eIF4E3 using anchorage-dependent foci formation assays in NIH 3T3 and U2OS cells. Consistent with its ability to repress target expression, eIF4E3 repressed even background levels of foci by approximately threefold (Fig. 4 D and E and Fig. S4E). This activity was cap dependent as the Trp98Ala mutant gave no repression relative to vector. By contrast, eIF4E1 overexpression led to increased foci relative to vector by approximately threefold and to eIF4E3 by approximately eightfold. To act in translation, eIF4E1 binds eIF4G and associated factors to recruit mRNAs to the ribosome. Our studies show that eIF4E3 does not associate with eIF4G in cells, and consistently, an eIF4G peptide binds eIF4E3 with 40-fold less affinity than eIF4E1 (20, 30). Analysis of the dorsal surface of eIF4E3 provides some insights into this reduced affinity (Fig. S5). Conserved residues that make key contacts to the peptide in eIF4E1, His37, Gln40, and Leu131, are replaced with Val, His, and Lys, respectively, in eIF4E3 (Fig. S5B). Structures of eIF4E1 and effector peptides reveal that residues spanning the small helix (α2′) in eIF4E1 contacts the longer peptide fragments in the N terminus (30). This helix in eIF4E3 points away from the peptide-binding site and constitutes one of the few conformational differences between eIF4E3 and eIF4E1 outside the cap-binding site. Furthermore, regions outside of the consensus binding site have been suggested to greatly increase the affinity of eIF4G for eIF4E1 (3). These interactions could also underlie the absence of interaction between eIF4E3 and endogenous eIF4G in cells. In summary, the reduction in affinity for eIF4G supports the notion that eIF4E3 is not involved in forming active translation complexes but rather forms inactive complexes sequestering the mRNA away from the active translation machinery. Thus, eIF4E3 represses target expression and oncogenic transformation, in direct contrast to eIF4E1, which stimulates these processes.
To address the clinical relevance of these findings, we monitored eIF4E3 mRNA levels in primary human blood specimens from healthy volunteers with normal eIF4E1 levels and M4/M5 AML patients with elevated eIF4E1 levels (11, 13, 31). Here, we observe that eIF4E3 mRNA levels are reduced by 3- to 10-fold in M4/M5 AML patients relative to healthy volunteers (Fig. S4B). These observations are consistent with a model whereby M4/M5 AML is characterized by not only elevation of the oncogenic activity of eIF4E1 but also with the concomitant loss of the repressive activity of eIF4E3. In summary, our data strongly suggest that eIF4E3 is, unexpectedly, a tumor suppressor and that it requires its atypical mode of cap recognition for this function.
Discussion
In conclusion, eIF4E3 interacts with the m7G cap uniquely dispensing with the traditional aromatic sandwich, but yet still discriminates between m7G and guanosine with at least a 1,000-fold greater affinity for the cap. In fact, eIF4E3 binds to m7GTP with an equal affinity to that reported for eIF4E2 (32), indicating that this atypical cap-binding strategy can be as effective as the use of an aromatic sandwich. eIF4E3 uses multiple distinct regions to form a pocket defined by Trp98, the aliphatic and backbone residues in the S1–S2 loop including Cys52, and the C terminus. Together, these provide the necessary negative charge to associate with the partially positively charged m7G moiety (Fig. 3F). This recognition strategy is not found in other cap-binding proteins to date but does suggest that the cap can be specifically recognized by broader structural scaffolds than previously thought. This cap-binding activity is functionally relevant and underlies, at least in part, the repressive activities of eIF4E3 we identified in cells. Our data strongly suggest that eIF4E3 is a cap-dependent tumor suppressor. In support of this, recent studies relate the loss of eIF4E3 to the malignant phenotype in oral cancers (33), and we observe a reduction of eIF4E3 in M4/M5 AML patients relative to healthy volunteers, whereas eIF4E1 is 3- to 10-fold more elevated here than in healthy volunteers (13). In cells, it is possible that binding key partner proteins increases the affinity of eIF4E3 for the cap, and also perhaps for other elements in the 5′-UTR, enabling it to more efficiently compete and thereby suppress eIF4E1 activity. For instance, the 5′-UTR elements may modulate interactions with eIF4E1 and binding factors (34), whereas other factors such as eIF4G increase the cap affinity of eIF4E1, which further increases with addition of PABP (3). Also, the C terminus in eIF4E3 undergoes large changes upon cap binding; this region could be involved in RNA recognition beyond the cap or with protein cofactors. Thus, in addition to the atypical cap binding we observe, association with other proteins or additional RNA elements could increase cap affinity to better enable competition with eIF4E1. This idea is supported by our findings that, although in vitro eIF4E3 has a lower affinity than eIF4E1 for the cap, in cells nearly all of the endogenous eIF4E3 is in the cap-bound fraction. This strongly suggests that factors in the cell increase the affinity of eIF4E3 for the cap in vivo. Further exploring these possibilities will be an important direction for future work. In summary, our studies strongly suggest that eIF4E3 uses a unique cap-binding activity to act as a tumor suppressor.
Materials and Methods
eIF4E3 protein (pET 15b vector) was purified from BL21 (DE3) cells. NMR studies were acquired at 600 MHz on a Varian INOVA spectrometer in 50 mM phosphate, pH 7.3, 100 mM NaCl, 100 μM tris(2-carboxyethyl)phosphine (TCEP), and 0.02% NaN3. For structure calculation, 3D NOESY spectra with a mixing time of 100 ms recorded at 800 and 600 MHz were used to obtain distance restraints. Structures were generated using the torsion angle dynamics program CYANA 2.1 (22). Coordinates and structural data have been deposited in the Protein Data Bank (PDB) with ID codes 4B6V (apo–eIF4E3) and 4B6U (m7GDP–eIF4E3). ITC was performed with a Microcal VP-ITC calorimeter, operating at 20 °C. The data were analyzed with Origin software. The different mutants were verified by NMR (Fig. S6). Standard methods for cell culture and transfections were used, as described (5). Immunoprecipitation was as follows: anti-Myc beads (Santa Cruz) were used to purify Myc-tagged eIF4E1, eIF4E3 wild type, or Trp98Ala mutant as in ref. 5. For anchorage-dependent foci assays, 500 cells were seeded per 10-cm plate for 14 d, and then stained with Giemsa (Sigma). For immunostaining, cells were fixed in paraformaldehyde, and results were visualized by confocal microscopy as previously described (5). RNA from primary human leukemia patients and normal bone marrow samples were isolated and analyzed as described by Assouline et al. (13). Detailed descriptions of protocols are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Quebec/Eastern Canada High Field NMR Facility. We are grateful to Drs. Pierre Thibault and Eric Bonneil (Proteomic Platform, Institute for Research in Immunology and Cancer). Research was supported by National Institutes of Health Grant R01 98571 and a Translational Research Program grant from the Leukemia and Lymphoma Society. The isothermal titration calorimetry studies were supported in part by a Natural Sciences and Engineering Research Council grant (to J.A.K.). K.L.B.B. holds a Canada Research Chair.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 4B6V (apo–eIF4E3) and 4B6U (m7GDP–eIF4E3)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216862110/-/DCSupplemental.
References
- 1.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: The role of the eukaryotic translation initiation factor eIF4E. Cell Cycle. 2007;6(1):65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 2.Graff JR, Zimmer SG. Translational control and metastatic progression: Enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20(3):265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- 3.von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol. 2004;11(6):503–511. doi: 10.1038/nsmb779. [DOI] [PubMed] [Google Scholar]
- 4.Calero G, et al. Structural basis of m7GpppG binding to the nuclear cap-binding protein complex. Nat Struct Biol. 2002;9(12):912–917. doi: 10.1038/nsb874. [DOI] [PubMed] [Google Scholar]
- 5.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175(3):415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell. 1997;89(6):951–961. doi: 10.1016/s0092-8674(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 7.Tomoo K, et al. Structural features of human initiation factor 4E, studied by X-ray crystal analyses and molecular dynamics simulations. J Mol Biol. 2003;328(2):365–383. doi: 10.1016/s0022-2836(03)00314-0. [DOI] [PubMed] [Google Scholar]
- 8.Volpon L, Osborne MJ, Topisirovic I, Siddiqui N, Borden KL. Cap-free structure of eIF4E suggests a basis for conformational regulation by its ligands. EMBO J. 2006;25(21):5138–5149. doi: 10.1038/sj.emboj.7601380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von der Haar T, et al. Folding transitions during assembly of the eukaryotic mRNA cap-binding complex. J Mol Biol. 2006;356(4):982–992. doi: 10.1016/j.jmb.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Quiocho FA, Hu G, Gershon PD. Structural basis of mRNA cap recognition by proteins. Curr Opin Struct Biol. 2000;10(1):78–86. doi: 10.1016/s0959-440x(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 11.Topisirovic I, et al. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23(24):8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen N, et al. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 2001;20(16):4547–4559. doi: 10.1093/emboj/20.16.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assouline S, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): A proof-of-principle clinical trial with ribavirin. Blood. 2009;114(2):257–260. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, et al. Design, synthesis and evaluation of analogs of initiation factor 4E (eIF4E) cap-binding antagonist Bn7-GMP. Eur J Med Chem. 2010;45(4):1304–1313. doi: 10.1016/j.ejmech.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi B, Cameron A, Jagus R. Characterization of mammalian eIF4E-family members. Eur J Biochem. 2004;271(11):2189–2203. doi: 10.1111/j.1432-1033.2004.04149.x. [DOI] [PubMed] [Google Scholar]
- 16.Joshi B, Lee K, Maeder DL, Jagus R. Phylogenetic analysis of eIF4E-family members. BMC Evol Biol. 2005;5:48. doi: 10.1186/1471-2148-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosettani P, Knapp S, Vismara MG, Rusconi L, Cameron AD. Structures of the human eIF4E homologous protein, h4EHP, in its m7GTP-bound and unliganded forms. J Mol Biol. 2007;368(3):691–705. doi: 10.1016/j.jmb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Hernández G, et al. Eukaryotic initiation factor 4E-3 is essential for meiotic chromosome segregation, cytokinesis and male fertility in Drosophila. Development. 2012;139(17):3211–3220. doi: 10.1242/dev.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, et al. Structural basis for nematode eIF4E binding an m(2,2,7)G-Cap and its implications for translation initiation. Nucleic Acids Res. 2011;39(20):8820–8832. doi: 10.1093/nar/gkr650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedzwiecka A, et al. Biophysical studies of eIF4E cap-binding protein: Recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol. 2002;319(3):615–635. doi: 10.1016/S0022-2836(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 21.Kentsis A, et al. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J Mol Biol. 2001;312(4):609–623. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J Mol Biol. 2002;319(1):209–227. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 23.Brunger A. XPLOR (Version 3.843): A System for X-Ray Crystallography and NMR. Yale Univ Press, New Haven, CT; 1996. [Google Scholar]
- 24.Zwahlen C, et al. Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: Application to a bacteriophage λ N-peptide/boxB RNA complex. J Am Chem Soc. 1997;119(29):6711–6721. [Google Scholar]
- 25.Fukuyo A, In Y, Ishida T, Tomoo K. Structural scaffold for eIF4E binding selectivity of 4E-BP isoforms: Crystal structure of eIF4E binding region of 4E-BP2 and its comparison with that of 4E-BP1. J Pept Sci. 2011;17(9):650–657. doi: 10.1002/psc.1384. [DOI] [PubMed] [Google Scholar]
- 26.Hodel AE, Gershon PD, Quiocho FA. Structural basis for sequence-nonspecific recognition of 5′-capped mRNA by a cap-modifying enzyme. Mol Cell. 1998;1(3):443–447. doi: 10.1016/s1097-2765(00)80044-1. [DOI] [PubMed] [Google Scholar]
- 27.Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage—structural studies of reovirus polymerase lambda3. Cell. 2002;111(5):733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 28.Gu M, et al. Insights into the structure, mechanism, and regulation of scavenger mRNA decapping activity. Mol Cell. 2004;14(1):67–80. doi: 10.1016/s1097-2765(04)00180-7. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996;93(3):1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell. 1999;3(6):707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- 31.Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011;25(7):1197–1200. doi: 10.1038/leu.2011.57. [DOI] [PubMed] [Google Scholar]
- 32.Zuberek J, et al. Weak binding affinity of human 4EHP for mRNA cap analogs. RNA. 2007;13(5):691–697. doi: 10.1261/rna.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi T, et al. cDNA microarray gene expression profiling of hedgehog signaling pathway inhibition in human colon cancer cells. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013054. e13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lall S, et al. Contribution of trans-splicing, 5′ -leader length, cap-poly(A) synergism, and initiation factors to nematode translation in an Ascaris suum embryo cell-free system. J Biol Chem. 2004;279(44):45573–45585. doi: 10.1074/jbc.M407475200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



