Significance
Fibrosis, a hallmark of many clinical disorders, occurs because of uncontrolled myofibroblast activity. We studied Dupuytren's disease, a common hereditable fibrotic condition that causes the fingers to irreversibly curl toward the palm. We found that freshly isolated tissue from Dupuytren's patients contained macrophages and released proinflammatory protein mediators (cytokines). Of the cytokines, only TNF selectively converted normal fibroblasts from the palm of patients with Dupuytren's disease into myofibroblasts via activation of the Wnt signaling pathway. Conversely, blockade of TNF resulted in reversal of the myofibroblast phenotype. Therefore, TNF inhibition may prevent progression or recurrence of Dupuytren's disease.
Keywords: musculoskeletal, scarring
Abstract
Dupuytren's disease is a very common progressive fibrosis of the palm leading to flexion deformities of the digits that impair hand function. The cell responsible for development of the disease is the myofibroblast. There is currently no treatment for early disease or for preventing recurrence following surgical excision of affected tissue in advanced disease. Therefore, we sought to unravel the signaling pathways leading to the development of myofibroblasts in Dupuytren's disease. We characterized the cells present in Dupuytren's tissue and found significant numbers of immune cells, including classically activated macrophages. High levels of proinflammatory cytokines were also detected in tissue from Dupuytren's patients. We compared the effects of these cytokines on contraction and profibrotic signaling pathways in fibroblasts from the palmar and nonpalmar dermis of Dupuytren's patients and palmar fibroblasts from non-Dupuytren's patients. Exogenous addition of TNF, but not other cytokines, including IL-6 and IL-1β, promoted differentiation into specifically of palmar dermal fibroblasts from Dupuytren's patients in to myofibroblasts. We also demonstrated that TNF acts via the Wnt signaling pathway to drive contraction and profibrotic signaling in these cells. Finally, we examined the effects of targeted cytokine inhibition. Neutralizing antibodies to TNF inhibited the contractile activity of myofibroblasts derived from Dupuytren's patients, reduced their expression of α-smooth muscle actin, and mediated disassembly of the contractile apparatus. Therefore, we showed that localized inflammation in Dupuytren's disease contributes to the development and progression of this fibroproliferative disorder and identified TNF as a therapeutic target to down-regulate myofibroblast differentiation and activity.
Dupuytren's disease is a common fibroproliferative disorder with a prevalence >7% in the United States (1). The classic description of disease progression is the initial appearance of palmar nodules characterized by high cellularity and cell proliferation, followed by the development of cords. This phase is followed by a final fibrotic stage that is associated with maturation of the cords and digital contractures resulting in significant impairment of hand function (2). Established flexion deformities of the digits are most commonly treated by surgical excision (fasciectomy) of the cord. The long recovery time following surgery has led to description of alternative techniques of disrupting the cord with a needle (percutaneous fasciotomy) (3) or enzymatic digestion using collagenase injections (4). However, even following surgery, patients often have significant residual dysfunction due to irreversible fixed flexion deformities of the joints. Currently, there is no specific treatment for early disease or prevention of recurrence following fasciectomy or fasciotomy of Dupuytren's cords. Therefore, we sought to unravel the cellular mechanisms leading to the development of this disease to reveal novel potential therapeutic targets.
The cell responsible for matrix deposition and contraction in Dupuytren's disease is the myofibroblast (5). Myofibroblasts characteristically express α-smooth muscle actin (α-SMA), which is the actin isoform typical of vascular smooth muscle cells (6). Fibroblast to myofibroblast differentiation is characterized by α-SMA expression, and exposure of the cells to stress leads to the incorporation of α-SMA protein into stress fibers (7). Unlike in granulation tissue, where the expression of α-SMA is transient (8), α-SMA expression is persistent in Dupuytren's disease. The development of myofibroblasts has been shown to be dependent on a number of different environmental cues, including tension in the matrix and exposure to a variety of different soluble mediators (7). The best studied of these is TGF-β1. The expression of TGF-β1 and associated signaling molecules is elevated in Dupuytren's disease (9), and Dupuytren's myofibroblasts or dermal fibroblasts from the same patients proliferated in response to 1 or 5 ng/mL TGF-β1 (10). Normal human dermal fibroblasts cultured in stressed collagen lattices also showed increased α-SMA expression when treated with 1 ng/mL TGF-β1 (11). Fibroblasts derived from the transverse carpal ligament of patients unaffected by Dupuytren's disease exposed to 2 ng/mL TGF-β1 showed no increase in α-SMA staining (12). However, these fibroblasts, as well as Dupuytren's myofibroblasts, did exhibit increased isometric contraction of collagen lattices when exposed to 12.5 ng/mL of TGF-β1, with higher doses being inhibitory (13). Therefore, TGF-β1 can promote cell proliferation and increase α-SMA expression and matrix contraction in a manner dependent on both the dose and the tissue of origin of the cells.
Inflammation is also known to play a crucial role in fibrosis, and various proinflammatory cytokines have been implicated in driving the progression of fibrotic diseases (14). Histological studies have identified the presence of immune cells in Dupuytren's disease; in particular, the number of macrophages was shown to correlate with the quantity of myofibroblasts (15). Intralesional steroid injections led to temporary softening and flattening of Dupuytren's nodules (16) and reduced recurrence following percutaneous needle fasciotomy (17). It has been suggested that this therapeutic benefit of steroids in early Dupuytren's disease may be due to diminished leukocyte recruitment (18), as well as increased apoptosis of macrophages and fibroblasts, with reduced proliferation of the latter (19). However, the link between disease progression and localized inflammation in Dupuytren's tissue remains unclear, and molecular mechanisms by which inflammatory cytokines directly drive myofibroblast differentiation remain unknown.
To explore these issues, we systematically studied the cytokines produced by freshly disaggregated cells from Dupuytren's nodules. TNF was identified as a key regulator of the myofibroblast phenotype, and TNF blockade in vitro led to down-regulation of the myofibroblast phenotype. Our data indicate that progression of early palmar nodules of Dupuytren's disease to deposition of extracellular matrix in cords and subsequent digital contractures in vivo may be prevented by local administration of a TNF inhibitor. This approach may also be effective in preventing recurrence following surgery, percutaneous needle fasciotomy, or collagenase treatment.
Results
Cells in Dupuytren's Nodules and the Cytokines They Produce.
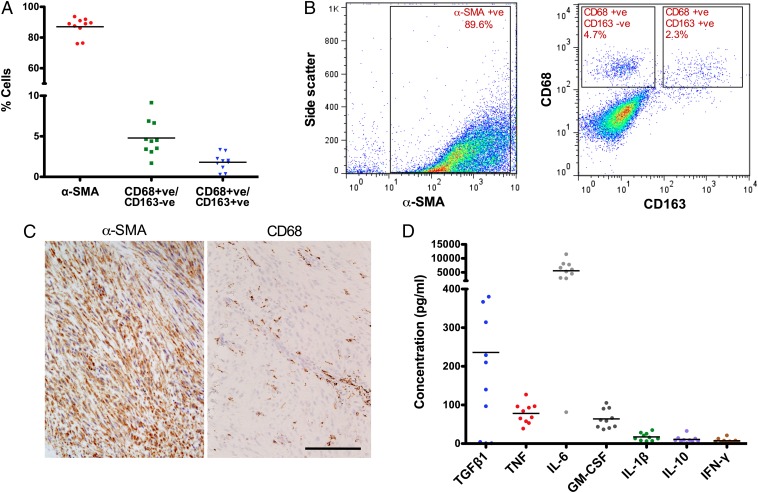
To quantify the presence of immune cells in Dupuytren's tissue, flow cytometric analysis was performed on cells directly disaggregated from Dupuytren's nodules. The majority (87 ± 6%) of cells were myofibroblasts, together with a significant number of macrophages (6.5 ± 2.3%), with the classically activated (CD68+/CD163−) M1 phenotype predominating (4.8 ± 2.2%; Fig. 1 A and B). Immunohistochemistry showed that the macrophages were distributed throughout the Dupuytren's nodules (Fig. 1C) and clustered around the blood vessels. Serial histological sections revealed no neutrophil elastase positive cells. Soluble cytokines produced by freshly disaggregated Dupuytren's nodule tissue included variable amounts of TGF-β1 (mean ± SD: 236 ± 248 pg/mL; range, 4–852 pg/mL), as well as TNF (mean ± SD: 78 ± 26 pg/mL), IL-6 (mean ± SD: 5,591 ± 3,215 pg/mL), and consistent with the presence of substantial numbers of classically activated M1 macrophages, GM-CSF (mean ± SD: 64 ± 24 pg/mL). Low levels of IL-1β (mean ± SD: 17 ± 10 pg/mL), IL-10 (mean ± SD: 11 ± 9 pg/mL), and IFNγ (mean ± SD: 7 ± 6 pg/mL) were observed (Fig. 1D). Cells from the same patient samples were also maintained in culture and examined for constituent cells and cytokine profiles at passage 2. Macrophages were absent, and the passaged cells produced greater amounts of TGF-β1 (mean ± SD: 654 ± 158 pg/mL) but less TNF (mean ± SD: 4 ± 4 pg/mL) and IL-6 (mean ± SD: 208 ± 312 pg/mL) than freshly isolated cells.
Fig. 1.
Inflammatory cells are present in Dupuytren's myofibroblast-rich nodules and resident cells in the nodules produce proinflammatory cytokines. (A) Flow cytometric analysis of cells isolated from freshly disaggregated Dupuytren's nodular tissue. Intracellular α-SMA–positive (myofibroblasts; mean ± SD: 87 ± 6.1%), cell surface CD68-positive CD163-negative (classically activated M1 macrophages; mean ± SD: 4.8 ± 2.2%), and CD68-positive CD163-positive (alternatively activated M2 macrophages; mean ± SD: 1.8 ± 1.0%) cells were quantified. (B) Representative flow cytometry scatter plots showing the proportion of α-SMA–positive cells and gating strategy for CD68+ and CD163+ cells in disaggregated Dupuytren's nodular tissue. (C) Serial histological sections of Dupuytren's nodular tissue stained for α-SMA+ (myofibroblasts) and CD68+ (monocytes) cells. CD68+ cells localized around blood vessels. (Scale bar, 100 μm.) (D) Cytokines released by freshly isolated nodular cells in monolayer culture using electrochemiluminescence. All data shown are from >10 patient samples.
Dermal Fibroblasts Treated with TGF-β1 Acquire the Myofibroblast Phenotype.
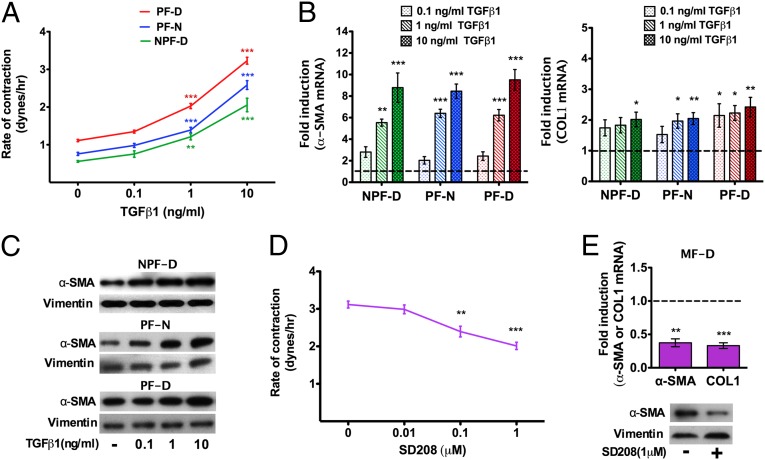
Recent data suggest that the tissues overlying the Dupuytren's nodules, including the dermis, are likely to constitute a source for myofibroblast precursors during disease progression (20). Therefore, we studied the effect of TGF-β1 on dermal fibroblasts from palmar and nonpalmar skin from patients with Dupuytren's disease, as well as palmar dermal fibroblasts from normal individuals unaffected by Dupuytren's disease. Cells were stimulated with 0.1, 1.0, and 10 ng/mL of TGF-β1. All cell types showed increased contractility in 3D collagen matrices when treated with 1.0 and 10 ng/mL TGF-β1 (Fig. 2A). These doses of TGF- β1 also resulted in increased levels of α-SMA and COL1 mRNA (Fig. 2B), as well as augmented α-SMA protein (Fig. 2C). Treatment of myofibroblasts from Dupuytren's nodules with SD208, a small molecule activin receptor-like kinase (ALK)5 inhibitor of the TGF-β1R1/Smad2/3 interaction (21), resulted in a dose-dependent decrease in contractility (Fig. 2D), with concomitant decrease in α-SMA and COL1 gene expression and α-SMA protein level (Fig. 2E). These data show that low doses of TGF-β1 (0.1 ng/mL) have no effect on dermal fibroblasts and that higher concentrations (1–10 ng/mL) stimulate myofibroblast differentiation of dermal fibroblasts, regardless of the source of origin. Furthermore, inhibition of TGF-β1 signaling in Dupuytren's myofibroblasts can reduce contraction and fibrotic gene expression.
Fig. 2.
TGF-β1 induces the myofibroblast phenotype indiscriminately in all types of dermal fibroblasts, an effect reversed with the TGF-β1R1/Smad2/3 inhibitor SD208. (A) TGF-β1 led to increased contractility of palmar (PF-D) and nonpalmar (NPF-D) dermal fibroblasts from patients with Dupuytren's disease, as well as in palmar dermal fibroblasts from normal individuals unaffected by Dupuytren's disease (PF-N). (B) All three cell types showed an increase in expression of α-SMA and COL1 assessed by quantitative RT-PCR (markers of myofibroblast contractility and matrix deposition, respectively) on exposure to TGF-β1 in a dose-dependent manner. Data are shown as relative expression compared with each cell type untreated. (C) Representative Western blots showing α-SMA protein expression increased in all dermal fibroblasts cultured with TGF-β1. Vimentin is shown as loading control and remained constant. (D) Dose-dependent reduction in contractility of Dupuytren's myofibroblasts on addition of SD208. (E) SD208 (1 μM) resulted in decreased α-SMA and COL1 expression in myofibroblasts from Dupuytren's nodules (MF-D) relative to untreated myofibroblasts. Representative Western blots showing α-SMA protein expression was reduced in Dupuytren's myofibroblasts treated with SD208 (1 μM). Vimentin is shown as loading control. All data are shown as the mean ± SEM from n ≥ 3 patients (each assay was performed in triplicate). *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of rhTNF on Dermal Fibroblasts of Different Origins and the Effect of Inhibiting TNF on Myofibroblasts.
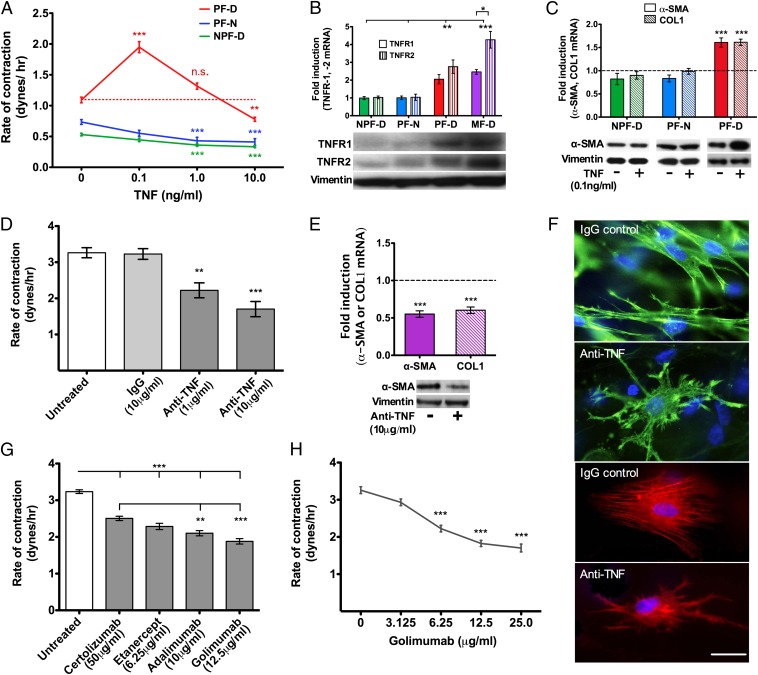
We examined the ability of other cytokines detected in Dupuytren's tissue to promote myofibroblast differentiation. Addition of recombinant human TNF (rhTNF) to palmar dermal fibroblasts from patients with Dupuytren's disease in 3D collagen lattices led to increased contraction. This effect peaked at 0.1 ng/mL TNF, a physiological concentration, and decreased thereafter to become inhibitory at 10 ng/mL, which is a very high concentration (Fig. 3A; Fig. S1A). In contrast, contraction of nonpalmar dermal fibroblasts from patients with Dupuytren's disease and palmar cells from normal individuals unaffected by Dupuytren's disease was dose-dependently inhibited by rhTNF (Fig. 3A). Interestingly, the freshly disaggregated cells from Dupuytren's nodules secreted TNF at levels near optimal (mean ± SD: 78 ± 26 pg/mL; Fig. 1D) for differentiation of palmar dermal fibroblasts into myofibroblasts. Expression of TNF receptors at message and protein levels were higher in palmar dermal fibroblasts from Dupuytren's patients and also in Dupuytren's myofibroblasts, where TNFR2 was especially raised (Fig. 3B). Addition of 0.1 ng/mL rhTNF led to enhanced expression of COL1 and α-SMA mRNA and also α-SMA protein only in palmar dermal fibroblasts from patients with Dupuytren's disease (Fig. 3C). Addition of rhIL-6 (Fig. S2A) or rhIL-1β (Fig. S2B) had no effect on contractility of palmar dermal fibroblasts from patients with Dupuytren's disease.
Fig. 3.
TNF selectively induces the myofibroblast phenotype in Dupuytren's palmar fibroblasts; anti-TNF reverses Dupuytren's myofibroblast phenotype. (A) Contraction of palmar fibroblasts from patients with Dupuytren's disease (PF-D) peaked on addition of 0.1 ng/mL TNF. In contrast, TNF treatment of PF-N and NPF-D led to a dose-dependent decrease in contractility. (B) Baseline gene expression of TNFR1 and TNFR2 was significantly higher in myofibroblasts and PF-D compared with both PF-N and NPF-D. In Dupuytren's myofibroblasts (MF-D), TNFR2 expression was significantly greater than TNFR1. Fold change was normalized to the baseline expression of NPF-D. (C) α-SMA and COL1 mRNA expression only increased in Dupuytren's palmar fibroblasts and not in other dermal fibroblasts (PF-N and NPF-D) when cultured with TNF (0.1 ng/mL) and compared with respective untreated fibroblasts. There was a corresponding increase in α-SMA protein expression in PF-D treated with TNF (0.1 ng/mL) but no difference in other dermal fibroblasts demonstrated by Western blotting. Vimentin is shown as loading control and remained constant. (D) Dose-dependent reduction in contractility of Dupuytren's myofibroblasts in response to neutralizing antibody to TNF. IgG isotype (10 μg/mL) antibody was used as a control. (E) Anti-TNF (10 μg/mL) led to a corresponding reduction in mRNA expression of α-SMA and COL1 in Dupuytren's myofibroblasts compared with the respective untreated cells. A concomitant reduction in α-SMA protein expression in Dupuytren's myofibroblasts was seen when treated with anti-TNF (10 μg/mL) demonstrated by Western blotting. Isotype IgG (0.1 ng/mL) control was used for anti-TNF, and vimentin is shown as loading control and remained constant. (F) Immunofluoresence staining of Dupuytren's myofibroblasts seeded in 3D collagen matrices. Cell morphology and actin cytoskeleton were visualized with phalloidin (green) or α-SMA (red) and nuclei stained with DAPI (blue). Neutralizing antibody to TNF (10 μg/mL) led to disassembly of the cytoskeleton. Isotype IgG control also used at 10 μg/mL (Scale bar, 30 μm.) (G) Comparison of current anti-TNF preparations approved by FDA for subcutaneous administration on the contractility of Dupuytren's myofibroblasts. Doses calculated based on 25% of recommended dose in rheumatoid arthritis (certolizumab 200 mg in 1 mL every 2 wk, etanercept 50 mg in 1 mL every week, adalimumab 40 mg in 0.8 mL every 2 wk, golimumab 50 mg in 0.5 mL every 4 wk). (H) Dose–response of Dupuytren's myofibroblasts to golimumab. All data shown are from n ≥ 3 patients (each in triplicate). Data expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. (not significant).
To determine whether inhibiting TNF activity would reduce contraction and fibrotic gene expression in Dupuytren's myofibroblasts, we used a number of means of TNF blockade. Addition of neutralizing antibody to TNF reduced isometric contraction of Dupuytren's myofibroblasts in a dose-dependent fashion (Fig. 3D), sustained for 72 h (Fig. S1B), which is the maximum time the cells could be maintained in culture in the culture force monitor (CFM). This downregulation of contractility was accompanied by reduced expression of COL1 and α-SMA at the message level, as well as α-SMA protein (Fig. 3E). Conversely, addition of neutralizing antibodies to IL-6 (Fig. S2C) or IL-1β (Fig. S2D) had no effect on myofibroblast contractility and neither did rhIL-10 (Fig. S2E). Inhibiting TNF did not affect myofibroblast viability (Fig. S1C). However, there was disassembly of the intracellular α-SMA contractile apparatus (Fig. 3F). There are four U.S. Food and Drug Administration (FDA)-approved anti-TNF agents suitable for subcutaneous administration. Although all were effective, adalimumab and golimumab were significantly more efficacious in inhibiting isometric myofibroblast contraction (Fig. 3G), and the latter was effective in a dose-dependent manner (Fig. 3H). Together, these data show that TNF blockade is equally effective in down-regulating the Dupuytren's myofibroblast phenotype as TGF-β1 inhibition.
TNF Acts via the Wnt Signaling Pathway in Dupuytren's Disease.
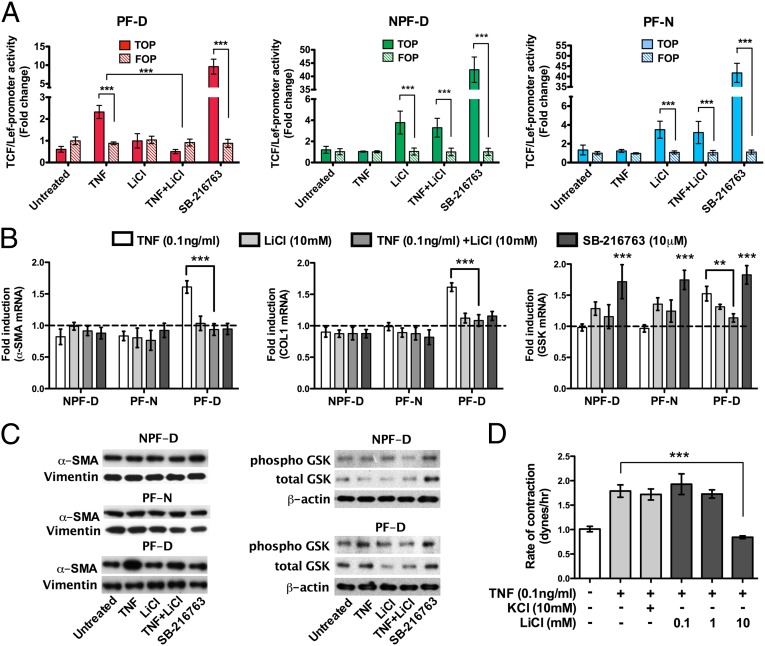
TNF derived from activated macrophages can promote Wnt/β-catenin activity in gastric cancer cells (22). Moreover, TNF activation of Wnt/β-catenin signaling pathways has been shown to control adipocyte differentiation (23). Ligation by Wnt of the receptor complex comprising Frizzled and LRP5/6 leads to accumulation of cytoplasmic β-catenin, which translocates to the nucleus, binding the transcription factors TCF/Lef, and promotes transcription of genes typically associated with myofibroblasts, including COL1 and α-SMA. Because a recent genomewide association study demonstrated that Wnt signaling may be involved in Dupuytren's disease (5), we explored the hypothesis that TNF may directly control myofibroblast differentiation via Wnt signaling. Addition of exogenous Dkk-1, an inhibititor of Wnt ligand binding to the Frizzled receptor complex, did not affect myofibroblast contractility (Fig. S2F), indicating that the myofibroblast phenotype is not dependent on binding of Wnt ligands to their receptor LRP5/6-Frizzled. Using the TOPflash TCF/Lef luciferase reporter assay, we found that stimulation of palmar dermal fibroblasts from Dupuytren's patients with TNF led to increased Wnt signaling. In contrast, this did not occur on TNF treatment of nonpalmar dermal fibroblasts from patients with Dupuytren's disease nor of palmar cells from normal individuals unaffected by Dupuytren's disease (Fig. 4A). These data are consistent with the cell type–specific effect of TNF on cell contractility and gene expression that we observed in Fig. 3A.
Fig. 4.
TNF acts via Wnt signaling pathway in Dupuytren's disease. (A) TNF (0.1 ng/mL) stimulated the activity of a TCF/Lef reporter assay, a downstream indicator of activation of the Wnt signaling pathway, in palmar fibroblasts from Dupuytren's patients (PF-D). This effect was reversed on addition of Li ions (10 mM), whereas LiCl alone had no effect on PF-D. In contrast, palmar fibroblasts from individuals unaffected by Dupuytren's disease (PF-N) or nonpalmar fibroblasts from Dupuytren's patients (NPF-D) treated with TNF (0.1 ng/mL) did not promote the activity of TCF/Lef reporter construct. LiCl led to increased TCF/Lef reported activity in nonpalmar dermal fibroblasts from Dupuytren's patients (NPF-D) and palmar fibroblasts from individuals unaffected by Dupuytren's disease (PF-N);. SB-216763 (10 μM), a selective inhibitor of GSK-3β (24), was used as a positive control and activated TCF/Lef in all cell types. (B) Only PF-D cultured with TNF (0.1 ng/mL) demonstrated an increase in α-SMA, COL1, and GSK-3β mRNA expression compared with respective untreated cells. The increase in GSK-3β expression by PF-D on exposure to TNF was reversed by LiCl (10 mM). SB-216763 (10 μM) was used as a positive control. (C) Palmar fibroblasts from patients with Dupuytren's disease (PF-D) treated with TNF (0.1 ng/mL) showed increased α-SMA protein expression and increased phosphorylated GSK-3β that was reversed by LiCl (10 mM), demonstrated by Western blotting. β-actin is shown as a loading control and remained constant. (D) Isometric contraction of PF-D was increased with TNF treatment (0.1 ng/mL) and reversed in a dose-dependent manner by LiCL (0.1–10 mM). KCl (10 mM) was used as a control for LiCl. All data shown for n ≥ 3 patients (each performed in triplicate). Data expressed as mean ± SEM. **P < 0.01, ***P < 0.001.
Following ligation of Frizzled and LRP5/6 by Wnt, glycogen synthase kinase (GSK-3β) is inactivated by phosphorylation, which prevents the degradation of β-catenin, preserving it within the cell. SB-216736 is a potent selective ATP-competitive GSK-3β inhibitor (24) and induces Wnt signaling pathways in the absence of Wnt ligands. Thus, SB-216736 drove TOPflash TCF/Lef luciferase indiscriminately in all three cells types, indicating that TCF/Lef can be activated in these cells and confirming the specific effect of TNF only in palmar fibroblasts from patients with Dupuytren's disease (Fig. 3A). Interestingly SB-216736 had no effect on α-SMA, COL1 mRNA or α-SMA protein expression in any cell type, whereas TNF led to increased α-SMA and COL1 mRNA and α-SMA protein only in palmar dermal fibroblasts from patients with Dupuytren's disease (Fig. 4B). These data indicate that inactivation of GSK-3β alone is insufficient to drive gene transcription downstream of TCF/Lef activation in contrast to cell stimulation with TNF, which promotes the expression of profibrotic genes. These data indicate that TNF induces additional signaling events required for effective gene expression.
We evaluated the effect of TNF on Wnt signaling events upstream of TOPflash TCF/Lef luciferase activation. Stimulation of palmar dermal fibroblasts from patients with Dupuytren's disease with TNF resulted in increased phosphorylation of GSK-3β (Fig. 4C), demonstrating that TNF drives inactivation of this key molecule in the Wnt signaling pathway. Again, treatment of cells with SB-216736 as a positive control induced phosphorylation of GSK-3β indiscriminately in both palmar and nonpalmar dermal fibroblasts from patients with Dupuytren's disease (Fig. 4C).
Lithium ions are well-established but nonselective modulators of GSK-3β activity; in the majority of cell types, LiCl inhibits GSK-3β and hence enhances Wnt signaling. However, in adipocytes, lithium ions stimulate the activity of GSK-3β by inhibiting phosphorylation of the enzyme (25). We found that in nonpalmar dermal fibroblasts from Dupuytren's patients and palmar fibroblasts from individuals unaffected by Dupuytren's disease, LiCI indeed stimulated TCF/Lef activation (Fig. 4A). In contrast, in palmar dermal fibroblasts from patients with Dupuytren's disease, LiCl had no effect on TCF/Lef activation (Fig. 4A). Moreover, in the latter cell type, we found that TNF-mediated TCF/Lef activation was reversed by LiCI. LiCl also inhibited TNF-mediated α-SMA and COL1 gene expression, α-SMA protein expression, and phosphorylation of GSK-3β (Fig. 4 B and C), and these effects translated to the functional outcome of collagen lattice contraction (Fig. 4D). Together, these data indicate that in palmar dermal fibroblasts from Dupuytren's patients LiCl activates GSK-3β activity and are consistent with the findings that, as previously demonstrated in adipocytes, TNF acts via the Wnt signaling pathway (23).
Discussion
Currently the management of established Dupuytren's disease once digital contractures have developed is predominantly surgical excision of the affected tissue or, less often, disruption of the cord with a needle or collagenase. Lack of understanding of the signaling pathways driving disease pathogenesis has meant that there is no specific therapeutic for treating early disease or for preventing recurrence following excision or division of the cord. The absence of valid targets has led to empirical treatment with modalities such as local steroid injection (16, 17) or radiotherapy (26, 27).
To identify the signaling mechanisms responsible for the development and persistence of the myofibroblasts in vivo, we studied freshly isolated cells, obtained from surgically excised Dupuytren's tissue. Using flow cytometry and immunohistology, we found that, although the majority of the cells in these specimens were myofibroblasts, ∼7% were macrophages, and the classical M1 proinflammatory phenotype predominated. Classical macrophages have previously been shown to be associated with fibrosis, whereas the role of alternatively activated M2 macrophages is more controversial (28). Inflammation is known to play a crucial role in fibrosis, and a variety of proinflammatory cytokines have been implicated, including TNF, IL-1β, and IL-6 (28). We found that cells freshly disaggregated from Dupuytren's tissue released appreciable amounts of TNF, IL-6, GM-CSF, and variable amounts of TGF-β1 (Fig. 1).
We documented the effect of these proinflammatory cytokines on dermal fibroblasts from different tissue sources. Our studies were restricted to using cells up to passage 2. Several factors informed our choice of experimental approach. Mature Dupuytren's disease is restricted to certain fibers of the palmar fascia. Many previous studies have compared cells from Dupuytren's nodules or cords with cells from the fascia in the region of the carpal tunnel or the transverse carpal ligament from affected or normal individuals or uninvolved transverse palmar fibers from patients with Dupuytren's disease. However, this approach has limitations. The palmar fascia over the carpal tunnel is rarely affected by Dupuytren's disease in susceptible individuals, and the transverse carpal ligament is always unaffected; hence, it is possible that the constituent cells are inherently different (29). Furthermore, with the exception of nodules in Dupuytren's disease, fascia is sparsely populated by cells and hence to obtain adequate numbers, most authors use cells to passage 5 (12). However, we and others have shown that at passage 5 the phenotypes of myofibroblasts and normal human dermal fibroblasts tend to merge (30, 31). In addition, whereas the cell of origin for Dupuytren's myofibroblasts remains controversial, there is accumulating evidence that the adjacent tissues, including the overlying dermis, make a significant contribution and that these cells are more akin to Dupuytren's myofibroblasts than fibroblasts from carpal tunnel fascia (20). Moreover, Dupuytren's disease is restricted to the palm of the hands in patients with a genetic predisposition. Therefore, it is important to avoid variations due to genetic and environmental factors. For these reasons, we compared dermal fibroblasts from palmar and nonpalmar sites from the same group of patients, using palmar dermal fibroblasts from individuals without Dupuytren's disease as controls.
It is well established that TGF-β1 induces the myofibroblast phenotype (7). Human dermal fibroblasts demonstrate enhanced α-SMA expression at low (1 ng/mL) concentrations of TGF-β1 (11, 32), whereas fibroblasts from the transverse carpal ligament only responded to higher doses (12, 13). We found that TGF-β1 increased the contractility in a dose-dependent manner of dermal fibroblasts from all three sources at concentrations of 1–10 ng/mL, which is in excess of the range we found released by freshly disaggregated cells from Dupuytren's nodular tissue. We also found that SD208, a small molecule inhibitor of TGF-β1R1/Smad2/3 interactions, led to a dose-dependent reduction in the myofibroblast phenotype. However, global inhibition of TGF-β1 is undesirable due to the important role of TGF-β1 in a wide range of physiological processes (21) and the increased inflammation, tumor promotion, and cardiac toxicity seen in animal studies (33). Although no adverse events specifically relating to inhibition of the TGF-β1 pathway have been reported in the limited clinical trials for fibrotic disorders to date, no late-phase studies to date have demonstrated efficacy (21, 34).
Fibrosis is a common pathological end point of many inflammatory disorders affecting critical visceral organs. In a murine model, TNF production was found to be essential for the development of bleomycin-induced pulmonary fibrosis, in part through up-regulation of TGF-β1 expression (35), and systemic administration of soluble TNF receptor led to reduction in fibrosis (36). The utility of these models may, however, be limited because not all strains of mice develop pulmonary fibrosis in response to bleomycin (35), emphasizing the importance of studying primary human tissues. There is no animal model for Dupuytren's disease, and in vivo conditions are most closely emulated in vitro by populating 3D collagen lattices with fibroblasts and maintaining the construct under tension under isometric conditions using a CFM (30, 37). Myofibroblasts only maintain their phenotype under stress, and loss of tension is associated with disassembly of α-SMA stress fibers within minutes (38). Using the CFM, we found that rhTNF led to a dose-dependent increase in contraction of palmar fibroblasts from patients with Dupuytren's disease. This effect peaked at 100 pg/mL, a concentration similar to that observed in Dupuytren's patient tissue. This effect was comparable to 1 ng/mL of TGF-β1, whereas rhIL-6 and rhIL-1β had no discernible effect. This increased contractility mediated by rhTNF was associated with increased expression of α-SMA and COL1 message and α-SMA protein. In contrast, contraction of palmar fibroblasts from individuals unaffected by Dupuytren's disease was inhibited by rhTNF, as were nonpalmar fibroblasts from patients with Dupuytren's disease, consistent with a previous report (11). The specific action of TNF is in contrast to the nonselective effect of TG-Fβ1, which induced the myofibroblast phenotype indiscriminately in all types of dermal fibroblasts. The response to TNF of dermal fibroblasts from different anatomical sites reflects the localization of Dupuytren's disease to the palm in susceptible patients, whereas TGF-β1 increases fibroblast contractility irrespective of the origin of the fibroblasts. It is possible that the dermal fibroblasts derived from palmar skin obtained from patient’s with Dupuytren's contracture undergoing dermofasciectomy may have included an appreciable number of myofibroblasts, and this might explain the differing response to TNF. However, this is unlikely because we have previously shown that palmar dermal fibroblasts behave like nonpalmar cells from the same patients in that they reach tensional homeostasis in the CFM, whereas nodule-derived myofibroblasts continue to contract (30).
Fibrosis induced by TGF-β1 has been shown to involve canonical Wnt signaling (39), and there is evidence of cross-talk between Wnt/β-catenin and TGF-β1 signaling pathways, including combinatorial transcriptional regulation of α-SMA for myofibroblast differentiation (40). Although it is possible that the induction of the myofibroblast phenotype in Dupuytren's palmar fibroblasts was due to downstream activation by TNF of TGF-β1 (41), the pathway would be specific for this cell type because TNF reduced the contractility of nonpalmar dermal fibroblasts from Dupuytren's patients and palmar dermal fibroblasts from normal individuals. This is in contrast to the similar effects of TGF-β1 on all three cell types. It is difficult to draw overall conclusions from the literature about interactions between signaling pathways because the source of cells varies widely between studies. The activation of TGF-β1 pathways was shown in 3T3 and murine pulmonary fibroblasts (41), whereas in human dermal fibroblasts from neonatal foreskins, TNF inhibited TGF-β/Smad signaling, again via activator protein 1 (AP-1) activation (42).
Wnt/β-catenin signaling has been shown to be intimately involved with fibrosis (43), and a recent genomewide association study showed that this pathway is likely involved in Dupuytren's disease (5). However, we found that exogenous addition of excess Dkk-1, a negative regulator of Wnt signaling, had no effect on myofibroblast contractility, suggesting that in the CFM model, contraction is not dependent on a Wnt ligand acting through the canonical pathway (44). We hypothesized that TNF may be acting via the Wnt/β-catenin signaling pathway, as previously demonstrated in adipocytes (23, 45, 46). In the unstimulated cell, β-catenin is phosphorylated by GSK-3β and undergoes ubiquitination and proteosomal degradation. Ligation by Wnt of the receptor complex comprising Frizzled and LRP5/6 leads to phosphorylation and inhibition of GSK-3β, preserving β-catenin from degradation and in turn promoting profibrotic gene expression via its binding to the transcription factors TCF/Lef (Fig. 5). We found that only palmar fibroblasts from patients with Dupuytren's disease exposed to TNF showed an increase in phosphorylated GSK-3β and TCF/Lef activation that was reversed by LiCl. Whereas lithium ions inhibit GSK-3β in the majority of cells, they selectively activate GSK-3β in specific cell types such as adipocytes (25) by inhibiting its phosphorylation (47). LiCl is an ATP noncompetitive inhibitor of GSK-3β and also acts indirectly by activating PKB, which in turn phosphorylates GSK-3β can also inhibit other protein kinases, including casein kinase-2, p38 kinase, and MAPK-activated protein kinase-2 (24). Therefore, it is possible that the apparent reversal of the effects of TNF by LiCl in palmar dermal fibroblasts from Dupuytren's patients may have been due to an activation of a pathway other than that mediated by GSK-3β. However, this is unlikely to account for the entire effect of LiCl in this system because it reduced total GSK-3β at both message and protein level and the level of phosphorylated GSK-3β induced by TNF. The downregulation of GSK-3β by LiCl was accompanied by reversal to baseline of the increased levels of mRNA for α-SMA and COL-1, as well as α-SMA protein. The latter translated to reduced contractility in the culture force monitor. These findings confirm that the TNF-induced development of the myofibroblast phenotype of palmar dermal fibroblasts from patients with Dupuytren's disease is mediated at least in part via the Wnt/β-catenin signaling pathway (Fig. 5). Conversely, LiCl led to increased TOPFlash activity in nonpalmar fibroblasts from Dupuytren's patients and palmar dermal fibroblasts from individuals unaffected with Dupuytren's disease. It has previously been shown that fibroblasts from different sources may show divergent responses to the same stimuli (43), and more recently (29), it was reported that Dupuytren's myofibroblasts and fibroblasts from uninvolved palmar fascia from the same patients both expressed genes related to Wnt/β-catenin pathway, unlike fascial-derived fibroblasts from individuals without Dupuytren's disease.
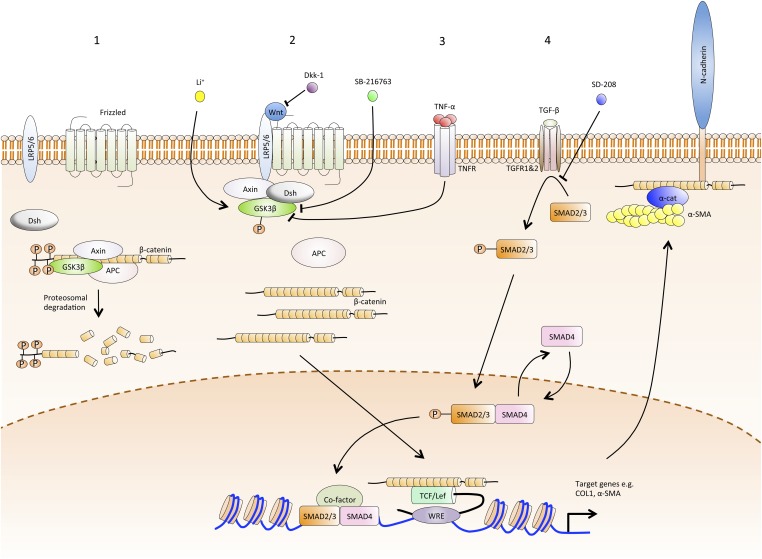
Fig. 5.
Schematic illustrating how TNF acts via the Wnt pathway leading to the development of the myofibroblast phenotype. [1] In the resting state, cytoplasmic β-catenin is phosphorylated by GSK-3β. This modification targets β-catenin for ubiquitination and proteosomal degradation. [2] Ligation by Wnt of the receptor complex comprising Frizzled and LRP5/6 leads to phosphorylation and inhibition of GSK-3β. Thus, β-catenin escapes modification and subsequent degradation, leading to accumulation of cytoplasmic β-catenin, which participates in cell–cell adherens junctions and also translocates to the nucleus. Here it binds to the transcription factors TCF/Lef and promotes the expression of genes typically associated with myofibroblasts: COL1 and α-SMA. Subsequent cytoskeletal assembly of α-SMA protein produces the contractile apparatus of the cell, with attachments to neighboring cells via cadherins and the matrix via integrins. Wnt ligand–receptor binding is competitively inhibited by Dkk-1, leading to resumption of β-catenin degradation. SB-216736 is a small molecule that increases the phosphorylation and thus specifically inhibits the activity of GSK-3β. [3] In palmar dermal fibroblasts from patients with Dupuytren's disease, TNF binding to TNFR leads to GSK-3β phosphorylation and inhibition, thereby releasing β-catenin from degradation and enabling the transcription of COL1 and α-SMA genes and assembly of an α-SMA–rich cytoskeleton. Li ions restore GSK-3β activity in these fibroblasts, thus enhancing β-catenin degradation and reversing the effect of TNF activation. [4] Binding of TGF-β1 to TGF-β1R1/2 leads to Smad2/3 phosphorylation and activation. The latter recruits Smad4 and, on entering the nucleus, leads to transcription of the same genes as the β-catenin TCF/Lef complex, namely COL1 and α-SMA. The interaction of TGF-β1R1/2 with Smad2/3 is selectively inhibited by SD-208.
The reason for the differential effects of TNF on the three types of dermal fibroblasts is of interest. It may have been due to the increased expression of TNF receptors by dermal fibroblasts from the palm of patients with Dupuytren's disease. TNF receptor (TNFR)1 is constitutively expressed by most cells, whereas endogenous expression of TNFR2 is restricted to a few cell types, including those of the hemopoietic lineage and mesenchymal stromal cells. TNFR2 can be induced by cytokines such as TNF (48). The levels of TNFR1 and especially TNFR2 were elevated compared with the other dermal fibroblasts. TNFR2 appears to be crucial in signaling a profibrotic response as intestinal myofibroblasts from WT or TNFR1-deficient mice showed increased proliferation and collagen production on treatment with TNF whereas myofibroblasts from TNFR2−/− or combined TNFR1−/−/TNFR2−/− were unresponsive (49). These findings would be consistent with our data that only palmar dermal fibroblasts from patients with Dupuytren's disease or Dupuytren's myofibroblasts showed increased expression of TNFR2 at both the message and protein level and may explain the selective effect of TNF on the former compared with nonpalmar Dupuytren's fibroblasts and non-Dupuytren's palmar fibroblasts. Myofibroblasts up to passage 2 in 3D collagen lattices exhibited a dose-dependent inhibition of contraction in the CFM on addition of neutralizing antibody to TNF. Inflammatory cells, in particular classically activated M1 macrophages, are likely to represent the major source of TNF in vivo. However, these cells were not present in the second passage myofibroblasts used in the CFM. We found that, at these early passages, myofibroblasts produced low levels of TNF that may continue to act in an autocrine or paracrine manner on cells expressing high levels of TNF receptors. Furthermore, TNF can up-regulate the expression of TNFR2, and in a murine model of pulmonary fibrosis, exposure to silica or bleomycin led to increased expression of TNFR2 but not TNFR1 (50). Neutralizing antibodies can bind transmembrane TNF and lead to reverse signaling (51). The latter can result in apoptosis or modulation of the response to external stimuli (52). We did not find any evidence of impaired cell viability over 24 h when myofibroblasts were exposed to neutralizing antibody to TNF. Instead, inhibition of myofibroblast contractility by blocking antibodies to TNF was associated with reduced expression of COL1 and α-SMA genes and α-SMA protein, together with disassembly of the α-SMA stress fibers. Therefore, anti-TNF effectively reverses the myofibroblast phenotype.
A deeper understanding of the signaling pathways that drive the development of the myofibroblast phenotype should lead to strategies for the treatment of early disease and prevention of further progression. Taken together, our data show that TNF is a rational therapeutic target for the treatment of early-stage Dupuytren's disease or for preventing recurrence. We found that nodules from patients with Dupuytren's disease contain significant numbers of classically activated macrophages, and the cells present in the nodules secrete biologically active amounts of TNF. Unlike TGF-β1, TNF acts specifically on palmar dermal fibroblasts from patients with the appropriate genetic background to induce a myofibroblast phenotype via the Wnt/β-catenin pathway, and these cells show higher expression of TNF receptors, especially TNFR2. The contractility of myofibroblasts was inhibited by anti-TNF and was associated with disassembly of the contractile apparatus. Therefore, we characterized Dupuytren's disease as a localized inflammatory fibroproliferative disorder and proposed TNF as a therapeutic target. It is possible that a similar mechanism pertains to other musculoskeletal fibrotic disorders such as frozen shoulder (adhesive capsulitis), which affects up to 2% of the population (53). In terms of translating these findings to clinical trials, the ideal anti-TNF agent would be injected locally into the nodules and ideally would remain there to minimize unwanted systemic effects while maximizing efficacy. Systemic inhibition of TNF is now recognized for the treatment of rheumatoid arthritis (54) and inflammatory bowel disease (55, 56). Unlike TGF-β1 inhibition, the safety profile of TNF inhibition is well established. Of the anti-TNF agents approved by the FDA for subcutanoeus administration, the two fully human complete IgG molecules, adalimumab and golimumab, were the most efficacious in our CFM model in term of inhibiting myofibroblast contractility, and golimumab may be preferred because of a longer half-life, necessitating less frequent injection. Unlike etanercept, both these agents are able to ligate both monomeric and trimeric TNF. Based on our findings, local injection of an anti-TNF to prevent the progression of early stages of Dupuytren's disease or avoid recurrence following surgery, needle fasciotomy, or collagenase digestion is an exciting possibility and is ready to test. If the proof of concept works, second-generation drugs that remain preferentially at the site of disease could be developed.
Materials and Methods
Patient Samples.
After approval by the local ethical review committee (REC 07/H0706/81), tissue samples were obtained with informed consent from patients with Dupuytren's disease. Dupuytren's nodular tissue, palmar skin (uninvolved skin overlying Dupuytren's tissue), and nonpalmar skin (full-thickness skin harvested from the groin or medial aspect of arm) were obtained from individuals with Dupuytren's disease undergoing dermofasciectomy. Tissue was also obtained from palmar skin of patients unaffected by Dupuytren's disease.
Cell Culture.
Fibroblasts from the palmar (PF-D) and nonpalmar dermis (NPF-D) of patients with Dupuytren's disease and palmar fibroblasts (PF-N) from individuals unaffected by Dupuytren's disease were isolated from full-thickness skin samples. Dupuytren's myofibroblasts (MF-D) were isolated from α-SMA–rich nodules (57). Tissue samples were dissected into small pieces and digested in DMEM (Lonza) with 1% penicillin–streptomycin (PAA), 5% (vol/vol) FBS (Gibco), and type I collagenase (Worthington Biochemical Corporation) + DNase I (Roche Diagnostics) for up to 2 h at 37 °C. Cells were cultured in DMEM with 10% (vol/vol) FBS and 1% penicillin–streptomycin at 37 °C in a humidified incubator with 5% (vol/vol) CO2. Cells up to passage 2 were used for experiments.
CFM.
Measurement of the isometric contractile forces generated by cells within 3D collagen matrices was performed as previously described (30). Briefly, 1.5 × 106 cells were seeded in 2.5 mL of type I collagen gel (FirstLink), and 3D matrices were tethered between two flotation bars and held stationary at one end while the other attached to a force transducer. Fibroblast-populated collagen matrix–generated tensional forces were continuously measured and data logged every minute (dynes: 1 × 10−5 N). Cell-populated matrices were cultured in DMEM with 10% (vol/vol) FBS and 1% penicillin–streptomycin at 37 °C in a humidified incubator for 24 h with 5% CO2 and treated with TGF-β1, TNF (Peprotech), rhIL-1β, rhIL-6, rhIL-10, anti–IL-1β, anti–IL-6, anti-TNF, anti-TNF (R&D Systems), LiCl (Invitrogen), TNF in combination with LiCl, SD208 (TOCRIS Bioscience), Dkk-1 (R&D Systems), adalimumab (Abbott), certolizumab (UCB), etanercept (Pfizer), or golimumab (Merck). Experiments using each patient sample were performed in triplicate.
Quantitative RT-PCR.
Cells were cultured in monolayer and treated with TGFβ1, SD208, TNF, anti-TNF, LiCl, SB216763, or TNF in combination with LiCl for 24 h, and total RNA was extracted from each sample using the QIAamp RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. Isolated RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). For real-time RT-PCR, Inventoried TaqMan Gene expression Assays were used for α-SMA (Hs00426835-g1), COL1a1 (Hs00164004-m1), GSK (Hs01047719-m1), TNFR1 (Hs01042313-m1), and TNFR2 (Hs00961749-m1; Applied Biosystems) with Reverse Transcriptase qPCR Mastermix No ROX (Eurogentec). Samples were run on the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). Expression was normalized to GAPDH (Hs02758991-g1; Applied Biosystems) and compared with the level of gene expression in either baseline respective cell types or with the level of gene expression in NPF-D, which was assigned the value of 1 using ΔΔCT analysis performed with SDS software (Applied Biosystems).
Western Blots.
Cells were cultured in monolayer and treated with TGF-β1, SD208, TNF, anti-TNF, LiCl, SB216763, or TNF in combination with LiCl for 30 min or 24 h before protein extraction. Cell lysates were prepared in lysis buffer [25 mM Hepes (pH 7.0), 150 mM NaCl, and 1% Nonidet P-40], containing protease inhibitor mixture (Roche Biochemicals) and phosphatase inhibitor mixture (Invitrogen), and then electrophoresed on 10% (wt/vol) SDS polyacrylamide gels, followed by electrotransfer of proteins onto PVDF transfer membranes (Perkin-Elmer). Membranes were blocked in 5% (wt/vol) BSA/TBS + 0.05% Tween and incubated overnight at 4 °C with primary antibodies against α-SMA primary antibody (Sigma), TNFR1, TNFR2, phosphorylated GSK-3β, total GSK-3β, β-actin (all from Cell Signaling), and vimentin (Abcam). HRP-conjugated anti-mouse IgG or anti-rabbit IgG (Amersham Biosciences) was used as secondary antibodies. Bound antibody was detected using the enhanced chemiluminescence kit (Amersham Biosciences) and visualized using Hyperfilm MP (Amersham Biosciences).
Flow Cytometry.
For detection of α-SMA, cells were permeabilized overnight at 4 °C with PBS containing 1% FCS, 0.01% sodium azide, and 0.05% saponin and then stained with anti–α-SMA (Abcam) and subsequently with phycoerythrin-conjugated goat anti-mouse IgG (Southern Biotechnology) for 10 min at 4 °C. For detection of macrophages, cells were stained for 30 min at 4 °C with Pacific blue–conjugated anti-CD1a (e-Bioscience) and phycoerythrin-conjugated anti-CD163 (R&D Systems), fixed in Cytofix (BD Bioscience), permeabilized overnight, and stained with fluorescein isothiocyanate–conjugated anti-CD68 (DAKO). For intracellular cytokine staining, cells were stimulated with brefeldin A (Sigma-Aldrich), permeabilized, and stained with allophycocyanin-conjugated anti-TNF (e-Bioscience). Samples were analyzed on a FACSCanto II (BD Bioscience), and data were analyzed using FlowJo software (TreeStar).
Immunohistochemistry.
All Dupuytren's tissue samples were fixed in formalin, longitudinally bisected, and embedded in paraffin wax, and 7-μm sections from the cut surface were processed for immunohistochemistry (57). Sequential sections were stained with mouse monoclonal anti–α-SMA antibody (Sigma), anti-CD68 antibody (DAKO), and mouse monoclonal anti-desmin (DAKO) antibody. Antibodies were detected using a two-staged polymer enhancer system (Sigma). Mouse serum at the same protein concentration as the monoclonal antibody solution was used as a control.
Mesoscale ELISA.
Freshly disaggregated cells from whole tissue samples were isolated as described previously (58). Dupuytren's nodular tissue samples were dissected into small pieces and digested in DMEM with 5% (vol/vol) FBS, 1% penicillin–streptomycin, and type I collagenase + DNase I for up to 2 h at 37 °C. Freshly disaggregated cells (5 × 105) were immediately plated in a six-well plate in 4 mL DMEM [5% (vol/vol) FBS and 1% penicillin–streptomycin]. Cells were incubated at 37 °C, 5% CO2, for 24 h, and then supernatants were harvested (passage 0). The remaining freshly disaggregated cells were cultured, and 5 × 105 cells at passage 1 or passage 2 from the same donor plated in a six-well plate with 4 mL DMEM [5% (vol/vol) FBS and 1% penicillin–streptomycin] and supernatants were harvested after 24 h. Supernatants were analyzed for IL-1β, IL-6, IL-8, IL-10, GM-CSF, IFN-γ, and TNF cytokines using a Human Pro-Inflammatory 9-Plex Ultra-sensitive kit (N05007A-1; Meso Scale Discovery) and for TGFβ1 using a MSD Human TGFβ1 kit (L451UA-1; Meso Scale Discovery).
Immunofluoresence.
Cells were cultured in 3D collagen matrices with DMEM, 10% (vol/vol) FBS, and 1% penicillin–streptomycin, treated with or without anti-TNF (10 μg/mL) for 24 h, and then fixed for 10 min with 3% (wt/vol) paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 (Sigma) for 30 min. Cells were stained with a mouse monoclonal α-SMA (Sigma) followed by Alexa Fluor 568–conjugated rabbit anti-mouse antibody (Invitrogen) and DNA with DAPI (Sigma). Secondary antibody alone was used as an immunolabeling control. Matrices were compressed to enhance visualization of cell morphology and intracellular α-SMA filaments. Images were acquired using confocal microscopy oil immersion objectives (60×), and the signal was analyzed by Ultraview confocal microscopy (PerkinElmer). Cell viability was assessed using a Live/Dead Viability/Cytotoxicity Kit (Invitrogen).
TOPflash.
Cells were transfected using the Amaxa Human Dermal Fibroblast Nucleofector Kit (Lonza) with the Wnt reporter plasmid TOPflash (Addgene Plasmid 12456) or the Wnt mutant reporter plasmid FOPflash (Addgene Plasmid 12457), both kindly donated by Randall Moon (Howard Hughes Medical Institute, University of Washington, Seattle) (59). Each Wnt vector was cotransfected with PRL-CMV Renilla Luciferase expression plasmid (Promega). After 24-h transfection in Opti-MEM reduced serum media (Life Technologies), cells were stimulated for a further 16 h with TNF (Peprotech), LiCl (Sigma), TNF with LiCl, or SB-2167613 (Sigma). The cells were then lysed, and Firefly and Renilla luciferase activities were analyzed with Dual-Luciferase Assay System (Promega) using a Micro-Beta Jet Luminescence Counter (Perkin-Elmer). Values of the Firefly luciferase reporter gene activity were normalized to Renilla luciferase activity.
Statistics.
The rate of fibroblast populated collagen lattice contraction (dynes/h) was calculated by measuring the average gradient of the curve between 6 and 24 h. A paired t test was used for comparison of TNFR1/2 expression in MF-D and to compare TOP and FOP TCF/Lef promoter gene activity in NPF-D, PF-N, and PF-D. Analysis of single variance was used for comparisons all other conditions. All statistical analyses were performed using software (GraphPad Software version 5.0c). Significance was achieved if P < 0.05.
Supplementary Material
Acknowledgments
This work is supported by the Kennedy Trust for Rheumatology Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301100110/-/DCSupplemental.
References
- 1.Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: Results from a population-based study. Hand (NY) 2011;6(2):149–158. doi: 10.1007/s11552-010-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rombouts JJ, Noël H, Legrain Y, Munting E. Prediction of recurrence in the treatment of Dupuytren’s disease: Evaluation of a histologic classification. J Hand Surg Am. 1989;14(4):644–652. doi: 10.1016/0363-5023(89)90183-4. [DOI] [PubMed] [Google Scholar]
- 3.Beaudreuil J, Lellouche H, Orcel P, Bardin T. 2012. Needle aponeurotomy in Dupuytren's disease. Joint Bone Spine 79(1):13–16.
- 4.Hurst LC, et al. CORD I Study Group Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 5.Dolmans GH, et al. Dutch Dupuytren Study Group German Dupuytren Study Group LifeLines Cohort Study BSSH-GODD Consortium Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011;365(4):307–317. doi: 10.1056/NEJMoa1101029. [DOI] [PubMed] [Google Scholar]
- 6.Skalli O, et al. A monoclonal antibody against alpha-smooth muscle actin: A new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 8.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–29. [PubMed] [Google Scholar]
- 9.Krause C, Kloen P, Ten Dijke P. 2011. Elevated transforming growth factor beta and mitogen-activated protein kinase pathways mediate fibrotic traits of Dupuytren's disease fibroblasts. Fibrogenesis Tissue Rep 4(1):14. [DOI] [PMC free article] [PubMed]
- 10.Kloen P, Jennings CL, Gebhardt MC, Springfield DS, Mankin HJ. Transforming growth factor-beta: Possible roles in Dupuytren’s contracture. J Hand Surg Am. 1995;20(1):101–108. doi: 10.1016/S0363-5023(05)80067-X. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J Invest Dermatol. 2007;127(11):2645–2655. doi: 10.1038/sj.jid.5700890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisson MA, McGrouther DA, Mudera V, Grobbelaar AO. The different characteristics of Dupuytren’s disease fibroblasts derived from either nodule or cord: Expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg [Br] 2003;28(4):351–356. doi: 10.1016/s0266-7681(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 13.Wong M, Mudera V. Feedback inhibition of high TGF-beta1 concentrations on myofibroblast induction and contraction by Dupuytren’s fibroblasts. J Hand Surg [Br] 2006;31(5):473–483. doi: 10.1016/j.jhsb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrew JG, Andrew SM, Ash A, Turner B. An investigation into the role of inflammatory cells in Dupuytren’s disease. J Hand Surg [Br] 1991;16(3):267–271. doi: 10.1016/0266-7681(91)90051-o. [DOI] [PubMed] [Google Scholar]
- 16.Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25(6):1157–1162. doi: 10.1053/jhsu.2000.18493. [DOI] [PubMed] [Google Scholar]
- 17.McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren contracture: A randomized, controlled study. J Hand Surg Am. 2012;37(7):1307–1312. doi: 10.1016/j.jhsa.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Meek RM, McLellan S, Crossan JF. Dupuytren’s disease. A model for the mechanism of fibrosis and its modulation by steroids. J Bone Joint Surg Br. 1999;81(4):732–738. doi: 10.1302/0301-620x.81b4.9163. [DOI] [PubMed] [Google Scholar]
- 19.Meek RM, McLellan S, Reilly J, Crossan JF. The effect of steroids on Dupuytren’s disease: Role of programmed cell death. J Hand Surg [Br] 2002;27(3):270–273. doi: 10.1054/jhsb.2001.0742. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal SA, et al. Identification of mesenchymal stem cells in perinodular fat and skin in Dupuytren’s disease: A potential source of myofibroblasts with implications for pathogenesis and therapy. Stem Cells Dev. 2012;21(4):609–622. doi: 10.1089/scd.2011.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oguma K, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27(12):1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi JK, Vidal-Puig A. Wnt signalling and the control of cellular metabolism. Biochem J. 2010;427(1):1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coghlan MP, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 25.Cheng K, Creacy S, Larner J. ‘Insulin-like’ effects of lithium ion on isolated rat adipocytes. II. Specific activation of glycogen synthase. Mol Cell Biochem. 1983;56(2):183–189. doi: 10.1007/BF00227219. [DOI] [PubMed] [Google Scholar]
- 26.Seegenschmiedt MH, Olschewski T, Guntrum F. Radiotherapy optimization in early-stage Dupuytren’s contracture: First results of a randomized clinical study. Int J Radiat Oncol Biol Phys. 2001;49(3):785–798. doi: 10.1016/s0360-3016(00)00745-8. [DOI] [PubMed] [Google Scholar]
- 27.Betz N, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186(2):82–90. doi: 10.1007/s00066-010-2063-z. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satish L, et al. Fibroblasts from phenotypically normal palmar fascia exhibit molecular profiles highly similar to fibroblasts from active disease in Dupuytren’s contracture. BMC Med Genomics. 2012;5:15. doi: 10.1186/1755-8794-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verjee LS, Midwood K, Davidson D, Eastwood M, Nanchahal J. Post-transcriptional regulation of alpha-smooth muscle actin determines the contractile phenotype of Dupuytren’s nodular cells. J Cell Physiol. 2010;224(3):681–690. doi: 10.1002/jcp.22167. [DOI] [PubMed] [Google Scholar]
- 31.Rehman S, et al. Dupuytren’s disease metabolite analyses reveals alterations following initial short-term fibroblast culturing. Mol Biosyst. 2012;8(9):2274–2288. doi: 10.1039/c2mb25173f. [DOI] [PubMed] [Google Scholar]
- 32.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budd DC, Holmes AM. Targeting TGFβ superfamily ligand accessory proteins as novel therapeutics for chronic lung disorders. Pharmacol Ther. 2012;135(3):279–291. doi: 10.1016/j.pharmthera.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011;29(4):140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz LA, et al. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res. 1998;24(6):721–743. doi: 10.3109/01902149809099592. [DOI] [PubMed] [Google Scholar]
- 36.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994;7(3):515–518. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- 37.Bisson MA, Mudera V, McGrouther DA, Grobbelaar AO. 2004. The contractile properties and responses to tensional loading of Dupuytren's disease–derived fibroblasts are altered: A cause of the contracture? Plast Reconstr Surg 113(2):611–621. [DOI] [PubMed]
- 38.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhmetshina A, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735–747. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafer SL, Towler DA. Transcriptional regulation of SM22alpha by Wnt3a: Convergence with TGFbeta(1)/Smad signaling at a novel regulatory element. J Mol Cell Cardiol. 2009;46(5):621–635. doi: 10.1016/j.yjmcc.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan DE, Ferris M, Pociask D, Brody AR. Tumor necrosis factor-alpha induces transforming growth factor-beta1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am J Respir Cell Mol Biol. 2005;32(4):342–349. doi: 10.1165/rcmb.2004-0288OC. [DOI] [PubMed] [Google Scholar]
- 42.Verrecchia F, Pessah M, Atfi A, Mauviel A. Tumor necrosis factor-alpha inhibits transforming growth factor-beta /Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem. 2000;275(39):30226–30231. doi: 10.1074/jbc.M005310200. [DOI] [PubMed] [Google Scholar]
- 43.Lam AP, Gottardi CJ. β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23(6):562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14(7):1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammarstedt A, Isakson P, Gustafson B, Smith U. Wnt-signaling is maintained and adipogenesis inhibited by TNFalpha but not MCP-1 and resistin. Biochem Biophys Res Commun. 2007;357(3):700–706. doi: 10.1016/j.bbrc.2007.03.202. [DOI] [PubMed] [Google Scholar]
- 47.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor receptor type 2. Curr Med Chem. 2004;11(16):2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 49.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280(43):36099–36109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz LA, et al. Upregulation of the p75 but not the p55 TNF-alpha receptor mRNA after silica and bleomycin exposure and protection from lung injury in double receptor knockout mice. Am J Respir Cell Mol Biol. 1999;20(4):825–833. doi: 10.1165/ajrcmb.20.4.3193. [DOI] [PubMed] [Google Scholar]
- 51.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: Reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15(5):353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Robinson CM, Seah KT, Chee YH, Hindle P, Murray IR. Frozen shoulder. J Bone Joint Surg Br. 2012;94(1):1–9. doi: 10.1302/0301-620X.94B1.27093. [DOI] [PubMed] [Google Scholar]
- 54.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 55.Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;((3):CD005112. doi: 10.1002/14651858.CD005112.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;((1):CD006893. doi: 10.1002/14651858.CD006893. [DOI] [PubMed] [Google Scholar]
- 57.Verjee LS, et al. Myofibroblast distribution in Dupuytren’s cords: Correlation with digital contracture. J Hand Surg Am. 2009;34(10):1785–1794. doi: 10.1016/j.jhsa.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Jain A, Brennan F, Nanchahal J. Treatment of rheumatoid tenosynovitis with cytokine inhibitors. Lancet. 2002;360(9345):1565–1566. doi: 10.1016/S0140-6736(02)11529-7. [DOI] [PubMed] [Google Scholar]
- 59.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13(8):680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.