Abstract
Cyanobacterial flavodiiron proteins (FDPs; A-type flavoprotein, Flv) comprise, besides the β-lactamase–like and flavodoxin domains typical for all FDPs, an extra NAD(P)H:flavin oxidoreductase module and thus differ from FDPs in other Bacteria and Archaea. Synechocystis sp. PCC 6803 has four genes encoding the FDPs. Flv1 and Flv3 function as an NAD(P)H:oxygen oxidoreductase, donating electrons directly to O2 without production of reactive oxygen species. Here we show that the Flv1 and Flv3 proteins are crucial for cyanobacteria under fluctuating light, a typical light condition in aquatic environments. Under constant-light conditions, regardless of light intensity, the Flv1 and Flv3 proteins are dispensable. In contrast, under fluctuating light conditions, the growth and photosynthesis of the Δflv1(A) and/or Δflv3(A) mutants of Synechocystis sp. PCC 6803 and Anabaena sp. PCC 7120 become arrested, resulting in cell death in the most severe cases. This reaction is mainly caused by malfunction of photosystem I and oxidative damage induced by reactive oxygen species generated during abrupt short-term increases in light intensity. Unlike higher plants that lack the FDPs and use the Proton Gradient Regulation 5 to safeguard photosystem I, the cyanobacterial homolog of Proton Gradient Regulation 5 is shown not to be crucial for growth under fluctuating light. Instead, the unique Flv1/Flv3 heterodimer maintains the redox balance of the electron transfer chain in cyanobacteria and provides protection for photosystem I under fluctuating growth light. Evolution of unique cyanobacterial FDPs is discussed as a prerequisite for the development of oxygenic photosynthesis.
Keywords: Mehler reaction, membrane inlet mass spectrometry, photorespiration, terminal oxidases
Flavodiiron proteins [FDPs; also called A-type flavoproteins (Flv)] comprise a family of proteins widespread among strict or facultative anaerobic Bacteria and Archaea but are also present in some anaerobic pathogenic protozoa, green algae, mosses, and lycophytes (1, 2). FDPs have been suggested to function as homodimers or homotetramers on detoxification of NO and/or O2 during nitrosative and/or oxidative stress. Some of the FDPs show high affinity toward NO and others to O2, whereas some enzymes catalyze both reactions but with different efficiency. FDPs share a common core comprised of two redox centers: the N-terminal β-lactamase–like domain with nonheme catalytic diiron center and the C-terminal flavodoxin-like domain with a flavin mononucleotide cofactor. Cyanobacterial FDPs have acquired an extra C-terminal flavin-reductase domain, thereby enabling the oxidation of NAD(P)H and reduction of a substrate within the same protein.
Cyanobacterial FDPs can be grouped into two clusters and seem to function as heterodimers with one monomer from each cluster (2). Some α-cyanobacteria and ancient eukaryotic species possess only two putative FDPs, whereas N2-fixing heterocystous cyanobacteria possess six FDPs. Unicellular non-N2-fixing cyanobacterium Synechocystis sp. PCC 6803 (hereafter, Synechocystis) has four genes encoding FDPs, Flv1–Flv4. Flv1 and Flv3 types of FDPs are present in all cyanobacteria, but the Flv2 and Flv4 FDPs are present only in β-cyanobacteria. We have recently shown that the Flv2/Flv4 heterodimer functions in photoprotection of photosystem (PS) II (2, 3). The Flv1 and Flv3 proteins have been shown to function in the light-induced O2 uptake in Mehler-like reaction that does not produce reactive oxygen species (ROS) (4, 5). It was shown that ∼20–30% of electrons released from water splitting by PSII are directed to O2 via Flv1 and Flv3 proteins upon high-light conditions. Under Ci-deprivation conditions the Flv1/Flv3 proteins, in cooperation with the photorespiratory pathway, might flux up to 60% of electrons to O2, functioning as a powerful electron sink (5). Elimination of the Flv1 and Flv3 proteins seems to have no detrimental effect on the growth and wellbeing of the Synechocystis cells under standard growth conditions (2, 4, 5). Impaired acclimation ability of the Δflv3 mutant has been reported only upon a shift of cells from slow growth mode (low light, air-level CO2), to rapid growth mode (standard growth light, 5% CO2) and also in combination with the ΔgcvT mutation that renders cells deficient in the photorespiratory 2-phosphoglycolate metabolism (6). Unlike Synechocystis, filamentous, N2-fixing, heterocystous cyanobacterium Anabaena sp. PCC 7120 (hereafter, Anabaena) has six genes encoding FDPs. Whereas flv1A, flv3A, flv2, and flv4 in Anabaena are highly homologous with genes from Synechocystis, the flv1B and flv3B genes encoding heterocyst-specific Flv1B and Flv3B proteins are likely to be involved in N2 metabolism (7).
Here we addressed the impact of FDPs upon growth of Synechocystis and Anabaena under fluctuating light (FL) conditions. We have recently identified in higher plants two proteins, the Proton Gradient Regulation 5 (PGR5) and the STN7 kinase of the light-harvesting complex II proteins, as absolute requirements for successful growth of plants under FL (8, 9). Because of light harvesting systems different from those in plant chloroplasts, cyanobacteria lack the gene coding for the STN7 kinase, and their pgr5-like gene is only a distant homolog of the plant pgr5 gene. As natural light conditions experienced by photosynthetic microorganisms in aquatic environments might be extremely variable in the light intensity, it is conceivable that cyanobacteria also harbor a mechanism to protect photosynthesis under such conditions. Likewise, the bioreactors that have so far been planned and tested for growth of cyanobacteria for biotechnology purposes are characterized by steep short-term fluctuations in light intensity upon rotation of the cells in bioreactors.
In contrast to the function of PGR5 as a safeguard of PSI in higher plants, we show that the PGR5-like protein in cyanobacteria is not important for survival under FL. Instead, we demonstrate an indispensable function of FDPs Flv1(A) and Flv3(A) in supporting photosynthesis and growth of cyanobacteria under FL conditions. The Flv1 and Flv3 proteins provide protection for sustainable function and protein stability of PSI upon abrupt high-light pulses by directing electrons to O2 in a harmless way without inducing damage in PSI.
Results
Growth Phenotype of Different Flv1 and Flv3 Mutants.
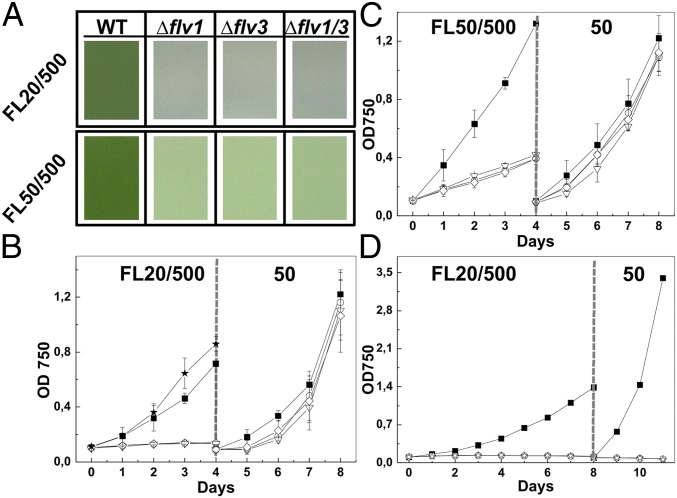
Flv1 and Flv3 proteins are ubiquitous in all cyanobacteria, yet no strong growth phenotype has been assigned for the mutants. Because the aquatic growth environments are typically characterized by fluctuations in the light intensity, we attempted to search for growth phenotypes in Synechocystis Δflv1, Δflv3, and Δflv1/Δflv3 mutants by exposing them to different intensities of constant light as well as to FL with low background illumination interrupted with repeating high-light pulses. Synechocystis mutants grown under constant low, normal-growth, and high-light conditions, 20, 50, and 500 µmol photons·m−2·s−1, respectively, did not show any significant growth phenotype compared with wild type (WT; Fig. S1 A–C). In contrast, FL produced a strong growth phenotype in all these mutants. Under the FL 20/500 regime (20 µmol photons·m−2·s−1 for 5 min and 500 µmol photons·m−2·s−1 for 30 s), the growth of the Δflv1, Δflv3, and Δflv1/Δflv3 mutant cells was completely inhibited (Fig. 1 A and B), whereas under FL 50/500 (50 µmol photons·m−2·s−1 for 5 min and 500 µmol photons·m−2·s−1 for 30 s) conditions, the mutant cells demonstrated drastic decrease of cell growth compared with WT (Fig. 1 A and C).
Fig. 1.
Growth phenotype of Synechocystis WT and mutant cells. (A) Cells were grown under FL 20/500 or 50/500 regime for 4 d. (B) Cells were grown under FL 20/500 regime for 4 d followed by adjustment of OD750 to 0.1 and shifting to constant light. (C) Cells were grown under FL 50/500 regime for 4 d followed by adjustment of OD750 to 0.1 and shifting to constant light. (D) Cells were grown under FL 20/500 regime for 8 d followed by adjustment of OD750 to 0.1 and shifting to constant light. Mean ± SD; n = 4. WT, ■; Δflv1::flv1 complementation strain, ★; mutant strains Δflv1, ○; Δflv3, ∇; Δflv1/Δflv3, ◇.
It was next examined whether the FL 20/500 and 50/500 regimes promote complete death of mutant cells or instead induce a growth arrest. To this end, the cells grown under two different FL regimes for 4 d were further diluted to OD750 = 0.1 and transferred to constant-light intensity of 50 µmol photons·m−2·s−1. Fig. 1 B and C shows that the mutant cells started to grow normally and reached nearly the same OD as WT cells after 4 d of growth at constant-light conditions. However, if the mutant cells were incubated under FL 20/500 regime for a longer period of time (8 d), the shift to constant light did not rescue the growth of the mutant cells (Fig. 1D). These results imply that mutant cells do not survive prolonged period of FL. Importantly, complementation of the flv1 deletion with the functional Flv1 protein (the Δflv1::flv1strain) fully abolished the observed phenotype, confirming that the effect was indeed caused by the flv1 deletion (Fig. 1B).
We likewise constructed the deletion mutants of Flv1A and Flv3A, the closest homologs of Synechocystis Flv1 and Flv3 proteins in filamentous Anabaena strains. Growth phenotypes of these Anabaena mutants (Fig. S1D), similar to those of the respective Synechocystis mutants, suggest a ubiquitous role for the Flv1 and Flv3 proteins in cyanobacteria in coping with FL conditions.
Real-Time Gas-Exchange Analysis of the Δflv1/Δflv3 Mutant.
To address the underlying molecular mechanisms for the cell growth arrest under FL in the absence of the Flv1 and Flv3 proteins, we next measured the photosynthetic gas exchange in the Δflv1/Δflv3 mutant. Real-time gas-exchange analyses with membrane inlet mass spectrometry (MIMS) were performed with WT and mutant cells under light conditions mimicking the fluctuating growth conditions (20/500, four cycles then dark).
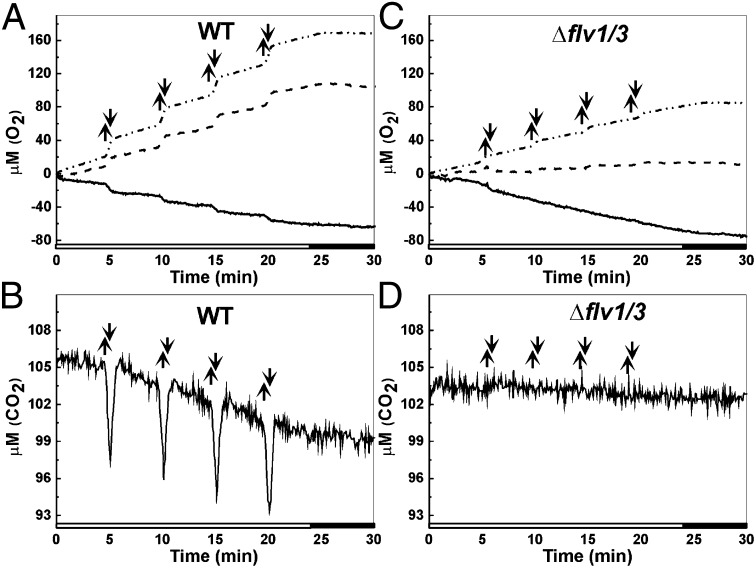
MIMS experiments with WT cells demonstrated, as expected, a strong signal of photosynthetic O2 evolution (Fig. 2A) and CO2 uptake (Fig. 2B) upon high-light pulses of FL, whereas the low-light phases repeatedly reduced the gas-exchange reactions to a significantly lower level. These signals showed conserved repetition in the amplitude of O2 and CO2 exchange from one high-light pulse to the next one (Fig. 2 A and B). 18O2 labeling was used to discriminate the evolution and uptake of O2 during illumination of the cells. In WT, the O2 uptake upon low-light phases was equivalent or even lower (0.6 µmol O2⋅L−1⋅min−1) than in the dark (0.7 µmol O2⋅L−1⋅min−1). During high-light pulses, a strong O2 photoreduction was observed in WT cells (12.6 µmol O2⋅L−1⋅min−1; Fig. 2A and Table 1), probably induced by the Flv1 and Flv3 proteins.
Fig. 2.
Real-time O2 (A and C) and CO2 (B and D) exchange measurements in Synechocystis WT and Δflv1/Δflv3 cells. The cells were grown under constant light of 50 μmol photons⋅m−2⋅s−1 and then set at OD750 = 0.4–0.5 and shifted to FL 20/500 for 2–3 d before the measurements. Actinic white light intensity of 20 μmol photons⋅m−2⋅s−1 was applied to the dark-adapted cells, and ∼30-s high-light pulses at an intensity of 500 μmol photons⋅m−2⋅s−1 was turned on (up arrows) and off (down arrows) every 4 min. Cumulative O2 evolution (dotted line), O2 uptake (solid line), and net photosynthesis (dashed line) were calculated from 18O2/16O2 exchange measurements.
Table 1.
Photosynthetic parameters of the WT and mutant cells
| Strains | FV/FM | O2 evolution rate | O2 photoreduction rate | Net photosynthesis | PM |
| WT | 0.43 ± 0.08 | 3.3 ± 0.3 LL | 0.6 ± 0.1 LL | 2.7 ± 0.5 LL | 0.33 ± 0.03 |
| 33.5 ± 0.8 HL | 12.6 ± 1.8 HL | 20.9 ± 1.1 HL | |||
| Δflv1/3 | 0.35 ± 0.05 | 2.4 ± 0.1 LL | 2.1 ± 0.1 LL | 0.3 ± 0.1 LL | 0.06 ± 0.02 |
| 11.4 ± 2.6 HL | 4.0 ± 1.9 HL | 7.4 ± 2.6 HL | |||
| WT+KCN | 1.7 ± 0.1 | ||||
| 25.1 ± 3.9 | |||||
| Δflv1/3 +KCN | 0.3 ± 0.1 | ||||
| 1.6 ± 0.7 | |||||
| Δflv1::flv1 | 0.42 ± 0.06 | 13.2 ± 1.1 | 0.38 ± 0.03 |
Cells were grown under constant light, then set at OD750 = 0.4–0.5 and shifted to FL 20/500 for 3 d before the measurements. O2 exchange values are presented as micromoles per liter per minute. LL, low-light phase (20 µmol photons·m−2·s−1) of FL; HL, high-light pulses (500 µmol photons·m−2·s−1) of FL. FV/FM recorded in the presence of 10 µM DCMU. The values are mean ± SD, n = 3.
In the Δflv1/Δflv3 mutant, the MIMS analyses (Fig. 2C) revealed drastically lower photosynthetic O2 evolution and net photosynthesis rates, being only approximately one-third of that in WT cells (Table 1). Moreover, no bursts of CO2 uptake were recorded in the Δflv1/Δflv3 mutant during the high-light pulses, indicating strong impairment in CO2 concentration/fixation in these cells (Fig. 2D). Paradoxically, the weak low-light-induced O2 uptake recorded in WT cells was even enhanced in the Δflv1/Δflv3 mutant cells (2.1 µmol O2⋅L−1⋅h−1; Fig. 2C), whereas the high-light-stimulated O2 uptake observed in WT was not detected in the Δflv1/Δflv3 mutant cells. To clarify the origin of the strong “background” O2 uptake in mutant cells, we applied iodoacetamide (IAC), an inhibitor of the photorespiratory pathway (4, 5, 10). Addition of IAC, however, further stimulated the rate of O2 photoreduction in both the WT and mutant cells (Fig. S2), suggesting that O2 photoreduction in the Δflv1/Δflv3 mutant did not originate from photorespiration.
Contribution of respiratory O2 uptake was estimated by treating the cells with KCN, an inhibitor of major respiratory terminal oxidases (11). In WT cells, KCN caused a significant decrease of dark O2 uptake but stimulated the low-light- as well as high-light–induced O2 photoreduction (Fig. 3 and Table 1). In contrast to WT, addition of KCN to the Δflv1/Δflv3 mutant cells abolished most of the light-induced O2 uptake (Fig. 3 and Table 1). Sensitivity of the O2 photoreduction to KCN (Fig. 3) suggests that a major terminal oxidase, the aa3-type cytochrome c oxidase, encoding by coxBAC operon and located in thylakoid and plasma membranes (11), is contributing to light-induced O2 uptake in the mutant cells. It should be noted that a small impact of true Mehler reaction, the leakage of electrons from PSI directly to O2, cannot be excluded.
Fig. 3.
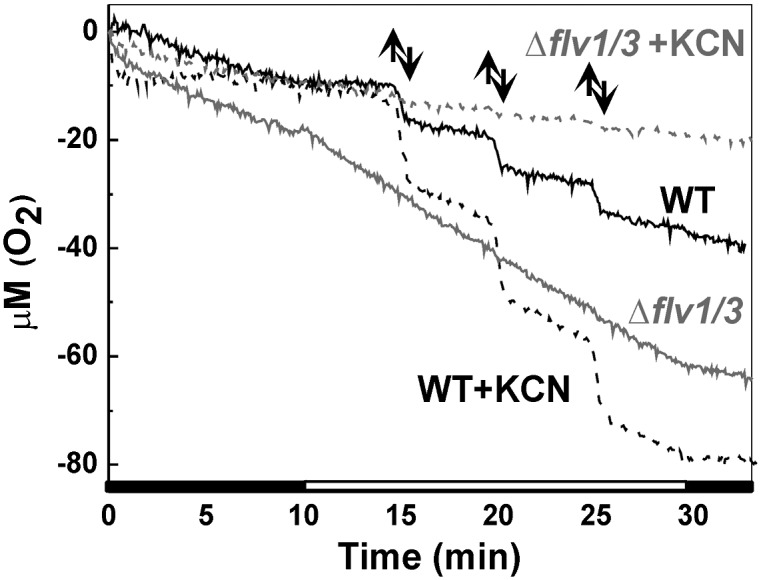
Effect of KCN on O2 uptake in the WT and ∆flv1/∆flv3 cells. O2 uptake was recorded from the WT (black line) and ∆flv1/∆flv3 cells (gray line) during 10-min darkness and upon exposure to FL in the absence (solid line) and in the presence (dashed line) of 1 mM KCN. Up and down arrows show on and off high-light pulses, respectively.
Influence of FL on the Functional Status of PS in the Δflv1/Δflv3 Mutants.
Real-time gas-exchange measurements (Fig. 2) provided evidence that the strongly impaired growth of the Δflv1/Δflv3 mutant cells under FL, compared with WT, is due to problems in the photosynthetic apparatus. To reveal the partial reactions of photosynthesis that specifically suffer under FL, we next addressed separately the function of PSII and PSI.
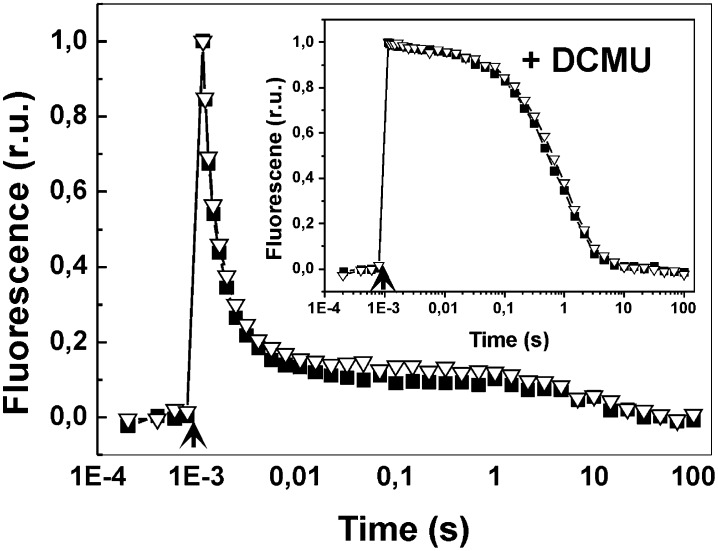
Both the acceptor and donor side status of PSII were assessed by recording a flash-induced fluorescence yield and its subsequent decay in darkness in the absence and presence of 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (DCMU), respectively. As shown in Fig. 4, functions of the acceptor and donor sides of the PSII complex were only marginally modified by FL conditions in the Δflv1/Δflv3 mutant compared with WT. Thus, the drastic effects of FL on photosynthesis in the Δflv1/Δflv3 mutant, as depicted by MIMS experiments (Fig. 2), cannot be attributed to malfunction of the donor or acceptor side of PSII.
Fig. 4.
Flash-induced increase in variable fluorescence and its subsequent decay in the WT and ∆flv1/∆flv3 cells in the absence and in the presence of DCMU (inset). WT (■) and Δflv1/Δflv3 (∇) were grown under constant-light conditions of 50 μmol photons⋅m−2⋅s−1, then set at OD750 = 0.4–0.5 and shifted to FL 50/500 for 3 d before the measurements. r.u., relative units.
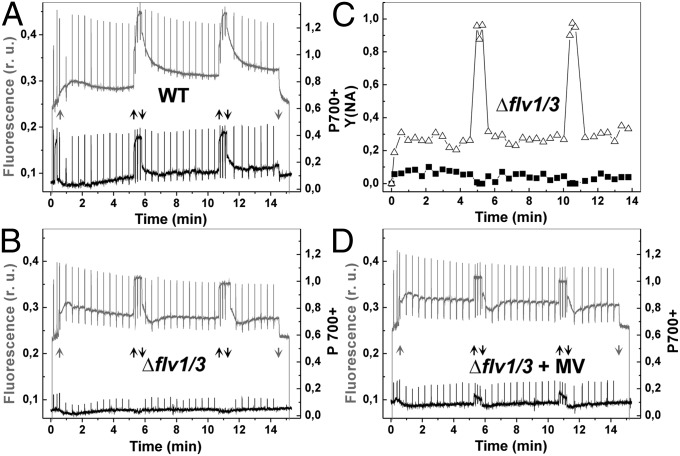
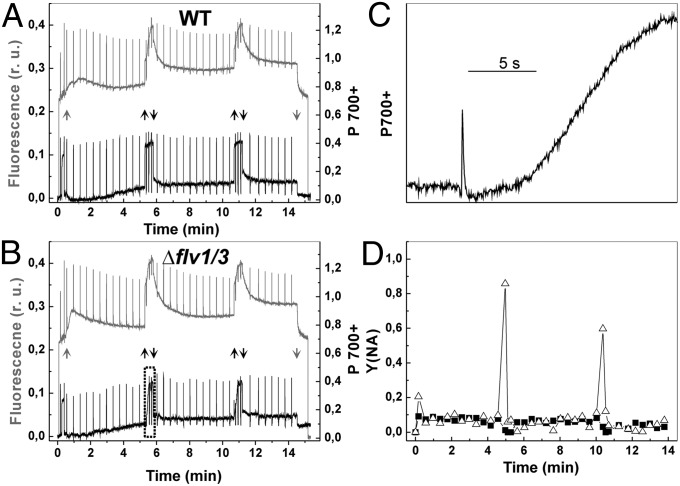
To get a more comprehensive picture of photosynthetic electron transfer properties in vivo, the chlorophyll (Chl) fluorescence and P700 redox state of WT and mutant cells were monitored simultaneously by DUAL-PAM using the fluctuating actinic light (Fig. 5). WT cells were dark-incubated for 10 min, and the first saturating pulse (5,000 μmol photons⋅m−2⋅s−1, 300 ms) was applied to monitor dark-level F0 and FM of Chl fluorescence. Cells were then illuminated with far-red light (75 W/m2) for 10 s, and the second saturating pulse was applied for determination of the PM value, a maximal change of the P700 signal from the fully reduced to the fully oxidized state. This light treatment also strongly increased FM value of Chl fluorescence due to state-2–state-1 transition (Fig. 5A). Then actinic red light, designed to mimic the fluctuating growth light condition, was turned on. Application of low actinic light resulted in gradual decrease of FM′. Switching on high light induced an increase in the Fs level, indicating strong reduction of the electron-transfer chain. Both the PSII yield, Y(II), and the PSI yield, Y(I), significantly decreased during fluctuating high-light cycles (Fig. 5A and Fig. S3 A and B). The Δflv1/Δflv3 mutant demonstrated lower Y(II), compared with WT, already at the onset of the illumination, and application of high-light pulses completely closed the PSII centers (Fig. 5B and Fig. S3A). During illumination with 50 µmol photons·m−2·s−1 actinic light, P700 remained partially reduced in both WT and mutant cells (Fig. 5 A and B). Subsequent application of the high-light pulses of 500 µmol photons·m−2·s−1 strongly oxidized P700 in the WT, whereas in the mutant P700 stayed completely reduced (Fig. 5 A and B). The same behavior of P700 was repeating during the entire experiment with FL. Moreover, application of the strong saturating pulse during the high-light phases of FL was not effective enough to oxidize P700 in the mutant, demonstrating a strong acceptor-side limitation of PSI during this phase (Fig. 5 B and C). The maximum oxidizable amount of PSI centers (PM) was approximately three times lower in the mutant cells compared with WT (Fig. 5 A and B and Table 1).
Fig. 5.
PSII and PSI properties of the WT and ∆flv1/∆flv3 cells grown under FL. (A, B, and D) Chl fluorescence (gray line) and P700 (black line) were monitored simultaneously in WT (A) and ∆flv1/∆flv3 in the absence (B) and presence (D) of MV. (C) Acceptor-side limitation of PSI was calculated from WT (■) and Δflv1/Δflv3 (△) in the absence of MV. The cells were grown under constant light, then set at OD750 = 0.4–0.5 and shifted to FL 50/500 for 2–3 d before the measurements. Low background actinic light (58 μmol photons⋅m−2⋅s−1; gray arrow) and high light (530 μmol photons⋅m−2⋅s−1; black arrow) was switched on and off (up and down arrow, respectively), mimicking FL. r.u., relative units.
Incubation of the Δflv1/Δflv3 mutants with methyl-viologen (MV), an artificial electron acceptor of PSI, resulted in only partial elimination of the acceptor side limitation of PSI, indicated by the appearance of the oxidized P700 fraction in the mutant cells upon a saturating light pulse fired during the high-light phase. MV also induced a small portion of open PSII centers during the high-light illumination phase of FL (Fig. 5D).
To reveal the molecular mechanism(s) behind such obscure modifications of the P700 oxidoreduction signals in the Δflv1/Δflv3 mutant upon growth under FL, we next exposed the cells grown under constant-light conditions of 50 µmol photons·m−2·s−1 to FL 50/500 and simultaneously recorded both the Chl fluorescence and the P700 oxidoreduction signals. During low-light phases of the FL experiment, the mutant cells grown under constant light did not show any distinct difference in the function of PSII or PSI compared with WT (Fig. 6 A and B and Fig. S4 A and B). The PM values were also similar in the mutant (0.41 ± 0.01; n = 3) and WT (0.40 ± 0.02; n = 3) growing at constant-light conditions. During the subsequent low- to high-light transition, P700 became strongly oxidized in the WT cells (Fig. 6A and Fig. S4C). In contrast, only a very fast and transient oxidation of P700, followed by prompt relaxation of P700+ occurred in the Δflv1/Δflv3 mutant cells at the onset of the high-light phase of the FL cycles (Fig. 6 B and C). After transient oxidoreduction, P700 remained completely reduced ∼5 s and reached a steady-state oxidation level only after ∼15 s of exposure to high light (Fig. 6C). Such a strong delay in P700 oxidation in mutants confirmed that the Flv1 and Flv3 proteins function as an important electron sink, and the absence of these proteins created strong and, importantly, repetitive acceptor-side limitation of PSI during the low- to high-light transitions of FL (Fig. 6D). Moreover, we monitored light-induced energization of membrane in WT and mutant cells treated with FL 50/500 for 3 d by using fluorescent pH indicator acridine yellow (AY). The mutant cells demonstrated approximately two times less light-induced changes in AY fluorescence (Fig. S5). Taking into account the fact that the relation between ΔpH and changes in amine fluorescence is linear, we can assume that low ΔpH values observed in the mutant might result in possible ATP starvation and consequently participate in causing the growth arrest in the Δflv1/Δflv3 mutant cells under FL.
Fig. 6.
PSII and PSI properties of the WT and ∆flv1/∆flv3 cells grown under constant light. (A and B) Chl fluorescence (gray line) and absorbance changes of P700 (black line) were recorded simultaneously in WT (A) and ∆flv1/∆flv3 (B). (C) Oxidation of 700 during low- to high-light transitions in ∆flv1/∆flv3. (D) Acceptor-side limitation of PSI in WT (■) and Δflv1/Δflv3 (△). The cells were grown under constant light, then set at OD750 = 0.4–0.5 and shifted to FL 50/500 for 2–3 d before the measurements. Low-background actinic light (58 μmol photons⋅m−2⋅s−1; gray arrow) and high light (530 μmol photons⋅m−2⋅s−1; black arrow) were switched on and off (up and down arrow, respectively), mimicking FL. r.u., relative units.
Response of Photosynthetic Proteins to FL.
To clarify whether the distinct modification of PSI function under FL in the absence of the Flv1 and Flv3 proteins is reflected at the level of photosynthetic proteins, the photosynthetic apparatus was examined after 4 d of growth under FL using an immunoblotting approach and different protein-specific antibodies. The steady-state level of PsaB, the core protein of PSI, decreased >50% in Δflv1, Δflv3, and Δflv1/Δflv3 mutant cells compared with WT, whereas D1, the PSII reaction center protein, was not significantly affected (Fig. 7A). No marked changes were observed in the amount of two different FNR isoforms and the Cytf subunit of the Cytb6f complex. Instead, a significant down-regulation of RbcL, representing ribulose-1,5-biphospate carboxylase/oxygenase, was evident in all studied mutant cells compared with WT. The Δflv1::flv1 complementation mutant behaved similarly to WT in respect to photosynthetic proteins, but the Flv1 protein was highly up-regulated.
Fig. 7.
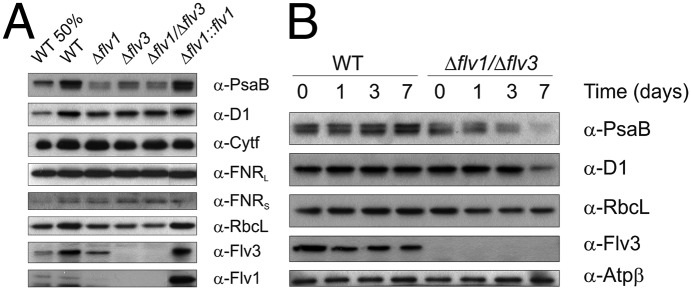
Immunoblots demonstrating the relative amounts of proteins in the WT; Δflv1, Δflv3, and Δflv1/Δflv3 mutants; and complementation strain. (A) Protein content of the WT and mutant strains grown 4 d under FL 50/500 regime was analyzed with corresponding antibodies. (B) Changes in the protein content of WT and the Δflv1/Δflv3 mutant upon shift from constant-growth light (day 0) to FL 50/500 for 1, 3, and 7 d. Proteins from total cell extracts (10 µg in each well) were separated by SDS/PAGE, and immunoblotting was performed by using specific antibodies. Flv1 and Flv3 proteins were detected from soluble samples (30 µg in each well).
Next, we assessed dynamic changes in photosynthetic proteins by shifting WT and the Δflv1/Δflv3 mutant from constant-growth light conditions to FL and by probing the total protein fraction with corresponding antibodies (Fig. 7B). In WT cells, the amount of PsaB started to increase after 3 d and was significantly up-regulated after 7 d of FL illumination. In contrast to this result, the Δflv1/Δflv3 mutant cells demonstrated a drastic decrease of the PsaB protein on the third day of FL treatment, and the protein was nearly depleted after 7 d. The shift from constant-growth light to FL conditions did not affect the level of the D1 protein in WT. In the Δflv1/Δflv3 mutant cells, the amount of the D1 protein was stable during the first 3 d of exposure to FL, but showed significant decrease on the seventh day. The protein amount of the RbcL started to decrease already from the first day of shift to FL in the Δflv1/Δflv3 mutant cells. Furthermore, the shift from constant-growth light to FL 50/500 conditions for 7 d significantly increased protein carbonylation in the mutant cells, demonstrating high ROS production and strong oxidative damage to proteins (Fig. S6).
Discussion
Cyanobacteria and Plants Have Distinctively Different Strategies to Cope with FL.
Natural light environment of photosynthetic organisms is highly dynamic, which inflicts a threat to the function of the photosynthetic apparatus. Key regulatory mechanisms of higher plants to cope with rapidly fluctuating light intensities were recently addressed (8, 9). The natural light environment of cyanobacteria, typically the oceans and lakes, is even more challenging, with predictable fluctuations in light intensity as well as in the frequency of high-light pulses (<1 Hz) far exceeding those occurring in the land. Indeed, a lens effect of waves produces excessively strong and also dim light, yet the intensity of light fluctuations decreases rapidly with increasing depth (12).
To elucidate cyanobacterial strategies to cope with FL, we tested whether one of the most important components involved in safeguarding the photosynthetic apparatus of higher plants under FL, the PGR5 protein, has a similar function in cyanobacteria. This hypothesis, however, turned out not to be the case. Indeed, deletion of the pgr5 homolog from the Synechocystis genome did not render the cells susceptible to FL (Fig. S1E), which contradicts with the behavior of the pgr5 mutant of Arabidopsis (9). Other proteins and mechanisms, so far identified as important for higher plants to cope with FL environments—for example, the STN7 kinase (8)—do not exist in cyanobacteria because of completely different architecture of the light harvesting systems.
Because mechanisms enabling plants to cope with abrupt fluctuations in light intensity are not present or functional in cyanobacteria, it is highly conceivable that the respective cyanobacterial mechanisms are not conserved in higher plants. Considering the possible cyanobacterial strategies to cope with FL, we addressed the role of FDPs, a unique family of cyanobacterial proteins that are involved in electron transfer processes. Two of the cyanobacterial FDPs (Flv2 and Flv4) function in photoprotection of PSII (2, 3), whereas the Flv1 and Flv3 proteins in Synechocystis function in accepting electrons after PSI and directing them to O2, in the so-called “Mehler-like” reaction that does not produce ROS (4, 5). Corresponding proteins in filamentous Anabaena cells are called Flv1A and Flv3A (7).
Flv1 and Flv3 Proteins Enable Growth and Photosynthesis of Cyanobacteria Under FL Conditions.
Despite earlier research showing the function of Flv1 and Flv3 as a light-induced O2 uptake mechanism (4, 5), attempts to find any environmental condition that would induce detrimental growth phenotype for the Δflv1 and/or Δflv3 mutants compared with WT Synechocystis have so far failed. Only the Δflv3 mutant has occasionally been shown to have a reduced capability to cope with high light (6).
Intriguingly, the exposure of the Δflv1(A) and/or Δflv3(A) mutants of Synechocystis and Anabaena to FL clearly demonstrated the indispensable role of these FDPs in survival and growth of cyanobacterial cells under conditions of abrupt changes in light intensity. Subjecting the cells to two different background illuminations, but keeping the intensity and frequency of high-light pulses constant, revealed that the background light intensity determines the severity of FL stress (Fig. 1 B and C). It is conceivable that the Flv1 and Flv3 proteins coevolved in cyanobacteria together with oxygenic photosynthesis to protect the photosynthetic apparatus upon strong fluctuations in light intensity characteristic to aquatic environments.
PSI Is the Primary Target of FL in the Absence of the Flv1/Flv3 Heterodimer.
The importance of the Flv1 and Flv3 proteins as an electron sink becomes evident only under FL—i.e., when the background low light is repeatedly interrupted with high-light pulses. More detailed analysis of the photosynthetic apparatus revealed that the function of PSI, but not of PSII, was strictly compromised in the Δflv1/Δflv3 mutant (Figs. 4 and 5). For further inspection of reasons behind such a severe phenotype, the Δflv1/Δflv3 mutant cells were grown under constant light and then subjected to actinic FL during measurements with DUAL-PAM. This study demonstrated a delay in oxidation of P700 upon the low- to high-light transition (Fig. 6C), due to strong acceptor-side limitation of PSI. In the thylakoid membrane of plants, strong PSI acceptor-side limitation results in leaking of electrons to O2, thus generating superoxide radicals not only at the stromal side, but also within the thylakoid membrane, most likely originating from the A1 electron acceptor under conditions when the Fe-S centers are reduced (13). Enhanced back-reactions due to the acceptor-side limitation of PSI also have a capacity to result in Chl triplet formation and consequently in formation of singlet oxygen that is known to be extremely hazardous for the protein environment (14). Cyanobacteria possess the Flv1 and Flv3 proteins, which are functioning as a strong and efficient electron sink during overreduction of electron transport chain by preventing the acceptor-side limitation of PSI and thus also ROS production. The Δflv1 and Δflv3 mutant cells grown under constant light do not show acceptor-side limitation of PSI during steady-state low light (Fig. 6D). Conversely, if the mutants were grown under constant light and shifted to FL conditions, the extreme and, importantly, repeated acceptor-side limitation of PSI, was observed during the low- to high-light transition phases (Fig. 6D), resulting in the strong and recurrent overreduction of electron-transport chain. At earlier stages of the FL treatment (up to 3 d), production of ROS and oxidative damage to proteins were not significant (Fig. S6), but at relatively lower levels, ROS might also function in triggering a signaling cascade to down-regulate the expression of PSI proteins. Indeed, a marked loss of PSI proteins was detected during 3 d of the FL treatment, whereas no changes in PSII proteins were observed (Fig. 7B). Upon prolonged FL treatment, a preferential loss of PSI complexes led to an increase in PSII-to-PSI ratio in the Δflv1/Δflv3 mutant. During low background light of FL, PSI could still cope with the relatively small amount of electrons coming from PSII. Upon abrupt high-light pulses, however, the overreduction of the intersystem electron-transfer chain kept P700 reduced, thus completely blocking linear electron flow and inhibiting photosynthesis. An impairment of net photosynthesis in the Δflv1/Δflv3 mutants, which implies decreased CO2 fixation, is in line with observed down-regulation of PSI complexes and RbcL subunits of RubisCO under FL. After prolonged incubation under FL, malfunction of PSI and, consequently, strongly increased ROS production and oxidative stress led to the irreversible cell damage and death (Fig. S6). Indeed, the Flv1 and Flv3 proteins in cyanobacteria are crucial to maintaining the redox balance of the electron-transfer chain, which provides protection for PSI under FL.
Finally, the complementation strain, by revealing the WT characteristics of growth and reverting the functional status of the photosynthetic apparatus, confirmed that the presence of the Flv1/Flv3 heterodimer provides cyanobacterial cells with a harmless way to dissipate excess electrons from PSI, which in turn is essential to sustaining photosynthesis under FL conditions (Figs. 1B and 7 and Table 1).
Concluding Remarks
We have demonstrated here an indispensable role of the Flv1 and Flv3 proteins in safeguarding of PSI and the survival of cyanobacteria under FL. Another FDP heterodimer, Flv2/Flv4, was previously shown to be crucial for photoprotection of PSII under high light and/or Ci-deprivation in β-cyanobacteria. Indeed, cyanobacteria, the progenitors of higher plant chloroplasts, have unique mechanisms to protect the photosynthetic apparatus against ROS. Intriguingly, the phylogenetic tree of the FDPs reveals gradual disappearance of FDPs upon evolution of higher plants. We postulate that the development of oxygenic photosynthesis in cyanobacteria was accompanied by evolution of the unique FDPs that provided a safe function of the photosynthetic machinery under oxygenic environment. Gradual disappearance of FDPs during evolution is apparently related to their futile function in storage of reducing equivalents, and they were gradually replaced by more “energy-efficient” mechanisms to regulate photosynthetic electron flow. It is conceivable that the movement of life from oceans to the land made such evolution possible.
Materials and Methods
Strains and Culture Conditions.
Synechocystis and Anabaena WT and the mutant strains were grown in BG-11 medium buffered with 20 mM TES N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid–KOH (pH 8.2) under continuous light (50 μmol photons⋅m−2⋅s−1) at 30 °C and 3% (vol/vol) CO2. Experiments were performed in growth chambers (AlgaeTron AG 130-ECO; PSI) equipped with LED white light, and the cells were shifted to air-level CO2. The following light regimes were applied: constant light of 20, 50, and 500 μmol photons⋅m−2⋅s−1 (dim, normal, and high light, respectively); FL 20/500 (20 μmol photons⋅m−2⋅s−1, 5 min/500 μmol photons⋅m−2⋅s−1, 30 s); and FL 50/500 (50 μmol photons⋅m−2⋅s−1, 5 min/500 μmol photons⋅m−2⋅s−1, 30 s). For all physiological experiments, the cells were first grown under constant light of 50 μmol photons⋅m−2⋅s−1, then inoculated to OD750 = 0.4–0.5 and shifted to FL 20/500 or 50/500 for 3–4 d before the measurements. For activity measurements, the cells were harvested and resuspended in a fresh BG-11 medium at Chl concentration of 10 or 15 μg⋅mL−1.
Mutant Strains.
The Δflv1 and Δflv3 single and Δflv1/Δflv3 double mutants have been described (4, 5). The construction of the Δflv1::flv1 complementation strain, pgr5 deletion mutant in Synechocystis, and flv1A and flv3A deletion mutants of Anabaena are described in SI Materials and Methods and Fig. S7.
MIMS.
Measurements of 16O2 (mass 32), 18O2 (mass 36), and CO2 (mass 44) exchange were performed simultaneously by MIMS directly from suspension cultures as described (ref. 5; SI Materials and Methods).
Fluorescence and P700 Measurement.
Chl fluorescence and P700 oxidoreduction were recorded by DUAL-PAM-100 (Walz). Mimicking of FL during measurements was performed by changing the intensity of applied red actinic light (635 nm) between 58 and 530 μmol photons⋅m−2⋅s−1.
Protein Isolation, Electrophoresis, and Immunodetection.
Total cell extract and the soluble fraction of Synechocystis cells were isolated as described in ref. 2. Proteins were separated by 12% SDS/PAGE containing 6 M urea. The proteins were electrotransferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore) and immunodetected by protein-specific antibodies. Additional information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the Academy of Finland Center of Excellence Project 118637, the European Union-Seventh Framework Programme ENERGY Project Energy-2010-1 Agreement 256808, the Finnish Doctoral Programme in Computational Chemistry and Molecular Spectroscopy (LasKeMo), and the Marie Curie Initial Training Network Harvest. Experimental support for MIMS experiments was provided by the HélioBiotec platform, funded by the European Union European Regional Development Fund, the Région Provence Alpes Côte d’Azur, the French Ministry of Research, and the Commissariat à l’Energie Atomique.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221194110/-/DCSupplemental.
References
- 1.Vicente JB, Justino MC, Gonçalves VL, Saraiva LM, Teixeira M. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 2008;437:21–45. doi: 10.1016/S0076-6879(07)37002-X. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM. Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE. 2009;4(4):e5331. doi: 10.1371/journal.pone.0005331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, et al. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell. 2012;24(5):1952–1971. doi: 10.1105/tpc.111.094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helman Y, et al. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol. 2003;13(3):230–235. doi: 10.1016/s0960-9822(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 5.Allahverdiyeva Y, et al. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J Biol Chem. 2011;286(27):24007–24014. doi: 10.1074/jbc.M111.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackenberg C, et al. Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC 6803. Planta. 2009;230(4):625–637. doi: 10.1007/s00425-009-0972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermakova M, Battchikova N, Allahverdiyeva Y, Aro EM. Novel heterocyst-specific flavodiiron proteins in Anabaena sp. PCC 7120. FEBS Lett. 2013;587(1):82–87. doi: 10.1016/j.febslet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM. Steady-state phosphorylation of light-harvesting complex II proteins preserves Photosystem I under fluctuating white light. Plant Physiol. 2012;160(4):1896–1910. doi: 10.1104/pp.112.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suorsa M, et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell. 2012;24(7):2934–2948. doi: 10.1105/tpc.112.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildner GF, Henkel J. Specific inhibition of the oxygenase activity of ribulose-1,5-bisphosphate carboxylase. Biochem Biophys Res Commun. 1976;69(1):268–275. doi: 10.1016/s0006-291x(76)80302-6. [DOI] [PubMed] [Google Scholar]
- 11.Hart SE, Schlarb-Ridley BG, Bendall DS, Howe CJ. Terminal oxidases of cyanobacteria. Biochem Soc Trans. 2005;33(Pt 4):832–835. doi: 10.1042/BST0330832. [DOI] [PubMed] [Google Scholar]
- 12.Iluz D, Alexandrovich I, Dubinsky Z. The enhancement of photosynthesis by fluctuating light. In: Najafpour M, editor. Artifical Photosynthesis. Rijeka, Croatia: InTech; 2012. pp. 115–134. [Google Scholar]
- 13.Mubarakshina MM, Ivanov BN. The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol Plant. 2010;140(2):103–110. doi: 10.1111/j.1399-3054.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford AW, Osyczka A, Rappaport F. Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O(2) FEBS Lett. 2012;586(5):603–616. doi: 10.1016/j.febslet.2011.12.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.