Abstract
The cell intrinsic factors that determine whether a neuron regenerates or undergoes apoptosis in response to axonal injury are not well defined. Here we show that the mixed-lineage dual leucine zipper kinase (DLK) is an essential upstream mediator of both of these divergent outcomes in the same cell type. Optic nerve crush injury leads to rapid elevation of DLK protein, first in the axons of retinal ganglion cells (RGCs) and then in their cell bodies. DLK is required for the majority of gene expression changes in RGCs initiated by injury, including induction of both proapoptotic and regeneration-associated genes. Deletion of DLK in retina results in robust and sustained protection of RGCs from degeneration after optic nerve injury. Despite this improved survival, the number of axons that regrow beyond the injury site is substantially reduced, even when the tumor suppressor phosphatase and tensin homolog (PTEN) is deleted to enhance intrinsic growth potential. These findings demonstrate that these seemingly contradictory responses to injury are mechanistically coupled through a DLK-based damage detection mechanism.
Axonal damage results in significant neuronal cell death and axon degeneration, often leading to permanent functional deficits. For example, optic nerve crush rapidly induces a stress response in retinal ganglion cells (RGCs) that includes profound alterations in gene expression patterns (1) and ultimately leads to apoptosis of these neurons (2). As axon injury may occur a significant distance from the cell body, it has been proposed that retrograde molecular motors play a critical role in conveying damage signals to the nucleus, allowing the cell to respond to damage (3). Attenuation of this transport mechanism has been shown to reduce degeneration, suggesting that the ability of the nucleus to detect an insult is an essential component of the injury response (4).
Recent data suggest that dual leucine zipper kinase (DLK) is an essential component of the neuronal response to axon damage. DLK protein is present in axons, and protein levels are increased in response to axonal injury (5). Loss of DLK has been shown to protect distal axons from Wallerian degeneration (6) and to abrogate stress-induced retrograde c-Jun N-terminal kinase (JNK) signaling through interaction with the scaffolding protein JNK-interacting protein 3 (JIP3) (7-9). In many instances, injury-induced JNK activation in neurons results in apoptosis through phosphorylation of activator protein 1 (AP-1) transcription factors such as c-Jun, which initiates a proapoptotic gene expression program (10, 11). Consistent with this, genetic deletion of JNK2 and/or JNK3 is sufficient to protect neurons from degeneration in a range of CNS injury models, including axotomy (12–14), although the role of DLK in these contexts is not known.
In contrast, DLK has been shown to regulate axon regeneration after axonal injury in adult peripheral nerves (9) and invertebrate systems (5, 15). The mechanism underlying the divergence between these apoptotic and regenerative phenotypes is unclear, but it could reflect distinct signaling pathways downstream of DLK in each system. Alternatively, this disparity may be a result of differences in the intrinsic or extrinsic factors that govern the potential for regrowth in the CNS and peripheral nerves.
In the current study, we use the optic nerve crush model in DLK-inducible knockout mice to investigate the role of this kinase after CNS axonal injury. Our results demonstrate that although neurodegeneration takes place during a period of weeks after nerve crush, initiation of a transcriptional stress response occurs rapidly in RGCs and includes both proapoptotic and proregenerative gene expression changes. DLK deletion broadly attenuates this response, provides substantial protection of RGCs from apoptosis, and eliminates the modest but reproducible axon regrowth observed after injury. These observations suggest a model in which optic nerve crush induces prolonged DLK-dependent stress signaling that coordinately primes RGCs for both apoptosis and regrowth but ultimately leads to cell death resulting from the absence of regenerative potential in the optic nerve. In this model, DLK-deficient neurons do not display either outcome, as they are largely unable to detect axonal injury.
Results
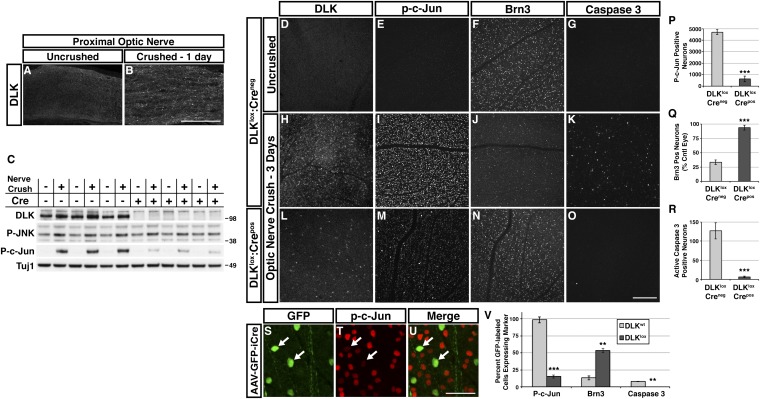
To determine whether DLK is activated in RGC axons after optic nerve crush, we stained sections of nerves and retinas for DLK and other markers 1–7 d after injury. DLK levels increased within 1 d in RGC axons, but not other cells (Fig. S1A), of the injured nerve (Fig. 1 A and B), and within 3 d in the ganglion cell layer (GCL) and nerve fiber layer of the retina (Fig. S1 B and C), implying that DLK signaling initiates in RGC axons after crush and remains limited to RGCs. Similarly, despite a high basal level of stress-independent phosphorylated JNK (p-JNK) in retinal neurons (16), injury-dependent p-JNK is increased in retina within 1 d (Fig. 1C), with elevated phosphorylation of its downstream AP-1 transcription factor c-Jun in the GCL during the first 3 d after nerve crush serving as a more sensitive readout of this stress-mediated JNK signaling (Fig. 1C and Fig. S1D). In contrast, expression of brain-specific homeobox/POU domain protein 3 (Brn3), a well-characterized RGC marker (17), was greatly reduced during this time (Fig. S1E). Only a small fraction of cells was actively undergoing apoptosis during this period, with the first detectable staining for active caspase 3 appearing 3 d after crush (Fig. S1F), which is consistent with previous studies (14). Thus, DLK up-regulation, JNK activation, and Brn3 down-regulation occur rapidly after nerve crush injury and precede neuronal apoptosis.
Fig. 1.
DLK is required for c-Jun phosphorylation and RGC apoptosis after nerve crush. Staining of proximal optic nerve 24 h after sham surgery (A) or nerve crush (B) reveals a crush-dependent increase in DLK protein. (Scale bar, 100 μm.) (C) Western blot from DLKlox:Creneg and DLKlox:Crepos retinas 24 h after nerve crush from three animals of each genotype. Nerve crush results in elevated p-JNK and p-c-Jun levels in DLKlox:Creneg retinas (lanes 1–6). DLK expression is significantly reduced in DLKlox:Crepos retinas, which attenuates the crush-induced increase in p-JNK and p-c-Jun (lanes 7–12). (D–G) Staining of whole-mount retinas from eyes with uncrushed optic nerves reveals many Brn3-positive RGCs (F) and no detectable DLK (D), p-c-Jun (E), or activated caspase 3 (G). (H–K) Retinas from DLKlox:Creneg mice 3 d after nerve crush. DLK is visible in many RGCs (H). A large number of RGCs are p-c-Jun positive (I), whereas the number of Brn3-positive RGCs is greatly reduced (J). A small fraction of cells show activation of caspase 3 (K). (L–O) Retinas from DLKlox:Crepos mice 3 d after nerve crush. Expression of DLK is visible in a small fraction of RGCs (L). A similarly small number of RGCs are brightly p-c-Jun positive, whereas the remainder show only very low levels of p-c-Jun staining (M). The number of Brn3-positive RGCs is comparable to uncrushed retinas (N), and only minimal activation of caspase 3 is observed (O). (Scale bar, 200 μm.) (P–R) Quantification of p-c-Jun staining shown in I and M. (P; n = 3/genotype), Brn3 staining shown in F, J, and N. (Q; n = 5/genotype), and caspase 3 staining shown in K and O. (R; n = 5/genotype; all error bars, SEM; ***P < 0.001). (S–U) Low-titer transduction of DLKlox:Creneg retinas with AAV-GFP-2A-iCre vector demonstrates cell-autonomous regulation of p-c-Jun by DLK. GFP-expressing DLK-null RGCs (arrows) exhibit greatly reduced p-c-Jun staining (red) compared with adjacent uninfected neurons 3 d after nerve crush. (Scale bar, 50 μm.) (V) Quantification of the proportion of AAV-GFP-2A-iCre–transduced RGCs displaying strong staining for p-c-Jun, Brn3, and activated caspase-3 3 d after nerve crush in DLKwild-type and DLKlox mice (**P < 0.01, ***P < 0.001; error bars, SEM; n = 3/genotype).
To investigate the role of DLK after optic nerve crush, we generated mice with a tamoxifen-inducible Cre recombinase-estrogen receptor (Cre-ERT) transgene driven by the chicken beta-actin-CMV hybrid (CAG) promoter, resulting in high levels of Cre-ERT in many tissues, including retina. CAG Cre-ERT:DLKlox/lox (referred to as DLKlox:Crepos) mice survive to adulthood with no evidence of the developmental abnormalities seen in DLK-null animals (8, 18). Dosing of DLKlox:Crepos mice at 10–12 wk of age with tamoxifen (Fig. S2A) results in elimination of the majority of DLK expression in retina (Fig. 1 C and L), with the small amount of remaining DLK protein varying slightly from animal to animal (Fig. 1C and Fig. S2B). No differences in health or behavior were observed between tamoxifen-treated DLKlox:Crepos mice and their tamoxifen-treated DLKlox:Creneg control littermates.
To examine the effect of DLK deletion on the stress response after optic nerve crush, DLKlox:Crepos and control animals were analyzed 3 d after injury in retina whole mounts. c-Jun phosphorylation and caspase 3 activation were significantly increased after nerve crush in control animals (compared with contralateral uncrushed retinas), accompanied by an increase in DLK and a decrease in Brn3-positive cells (Fig. 1 D–K). Evaluation of DLKlox:Crepos retinas revealed attenuation of all these injury-induced changes. Up-regulation of DLK protein after crush was absent in all but a small fraction of GCL neurons (Fig. 1L). The number of brightly p-c-Jun positive cells was also reduced to 13% of that seen in littermate controls (Fig. 1 E, I, M, and P). The persistence of some p-c-Jun labeled nuclei in DLK-deficient retinas likely reflects incomplete knockout, given that p-c-Jun-positive cell number is variable from animal to animal and levels of p-c-Jun correlate with the amount of DLK protein remaining (Fig. S2 B and C). Brn3 expression in DLKlox:Crepos retinas was maintained in 84% of RGCs after injury, whereas only 13% retained expression in DLKlox:Creneg retinas (Fig. 1 F, J, N, and Q), and caspase 3 activation in DLKlox:Crepos retinas was nearly eliminated (5% of littermate controls; Fig. 1 G, K, O, and R). Intravitreal injection of DLKlox:Creneg retinas with a low-titer adeno-associated viral vector (AAV) driving 2A peptide-mediated bicistronic neuronal expression of cytoplasmic GFP and codon-improved nuclear Cre (AAV-GFP-2A-iCre) resulted in similar attenuation of these injury-induced changes, specifically in transduced RGCs, demonstrating that DLK acts cell-autonomously to direct the neuronal injury response after nerve crush (Fig. 1 S–V).
We next addressed whether the reduced caspase activation observed at early points after optic nerve crush in DLKlox:Crepos retinas results in persistent protection of RGC axons and cell bodies. As Brn3 is rapidly down-regulated upon injury, we evaluated γ-synuclein as a marker based on the specific RGC expression observed by in situ hybridization (19, 20). An antibody against γ-synuclein displayed strong nuclear staining that colabeled, but was not limited to, all Brn3-positive nuclei (Fig. S3A). Further analysis revealed a strong correlation between γ-synuclein and the neuronal nuclei antigen (NeuN) (Fig. S3A), a pan-neuronal marker that labels RGCs and displaced amacrine cells, which make up ∼50% of total GCL neurons (21). Despite the lack of specificity for RGCs, the nuclear staining and uniform intensity enabled reliable quantification of GCL neurons and complemented assessment of RGC axons with an antineurofilament antibody. Unlike Brn3, neither of these markers displayed obvious changes in staining 3 d after nerve crush (Fig. S3B).
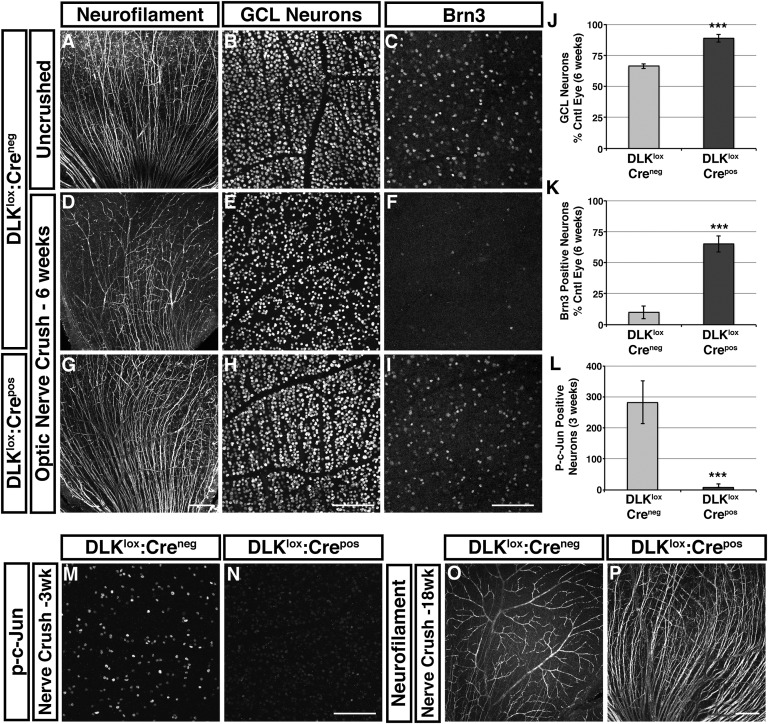
Six weeks after optic nerve crush, a substantial reduction in the number of neurofilament-labeled axons and a 44% reduction in GCL neurons were observed in DLKlox:Creneg retinas, whereas DLKlox:Crepos retained their axons and lost only 11% of GCL neurons (Fig. 2 A, B, D, E, G, H, and J). Brn3 was largely absent from DLKlox:Creneg retinas, but 65% of RGCs still maintained expression in DLKlox:Crepos retinas, even at this later time (Fig. 2 C, F, I, and K). Consistent with this finding, DLKlox:Crepos retinas displayed only 3% of the p-c-Jun labeled cells seen in littermate controls 3 wk after nerve crush (Fig. 2 L–N), indicating that DLK deficiency does not simply delay the injury response. Robust protection of RGC axons and GCL neurons, as well as Brn3 staining, persisted even 18 wk after injury, a time by which neurofilament labeled axons were nearly absent in control animals (Fig. 2 O and P and Fig. S3C).
Fig. 2.
Loss of DLK results in sustained protection of RGC axons and cell bodies from degeneration. (A–C) Retinas from DLKlox:Creneg mice with uncrushed optic nerves. Neurofilament staining labels RGC axons (A). GCL neurons (B) and Brn3-positive RGCs (C) from the same retina are shown in higher magnification. (D–F) Retinas from DLKlox:Creneg mice 6 wk after nerve crush. The majority of neurofilament-positive RGC axons have degenerated at this time (D). A concordant reduction in the number of GCL neurons is observed (E), and very few Brn3-positive cells are visible (F). (G–I) Retinas from DLKlox:Crepos mice 6 wk after nerve crush. Neurofilament-positive RGC axons remain largely intact (G). Only a small reduction in the number of GCL neurons is observed (H), and Brn3 expression is retained in many RGCs (I). (Scale bars, 200 μm for NF and 100 μm for Brn3 and GCL.) (J–L) Quantification of GCL neuron staining shown in E and H relative to contralateral control eye (J; n = 5/genotype), Brn3 staining shown in F and I relative to control (K; n = 5/genotype), and p-c-Jun staining shown in M and N (L; error bars, SEM; n = 3/genotype; ***P < 0.001). (M and N) p-c-Jun staining of retinas 3 wk after nerve crush. DLKlox:Creneg retinas have many p-c-Jun positive cells, whereas there are very few positive cells in DLKlox:Crepos retinas. (O and P) Neurofilament staining of retinas from DLKlox:Creneg and DLKlox:Crepos mice 18 wk after nerve crush. RGC axons are almost completely degenerated in DLKlox:Creneg but are still present in DLKlox:Crepos retinas. Most labeling in DLKlox:Creneg retinas reflects nonspecific staining of the retinal vasculature and a few remaining RGC axons in the lower left corner (n = 4 DLKlox:Creneg; n = 2 DLKlox:Crepos).
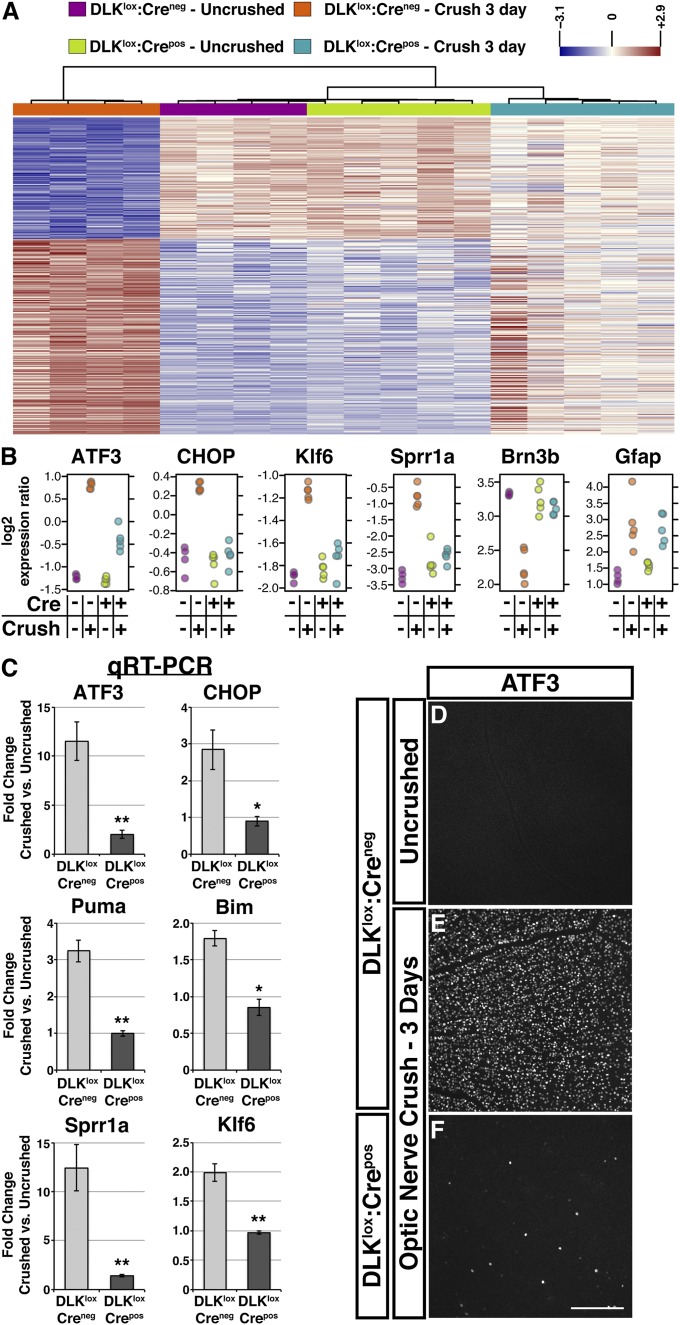
To better understand the mechanisms underlying DLK-dependent cell death, we next evaluated injury-induced gene expression changes by microarray in whole retina from DLKlox:Crepos mice and littermate controls 3 d after nerve crush. Acute isolation of RNA from whole retina avoids stimulation of a stress response during retinal dissociation yet still detects many of the expression changes found in purified RGCs after nerve crush (1, 22). In agreement with these previous studies, optic nerve injury in DLKlox:Creneg controls induced widespread changes in the retinal expression profile, with 342 genes up- or down-regulated (P < 0.01; Fig. 3A and Dataset S1). Unbiased pathway analysis revealed that the most highly significant genes up-regulated by injury were involved in biological processes enriched for terms such as stress response, cell death, and inflammation (Dataset S2). These include the proapoptotic genes Harakiri (Hrk) and C/EBP homologous protein (CHOP), both of which are required for neuronal cell death after axonal injury (23, 24), and suggest that many of the genes that regulate the apoptosis of RGCs are induced within 3 d of nerve crush.
Fig. 3.
DLK broadly regulates the transcriptional response to axonal injury. (A) Heat map of injury-induced gene expression changes (P < 0.01) between uncrushed and crushed control and DLK-deficient retinas observed 3 d after optic nerve crush (n = 342). Groups are clustered on the basis of similarity analysis. (B) Selected genes showing significant up- or down-regulation after nerve crush. The increases in ATF3, CHOP, Klf6, and Sprr1a expression and the decrease in Brn3b were attenuated in DLKlox:Crepos retinas. The increase in GFAP was genotype-independent. (C) Quantitative RT-PCR of selected expression changes observed in microarray. Numbers represent fold increase compared with uncrushed eyes of same genotype (error bars, SEM; n = 3/genotype; *P < 0.05, **P < 0.01). (D–F) Retinas stained for ATF3. Expression is negligible in uncrushed retinas (D) but is significantly increased throughout the GCL after nerve crush in DLKlox:Creneg mice (E). DLKlox:Crepos retinas display ATF3 in only a few scattered cells after crush (F). (Scale bar, 200 μm.)
Interestingly, the expression-profiling data also provided evidence of a proregenerative response to nerve crush in DLKlox:Creneg retinas. Among the up-regulated genes were a number that are also induced after sciatic nerve lesion (25) and have demonstrated roles in promoting axon regeneration in other systems (Dataset S3). These include small proline-rich repeat protein 1A (Sprr1a), fibroblast growth factor-inducible 14 (Fn14), and heat shock protein beta-1 (Hspb1) (26-28), as well as the transcription factors activating transcription factor 3 (ATF3) and Kruppel-like factor 6 (Klf6) (29, 30). Gene ontology and pathway analysis of the most significantly down-regulated genes revealed enrichment for terms relevant for the maturation of neurons (Dataset S2), including Brn3a and Brn3b, which may reflect a broader pattern of RGC dedifferentiation that could enhance regenerative capacity (31).
To identify which gene expression changes depend on DLK, expression profiles after injury were compared between DLKlox:Crepos and littermate DLKlox:Creneg retinas. Of 342 highly significant injury-induced changes, 201 (59%) displayed significant differences (P < 0.05) and 151 (44%) displayed highly significant differences between genotypes (P < 0.01). These values may represent an underestimation resulting from variance and incomplete excision (95%–99%) of DLK (Fig. 1L and Fig. S4 A and B), as evaluation of the top 100 most unambiguous injury-induced changes revealed that 92 of them exhibited dependence on DLK at P < 0.05, and assessment across a range of P values yields similar results (Datasets S1 and S3). Furthermore, hierarchical clustering of all samples across the top 342 genes indicated that crushed DLKlox:Crepos retinas are more similar to uninjured than injured control retinas (Fig. 3A). The list of DLK-dependent expression changes includes many genes with AP-1 transcription factor binding sites (Dataset S3) and genes implicated in both neuronal cell death and axon regeneration (Fig. 3B and Dataset S3).
To validate these results, we performed quantitative RT-PCR (qPCR) on independent samples for a number of prominent injury-induced genes. The results were consistent with those from the microarray, with the expression of both proapoptotic [CHOP, p53 upregulated modulator of apoptosis (Puma), Bcl-2 interacting mediator of cell death (Bim)] and regeneration-associated genes (ATF3, Sprr1a, Klf6) displaying clear DLK-dependence after injury (Fig. 3C). For ATF3, DLK-dependence was further confirmed by immunostaining of whole-mount retinas, which showed that up-regulation in DLKlox:Crepos retinas is restricted to a few scattered RGCs (Fig. 3 D–F). Low-titer transduction of DLKlox:Creneg RGCs with AAV-GFP-2A-iCre confirmed that injury-induced up-regulation of nuclear ATF3 is cell-autonomous (Fig. S4 C–F).
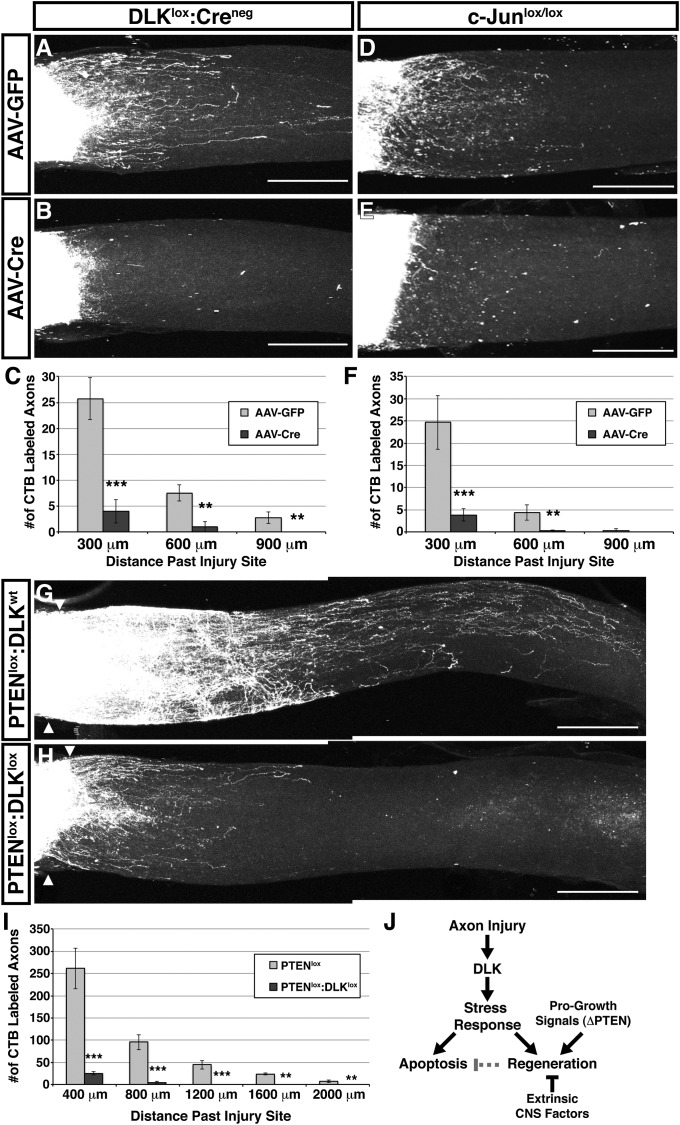
The lesion-induced elevation of regeneration-associated genes led us to investigate the extent to which RGC axons regrow past the injury site and whether this regeneration is dependent on DLK. Because axon regrowth after optic nerve crush is sparse (32), we used two-photon microscopy for optical sectioning through the entire depth of whole-mounted nerves to observe all of the axons past the lesion site (Fig. S5 A and B). Maximum projections of the resulting Z-stacks revealed that RGCs mount a modest but reproducible regenerative response (Fig. 4A). To determine whether the DLK-mediated transcriptional response is necessary for this regrowth, we evaluated axon regeneration 2 wk after optic nerve crush in DLKlox mice and c-Junlox mice injected intravitreally with AAV-Cre (32) (Fig. S5C). Disruption of either DLK or c-Jun reduced the regrowth of axons by greater than 85% compared to that observed in littermates injected with a control AAV-GFP vector (Fig. 4 A–F). Although the regrowth of RGCs axons after crush injury is limited, these findings show that they do initiate a regenerative response that requires both DLK and one of its downstream transcription factors, c-Jun. Together, these results argue that the DLK-mediated transcriptional response is necessary for the regrowth of injured RGC axons, but it is not certain whether activation of c-Jun alone would be sufficient to rescue the regenerative defects observed in the absence of DLK.
Fig. 4.
DLK is required for axon regeneration after optic nerve crush. (A and B) Cholera toxin β-Alexa 594 (CTB)-labeled axons growing past the injury site in DLKlox optic nerves 2 wk after crush. A maximum projection of a two-photon Z-series through the whole nerve reveals modest axon regrowth from RGCs previously transduced with a control AAV-GFP vector (A), but this regeneration is reduced after DLK deletion mediated by intravitreal injection of AAV-Cre (B). (Scale bars, 300 μm.) (C) Quantification of labeled axons that have grown past the injury site in A and B (error bars, SEM; n = 4 for AAV-GFP, n = 6 for AAV-Cre; **P < 0.01, ***P < 0.001). (D and E) CTB-labeled RGC axons growing past the injury site in Z-series maximum projections of c-Junlox optic nerves. Intravitreal AAV-Cre–mediated knockout of c-Jun (E) reduces the low level of basal regeneration observed 2 wk after crush (D). (F) Quantification of labeled axons that have grown past the injury site in D and E (error bars, SEM; n = 6 for AAV-GFP, n = 5 for AAV-Cre; **P < 0.01, ***P < 0.001). (G and H) CTB-labeled regenerating axons of PTEN-deficient RGCs in the presence or absence of DLK 2 wk after optic nerve crush. Prior intravitreal injection of AAV-Cre enables enhanced regeneration in PTENlox mice (G) that is reduced in PTENlox:DLKlox mice (H). Triangles mark the injury sites in these maximum projections of whole-nerve two-photon Z-stacks. (I) Quantification of axons growing past the injury site in G and H (error bars, SEM; n = 4/genotype; **P < 0.01, ***P < 0.001). (J) Model for coordinated regulation of apoptosis and axon regeneration by DLK. Axonal injury results in activation of DLK, which engages a transcriptional stress response that primes for both apoptosis and regrowth. DLK signaling combined with progrowth signaling via PTEN deletion results in axon regeneration, whereas DLK activation alone results in neuronal cell death resulting from factors that limit regeneration in the CNS. Active regeneration may suppress apoptosis and thus improve neuronal survival.
DLK transiently delays Wallerian degeneration of distal axons after sciatic nerve injury (6), so we next assessed whether failure of distal axon degeneration might contribute to the lack of axon regrowth in DLK-deficient optic nerves. We evaluated axon regeneration in an nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1)-overexpressing transgenic mouse line in which distal RGC axons display strong protection 7 d after crush (Fig. S5 D and E). Despite this protection, we observed no significant difference in regrowth past the lesion (Fig. S5 F–H).
To determine whether manipulations that relieve the cell-intrinsic limitations on axon regeneration in the CNS are able to override the requirement for DLK, we next evaluated regrowth after deletion of the tumor suppressor phosphatase and tensin homolog (PTEN), a negative regulator of the progrowth mammalian target of rapamycin (mTOR) pathway (32). As has been observed in previous studies (32), enhanced axon regeneration was enabled by AAV-Cre–mediated or AAV-GFP-2A-iCre–mediated knockout of PTEN in RGCs (Fig. 4D and Fig. S4I). However, this regeneration was reduced by more than 90%, but not eliminated, in mice harboring conditional alleles of both PTEN and DLK (Fig. 4 E and F and Fig. S4 J and K). Although differential knockout of these genes in a small number of RGCs cannot be entirely excluded, these results suggest that deletion of PTEN may provide a small DLK-independent enhancement of axon growth, but that substantial regeneration of growth-enabled RGCs requires the DLK-mediated neuronal stress response.
Discussion
In this study, we demonstrate that DLK is required for both the proapoptotic and proregenerative responses that occur after optic nerve lesion through broad regulation of injury-induced alterations in gene expression. Our results demonstrate that DLK is an essential component of the initial injury response apparatus in RGC axons. Four lines of evidence support this conclusion: (i) DLK protein is elevated before the majority of injury-dependent changes in RGC cell bodies; (ii) DLK is required for JNK activation, which is known to initiate in axons (7, 8); (iii) the diversity of DLK-dependent gene expression changes is consistent with a function as a common upstream signal for multiple stress-induced pathways, as suggested by its role in enabling retrograde signaling of both phosphorylated STAT3 and JNK after sciatic nerve lesion (9); and (iv) the persistence of Brn3 expression and absence of c-Jun phosphorylation in DLK-deficient RGCs several weeks after crush suggests that DLK cannot be circumvented by alternative signals. Taken together, these observations suggest that DLK acts at an early stage of the injury response and is essential for nuclear detection of axonal damage.
DLK-dependent induction of a number of proapoptotic genes occurs within 3 d of injury, including the unfolded protein response transcription factor CHOP and the BH3-only Bcl-2 family member Bim, both of which contribute to the death of RGCs (23, 33). Despite this early priming for apoptosis, many RGCs have not yet degenerated 3 wk after injury and remain p-c-Jun positive. These observations suggest that RGCs do not undergo a rapid degeneration after insult but, rather, enter a prolonged period in which stress signals are elevated before their eventual apoptosis. The type of slow, progressive degeneration may more accurately reflect the mechanism of RGC loss that occurs in the context of neurodegenerative diseases such as glaucoma, and this study and others suggest that DLK inhibition may represent an attractive therapeutic target in this indication (34).
Injury to peripheral nerves not only promotes apoptosis but also initiates a transcriptional program for axon regeneration in sensory neurons, whereas regeneration is poorly activated by lesions of the central branches of the same axons (35). This observation, combined with the lack of regeneration in the CNS, has led to a model in which CNS injury fails to robustly stimulate the intrinsic regrowth program. However, our results reveal that the DLK-mediated stress response involves a substantial proregenerative component in injured RGCs, including activation of c-Jun (36).
Our whole-nerve evaluation of regeneration after injury uncovered modest but significant DLK-dependent axon regrowth that remains limited because of the barriers on CNS regeneration. Moreover, we found that DLK is required for RGC axon regeneration even in the context of PTEN deletion, indicating that improved mTOR signaling is not sufficient for regeneration in the absence of this endogenous DLK-mediated stress response. Together these findings indicate that DLK directs a previously underappreciated proregenerative response after optic nerve injury, just as it does for the more familiar regeneration stimulated by peripheral nerve lesion (9) (Fig. 4J).
Previous studies indicate that neuronal stress can promote cell death in some contexts or axon regrowth in others (36, 37). However, the control of both responses in RGCs revealed by our current work demonstrates that DLK mechanistically couples these disparate reactions at an early stage of the injury response. It is possible that DLK may in fact coordinately prime for both responses across many systems, with the primary outcome determined by distinct features of each context that control regenerative potential. This unexpected coupling of seemingly opposing pathways is reminiscent of cell growth regulation, in which a number of potential oncogenes not only stimulate proliferation but, paradoxically, also predispose cells to apoptosis. This connection is thought to act as a safeguard against malignancy, such that the survival of dividing cells is dependent on additional feedback that affirms that the growth is appropriate for the context (38). The linking of axon regeneration and neurodegeneration by DLK may represent a similar phenomenon in which stimulation of growth is coupled to increased vulnerability to apoptosis.
Given that deletion of PTEN and other manipulations that enhance optic nerve regeneration also improve RGC survival after optic nerve crush (32, 39, 40), it is tempting to speculate that apoptosis of DLK-primed neurons may in fact ultimately be triggered by inadequate or inappropriate regeneration. In the simplest form of this model (Fig. 4J), axon injury coordinately primes neurons for both regeneration and apoptosis through activation of DLK. In circumstances that permit regeneration (e.g., PTEN knockout), feedback from extrinsic or intrinsic signals may serve to attenuate the proapoptotic signaling initiated by DLK. In cases in which regeneration fails (e.g., wild-type optic nerve), the absence of this feedback would lead to extensive cell death over time. As in the “fail-safe” response of mitotic cells to oncogenic signals, lack of an appropriate growth response results in cell death (41). The consequences of regeneration for survival in the PNS appear to further substantiate this view. Preventing regeneration of injured peripheral nerves reduces long-term sensory neuron survival (42), whereas nerve repair that enables regeneration after transection reduces cell death (43).
Our current study reveals that the susceptibility of RGCs to apoptosis in the weeks after axonal injury is mechanistically coupled through DLK to an attempt to regrow via the activation of proregenerative pathways. The necessity of DLK for the nuclear detection of axotomy therefore makes it a master integrator of the neuronal stress response, controlling both degenerative and regenerative reactions to axonal injury.
Materials and Methods
Details on all procedures used in this study, including mouse lines, optic nerve crush, tissue processing, immunohistochemistry, imaging, quantification, microarray analysis, and qPCR validation can be found in the SI.
Supplementary Material
Acknowledgments
T.A.W., B.W., S.H.R, Z.J., J.K., and J.W.L. are employees of Genentech. We thank the Ben A. Barres laboratory at Stanford University for assistance with the nerve crush technique and Robby Weimer, Dara Kallop, Jin-Wu Tsai, Hilda Solanoy, Joy Zuchero, and Tiffany Wu for technical assistance.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211074110/-/DCSupplemental.
References
- 1.Yang Z, et al. Changes in gene expression in experimental glaucoma and optic nerve transection: The equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48(12):5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–786. [PubMed] [Google Scholar]
- 3.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18(3):276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlson E, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29(31):9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191(1):211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12(4):387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168(5):775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh AS, et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol. 2011;194(5):751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74(6):1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ham J, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14(5):927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29(3):629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang DD, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389(6653):865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 13.Kuan CY, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci USA. 2003;100(25):15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes KA, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis. 2012;46(2):393–401. doi: 10.1016/j.nbd.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffey ET, et al. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J Neurosci. 2002;22(11):4335–4345. doi: 10.1523/JNEUROSCI.22-11-04335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan L, et al. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93(9):3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirai S, et al. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26(46):11992–12002. doi: 10.1523/JNEUROSCI.2272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soto I, et al. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28(2):548–561. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckingham BP, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28(11):2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18(21):8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: Gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24(40):8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73(3):445–452. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imaizumi K, et al. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci. 2004;24(15):3721–3725. doi: 10.1523/JNEUROSCI.5101-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stam FJ, et al. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25(12):3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 26.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22(4):1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23(29):9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma CH, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121(11):4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312(2):596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27(30):7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weishaupt JH, Klöcker N, Bähr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J Mol Neurosci. 2005;26(1):17–25. doi: 10.1385/JMN:26:1:017. [DOI] [PubMed] [Google Scholar]
- 32.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKernan DP, Cotter TG. A Critical role for Bim in retinal ganglion cell death. J Neurochem. 2007;102(3):922–930. doi: 10.1111/j.1471-4159.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- 34. Welsbie DS, et al. (2013) Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci USA 110:4045–4050. [DOI] [PMC free article] [PubMed]
- 35.Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat Rev Neurosci. 2012;13(3):183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- 36.Raivich G, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43(1):57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Palmada M, et al. c-jun is essential for sympathetic neuronal death induced by NGF withdrawal but not by p75 activation. J Cell Biol. 2002;158(3):453–461. doi: 10.1083/jcb.200112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281(5381):1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 40.Smith PD, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64(5):617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrington EA, Fanidi A, Evan GI. Oncogenes and cell death. Curr Opin Genet Dev. 1994;4(1):120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 42.Rich KM, Disch SP, Eichler ME. The influence of regeneration and nerve growth factor on the neuronal cell body reaction to injury. J Neurocytol. 1989;18(5):569–576. doi: 10.1007/BF01187077. [DOI] [PubMed] [Google Scholar]
- 43.McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: Timecourse of cell death and elimination. Exp Brain Res. 2002;142(3):308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.