Abstract
Membrane fusion along the endocytic pathway occurs in a sequence of tethering, docking, and fusion. At endosomes and vacuoles, the CORVET (class C core vacuole/endosome tethering) and HOPS (homotypic fusion and vacuole protein sorting) tethering complexes require their organelle-specific Rabs for localization and function. Until now, despite the absence of experimental evidence, it has been assumed that CORVET is a membrane-tethering factor. To test this theory and understand the mechanistic analogies with the HOPS complex, we set up an in vitro system, and establish CORVET as a bona-fide tether for Vps21-positive endosome/vacuole membranes. Purified CORVET binds to SNAREs and Rab5/Vps21-GTP. We then demonstrate that purified CORVET can specifically tether Vps21-positive membranes. Tethering via CORVET is dose-dependent, stimulated by the GEF Vps9, and inhibited by Msb3, the Vps21-GAP. Moreover, CORVET supports fusion of isolated membranes containing Vps21. In agreement with its role as a tether, overexpressed CORVET drives Vps21, but not the HOPS-specific Ypt7 into contact sites between vacuoles, which likely represent vacuole-associated endosomes. We therefore conclude that CORVET is a tethering complex that promotes fusion of Rab5-positive membranes and thus facilitates receptor down-regulation and recycling at the late endosome.
Keywords: endolysosomal system, Rab GTPase
The endocytic pathway of eukaryotic cells consists of highly interconnected organelles. Early endosomes form a hub for several pathways. These hubs receive cargo from the plasma membrane, such as ligand-bound receptors, which are sorted back to the surface after being discharged from associated ligands. Alternatively, receptors and transporters may be ubiquitinated at the plasma membrane and then sorted to the lysosome for degradation. In addition, Golgi-derived vesicles transport selected cargo, such as lysosomal hydrolases or membrane proteins, to the early endosome. To bring cargo to the lysosome, early endosomes mature into late endosomes (1, 2). This process is highly coordinated, and requires: (i) sorting of ubiquitinated cargo into intraluminal vesicles, (ii) recycling of receptors that were initially bound to hydrolases, and (iii) a replacement of the fusion machinery to enable the mature endosome to fuse with the lysosome. For each of these processes, dedicated machineries have been identified. Sorting of cargo into the lumen requires five ESCRT (endosomal sorting complex required for transport) complexes, whereas recycling of receptors occurs via the retromer complex (3, 4).
Fusion of mature endosomes with the vacuole depends on initial tethering, mediated by the Rab7 GTPase Ypt7 and the HOPS (homotypic fusion and vacuole protein sorting) complex, and subsequent SNARE-driven bilayer mixing (5, 6). Ypt7, like any Rab, is a switch-like protein that binds to its effector only in the active GTP-form. Because Rabs are poor catalysts, nucleotide exchange via a guanine nucleotide exchange factor (GEF) is necessary to generate Ypt7-GTP (7, 8). We recently identified the Mon1-Ccz1 complex as an endosomal GEF complex that activates Ypt7 (9), in agreement with Mon1/SAND-1 function in metazoan cells (10–12). Active Ypt7 then drives fusion of late endosomes with the vacuole (5, 9). This reaction requires HOPS, a hexameric tethering complex (13, 14). HOPS has four subunits that are shared with the endosomal CORVET (class C core vacuole/endosome tethering) complex. This class C core complex consists of Vps11, Vps16, Vps18, and Vps33 (15). The Munc18/Sec1-like Vps33 likely binds to SNAREs (16, 17). We recently solved the overall structure of HOPS and could show that the Rab-specific subunits Vps39 and Vps41 are localized at opposite ends of a seahorse-like structure, suggesting that HOPS bridges Ypt7-positive membranes before their SNARE-mediated fusion (6), in agreement with previous in vitro data (18, 19).
Less is known about the homologous hexameric CORVET complex. CORVET is found at endosomes, is required for efficient endocytosis, and binds Vps21, presumably via its Vps3 and Vps8 subunits (20–25). Vps3 has high homology to Vps39 and can compete with Vps39 for Vps11 binding, whereas Vps8 is homologous to Vps41 (20, 22, 26). Even though structural data are lacking, it is likely that Vps3 and Vps8 are present at opposite ends on CORVET, similar to the arrangement found in HOPS (6).
We recently showed the CORVET function is required in an independent reaction at the endosome (23). In the absence of CORVET subunits, endosomes have a defect in protein sorting along the endocytic pathway, endosomal biogenesis, and ESCRT function, likely because of insufficient endosome-endosome fusion (23, 27). To analyze CORVET function beyond localization, we purified CORVET to test its function directly. Our data now show that CORVET is a Vps21-dependent tethering factor that promotes tethering and fusion of Rab5/Vps21-positive membranes.
Results
Purification and Characterization of CORVET.
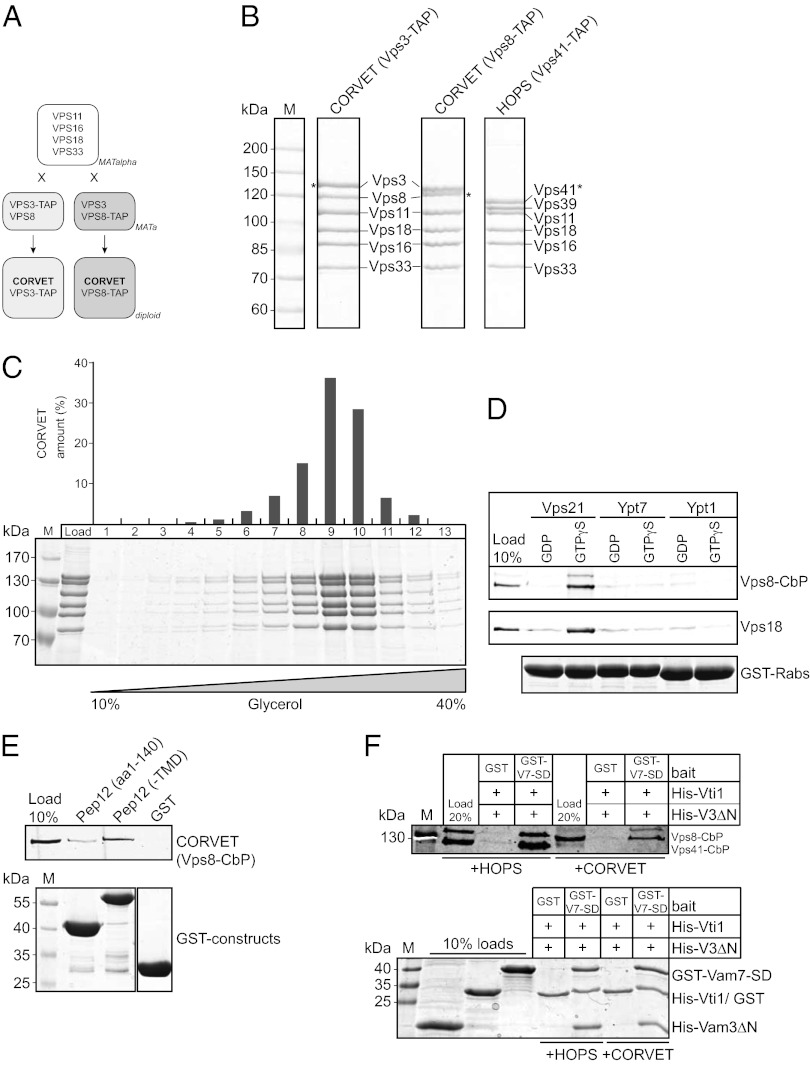
Previous assays on CORVET interaction relied largely on cellular lysates from wild-type or mutant strains (20–22). Because intermediate complexes between HOPS and CORVET exist (20, 26), it was not clear if indeed CORVET or just one subunit was analyzed under these conditions. We therefore decided to isolate and analyze CORVET from an overexpression strain to overcome these limitations (Fig. 1A). We purified CORVET via a C-terminal tandem affinity purification (TAP) tag on the CORVET-specific Vps3 and Vps8 subunits and obtained the purified hexamer, very similar to the previously characterized HOPS complex (Fig. 1B). We did not find any evidence for contaminations with HOPS subunits. To determine its size, we applied the purified complex to glycerol gradients and recovered CORVET in fractions 9 and 10 (Fig. 1C), which correspond to the molecular weight of about 700 kDa (6). Our overexpressed complex thus has the same stoichiometry and mass as the previously purified native CORVET (20).
Fig. 1.
Purification and characterization of CORVET. (A) Construction of CORVET overexpression strains. Overproduction strains with a C-terminal TAP-tag on the CORVET specific subunits were created by mating of haploid strains overexpressing the class C core proteins (MATalpha) or tagged CORVET specific subunits (MATa), respectively. (B) Tandem affinity purification of CORVET and HOPS. Subunits of the Vps tethers were co-overexpressed and purified from yeast lysate with the TAP method, using Vps3, Vps8 (CORVET), and Vps41 (HOPS) as bait proteins (for details see Materials and Methods). Next, 2.5% of the eluates were mixed with SDS sample buffer, resolved on gradient 7.5% SDS/PAGE gels, and stained with Coomassie. Asterisks (*) indicate subunits with remaining calmodulin-binding peptide (CbP). (C) Purification of CORVET via a glycerol gradient. The CORVET complex was isolated via TAP-tagged Vps8 (as described in B), and the TEV-eluate was applied on a 10–40% (wt/vol) gradient. Fractions were harvested, TCA precipitated, and analyzed by SDS-PAGE and Coomassie staining. A quantification of the protein amount is shown above the SDS-PAGE. (D) Purified CORVET interacts with Vps21-GTP. GST-Rab fusion proteins of Vps21, Ypt7, and Ypt1 were loaded with the respective nucleotides, bound to GSH beads, and used for the Rab pull-down experiment. CORVET was purified from overexpression strain via the TAP-tagged Vps8 subunit (as shown in B and C) and added to immobilized Rabs. After a 1.5-h incubation, eluted proteins and GST-Rabs were analyzed by SDS/PAGE and Western blotting against the CbP-tag (Vps8) and Vps18. (E) Interaction of CORVET with the endosomal SNARE Pep12. Purified CORVET (B–D) was added to immobilzed (SNARE-) GST constructs. Samples were incubated for 2 h, then washed and eluted by boiling in SDS sample buffer. Proteins were analyzed as described in D. (F) CORVET interacts with assembled vacuolar SNAREs. GST-Vam7 SNARE domain (GST-V7-SD) or GST were incubated with His-Vti1 and His-Vam3∆N (His-V3∆N) in the presence of CORVET or HOPS for 2 h. After coupling to GSH beads and washing, the assembly of the SNAREs was confirmed by SDS-PAGE and Coomassie staining (Lower), whereas CORVET (and HOPS) binding was shown by Western blotting (Upper) and antibody decoration against the Calmodulin peptide (on Vps8 in CORVET and on Vps41 in HOPS).
As with HOPS, we expected that CORVET would be able to bind SNAREs and its respective Rab Vps21. To analyze the binding to Rabs, we applied purified CORVET to GST-Rabs that were preloaded with GDP or GTP, and observed a specific interaction only with Vps21-GTP (Fig. 1D), as shown by the signals of the structurally critical Vps18 and Vps8 proteins. In addition to Rabs, CORVET should also interact with SNAREs, most likely via its Vps33 subunit (16, 28). Here, we used the endosomal Pep12 or just the N-terminal Habc domain of Pep12 for the analysis, and observed specific binding to full-length Pep12, and much less so to the Habc domain (Fig. 1E). This finding is analogous to the previously described interaction of monomeric Vps33 with the vacuolar SNARE Vam3 (16, 28, 29). Furthermore, CORVET was able to bind assembled SNAREs, although with lower efficiency than HOPS (17) (Fig. 1F), which indicates that Vps33 in both complexes has similar binding properties. We therefore conclude that purified CORVET behaves similar to HOPS in Rab (Vps21-GTP) and SNARE binding.
CORVET Promotes Membrane Tethering.
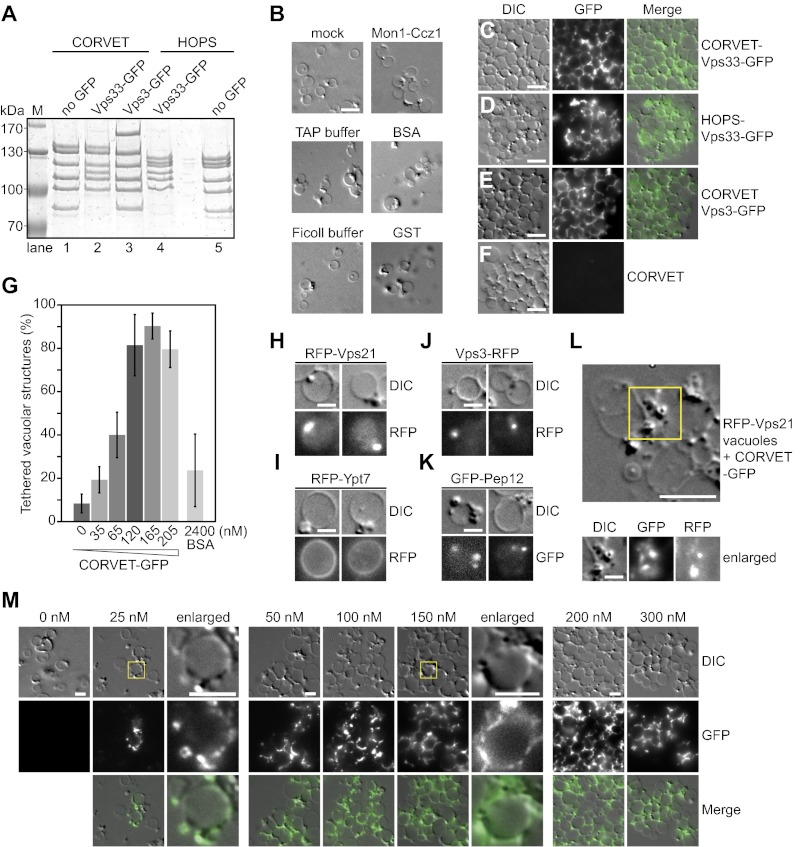
Besides interaction studies, no functional assay for CORVET activity had been developed. We therefore set out to establish a CORVET-specific tethering assay. As purified vacuoles are tightly associated with Vps21-positive endosomes (30), we asked if CORVET might trigger their association in vitro. For this assay, we generated CORVET and HOPS with C-terminal GFP-tags on Vps33 or Vps3 (CORVET) to monitor both tethering and tether. Purified complexes were isolated via a TAP-tag on Vps8 (CORVET) and Vps41 (HOPS), and showed a specific shift because of the GFP-tag. We did not notice any significant cross-contamination on Coomassie-stained gels (Fig. 2A, compare lanes 2 and 4, or 1 and 5). To monitor tethering, but not fusion, we incubated vacuoles for 10 min at 26 °C in the absence of ATP. To control for nonspecific clustering, vacuoles were preincubated in the presence of the CORVET purification buffer (TAP-buffer), reaction buffer (Ficoll buffer), excess of Mon1-Ccz1, BSA, or GST, which did not cause tethering (Fig. 2B). In contrast, both CORVET and HOPS triggered massive clustering of vacuoles (Fig. 2 C–F). Tethering was equally efficient, if the GFP tag was on Vps33, Vps3, or omitted (Fig. 2 C–F), and GFP-tagged HOPS and CORVET were found between clustered vacuoles. Furthermore, the observed tethering was dose-dependent (Fig. 2G), and optimal with 165 nM CORVET. This result was very obvious when we quantified CORVET-induced contacts with more than 900 vacuoles per condition (Fig. 2G). Of note, the optimal CORVET concentration is similar to the amount of HOPS required to promote vacuole and liposome fusion (6, 19).
Fig. 2.
Tethering of endolysosomal membranes by the CORVET complex. (A) Purified HOPS and CORVET variants. Overexpressed CORVET and HOPS with or without GFP-tags on the indicated subunits were purified via TAP-tags on Vps8 (CORVET) or Vps41 (HOPS), as described in Materials and Methods, separated on SDS-PAGE gel, and stained with Coomassie. The lane between 4 and 5 was not loaded with protein. (B) Tethering controls. Isolated vacuoles were visualized directly by differential interference contrast (DIC) optics (mock) or incubated with Ficoll buffer (25% of volume, 10 mM Pipes/KOH, pH 6.8, 200 mM sorbitol), TAP buffer (25% of volume), Mon1-Ccz1 [1.1 µM, purified via Ccz1 (9)], BSA (2.4 µM), or GST (40.0 µM). (C–F) Induction of tethering by HOPS and CORVET. Tethering was monitored via microscopy by DIC optics or by following GFP-fluorescence. Purified CORVET (200 nM) and HOPS (800 nM) had a C-terminal GFP-tag on Vps33 (C and D) or Vps3 (E). (F) CORVET without a GFP-tag was added. (G) Quantification of tethering mediated by CORVET-GFP. CORVET with Vps33-GFP was added at the indicated concentrations, and tethered vacuoles were counted. To quantify the results, vacuoles were counted for each experimental condition (n > 900) and mean values ± SDs were calculated. For details see Materials and Methods. (H–K) Localization of Rabs and endosomal proteins that are associated with purified vacuoles. Vacuoles were purified from cells expressing RFP-tagged Vps21 (H), Vps3 (J), or Ypt7 (I), or GFP-tagged Pep12 (K), and visualized by DIC optics and fluorescence microcopy. (L) Colocalization of CORVET and Vps21. RFP-Vps21-positive vacuoles were incubated with CORVET-GFP (Vps33). Dot-like structures observed by DIC optics (Upper) were monitored by fluorescence microscopy (enlarged structures, Lower). (M) Titration of CORVET. CORVET-GFP (Vps33) was added at the indicated concentrations to the tethering assay. All reactions contained the same salt concentration (110 mM NaCl). Vacuoles were monitored as before. Yellow squares mark enlarged images. (Scale bars: 5 µm in B–F; 2 µm in H–K and M, 3 µm in L)
We asked if the localization of CORVET could be correlated with its binding partner Vps21 and other endosomal proteins. Isolated vacuoles are associated with a fraction of endosomes, and we indeed found dot-like structures around isolated vacuoles that were isolated from cells expressing RFP-Vps21, Vps3-RFP, or GFP-Pep12 (Fig. 2 H, J, and K). This finding demonstrates that vacuoles are isolated with attached endosomes. In contrast, vacuoles from cells expressing RFP-Ypt7 showed a Ypt7-positive ring round the vacuole (Fig. 2I). We then colocalized CORVET and Vps21, and observed both in the same dot-like structures between clustered vacuoles (Fig. 2L). Thus, CORVET-mediated tethering correlates at sites that are positive for Vps21 and other endosomal tethering/fusion markers on membranes.
This dot-like appearance of CORVET was also obvious when we titrated CORVET-GFP into the tethering assay and monitored clustering on vacuoles (Fig. 2M). Low amounts of CORVET associated with dot-like structures proximal to the vacuolar rings, which likely correspond to late endosomes. When we titrated more CORVET into the assay, it accumulated in these regions. Interestingly, excess CORVET seem to reduce tethering again (Fig. 2 G and M). If CORVET, like HOPS, would tether membranes by bridging two Rab-containing membranes, excess CORVET might saturate the available Vps21-GTP on either membrane and thus inhibit further tethering.
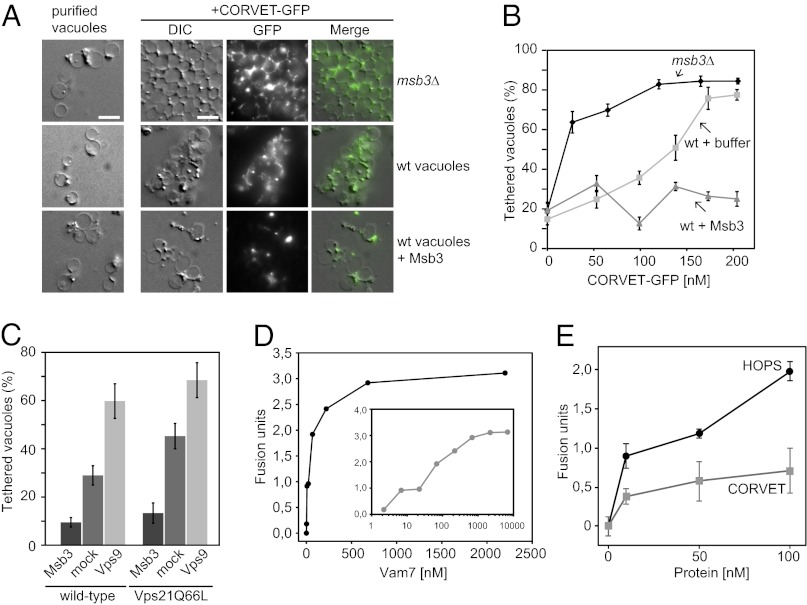
To address specificity of the assay further, we used vacuoles/endosomes isolated from msb3∆ cells. Msb3 was recently identified as the Vps21-specific GAP (27, 30). In the absence of Msb3, Vps21-GTP accumulates on the surface of vacuoles and might thus provide additional binding sites for CORVET. We would therefore expect a higher amount of available Vps21-GTP on vacuoles from msb3∆ cells and therefore more efficient tethering upon CORVET addition. This result was indeed observed (Fig. 3A). Whereas wild-type vacuoles/endosomes required 150 nM of CORVET, vacuoles/endosomes from msb3∆ cells showed already with less than 50 nM very efficient tethering (Fig. 3B). Moreover, if purified recombinant MBP-Msb3 was added to the tethering reaction, CORVET did neither localize efficiently to vacuoles/endosomes, nor did it promote tethering (Fig. 3 A and B). We further substantiated this observation by comparing the effect of Msb3 and the Vps21-specific GEF Vps9 on tethering (Fig. 3C). When Vps9 was added to wild-type vacuoles/endosomes, CORVET-mediated tethering was strongly stimulated, indicating that additional Vps21-GTP was present (Fig. 3C). Furthermore, CORVET stimulated tethering on vacuoles/endosomes carrying the GTP-locked Vps21Q66L even better, and this was further enhanced by the addition of Vps9 (Fig. 3C). Of note, the locked Vps21 mutant is still sensitive to Msb3 as shown for other Rabs before (31). We therefore conclude that CORVET requires Vps21-GTP for efficient tethering of membranes.
Fig. 3.
CORVET promotes tethering and fusion in a Rab-dependent manner. (A and B) Effect of Msb3 on CORVET-mediated fusion. (A) Tethering in the presence and absence of Msb3. Vacuoles/endosomes isolated from wild-type (wt) or msb3∆ cells were preincubated for 5 min with Ficoll buffer or, where indicated, with Msb3 (140 nM). After addition of CORVET-GFP (Vps33, 135 nM), vacuoles were analyzed via microscopy. (Scale bar, 5 µm.) (B) CORVET-GFP titration and quantification of tethered vacuolar structures. CORVET was added as described in A, in total n = 12,800 vacuoles were analyzed (> 500 vacuoles at each concentration), mean values and ± SEM were calculated. (C) Effect of Vps9 on CORVET-mediated tethering. Vacuoles were purified from wild-type or cells expressing Vps21 Q66L, and subjected to the tethering assay in the presence of 125 nm CORVET. Where indicated, recombinant purified Vps9 (1.5 µM) or Msb3 (140 nM) were added. All reactions were supplied with 0.1 mM GTP. Quantification was done as in B. (D) Fusion of vacuoles/endosomes from msb3∆ cells in the presence of Vam7. Vacuoles/endosomes from the two tester strains were isolated and incubated in the presence of indicated amounts of recombinant Vam7. Fusion was determined after 90 min at 26 °C (Materials and Methods). (Inset) Titration of Vam7 was plotted against a logarithmic scale of protein concentration. (E) CORVET stimulates the Vam7-dependent fusion reaction in vitro. The same vacuoles/endosomes as in D were incubated with 6.8 nM Vam7 in the presence of indicated amounts of the purified CORVET complex, HOPS complex, or buffer as a control. The extent of fusion was determined after 90 min at 26 °C. For every titration curve, three different experiments were carried out. Mean values with SDs were plotted against protein concentrations after subtraction of respective buffer values. D shows a representative experiment (n = 3).
CORVET Can Promote Fusion of Vacuoles.
To address if CORVET is able to promote fusion of membranes, we again used isolated vacuoles/endosomes from msb3∆ cells, which also efficiently recruit CORVET to tethering sites between vacuoles (Fig. 3A). As CORVET was able to promote efficient tethering and might recognize vacuolar SNAREs similar to HOPS, we reasoned that it might also be able to promote fusion. For this reason we took advantage of the established vacuole fusion assay. We used two vacuole types: one lacks alkaline phosphatase (Pho8); the other is deleted for the activating protease Pep4 and carries inactive pro-Pho8. Fusion of vacuoles results in mixing of the lumina and conversion of pro-Pho8 to active Pho8 by Pep4, which can be measured spectrophotometrically (32). Because pro-Pho8 is sorted via the AP-3 pathway to vacuoles and Pep4 is activated in the vacuole lumen (2), the fusion assay will measure primarily vacuole-vacuole fusion. Here, we used isolated vacuoles/endosomes from msb3∆ cells, which show higher fusion activity because Msb3 also acts as a Ypt7-GAP and vacuoles therefore have more Ypt7-GTP on their surface (27, 30). HOPS-dependent fusion is seen best if vacuole fusion is promoted by limiting amounts of the SNARE Vam7. Addition of HOPS then further triggers fusion strongly (17). We thus titrated Vam7 into the fusion reaction of vacuoles/endosomes from msb3∆ cells (Fig. 3D), selected a limiting amount of Vam7, and then used this concentration to test for the additional stimulatory effects by HOPS or CORVET (Fig. 3E). Stimulation of fusion by CORVET reached about 50% of HOPS-stimulated activity at concentrations of 50 nM, and about 30% at 100 nM (Fig. 3E). This result indicates that CORVET is able to partially substitute for HOPS in the fusion reaction if Vps21-GTP is available on vacuoles.
CORVET Localizes to Docked Vps21-positive Structures in Vivo.
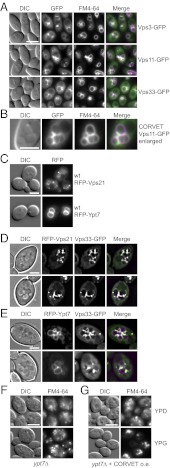
We then asked if we could observe CORVET function also in vivo. To this end, we followed GFP-tagged subunits in the CORVET overexpression strain. Similar to our in vitro observations, we detected the CORVET subunits at contact zones between vacuoles, here marked by the lipophilic dye FM4-64 (Fig. 4 A and B). To monitor the relative distribution of CORVET to the Rabs simultaneously, we tagged both Vps21 and Ypt7 with RFP. RFP-Vps21 was detected on multiple dots proximal to the vacuole, whereas Ypt7 was located on vacuolar rings (Fig. 4C). When we followed both Rabs in the presence of overexpressed CORVET, we obtained a clear change. Vps21 now accumulated in the same bright dots as CORVET-GFP proximal to and at contact sites of vacuoles, which most likely are clustered endosomes (Fig. 4D). In contrast, Ypt7 localization on the vacuolar surface was not disturbed by CORVET overproduction. Ypt7 remained distributed evenly over the surface of the vacuole, whereas CORVET was found in dots at the interfaces as observed before (Fig. 4E). It is possible that the additional slight fragmentation of the vacuole (Fig. 4E) is caused by the RFP-tagging of Ypt7, which impairs its function, but not localization, in vivo (33, 34). To exclude that vacuole-bound Ypt7 contributes to the CORVET activity, we deleted the protein in the diploid CORVET overexpression strain. When we then monitored the behavior of fragmented FM4-64 labeled vacuoles/endosomes, which are dispersed in wild-type (Fig. 4F), we observed again CORVET-driven clustering of these fragments in vivo when CORVET was overproduced (Fig. 4G). We conclude that CORVET triggers clustering of Vps21-positive structures in vivo, consistent with the described in vitro data and its proposed role as an endosomal tethering factor.
Fig. 4.
Relocalization of Vps21 upon CORVET overproduction. (A and B) Accumulation of overproduced CORVET at vacuole-vacuole contact sites. Cells expressing all CORVET subunits under control of the GAL1 promoter were grown in YPG medium to midlogarithmic phase, stained with FM4-64, harvested, washed, and analyzed by fluorescence microscopy. The GFP-tag was fused C-terminally to the indicated overproduced subunits. (B) Enlarged image section of Vps11-GFP in the CORVET overexpression strain (A). (C) Localization of RFP-tagged Rab proteins Vps21 and Ypt7 in wild-type (wt) cells. Cells were grown in (SRC-Met + 2% gal) for plasmid maintenance and analyzed as described. Exposure times were kept constant for all strains and approaches in A–C. (D and E) Colocalization of RFP-Rabs and overproduced CORVET-GFP. Cells were incubated in the same selective medium as described in C to induce overexpression of CORVET and maintenance of RFP-Rab encoding plasmids. The GFP-tag was fused to the CORVET subunit Vps33. Exposure times were kept constant for all strains and approaches. (A–E) Depicted fluorescence images are sum projections of four z-slices (0.25-µm spacing), after 3D deconvolution. (F and G) Clustering of endolysosomal structures upon CORVET overexpression. YPT7 was deleted in wt (F) and CORVET overproduction (G) strains that lack an additional GFP-tag. Cells were grown in YPD to repress, or YPG to induce the GAL1 promoter-driven overexpression of CORVET, and analyzed as described in A. (Scale bars, 5 µm in A, F, and G; 3 µm in B–E.)
Discussion
Here, we demonstrate that CORVET is a Vps21-specific tethering complex that promotes fusion. For this study, we established the purification of this heterohexamer, its binding to SNAREs and Rab5/Vps21-GTP and analyzed its ability to tether isolated vacuoles and the associated endosomes. CORVET is able to bind to Pep12 and assemble vacuolar SNAREs, indicating a promiscuous function of the SM protein Vps33, which is also present in HOPS. However, binding was not as efficient as for HOPS, suggesting some regulation of Vps33 because of its incorporation into each complex. CORVET-mediated tethering is dose-dependent and is promoted by raising the Vps21-GTP concentrations, as observed on vacuoles isolated from msb3∆ cells. In agreement, CORVET accumulates at Vps21-positive contact sites in vivo and clusters endosomal/vacuolar structures in ypt7∆ cells. This finding suggests that CORVET is a tether that bridges Vps21-GTP–containing membranes before their SNARE-mediated fusion. In essence, CORVET and HOPS then likely function similarly in first recognizing their activated Rab, which is required for their localization to endosomes or vacuoles (6), before tethering and fusion. Our data are thus unique in providing direct evidence for the tethering function of CORVET, which was implicated in our previous in vivo analysis and suggested by its similarity to HOPS (6, 20, 21).
Why is CORVET then essential for endosomal trafficking if other tethers also function along the endocytic pathway? We recently showed that CORVET is an independent tethering complex that functions downstream of the EEA1-like Vac1 protein (23). The Vac1 tether likely mediates fusion of endocytic vesicles with endosomes (35, 36). Endosomal maturation is, however, accompanied by multiple fusion events, and will require in addition endosome-endosome fusion. Indeed, deletion of all Rab5 proteins in yeast will strongly impair endosomal biogenesis (23, 27). Similarly, the down-regulation of all Rab5 proteins in mouse liver inhibits late endosome biogenesis altogether (37). These authors conclude that endosomal biogenesis relies on multiple fission and fusion events (37). Indeed, several of the shared subunits of CORVET and HOPS were previously isolated via immobilized mammalian Rab5-GTP (38). This finding indicates that endosome-endosome fusion is an essential part of the maturation process in vivo, which is impaired if any of the CORVET subunits are deleted (23, 27, 38, 39).
The requirement of efficient endosome-endosome fusion becomes quite obvious when considering the entire membrane surface of a multivesicular endosome. A mature late endosome is also called multivesicular body (MVB), and is filled with numerous (>50) small vesicles (r = 23 nm). MVBs can be as large as 200 nm in diameter (40). To generate one MVB with this amount of vesicles, the endosome would have to be as large as 400 nm in diameter. It is thus likely that fusion of endosomes via CORVET is a necessary part to also generate enough surface area for intraluminal vesicle generation. This calculation may underestimate the endosomal fusion requirement as it does not yet take into account that endosomes constantly lose membrane because of receptor recycling via the retrieval pathway (41). Consequently, if endosomes stay too small, ESCRT and retromer could function insufficiently, which could explain the observed protein-sorting defects of CORVET and Vps21 mutants (23, 27). In contrast, if just one CORVET subunit is overproduced in cells, tethering can still occur, but endosomal fusion may not occur because of the lack of the remaining subunits. We indeed observed then the accumulation of multiple small MVBs in clusters, which accumulated cargo destined for the vacuole lumen (21). We thus believe that CORVET is the Rab5-dependent tether required for fusing endosomes in yeast and mammalian cells (23, 37, 38).
Our study thus provides a conclusive explanation of CORVET function as a Rab5-dependent tethering complex, as shown by our in vitro analysis. It nevertheless leaves the question open as to why CORVET and HOPS are so similar, and can even substitute missing subunits. At present, we consider it unlikely that HOPS necessarily forms out of preexisting CORVET. Our data are, however, consistent with the idea that CORVET as HOPS assemble SNAREs via Vps33 (16, 17, 28), although specific adjustments in Vps33 activity because of intersubunit interactions are expected. Indeed, HOPS was more active in fusion than CORVET on vacuoles from msb3∆ cells, despite the respective active Rab (Ypt7 and Vps21) was present for either complex on these vacuoles. Future assays will need to resolve the precise function of these tethers in the fusion cascade, their turnover, and regulation.
Materials and Methods
Yeast Strains and Molecular Biology.
Details on additional methods and all used Saccharomyces cerevisiae strains and plasmids are listed in Tables S1 and S2. In general, overexpression, deletion, and C-terminal tagging was carried out by homologous recombination of PCR fragments into BY strains (42).
Microscopy.
For microscopy of S. cerevisiae cells expressing a GFP or RFP tag, overnight cultures were diluted and grown to logarithmic phase in YP medium containing 2% (wt/vol) glucose or galactose, respectively. For maintenance of RFP-Rab plasmids, cells were grown in synthetic selective medium with raffinose and 2% (wt/vol) galactose (SRC-Met + 2% gal). Staining of endolysosomal compartments with FM4-64 (Invitrogen) was performed as described previously (43). Cells were analyzed in synthetic medium (SDC or SGC) using a Leica DM5500 microscope setup (Leica) with a SPOT Pursuit camera (Diagnostic Instruments) and with filters for DIC, GFP, RFP, and FM4-64. Where indicated, z-stacks (8 z-sections; 0.25-µm section spacing; sample thickness 2.0 µm) were acquired with an Olympus IX-71 microscope with a CoolSNAP HQ2 CCD camera (Photometrics) and subjected to 3D deconvolution using Softworx v5.5 software (Applied Precision). Processing and arrangement of pictures was performed with ImageJ (http://imagej.nih.gov/ij/), Adobe Photoshop and Adobe Illustrator (version CS4, Adobe Systems).
CORVET Purification and Analysis.
CORVET(-GFP) overexpression strains were grown to a OD600 ∼ 8–10 in yeast peptone medium (YP) containing 2% (wt/vol) galactose to induce the GAL1 promoter. TAP (44) of CORVET constructs was performed as described previously for HOPS (26), omitting the CaM-bead purification step. The following adjusted buffer was used for the preparation: 50 mM Hepes/NaOH (pH 7.4), 300 mM NaCl, 1.5 mM MgCl2, 10% glycerol (vol/vol) and, where indicated 0.15% (vol/vol) Nonidet P-40 (IGEPAL CA-630; Sigma-Aldrich). For glycerol gradient analysis, purified CORVET complex was loaded onto a continuous glycerol gradient with 10–40% (vol/vol) glycerol (Gradient Master, Biocomp Instruments) using 11 × 60-mm polyallomer tubes (Beckman Coulter). After centrifugation in a SW40 rotor (Beckman Coulter) for 18 h at 257,000 × g, fractions were harvested, TCA-precipitated, and analyzed on a 7.5% Coomassie stained acrylamid-gel. Protein amounts were quantified using a gel documentation system (VersaDoc) with the Quantity One application software (Bio-Rad Laboratories).
Vacuole Fusion Assay.
Vacuoles were purified from the reporter strains BJ3505 (pep4∆) and DKY6281 (pho8∆) by DEAE-dextran lysis and Ficoll density gradient flotation as described previously (32). MSB3 was deleted in both strain backgrounds (30). Reactions were set up with 3 µg of each vacuole population in fusion reaction buffer (10 mM Pipes/KOH, pH 6.8, 125 mM KCl, 5 mM MgCl2, 0.2 M sorbitol), supplemented with 10 µM CoA and 6.8 nM Vam7. CORVET and HOPS were titrated as indicated. After an incubation for 90 min at 26 °C and addition of p-nitrophenyl phosphate, fusion activity could be monitored by measuring the absorbance of generated nitrophenol at 400 nm (32).
Tethering Assay.
Vacuoles were isolated from BY wild-type and msb3∆ strains as described above. Isolated organelles from different strain backgrounds were diluted in 0% (wt/vol) Ficoll buffer to a concentration of 0.5–0.6 mg/mL and immediately used for the following assays. Tethering reaction was set up by incubating 10-µL vacuoles with the respective amount of purified tethering complexes (standard concentration 0.5–0.6 mg/mL) with and without inhibitors. Each incubation was performed for 10 min at 26 °C and mild agitation (800 rpm, Thermomixer comfort; Eppendorf). Tethering was similar at salt concentrations between 50 and 150 mM NaCl. Pictures of vacuoles were taken (random fields, n ≥ 9) with constant exposure times (DIC = 30 ms, GFP = 300 ms) using the same camera setup as described above. The ratio between untethered and tethered vacuolar structures was determined by counting vacuoles, the surface of which was covered to more than 50% by another vacuole. For each quantified condition, more than 900 vacuoles were counted with ImageJ (http://imagej.nih.gov/ij/). Mean values, standard variances and SEs were calculated using Origin software (OriginLab) and Excel (Microsoft).
Supplementary Material
Acknowledgments
We thank Fulvio Reggiori and Francis Barr for discussions, and Daniel Orban and Clemens Ostrowicz for experimental support in the initial phase of this project; Dieter Langosch and Francis Barr for plasmids; Nadine Epp for providing the RFP-Vps21 and Ypt7 plasmids; and Henning Arlt for technical support. This work was funded by the Fritz-Thyssen foundation (to C.B.), the Deutsche Forschungsgemeinschaft (SFB 944-P11, UN111/5-2), and by the Hans-Mühlenhoff foundation (to C.U.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221785110/-/DCSupplemental.
References
- 1.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epp N, Rethmeier R, Krämer L, Ungermann C. Membrane dynamics and fusion at late endosomes and vacuoles—Rab regulation, multisubunit tethering complexes and SNAREs. Eur J Cell Biol. 2011;90(9):779–785. doi: 10.1016/j.ejcb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20(4):427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balderhaar HJK, et al. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci. 2010;123(Pt 23):4085–4094. doi: 10.1242/jcs.071977. [DOI] [PubMed] [Google Scholar]
- 6.Bröcker C, et al. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci USA. 2012;109(6):1991–1996. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22(4):461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachmann J, Ungermann C, Engelbrecht-Vandré S. Rab GTPases and tethering in the yeast endocytic pathway. Small GT{ases. 2011;2(3):182–186. doi: 10.4161/sgtp.2.3.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann M, et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20(18):1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141(3):497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464(7289):778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22(22):2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97(17):9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151(3):551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: Gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21(4):543–551. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nat Struct Mol Biol. 2010;17(6):710–717. doi: 10.1038/nsmb.1809. [DOI] [PubMed] [Google Scholar]
- 17.Krämer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011;22(14):2601–2611. doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21(13):2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27(15):2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12(5):739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Markgraf DF, et al. The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments. Mol Biol Cell. 2009;20(24):5276–5289. doi: 10.1091/mbc.E09-06-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plemel RL, et al. Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic. Mol Biol Cell. 2011;22(8):1353–1363. doi: 10.1091/mbc.E10-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrera M, et al. Functional separation of endosomal fusion factors and the CORVET tethering complex in endosome biogenesis. J Biol Chem. 2013;288:5166–5175. doi: 10.1074/jbc.M112.431536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawelec A, Arsić J, Kölling R. Mapping of Vps21 and HOPS binding sites in Vps8 and effect of binding site mutants on endocytic trafficking. Eukaryot Cell. 2010;9(4):602–610. doi: 10.1128/EC.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abenza JF, et al. Aspergillus RabB Rab5 integrates acquisition of degradative identity with the long distance movement of early endosomes. Mol Biol Cell. 2010;21(15):2756–2769. doi: 10.1091/mbc.E10-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrowicz CW, et al. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11(10):1334–1346. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 27.Nickerson DP, et al. Termination of isoform-selective Vps21/Rab5 signaling at endolysosomal organelles by Msb3/Gyp3. Traffic. 2012;13(10):1411–1428. doi: 10.1111/j.1600-0854.2012.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobingier BT, Merz AJ. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol Biol Cell. 2012;23(23):4611–4622. doi: 10.1091/mbc.E12-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulubova I, Yamaguchi T, Wang Y, Südhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8(3):258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 30.Lachmann J, Barr FA, Ungermann C. The Msb3/Gyp3 GAP controls the activity of the Rab GTPases Vps21 and Ypt7 at endosomes and vacuoles. Mol Biol Cell. 2012;23(13):2516–2526. doi: 10.1091/mbc.E11-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Antoni A, Schmitzová J, Trepte H-H, Gallwitz D, Albert S. Significance of GTP hydrolysis in Ypt1p-regulated endoplasmic reticulum to Golgi transport revealed by the analysis of two novel Ypt1-GAPs. J Biol Chem. 2002;277(43):41023–41031. doi: 10.1074/jbc.M205783200. [DOI] [PubMed] [Google Scholar]
- 32.Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci. 1995;17(4):283–294. [Google Scholar]
- 33.Cabrera M, et al. Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering. J Cell Biol. 2010;191(4):845–859. doi: 10.1083/jcb.201004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrera M, et al. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20(7):1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson MR, Burd CG, Emr SD. Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr Biol. 1999;9(3):159–162. doi: 10.1016/s0960-9822(99)80071-2. [DOI] [PubMed] [Google Scholar]
- 36.Tall GG, Hama H, DeWald DB, Horazdovsky BF. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10(6):1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeigerer A, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485(7399):465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 38.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122(5):735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 39.Russell MRG, Shideler T, Nickerson DP, West M, Odorizzi G. Class E compartments form in response to ESCRT dysfunction in yeast due to hyperactivity of the Vps21 Rab GTPase. J Cell Sci. 2012;125(Pt 21):5208–5220. doi: 10.1242/jcs.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luhtala N, Odorizzi G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J Cell Biol. 2004;166(5):717–729. doi: 10.1083/jcb.200403139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142(3):665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21(11):947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 43.LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol. 2005;168(3):401–414. doi: 10.1083/jcb.200407141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig O, et al. New constructs and strategies for efficient PCR-based gene manipulations in yeast. Yeast. 1998;14(12):1139–1146. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1139::AID-YEA306>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.