Abstract
Chronic drug administration induces neuroplastic changes within brain circuits regulating cognitive control and/or emotions. Following repeated pairings between drug intake and environmental cues, increased sensitivity to or salience of these contextual cues provoke conscious or unconscious craving and enhance susceptibility to relapse. To explore brain circuits participating in such experience-induced plasticity, we combined functional MRI with a preclinical drug vs. food self-administration (SA) withdrawal model. Specifically, two groups of rats were trained to associate odor cues with the availability of i.v. cocaine or oral sucrose, respectively. After 20 d of cocaine or sucrose SA followed by prolonged (30 d) forced abstinence, animals were presented with odor cues previously associated with or without (S+/S−) reinforcer (cocaine/sucrose) availability while undergoing functional MRI scans. ANOVA results demonstrate that a learning effect distinguishing S+ from S− was seen in the insula and nucleus accumbens, with the insula response reflecting the individual history of cocaine SA intake. A main effect of group, distinguishing cocaine from sucrose, was seen in the medial prefrontal cortex (infralimbic, prelimbic, and cingulate cortex) and dorsolateral striatum. Critically, only the dorsomedial striatum demonstrated a double dissociation between the two SA groups and learning (S+ vs. S−). These findings demonstrate altered cortico-limbic-striatal reward-related processing to learned, environment reward-associated contextual odor cues, which may serve as potential biomarkers for therapeutic interventions.
Keywords: context cues, neuroplasticity, drug addiction
Preventing or reducing the incidence of relapse remains the major challenge for the treatment of addiction, as recidivism rates can range as high as 90%. Both preclinical (1) and human addiction studies (2) have demonstrated that following repeated pairings with drug administration, various contextual and environmental stimuli when subsequently presented alone can elicit drug-seeking and drug-taking behaviors. Further, dysregulation within specific brain circuits regulating cognitive control and/or emotional regulation (3) can amplify the perceived salience of environmental contexts and cues that provoke craving (4) and thereby enhance susceptibility to relapse (2). These observations speak to the important role of reward learning in addictive processes (5). As such, it is critical to better understand the neural mechanisms and circuitry underlying the retrieval of cue-induced memories as an important intermediary in developing more efficacious interventions to decrease recidivism.
The amygdala, nucleus accumbens (NAc), and various prefrontal cortical regions respond to drug-associated cues in human cocaine addicts (6, 7). Supporting their critical roles in the neural circuitry of drug-seeking behavior, these regions have also been identified using preclinical reinstatement models (8, 9). For example, NAc neurons exhibit conditioned responses to drug-associated environmental stimuli even after prolonged drug abstinence, suggesting persistent alterations in reward-related information processing (10), whereas inactivation of the dorsal prefrontal cortex (PFC) or basolateral amygdala blocks cue-induced reinstatement (11).
During the development of drug addiction, drug-seeking behavior becomes progressively established as a maladaptive stimulus-response habit (12). The transition from goal-directed action to habit-induced response is accompanied by a processing shift from ventral to more dorsal striatal control over drug-seeking and -taking behavior (13). Inter- and intracircuit processing between the amygdala, the NAc, and ascending striatal circuitry link this system to the dorsal striatum (14), contributing to habit formation.
Preclinical studies have not as yet examined the neural circuitry underlying conditioned cue responses at a systems level. As such, we adapted a model of contextual cued drug self-administration (SA) and enforced abstinence coupled with functional MRI (fMRI) to simulate environmental cue-induced craving and relapse in humans. To control for motor behavior and nonspecific learning effect resulting from contextual cues (such as the operant chamber and housing light) and to investigate circuit neuroplasticity that is specific to distinct reward types, we included a group of identically treated rats trained to self-administer oral sucrose. Using fMRI, we tested the hypotheses that (i) cue responsivity circuitry can be elucidated in an anesthetized animal; (ii) variations in the emotional valence of learning would elicit differential responses in frontal cortex, amygdala, and ventral striatum, regions strongly implicated in appetitive and aversive learning (15); and (iii) selective and specific cue response circuitry will differentiate stimuli previously paired with drug vs. natural rewards.
Results
Behavioral Analyses.
Two groups of rats were trained for 20 d to SA either i.v. cocaine or oral sucrose in the presence of an odor (lemon or vanilla) in a discriminative cue conditioning paradigm. Subsequently, rats self-administered cocaine or sucrose for an additional 20 d in a 6 h, long-access (LgA) SA paradigm. Following ∼30 d of drug abstinence, rats were anesthetized and subjected to cue presentations while whole brain fMRI data were acquired (Materials and Methods).
Both SA groups learned to self-administer oral sucrose or i.v. cocaine and to behaviorally discriminate odors paired (S+) or not paired (S−) with reinforcer presentations (Figs. S1 and S2). As expected, the cocaine SA group also escalated their responding over the course of the 20 SA sessions (Fig. S3; SI Materials and Methods).
Olfactory Processing.
Odor stimuli produced distinct response patterns in the olfactory bulb following vanilla and lemon odor delivery, irrespective of whether they served as S+ or S− cues during SA training or were presented for the first time to the training naïve, housing control group (Figs. S4 and S5), suggesting that anesthesia did not preclude odor-specific sensory processing and prior exposure to the odors per se did not alter sensory reception and processing.
Main and Interaction Cue Responses in SA Groups.
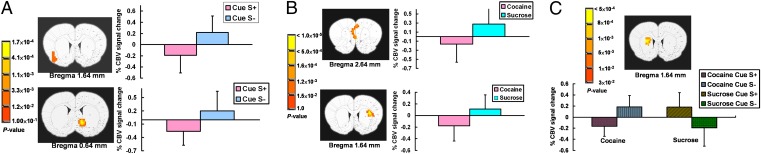
A 2 (group) × 2 (cue type; S+ or S−) whole brain ANOVA revealed main effects of group, cue, and a group × cue interaction. Linear mixed-effects (LME) model analysis of the main effect of learning (differential response between cues collapsing across SA groups) showed significant activation differences in insula and NAc to odors previously serving as S+ and S− (Fig. 1A). In both regions, the S+ vs. S− cues led to neuronal responses in the opposite direction. Exploratory post hoc analysis of this learning effect revealed a significantly greater response to the S+ cue in the NAc (two-sample t test, P = 0.01) but not the insula in the cocaine SA vs. sucrose group (Fig. S6). There was no difference between these two groups in responses to the S− cues in either area.
Fig. 1.
Learning (A), group (B), and learning × group interaction effects (C) revealed by a liner mixed effect model (LME) to olfactory stimulus presentation. Activation regions are overlaid onto a standard rat atlas to facilitate anatomical localization and show statistically significant differences in (A) response to S+ vs. S− in insula (spatial extent 10 voxels) and NAc (9 voxels), (B) difference between cocaine and sucrose groups in medial prefrontal (8 voxels) (infralimbic, prelimbick and cingulate cortices) and dorsal striatum (9 voxels), and (C) double dissociation response pattern in dorsal striatum (7 voxels) to S+ and S− in cocaine and sucrose groups. Note, spatial extent of significant clusters for Figs. 1 and 2 is based on a resampled voxel size of 0.14 × 0.14 × 1 mm3 for coregistration to a brain atlas and 0.14 × 0.14× 0.18 mm3 for coregistering to T1 images, respectively.
The main effect of group (collapsing across cue conditions) revealed two brain regions: the medial prefrontal cortex (mPFC, cingulate and prelimbic cortex) and dorsolateral striatum (DLS; Fig. 1B) wherein odor responses differentiated the two SA groups, manifest as a negative fMRI signal change in the cocaine SA group but positive fMRI signal response in the sucrose SA group. Finally, only one region, the dorsomedial striatum (DMS), demonstrated a significant learning × group interaction (Fig. 1C) and did so in a double dissociation fashion such that the cocaine SA animals showed negative fMRI signal change to the S+ cue and positive fMRI signal response to the S− cue, whereas the sucrose SA animals showed an opposite pattern of S+/ S− cue responses.
Spatiotemporal Processing of Cues and Relationship to SA History.
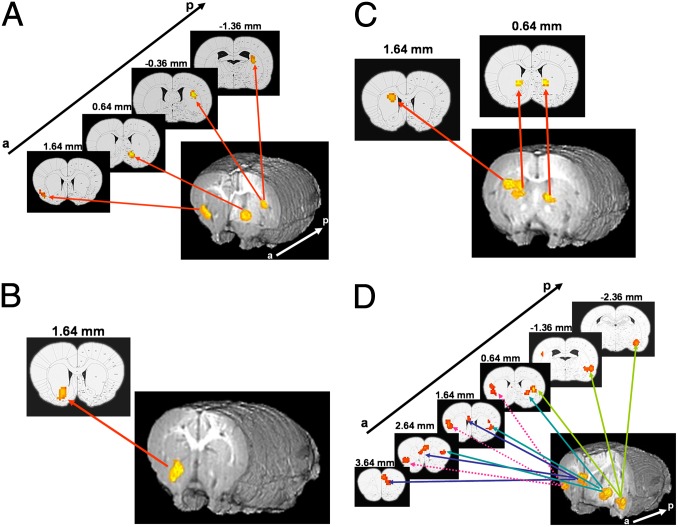
Because it is possible that conditioned cue responses might have different temporal profiles when paired to a natural or a drug reinforcer (16, 17), we reanalyzed our data into early (first minute) and late (second minute) phases of cue presentation using a separate 2 (learning) × 2 (group) ANOVA. LME analysis revealed a significant learning main effect in the insula, NAc, and dorsal striatum (Fig. 2A), a significant group main effect in the NAc (Fig. 2B), and a learning × group interaction in the DMS and central striatum during the first 1-min cue early phase (Fig. 2C). In contrast, whereas a group effect was seen in the prefrontal cortex, insula, striatum, and amygdala (Fig. 2D), no learning or interaction effects were seen when only considering the late phase. Table S1 summarizes the results from this whole brain temporal analysis. These data suggest that initial cue presentation engages processes mostly related to reward anticipation (regardless of reward specificity), whereas information about reward outcome (cocaine vs. sucrose) appears to be processed with an additional temporal delay.
Fig. 2.
Olfactory cue induced activation during early (A–C) and late (D) phases of cue presentation. Activation maps show statistically significant early (A) learning effect in insula (8 voxels), NAc (7 voxels), and dorsolateral striatum (11 voxels), (B) group effect in NAc (10 voxels), and (C) learning × group effect in dorsomedial (bregma, 1.64; 12 voxels) and central striatum (bregma 0.64, R/L 8/7 voxels), (D) Late group effects in mPFC (37 voxels), insula (27 voxels), dorsolateral striatum (19 voxels), and amygdala (16 voxels). The numbers above each panel indicate coronal slice location relative to bregma. Blue arrows indicate anterior (a) to posterior (p) localization. Each panel depicts both a rendered whole brain reconstruction and the corresponding single atlas slice for anatomical localization.
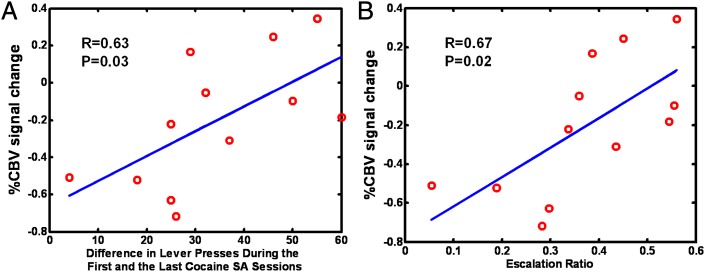
Finally, we assessed whether the neuronal responses seen were related to the amount of cocaine or sucrose obtained during the SA training. Only the neuronal response in insula, which demonstrated a significant learning main effect, predicted the amount of cocaine intake during the training sessions. Specifically, both the difference in cocaine intake (infusions during the last SA session minus the first SA session; P = 0.03; Fig. 3A) and the escalation ratio of cocaine SA (P = 0.02; Fig. 3B) were positively correlated with the S+ cue response, suggesting that this learning related neuronal readout may reflect the magnitude of addiction severity or cued conditioned strength. There were no correlations between neuronal activity and amount of sucrose intake.
Fig. 3.
Cue-associated insula response reflects prior drug exposure history. Scatter plots of percent rCBV signal change and (A) difference in cocaine intake during the last SA session minus that during the first SA session and (B) the escalation ratio of cocaine SA intake.
Discussion
Neuronal responses in the insula and NAc distinguished reward-associated odor stimuli previously paired during cocaine and sucrose SA (S+; Fig. 1A), regardless of reward type (i.e., a learning response) and did so with a greater neuronal response to S+ (vs. S−). In contrast, the odor response in the mPFC (prelimbic, infralimbic cortex, CG1/CG2) and DLS differentiated the cocaine- from the sucrose-associated olfactory stimuli (i.e., a group response), but did not distinguish S+ from S− (Fig. 1B). Intriguingly, only the DMS, an important convergent region linking reward pathway with those associated with cognitive processing and motor control circuits, demonstrated a group × cue response interaction, with the signal following the S+ and S− responses inverted between the sucrose and cocaine groups (Fig. 1C), suggesting a functional double dissociation calculation that produced a readout signaling both reward anticipation coupled with prediction of reward specificity or incentive salience (reward outcome, in this case, cocaine vs. sucrose). Finally, neuronal processing potentially related to reward anticipation and reward outcome appear to have distinct temporal profiles, with earlier cue processing in the NAc, DLS, and insula (Fig. 2 A and B) most sensitive to reward presence or absence (the difference between S+ and S−) regardless of reward type, whereas somewhat more delayed computations (Fig. 2D), mostly within the amygdala, insula, mPFC, and striatum, differentiated between reward types, i.e., cocaine vs. sucrose. Both the DMS and dorsal central striatum produced a learning × group odor discrimination response during the first minute of cue presentation (Fig. 2C). Finally, only the cue response magnitude in insula predicted the escalation of cocaine SA over the course of training (Fig. 3).
Cue Response as a Function of Learning.
Consistent with the extant literature, we observed learning-related cue responses (S+ vs. S−) in the NAc and insula (Fig. 1A). Single-unit recording in rodents and nonhuman primates have implicated ventral striatal neuronal encoding of stimulus–reward associations (18, 19) and cue-induced reward expectation (20, 21). Human functional neuroimaging data also demonstrate ventral striatal activity to cue presentation following appetitive and aversive Pavlovian conditioning (22). The NAc, under the influence of dopamine (DA) release from the ventral tegmentum and glutamatergic afferents from the PFC, amygdala, and hippocampus, integrates limbic information related to memory, drive, and motivation with the generation of goal-directed motor behaviors (23). This area is also activated by conditioned cues in rats undergoing a variety of extinction manipulations (24–26).
The insula, a structure recently implicated in addiction (27), was the only other region showing a differential response to S+ and S−. Projections from anterior insular cortex to ventral striato-pallidum and extended amygdala are thought to contribute to learned- and motivation-driven behaviors, especially those requiring emotional processing (28, 29). Human imaging studies suggest that the anterior insula provides a rich interoceptive foundation within which emotionally salient activity from forebrain regions, including the orbitofrontal, dorsolateral prefrontal, and anterior cingulate cortices may be integrated (27). Interestingly, greater activation to unexpected versus expected stimuli in anterior insula, ventral striatum, and orbitofrontal cortex is also seen in humans to taste stimuli (30), consistent with the observed signal difference in these same areas to S− (vs. S+) presentation. Recent theoretical models suggest that anterior insula together with cingulate forebrain regions process the relative salience of stimuli signaling to the organism to attend to external vs. internal homeostatically relevant stimuli (31). Our data support a common role for the insula in responding to learned environmental cues that signal both food and drug (32).
Cue Response as a Function of Group.
Cue responses in the mPFC and DLS discriminated the cocaine from the sucrose SA groups. The DLS is critical for both the acquisition and the expression of goal-directed behaviors (33). Moreover, the DLS, analogous to the putamen in primates, is involved in relatively inflexible response learning that is insensitive to outcome devaluation (34) a hallmark of drug addiction (35). Habit learning has been implicated in the transition from early drug use to the compulsive drug seeking that characterizes addiction (12, 13). Lesions of the DLS attenuate cue-controlled drug seeking (36). Pavlovian-instrumental transfer is thought to underlie the role of cues in drug seeking (37) and may reflect cortical–basal ganglia interactions. Our infralimbic–dorsal striatal cue response is consistent with the role these structures are thought to play in regulating habit behavior.
How might the dorsal striatum distinguish between cocaine vs. food reinforcers (38)? One possible mechanism is the more robust DA release (39, 40) and neuronal firing seen from drug- vs. non–drug-rewarded contexts. Distinct populations of NAc neurons differentially process information about goal-directed behaviors for cocaine versus natural reward (41, 42). Further, cocaine administration has been suggested to more rapidly accelerate the development of habits than that of a food reinforcer (13).
Differential cue responses as a function of group were also seen in the mPFC. A major aspect of addiction is the pathological narrowing of goal selection to those that are drug related to the exclusion or minimizing of natural or biological reinforcers, including food. Regions of the PFC are associated with the cognitive control that permits goal-directed behavioral selection via the active maintenance of goal representations (43). Phasic DA release (44) during repeated drug intake would likely produce neuroadaptations in the PFC, ventral, and dorsal striatum reflected in the observed differential cue response.
Double Dissociation of Learning and Reward Type.
Perhaps our most provocative finding is the DMS response that identified both the cued learned association (S+ vs. S−) and reward type (cocaine vs. sucrose) in a double disassociation fashion (Fig. 1C). The basal ganglia are known to integrate the learning and motivational processes through which actions are acquired, selected, and implemented to determine adaptive decision-making (45). In this context, our results suggest that DMS computes information (or receives partially or fully computed information) associated with reward anticipation and reward outcome values and that striatal neuroadaptations, perhaps manifest during the incubation period that followed prolonged cocaine and sucrose SA, may have altered cue information processing to differentiate responses to S+ and S− in the two SA groups.
That the same learning experience can result in differential synaptic changes in the DLS and DMS has been previously reported (33). The DMS, part of a circuit incorporating the mPFC, medial dorsal thalamic nucleus, and substantia nigra, mediates goal-directed actions that involve a DA-mediated reward process (45). In contrast, habits appear to be encoded in a network involving sensory-motor cortical inputs to the DLS. In addition, dual cortico–basal ganglia systems permit both parallel and integrative processing (14), such that convergence zones link reward pathway with those associated with cognitive function. A recent study by Murray et al. (46) provides further evidence suggesting differential roles for the DMS and DLS in goal-directed vs. habitual performance during cocaine-seeking behavior. Our data are highly consistent with the proposed distinct neural networks that mediate the acquisition of goal-directed actions and habits and the role of goal values and learned rewards in action selection (45).
Interaction Between Striatal and Other Circuits.
Our data are consistent with corticolimbic-striatal mechanisms associated with discriminative cue response to cocaine vs. natural rewards. The primate and rodent striatum has been organizationally described as cascading serial circuits that link the ventral medial with progressively more dorsal lateral regions of the striatum with midbrain DA neurons (12). This anatomical pattern has been hypothesized to underlie the development of addiction, as the pursuit of drugs become progressively less goal directed and more habitual, such that neuroadaptations that are initially restricted to more ventral striatal areas at the earliest stages of drug use progressively involve more dorsal and lateral striatal activity (12). Cocaine intake has been postulated to shift the balance of associative encoding between these two regions, leading to regionally specific effects on neural processing in striatum (47).
Our data suggest that NAc plays an important role in differentiating not only the conditional stimuli but also reward types, perhaps as a function of altered activity from dopaminergic and glutamatergic neurons projecting from VTA and amygdala/prefrontal cortex, respectively (48). This speculation is consistent with the differential response to cues in amygdala and prefrontal cortex in the cocaine and sucrose groups. The amygdala is particularly implicated in the acquisition, storage, and expression of emotional memories and in reward processing and stimulus–reward associations through its interactions with the ventral striatum (49, 50). The amygdala also projects to a variety of cortical–basal ganglia network structures implicated in the control of goal-directed action. Finally, the amygdala is necessary for encoding outcome values within the insula cortex (51), with these data then distributed to regions of prefrontal cortex and striatum to control action (52). Critically, these regions, including the prelimbic cortex and dorsal striatum, demonstrated differential olfactory cue responses in the cocaine and sucrose SA groups.
Early-Late Cue Responding.
Drug-related salient stimuli induce the reinstatement of drug seeking behavior in preclinical models of addiction, as human studies have consistently pointed toward the role of cues to enhance motivation to seek and take drugs, either via conscious or unconscious craving (13). Our data suggest that information readout following environmental cues have distinct temporal and regional profiles. The earlier cue readout (Fig. 2 A–C) involving the NAc, dorsal striatum, and insula may reflect the presence/absence of a reward, with the NAc also processing reward specificity. Contemporaneously, the DMS may calculate an interaction between learning and motivational processes into performance. In contrast, relatively delayed computations (Fig. 2D) within the amygdala, insula, mPFC, and striatum may signal reward types. These regional cue responses are consistent with a priming model that connectivity among the amygdala, ventral striatum, and anterior cingulate cortex could be a core neural circuit of drug cue- and stress-related drug reinstatement (53).
Limitations and Technical Considerations.
Rats in this study were anesthetized and thus unable to demonstrate outcome selection to cue presentation (although they demonstrated odor discrimination during earlier SA training; Fig. S2). Thus, the implications and behavioral relevance of our findings await confirmation in a behaving model. Nevertheless, several observations speak to the minimal role played by anesthesia in our cue responses and the integrity of the model. For example, anesthesia did not preclude olfactory bulb activation to odor presentation. Indeed, a similar pattern of activation was seen for both odors (lemon and vanilla), whether they served previously as S+ or S− in SA-trained rats or were presented for the first time in naïve animals (Fig. S4). Anesthesia does not seem to significantly alter brain networks revealed during the resting state (54, 55), and our whole brain ANOVA analysis produced brain regions compellingly consistent with those seen in awake, behaving models of drug addiction.
It should also be noted that our naïve, handling control group was not perfectly matched to our SA treatment groups in that animals were not subjected to sham catheter surgery (nor was the sucrose SA group) nor did they receive odors before imaging. However, the many months between catheter surgery, SA training, and subsequent imaging should have been sufficient to preclude surgery-related issues.
The above comments on temporal difference processing of reward anticipation and outcome must be tempered by the very different time scales between the fast, single cell electrophysiology signal and that of the slower and more delayed fMRI response measured herein (56). Although the precise relationship of the fMRI signal has yet to be causally attributed to a neuronal computation leading to a behavioral response, it should be noted that it has been repeated shown to correlate with human subject processing of perceived reward value and incentive salience (57, 58).
An additional unanswered question relates to the neurobiological significance of the regional cerebral blood volume (rCBV) response polarity. The NAc learning effect was manifest as reduced rCBV to the cue, which may reflect inhibition of NAc neural activity (59). However, interpretation requires caution as many neural and nonneural factors may contribute to the hemodynamic signal (60–62).
Conclusion
In conclusion, using fMRI and a preclinical SA + incubation model of drug addiction, we provide evidence of differential responding to reward-associated olfactory cues in rats with a history of chronic cocaine and sucrose SA along with a functional interaction of information processing in a cortico-limbic-striatal system involved in goal-directed rewarded and instrumental behavior. We demonstrate that several components of this system are coactivated by cues to both potentially anticipate reward and encode reward outcome values, presumably as a result of convergence of distinct cortico-striatal projections. Our data suggest that cues associated with cocaine-reinforced SA behavior engage motivation-related brain circuits in the ventral and dorsal striatum and mPFC that are also activated by natural reward associated cues, although the neuronal encoding appears distinct.
Materials and Methods
Subjects.
Forty male 12-wk-old Long-Evans rats (Charles River Laboratories) underwent surgical procedures, behavioral training, and 11–17 d of drug abstinence at the University of Pennsylvania. Animals were then transferred to the National Institute on Drug Abuse, Intramural Research Program, where they were maintained for an additional period (total abstinence period, 30–34 d), followed by brain imaging procedures. All food, caging, bedding, and the reverse light–dark cycle were identical at the two facilities. Protocols were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the University of Pennsylvania and the NIDA-IRP.
Cocaine SA.
Following jugular catheter implantation, animals in the cocaine SA group (n = 12) were trained to self-administer cocaine (0.75 mg/kg per infusion) in daily 1-h sessions under a fixed-ratio 1 (FR1) schedule of reinforcement for 4 d. Before placement of an animal into the chamber each day, a contextual odor cue (serving as a S+) in the form of a cotton swab soaked in either a lemon or a vanilla scent (Pure Lemon Extract and Pure Vanilla Extract; McCormick) was inserted underneath the chamber floor; one half of the animals receiving the lemon and half received the vanilla as their S+. Each lever press was followed by delivery of a cocaine infusion, which was paired with a 7-s illumination of the lever light. An 8-s time-out period followed each reinforced lever press, during which responding was recorded but not reinforced (see SI Materials and Methods for details of surgery and SA apparatus).
Odor Discrimination Training.
After the initial 4 d of cocaine SA training, animals were switched to an odor discrimination training phase consisting of daily S+ sessions (7 d/wk for 2 wk) identical to the previous training sessions, except that cue lights were now no longer presented. In addition, an S− session was conducted each day 15 min before or after the S+ session.
Odor Discrimination Test Sessions.
Odor discrimination performance was assessed in two extinction test sessions conducted on successive days (one S+ and one S− odor session). Animals were placed in the operant chamber with the S+ or the S− odor and allowed to press the lever for 15 min, during which time the lever presses were recorded but not reinforced. The order of the S+ and S− sessions were counterbalanced across animals.
Long-Access SA Sessions.
Following discrimination sessions, the duration of daily SA sessions was extended to 6 h/d (referred to as LgA sessions). Animals were trained under LgA sessions 5 d/wk with the S+ odor for an additional 20 d. To maintain the discrimination, rats also received S− odor training for 15 min for 2 d/wk.
Sucrose and Housing Control Groups.
A separate group of rats (n = 14), serving as a positive control, was trained to self-administer sucrose instead of cocaine. A third group (n = 12) of animals served as a negative control group and remained in the colony room. They were not exposed to the operant chambers or operant procedures but were otherwise handled and treated as the two SA groups.
MRI Experiments.
Rats were anesthetized with 2% isoflurane in a 1:1 mixture of O2:air, and both femoral veins and one femoral artery were catheterized for drug delivery and monitoring arterial blood gases and blood pressure, respectively (SI Materials and Methods). They were then intubated and placed on artificial ventilation. Intravenous propofol (35 mg/kg/h) was initiated and used to maintain a stable anesthetic level throughout MRI data acquisition.
Scanning Procedures.
MRI experiments were performed on a Bruker Biospin 9.4-T scanner (Bruker) equipped with an active-shielded gradient coil. A birdcage coil driven in linear mode was used for radio frequency excitation, and a single-turn circular surface coil (2.5 cm in diameter) was used for signal reception (55). Anatomical localization of slices was standardized using T2-weighted images (T2WI) with a rapid acquisition with relaxation enhancement (RARE) sequence. The anterior commissure (−0.36 mm from the bregma) was used as the anatomical fiducial for standardizing slice localizations. Cerebal blood volume (CBV)-weighted data were acquired following i.v. administration of 20 mg/kg iron-oxide contrast agent, a dose shown to enhance the sensitivity of and offset the positive fMRI effect in CBV-weighted fMRI signals (63). A fast low-angle single shot gradient-echo imaging sequence was used with the following parameters: field of view = 3.0 cm, matrix size = 64 × 64 (in-plane resolution of 470 μm), slice thickness = 1.5 mm, number of slice = 15, flip angle = 90°, repetition time = 240 ms, and echo time = 4.5 ms. A total of 80 time points were collected with a temporal resolution of 15.36 s.
Experimental Paradigm.
Each rat was exposed to both S+ and S− odors in a single MRI session, which consisted of 10 2-min epochs. Two S+ and two S− epochs were delivered consecutively, separated by two 2-min epochs of pure air. The order of S+ and S− presentation was counterbalanced, giving two vanilla scent presentations always first and two lemon scent presentations second. The odor and/or air were delivered at a rate of 1 L/min through two pieces of tubing placed bilaterally within the nares and connected to a custom-built MRI compatible flow olfactometer. Fig. S7 shows details of the experiment and the stimulus presentation.
CBV Data Analysis.
Image analysis was performed offline using analysis of neuroimages (64). Images were first motion corrected by registering each 3D volume to a base volume and then manually registered onto a common 3D space to facilitate group comparisons. Before linear model fitting, the time series at each voxel was low-pass filtered to eliminate high-frequency noise and removal of linear and quadratic trends. A Gaussian filter of 0.6 mm full width at half maximum was applied for spatial filtering. Session baseline data (i.e., the first eight time points consisting of air before odor presentations) were averaged to represent the baseline signal, which was subsequently subtracted from the images obtained during S+, S−, or air epochs at each time point on a pixel-by-pixel basis.
Iron oxide particles were used as a long half-life intravascular contrast agent. After infusion, the paramagnetic property of the contrast medium causes local magnetic field changes that result in a decrease in the relaxation time and, in turn, leads to reduced signal intensity. For example, an increase in CBV will induce an increase in the intravoxel contrast agent and consequently a decrease in MRI signal. In the present study, the percent rCBV change (% ∆rCBV) is thus defined as a negative fMRI signal change:
where S is the measured signal, and S0 is the signal from baseline epoch.
Statistical Analysis.
We used a general linear model (GLM) with a boxcar design representing the 2-min on- and off-periods to fit individual signal time series. Before the GLM, we first corrected for signal drift and subtracted the preliminary baseline value (i.e., the averaged value of the first eight time points consisting of air before odor presentations) from the fMRI raw time series and normalized the signal changes by dividing by the preliminary baseline value. Further, the baseline of the periods in between odors was modeled by using a polynomial function, which models the baseline with a constant value, a linear drift, and a higher order-shaped curve (quadratic, cubic and quartic) baseline, which captures most signal drift in each scan run.
The voxelwise average fMRI signal change (β value) amplitude produced by each odor condition was determined relative to baseline and submitted to a group level analysis. The statistical clusters were resampled to 0.14 × 0.14 × 1 mm3 for coregistration to a brain atlas (65) and 0.14 × 0.14× 0.18 mm3 for coregistering to T1 images. A cluster-based analysis (Monte Carlo simulation) was applied to account for multiple comparisons. All of the group statistical maps below were thresholded at a whole brain, multiple comparison-corrected α level of P ≤ 0.05 using a voxelwise (cluster size, seven voxels) α of P = 0.05.
To confirm that the scent was appropriately delivered and neuronally processed in this anesthetized preparation, we first examined brain activation following olfactory stimuli using a 2 (scent: vanilla and lemon) × 3 (group: control, cocaine, and sucrose self-administering) ANOVA. To account for learning, two-sample t tests were conducted in the cocaine and sucrose SA groups for S+ (lemon S+ vs. vanilla S+) and S− (lemon S− vs. vanilla S−) stimuli.
In subsequent statistical analysis in the cocaine and sucrose SA groups, two contrasts acquired during S+ (vanilla or lemon) and S− (vanilla or lemon) presentation were used. Because half of each SA group was trained with lemon and half with vanilla as S+ (the other odor serving as the respective S−), data from each group (cocaine or sucrose) under the same condition (S+ or S−) but with different scent stimuli (vanilla or lemon) were combined for subsequent statistical LME model analysis (Fig. S7A). LME modeling consisted of 2 group (cocaine vs. sucrose) × 2 learning conditions (S+ vs. S−).
To examine potential temporal differences in the response between sucrose- and cocaine-related stimuli that might be reflected in the relatively long-duration odors, data from each 2-min odorant presentation were separated into an early (first minute) and late (second minute) phases (Fig. S7B).
The relationship between rCBV changes and SA behavior was evaluated using the Pearson correlation coefficient in regions showing significant statistical effect from the LME analyses. Three separate metrics were used to quantify cocaine and sucrose SA behavior: (i) the difference in intake during the last minus the first SA session (D); (ii) escalation ratio, defined as D/intake during the last SA session; and (iii) the sum of all SA sessions.
Supplementary Material
Acknowledgments
We thank Dr. Anna Rose Childress for her helpful comments and insights on the study. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), and by an NIH Director’s Bench-to-Bedside grant (to L.P. and E.A.S.). The project was also partially supported by the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania and National Center for Research Resources Grant UL155024134.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207531110/-/DCSupplemental.
References
- 1.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 4. Childress AR, et al. (2008) Prelude to passion: Limbic activation by “unseen” drug and sexual cues. PLoS One 3(1):e1506. [DOI] [PMC free article] [PubMed]
- 5.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 6.Garavan H, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 7.Kilts CD, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35(2):276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27(13):3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1-2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 12.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 14.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9(2):223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 16.Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(24):10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olofsson JK, Bowman NE, Khatibi K, Gottfried JA. A time-based account of the perception of odor objects and valences. Psychol Sci. 2012;23(10):1224–1232. doi: 10.1177/0956797612441951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opris I, Hampson RE, Deadwyler SA. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: Categories with different activations. Neuroscience. 2009;163(1):40–54. doi: 10.1016/j.neuroscience.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fudge JL, Emiliano AB. The extended amygdala and the dopamine system: another piece of the dopamine puzzle. J Neuropsychiatry Clin Neurosci. 2003;15(3):306–316. doi: 10.1176/jnp.15.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: Evidence from an autoshaping paradigm. Eur J Neurosci. 2006;23(5):1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- 21.Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89(5):2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 22.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 23.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 24.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kufahl PR, et al. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63(10):823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotsenpiller G, Horak BT, Wolf ME. Dissociation of conditioned locomotion and Fos induction in response to stimuli formerly paired with cocaine. Behav Neurosci. 2002;116(4):634–645. [PubMed] [Google Scholar]
- 27.Goldstein RZ, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD. The sentient self. Brain Struct Funct. 2010;214(5–6):563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25(50):11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J Neurosci. 2011;31(41):14735–14744. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5-6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 34.Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 36.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30(46):15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 41.Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23(35):11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koya E, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12(8):1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27(15):4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 45.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37(11):2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Front Integr Neurosci. 2007;1:1–10. doi: 10.3389/neuro.07.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow ND, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter MGME, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez DR, Savage LM. Differential involvement of the basolateral amygdala, orbitofrontal cortex, and nucleus accumbens core in the acquisition and use of reward expectancies. Behav Neurosci. 2007;121(5):896–906. doi: 10.1037/0735-7044.121.5.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive memory. J Neurosci. 2000;20(23):8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 54.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 55.Lu H, et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104(46):18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 57.Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29(50):15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 59.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9(4):569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 60.Shih YY, et al. A new scenario for negative functional magnetic resonance imaging signals: Endogenous neurotransmission. J Neurosci. 2009;29(10):3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30(3):700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron. 2012;76(3):629–639. doi: 10.1016/j.neuron.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4T. Magn Reson Med. 2007;58(3):616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- 64.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 65.Lu H, et al. Registering and analyzing rat fMRI data in the stereotaxic framework by exploiting intrinsic anatomical features. Magn Reson Imaging. 2010;28(1):146–152. doi: 10.1016/j.mri.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.