Abstract
Transforming growth factor (TGF)-β-activated kinase 1 (TAK1) and Nemo-like kinase (NLK) function in Xenopus, Drosophila, and Caenorhabditis elegans development. Here we report that serine phosphorylation of STAT3 induced by TAK1-NLK cascade is essential for TGF-β-mediated mesoderm induction in Xenopus embryo. Depletion of TAK1, NLK, or STAT3 blocks TGF-β-mediated mesoderm induction. Coexpression of NLK and STAT3 induces mesoderm by a mechanism that requires serine phosphorylation of STAT3. Activin activates NLK, which in turn directly phosphorylates STAT3. Moreover, depletion of either TAK1 or NLK inhibits endogenous serine phosphorylation of STAT3. These results provide the first evidence that TAK1-NLK-STAT3 cascade participates in TGF-β-mediated mesoderm induction.
Keywords: TAK1, NLK, STAT3, TGF-β signal, mesoderm induction
During embryogenesis, specification of cell fate is under the control of many secreted signaling molecules. Several members of the transforming growth factor (TGF)-β family of ligands, including activin, Vg1, and nodal-related proteins, have been identified as determinants of cell fate in mesoderm induction (Thomsen and Melton 1993; Symes et al. 1994; Smith 1995; Agius et al. 2000). TGF-β-activated kinase 1 (TAK1), a member of the MAP kinase kinase kinase (MAPKKK) family, is activated by various cytokines, including TGF-β family ligands (Yamaguchi et al. 1995; Shibuya et al. 1996). Nemo-like kinase (NLK) is a MAPK-related kinase that functions downstream of TAK1 in a pathway that inhibits Wnt signaling (Ishitani et al. 1999; Meneghini et al. 1999). NLK binds to and phosphorylates TCF/LEF transcription factors, which prevents them from binding to their DNA targets (Ishitani et al. 1999). Recent studies indicate that NLK binds not only to TCF/LEF but also to other transcription factors, including Sox11 (Hyodo-Miura et al. 2002) and HMG2L1 (Yamada et al. 2003). This raises the possibility that other transcription factors and signaling pathways may be regulated by the TAK1-NLK cascade.
The JAK-STAT signaling pathway transduces signals induced by a large number of cytokines, hormones, and growth factors. Signaling is initiated by JAK tyrosine kinases, which phosphorylate the C-terminal tyrosine residue of STAT transcription factors. These tyrosine-phosphorylated STATs then dimerize through their SH2 domain and translocate into the nucleus, where they directly activate target genes (Darnell 1997; O'Shea et al. 2002). It has previously been reported that phosphorylation of the C-terminal serine residue of STAT3 is required for maximal transcriptional activity (Decker and Kovarik 2000; O'Shea et al. 2002). However, neither the biological significance of this phosphorylation nor the endogenous kinase(s) responsible for it has been demonstrated.
In this study, we show that NLK binds to STAT3 and directly phosphorylates its C-terminal serine residue, and that this phosphorylation requires the activity of the TAK1-NLK cascade. In addition, we demonstrate that serine phosphorylation of STAT3 induced by TAK1-NLK cascade is indispensable for TGF-β-mediated mesoderm induction in early Xenopus development. Taken together, these results provide the first evidence that the TAK1-NLK-STAT3 cascade functions in the TGF-β family signal transduction pathway.
Results and Discussion
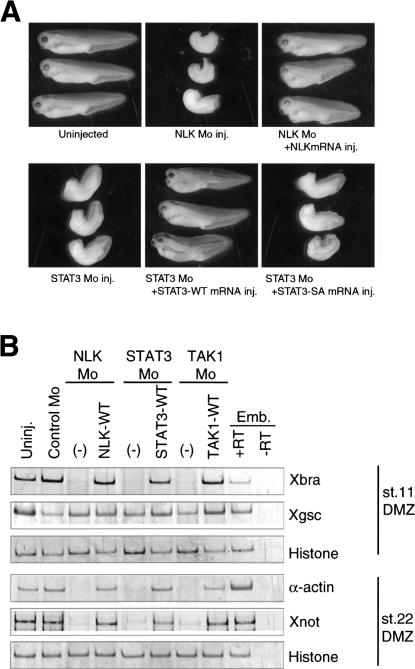
To address the roles of NLK in early Xenopus embryos, we inhibited NLK expression in developing embryos by using a morpholino-antisense oligonucleotide (NLK Mo), which is a specific translational inhibitor, and analyzed the phenotypes of the resulting embryos. Injection of NLK Mo into Xenopus four-cell-stage embryos resulted in incomplete gastrulation and a shortened anterior–posterior axis (Fig. 1A, upper middle panel). The NLK Mo-induced phenotype was rescued by coinjection of NLK mRNA (Fig. 1A, upper right panel). This phenotype appears to result from the inhibition of either mesoderm induction or gastrulation movement (Kimelman and Griffin 1998; Smith et al. 2000; Wallingford et al. 2002). To examine each of these possibilities, we injected NLK Mo into the prospective dorsal mesodermal region of four-cell-stage embryos, and determined the expression patterns of several mesodermal marker genes at stage 11 and 22. Injection of NLK Mo inhibited the expression of a pan-mesodermal marker, Xbra, and the late mesodermal markers, α-actin and Xnot, but had no effect on the expression of a dorsal mesodermal marker, goosecoid (Xgsc; Fig. 1B). Inhibition of these markers was rescued by coinjection of NLK mRNA (Fig. 1B). These results demonstrate that endogenous NLK is involved in mesoderm induction.
Figure 1.
NLK and STAT3 are involved in mesoderm induction. NLK Mo (8.4 ng), STAT3 Mo (10 ng), control Mo (20 ng), TAK1 Mo (20 ng), and synthetic mRNA of NLK-WT (40 pg), STAT3-WT (50 pg), STAT3-SA (50 pg), or TAK1-WT (100 pg) were injected into the dorsal equatorial region of four-cell-stage embryos as indicated. (A) The embryos were cultured to stage 35/36. Control uninjected embryos are shown in the top left panel. (B) Expression of Xbra (a pan mesodermal marker), Xgsc (a dorsal mesodermal marker), α-actin, Xnot (late mesodermal markers), and histone (a ubiquitous control) in dorsal marginal zone (DMZ) was analyzed by RT–PCR. Emb indicates whole-embryo control with (+RT) or without (–RT) the RT step.
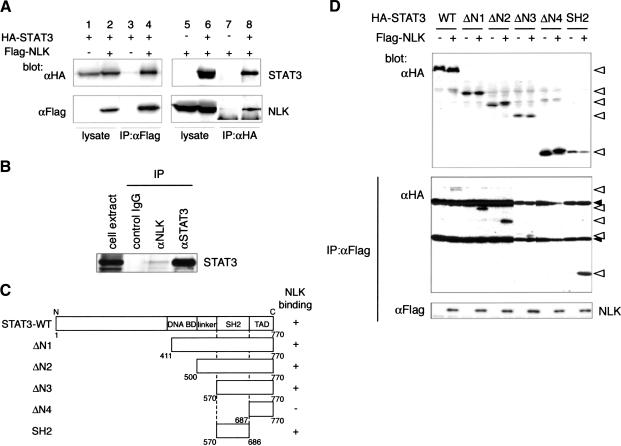
In an attempt to understand how NLK regulates mesoderm induction in Xenopus embryos, we isolated NLK-binding proteins from a Xenopus oocyte cDNA library by the yeast two-hybrid screening method, using the C-terminal domain of NLK (202–447 amino acids of NLK) as bait (Yamada et al. 2003). From this screen, we isolated the cDNA encoding Xenopus STAT3, a member of the STAT family of transcription factors (Darnell 1997; Nishinakamura et al. 1999; Hirano et al. 2000). To examine whether STAT3 associates with NLK in vivo, we constructed expression vectors encoding Flag-tagged NLK (Flag-NLK) and HA-tagged STAT3 (HA-STAT3). We found that Flag-NLK could be coimmunoprecipitated with HA-STAT3, and vice versa, when cotransfected into COS7 cells (Fig. 2A, lanes 4,8). STAT3 could also be coimmunoprecipitated with a kinase-negative mutant of NLK, NLK-KN (data not shown). This indicates that the association between NLK and STAT3 does not require NLK kinase activity. To prove for the existence of endogenous NLK and STAT3 complex in cells, we isolated extracts from untransfected mouse embryonic fibroblast, and performed immunoprecipitation analysis by using anti-NLK antibody. We found that endogenous STAT3 was coimmunoprecipitated with NLK (Fig. 2B). We next constructed a series of STAT3 deletion mutants (Fig. 2C) and characterized them for binding to NLK (Fig. 2D). Deletion of the STAT3 DNA-binding domain (DNA BD), linker domain (linker), or transactivation domain (TAD) had no effect on binding to NLK, whereas deletion of the SH2 domain abolished NLK binding (Fig. 2D). Consistent with this, the minimal region of STAT3 required for the binding to NLK was found to reside in the SH2 domain (Fig. 2D). Thus, STAT3 interacts with NLK via its SH2 domain.
Figure 2.
STAT3 associates with NLK via its SH2 domain. (A) COS7 cells were transfected as indicated. Immunoprecipitates obtained by using anti-Flag (lanes 3,4) or anti-HA (lanes 7,8) antibody were subjected to Western blotting with the indicated antibodies. Expression of HA-STAT3 or Flag-NLK was monitored (lanes 1,2,5,6). (B) Immunoprecipitates obtained from mouse embryonic fibroblast extracts by using control IgG, anti-NLK antibody, or anti-STAT3 antibody were subjected to Western blotting with anti-STAT3 antibody. (C) Diagram of STAT3 deletion mutants. (D) 293 cells were transfected as indicated. Immunoprecipitates obtained with anti-Flag antibody were subjected to Western blotting with anti-HA antibody (middle) or anti-Flag antibody (bottom). Expression of HA-STAT3 deletion mutants was monitored (top). Open triangles and closed triangles indicate the migrations of STAT3 deletion mutants and IgG proteins, respectively.
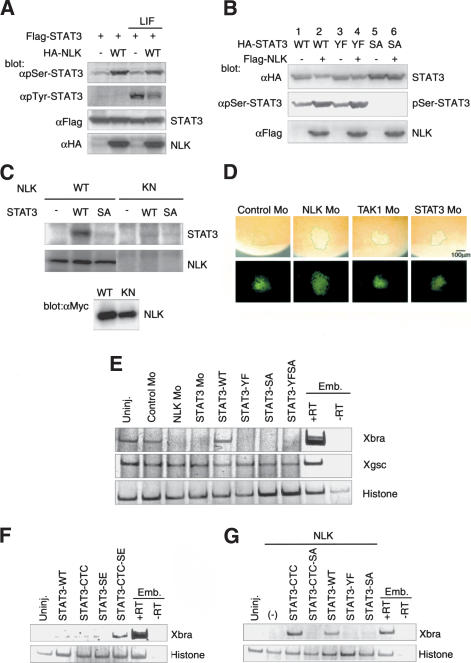
C-terminal serine residue in STAT3 (Ser 727 in mouse and Ser 728 in Xenopus STAT3) is phosphorylated by several kinases, including those of the MAPK family, and this phosphorylation modulates its transcriptional activity (Decker and Kovarik 2000; O'Shea et al. 2002). We thus tested whether NLK phosphorylates the corresponding serine residue of STAT3. Flag-STAT3 was cotransfected with or without HA-NLK into 293 cells, and the phosphorylation of STAT3 was monitored by Western blotting with antibodies that specifically recognize the phosphorylated form of STAT3 at either the Ser 727 or Tyr 705 residue. Western blot analysis revealed that coexpression of NLK resulted in increased levels of Ser 727, but not Tyr 705, phosphorylation (Fig. 3A). Moreover, coexpression of NLK caused a mobility shift in the STAT3 protein on SDS-PAGE (Fig. 3B, lane 2). To test whether this mobility shift is a consequence of Ser 727 phosphorylation, we used a mutant, STAT3-SA, in which Ser 727 is replaced with alanine. As expected, no serine phosphorylation of STAT3-SA was detected, and the migration of this mutant protein was unaffected by coexpression of NLK (Fig. 3B, lanes 5,6). We next examined whether NLK directly phosphorylates the Ser 727 residue in STAT3 by using bacterially expressed wild-type STAT3 and STAT3-SA proteins. In vitro kinase assays showed that NLK could phosphorylate wild-type STAT3 in a kinase-dependent manner but failed to phosphorylate STAT3-SA (Fig. 3C). Taken together, these results clearly demonstrate that NLK directly and specifically phosphorylates the C-terminal serine residue of STAT3.
Figure 3.
NLK phosphorylates the C-terminal serine residue of STAT3. (A,B) 293 cells were transfected as indicated and treated with LIF or left untreated, and whole-cell lysates were subjected to Western blotting by using the indicated antibodies. (C) 293 cells were transfected with Myc-tagged wild-type NLK (WT) or kinase-negative NLK (KN). Immunoprecipitates obtained with anti-Myc antibody were incubated with bacterially expressed STAT3 and [γ-32P] ATP. Phosphorylation of STAT3 (top) and autophosphorylation of NLK (middle) are shown. Expression of Myc-NLK was monitored (bottom). (D) The indicated Mo and lineage tracer FLDx (green; bottom) were injected into the marginal zone of 32-cell-stage embryos. Whole-mount immunostaining with anti-pSer-STAT3 antibody was performed at stage 11 (top). Green lines indicate the boundary of the injected area. (E) RT–PCR analysis of the expression of mesodermal markers in DMZ. Control Mo (10 ng), NLK Mo (8.4 ng), STAT3 Mo (10 ng), or synthetic mRNA of wild-type STAT3 or its mutants (2 ng) were injected as indicated. (F,G) RT–PCR analysis of expression of Xbra in animal caps. (F) Wild-type STAT3 or mutant mRNAs (5 ng) were injected as indicated. (G) NLK mRNA (2.5 ng) was injected with or without STAT3 mutant mRNAs (2.5 ng) as indicated.
We next tested whether NLK is involved in the serine phosphorylation of endogenous STAT3 in Xenopus embryos. We performed whole-mount immunostaining by using an antibody that specifically recognizes the Ser 728 phosphorylated form of STAT3. Serine-phosphorylated STAT3 was detected in ectodermal and mesodermal regions at the gastrula stage (Fig. 3D; data not shown). As a control, when STAT3 Mo was injected into the prospective mesodermal regions of the embryos, serine phosphorylation of STAT3 was greatly reduced in the injected region (Fig. 3D). Similarly, when embryos were injected with NLK Mo, serine phosphorylation of STAT3 was significantly reduced (Fig. 3D). These results suggest that NLK is involved in the serine phosphorylation of endogenous STAT3 in early Xenopus embryos.
To investigate the role of STAT3 in mesoderm induction, we injected STAT3 Mo into the prospective dorsal mesodermal regions of four-cell-stage embryos, and later examined their phenotypes and mesodermal marker gene expression. Similar to NLK Mo, injection of STAT3 Mo resulted in incomplete gastrulation and a shortened anterior–posterior axis, and inhibited Xbra, α-actin and Xnot expression in the dorsal marginal zone, but did not affect Xgsc expression (Fig. 1A,B). These inhibitions were also rescued by coinjection of STAT3 mRNA (Fig. 1A,B). We also injected mRNAs encoding several STAT3 mutants, STAT3-YF, STAT3-SA, or STAT3-YFSA, each of which substitutes C-terminal tyrosine or/and serine residue(s) of STAT3 with alanine or/and phenylalanine residue(s), respectively. Injection of these mutant mRNAs into the dorsal marginal zone resulted in the inhibition of Xbra expression (Fig. 3E). Moreover, the STAT3 Mo-induced phenotype was not rescued by coinjection of STAT3-SA mRNA (Fig. 1A). These results suggest that mesoderm induction requires the phosphorylation of both tyrosine and serine residues in STAT3.
To further address the roles of NLK and STAT3 in mesoderm induction, we constructed the STAT3 mutants STAT3-CTC and STAT3-SE. The STAT3-CTC mutant replaces the Ala-Thr-Asp residues at position 662–664 of Xenopus STAT3 to Cys-Thr-Cys. It has been reported that substitution with these cysteine residues within the C-terminal loop of the STAT3 SH2 domain results in the generation of a constitutively active form of STAT3, due to Cys-mediated dimerization that is independent of tyrosine phosphorylation (Bromberg et al. 1999). Thus, the STAT3-CTC mutant would be expected to bypass the requirement for tyrosine phosphorylation. The STAT3-SE mutant replaces the C-terminal Ser 728 of Xenopus STAT3 with a glutamic acid residue, which mimics the phosphorylated serine. We found that injection of wild-type STAT3, STAT3-CTC, or STAT3-SE mutant mRNA did not induce Xbra expression in animal caps (Fig. 3F). However, injection of the STAT3-CTC-SE double-mutant mRNA did induce Xbra expression (Fig. 3F). This suggests that both dimerization and serine phosphorylation of STAT3 are required for induction of Xbra. Consistent with this, coinjection of STAT3-CTC mRNA with NLK mRNA strongly induced Xbra expression (Fig. 3G). Xbra expression in this case was dependent on the serine phosphorylation of STAT3, because coinjection of STAT3-CTC-SA mRNA with NLK mRNA did not induce its expression. Coinjection of wild-type STAT3 mRNA with NLK mRNA also induced Xbra expression moderately, and this expression was dependent on both the serine and tyrosine phosphorylation of STAT3 (Fig. 3G). These results indicate that both serine and tyrosine phosphorylation of the STAT3 C-terminal domain are required for mesoderm induction.
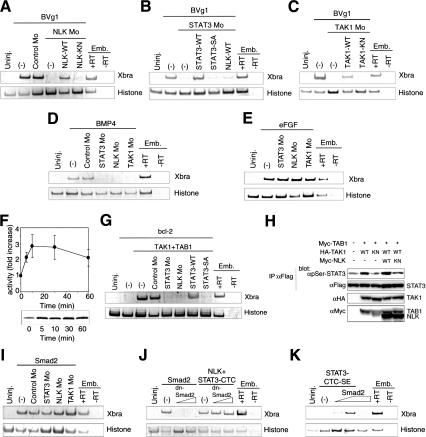
Members of both the TGF-β and FGF family of ligands have the ability to induce mesoderm development (Amaya et al. 1993; Thomsen and Melton 1993; Symes et al. 1994; Smith 1995; Agius et al. 2000). We thus examined whether NLK and STAT3 function in the induction of mesoderm by these TGF-β and/or FGF family ligands. Vg1, BMP, or FGF has been shown to induce Xbra expression in animal caps. We found that injection of NLK Mo or STAT3 Mo could block induction of Xbra expression by Vg1 or BMP (Fig. 4A,B,D) but had no effect on induction by FGF (Fig. 4E). Inhibition of Vg1-induced Xbra expression by NLK Mo could be rescued by coinjection with wild-type NLK mRNA but not by a kinase-negative mutant, NLK-KN (Fig. 4A). Similarly, coinjection of wild-type STAT3 mRNA, but not STAT3-SA mRNA, rescued the inhibition of Vg1-induced Xbra expression by STAT3 Mo (Fig. 4B). These results suggest that NLK and STAT3 function in a pathway downstream of the TGF-β family of ligands in early Xenopus embryos.
Figure 4.
TGF-β family ligands induce the expression of Xbra through the TAK1-NLK-STAT3 pathway. (A–E,G,I–K) RT–PCR analysis in animal caps. Synthetic mRNAs encoding BVg1 (15 pg), BMP4 (100 pg), eFGF (100 pg), NLK-WT (A,B, 40 pg; J, 1.25 ng), NLK-KN (40 pg), STAT3-WT (50 pg), STAT3-SA (50 pg), STAT3-CTC (1.25 ng), STAT3-CTC-SE (2.5 ng), TAK1-WT (100 pg), TAK1-KN (100 pg), TAB1 (1 ng), bcl-2 (250 pg), Smad2 (I, 2 ng; J, 1.25 ng; K, 500–750 pg), and dominant-negative Smad2 mutant (dnSmad2, 0.5–1 ng) were injected as indicated. Control Mo (20 ng), NLK Mo (8.4 ng), STAT3 Mo (10 ng), and TAK1 Mo (20 ng) were also injected as indicated. (F) 293 cells were treated with recombinant human activin A (10 ng/mL) for the indicated times, and endogenous NLK was immunoprecipitated and subjected to in vitro kinase assay. (Top) Activity was expressed as fold increase relative to that of NLK from unstimulated cells and is presented as mean ± S.E.M. from at least three experiments. (Bottom) Autoradiogram shows the phosphorylation of LEF-1. (H) 293 cells were transfected with a Flag-tagged STAT3 expression vector together with the indicated expression vectors. Immunoprecipitates obtained using anti-Flag antibody were subjected to Western blotting with indicated antibodies. Expression of HA-TAK1, Myc-TAB1, and Myc-NLK was monitored (αHA and αMyc).
To further confirm the involvement of NLK in TGF-β family signaling, we examined whether TGF-β family ligands can activate NLK kinase activity. 293 cells were treated with recombinant human activin A, and endogenous NLK was immunoprecipitated from the cell lysates with anti-NLK antibody. These immunoprecipitates were subjected to an in vitro kinase assay by using bacterially expressed LEF1 protein as a substrate (Ishitani et al. 1999). NLK kinase activity was found to increase within 5 min after activin A treatment, reach a maximum after 10–30 min, and decline thereafter with time (Fig. 4F). Activin A stimulated NLK kinase activity in a dose-dependent manner (data not shown). Thus, a TGF-β family ligand is able to activate NLK.
Our previous studies have shown that TAK1 functions as an upstream MAPKKK acting on the MAPK-related kinase, NLK (Ishitani et al. 1999; Meneghini et al. 1999). In addition, TAK1 can be activated by TGF-β and BMP stimulation (Yamaguchi et al. 1995). Together, these results raise the possibility that the mesoderm induction during embryogenesis by TGF-β family ligands involves activation of TAK1, which in turn regulates STAT3 via its action on NLK. To test this possibility, we examined the effects of injection of Xenopus TAK1 Mo into early Xenopus embryos. Injection of TAK1 Mo also inhibited Xbra, α-actin and Xnot expression in the dorsal marginal zone but did not affect Xgsc expression (Fig. 1B). When TAK1 Mo was injected into the prospective mesodermal region of embryos, serine phosphorylation of STAT3 was significantly reduced in the injected regions (Fig. 3D). In an animal cap assay, Vg1- and BMP-induced Xbra expression were also found to be inhibited by coinjection of TAK1 Mo (Fig. 4C,D), whereas FGF-induced Xbra expression was not inhibited (Fig. 4E). Inhibition of Vg1-induced Xbra expression by TAK1 Mo was rescued by coinjection with wild-type TAK1 mRNA but not with a kinase-negative TAK1-KN mutant mRNA (Fig. 4C). These results demonstrate that TAK1 is indispensable for both serine phosphorylation of STAT3 and mesoderm induction by TGF-β family ligands. Activation of TAK1 by coexpression with TAB1 (Shibuya et al. 1996) resulted in the induction of Xbra expression (Fig. 4G; Shibuya et al. 1998), and this induction was inhibited by injection of NLK Mo, STAT3 Mo or STAT3-SA mRNA (Fig. 4G). Moreover, we found that activation of TAK1 potentiates serine phosphorylation of STAT3 in 293 cells, and this potentiation was inhibited by coexpression of NLK-KN (Fig. 4H). Taken together, these results suggest that the TAK1-NLK-STAT3 pathway regulates TGF-β signaling in both Xenopus early embryos and mammalian cells.
The Smad transcription factors are known to be crucial intracellular transducers of TGF-β family signaling (Heldin et al. 1997; Attisano and Wrana 1998; Derynck et al. 1998; Massagué 2000), and Smad-mediated signaling is important in the early patterning of mesoderm (Whitman 1998). We further examined the relationship between TAK1-NLK-STAT3 and Smad pathways in mesoderm induction. Injection of Smad2 mRNA induced Xbra expression in animal caps, and coinjection of STAT3 Mo, NLK Mo or TAK1 Mo with Smad2 did not inhibit the expression of Xbra (Fig. 4I). On the other hand, coinjection of the dominant-negative mutant of Smad2 (Candia et al. 1997) had no effect on Xbra expression induced by coinjection of NLK mRNA with STAT3-CTC mRNA (Fig. 4J). Moreover, although the injection of Smad2 or STAT3-CTC-SE mRNA at lower dose did not induce Xbra expression, coinjection of both mRNAs did induce Xbra expression (Fig. 4K). These results demonstrate that TAK1-NLK-STAT3 and Smad pathways function independently and synergistically in TGF-β-mediated mesoderm induction. A previous report showed that STAT3 and Smad1 physically interact via p300/CBP to induce differentiation of neural progenitor cells that have been treated with both LIF and BMP (Nakashima et al. 1999). STAT3 and Smads may also interact and cooperate in mesoderm induction in early Xenopus development.
Although the role of the C-terminal tyrosine phosphorylation of STAT3 is well understood, the molecular mechanisms and in vivo functions of the C-terminal serine phosphorylation are unclear. Our results suggest that the C-terminal serine is phosphorylated in vivo through the TAK1-NLK pathway. Furthermore, we demonstrate that the serine phosphorylation, as well as tyrosine phosphorylation, is indispensable for endogenous mesoderm induction by TGF-β family signaling. These findings provide the first evidence identifying a kinase that acts on the STAT3 C-terminal serine in vivo, and demonstrate the significance of STAT3 serine phosphorylation in embryogenic development. Because NLK is unable to phosphorylate the C-terminal tyrosine residue, another tyrosine kinase(s) is also responsible for phosphorylation of STAT3 in early Xenopus development. Although the exact identity of the cognate tyrosine kinase and its activator are unclear at present, it is clear that two signal transduction pathways function together to activate STAT3: the TAK1-NLK pathway activated by TGF-β family ligands and a signaling pathway using a yet-to-be-identified tyrosine kinase.
Materials and methods
Mutant plasmid construction
Mutants of Xenopus STAT3 (xSTAT3) were generated by site-directed mutagenesis using the polymerase chain reaction (PCR). In xSTAT3-YF, xSTAT3-SA, and xSTAT3-YFSA, Tyr 706 and Ser 728 were mutated to phenylalanine and alanine, respectively. In xSTAT3-CTC, xSTAT3-SE, and xSTAT3-CTC-SE, alanine–threonine–asparagic acid at 662–664 and Ser 728 were mutated to cysteine–threonine–cysteine and glutamic acid, respectively. The dominant-negative mutant of Smad2 was generated as described previously (Candia et al. 1997).
Embryo handling, morpholino oligonucleotides
Embryo manipulation, microinjection, and RT–PCR were as described (Shibuya et al. 1998). The morpholino oligonucleotides (Mo) used here were 5′-GCCCTTCCCTACACGGATGTCCCCC-3′ (xNLK), 5′-GGTTC CACTGCGCCATTGTGTGGGC-3′ (xSTAT3), and 5′-TCATTTCAG CAGAGGTGGCAGACAT-3′ (xTAK1; Gene Tools, LLC).
Immunoprecipitation and in vitro kinase assay
Cell lysates were precleared with protein G-Sepharose beads and incubated with the appropriate antibodies coupled to protein G-Sepharose beads. Immunoprecipitates were washed four times with 1 mL lysis buffer or phosphate-buffered saline and subjected to Western blotting or in vitro kinase assay. Endogenous NLK was immunoprecipitated by rabbit polyclonal anti-NLK antibody (Ishitani et al. 2003). Immunoprecipitates were incubated with 1 μg bacterially expressed recombinant LEF-1 (Ishitani et al. 1999; Meneghini et al. 1999) or STAT3 in 20 μL kinase buffer for 2.5 min at 25°C (endogenous NLK) or for 10 min at 30°C (Myc-NLK). Phosphorylated substrates were subjected to SDS-PAGE and quantitated by using an image analyzer (Fujix BAS 2500).
Acknowledgments
We thank E. Nishida for helpful discussions, M. Lamphier for critical reading of the manuscript, and M. Yamada and K. Nakamura for technical assistance. This work was supported by Grants-in-Aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1166904.
References
- Agius E., Oelgeschlager, M., Wessely, O., Kemp, C., and De Robertis, E.M. 2000. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E., Stein, P.A., Musci, T.J., and Kirschner, M.W. 1993. FGF signalling in the early specification of mesoderm in Xenopus. Development 118: 477–487. [DOI] [PubMed] [Google Scholar]
- Attisano L. and Wrana, J.L. 1998. Mads and Smads in TGFβ signalling. Curr. Opin. Cell. Biol. 10: 188–194. [DOI] [PubMed] [Google Scholar]
- Bromberg J.F., Wrzeszczynska, M.H., Devgan, G., Zhao, Y., Pestell, R.G., Albanese, C., and Darnell Jr., J.E. 1999. Stat3 as an oncogene. Cell 98: 295–303. [DOI] [PubMed] [Google Scholar]
- Candia A.F., Watabe, T., Hawley, S.H., Onichtchouk, D., Zhang, Y., Derynck, R., Niehrs, C., and Cho, K.W. 1997. Cellular interpretation of multiple TGF-β signals: Intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124: 4467–4480. [DOI] [PubMed] [Google Scholar]
- Darnell J.E. 1997. STATs and gene regulation. Science 277: 1630–1635. [DOI] [PubMed] [Google Scholar]
- Decker T. and Kovarik, P. 2000. Serine phosphorylation of STATs. Oncogene 19: 2628–2637. [DOI] [PubMed] [Google Scholar]
- Derynck R., Zhang, Y., and Feng, X.H. 1998. Smads: Transcriptional activators of TGF-β responses. Cell 95: 737–740. [DOI] [PubMed] [Google Scholar]
- Heldin C.H., Miyazono, K., and ten Dijke, P. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471. [DOI] [PubMed] [Google Scholar]
- Hirano T., Ishihara, K., and Hibi, M. 2000. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19: 2548–2556. [DOI] [PubMed] [Google Scholar]
- Hyodo-Miura J., Urushiyama, S., Nagai, S., Nishita, M., Ueno, N., and Shibuya, H. 2002. Involvement of NLK and Sox11 in neural induction in Xenopus development. Genes Cells 7: 487–496. [DOI] [PubMed] [Google Scholar]
- Ishitani T., Ninomiya-Tsuji, J., Nagai, S., Nishita, M., Meneghini, M., Barker, N., Waterman, M., Bowerman, B., Clevers, H., Shibuya, H., et al. 1999. The TAK1-NLK-MAPK-related pathway antagonizes signaling between β-catenin and transcription factor TCF. Nature 399: 798–802. [DOI] [PubMed] [Google Scholar]
- Ishitani T., Kishida, S., Hyodo-Miura, J., Ueno, N., Yasuda, J., Waterman, M., Shibuya, H., Moon, R.T., Ninomiya-Tsuji, J., and Matsumoto, K. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signaling. Mol. Cell. Biol. 23: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. and Griffin, K.J. 1998. Mesoderm induction: A postmodern view. Cell 94: 419–421. [DOI] [PubMed] [Google Scholar]
- Massagué J. 2000. How cells read TGF-β signals. Nat. Rev. Mol. Cell. Biol. 1: 169–178. [DOI] [PubMed] [Google Scholar]
- Meneghini M.D., Ishitani, T., Carter, J.C., Hisamoto, N., Ninomiya-Tsuji, J., Thorpe, C.J., Hamill, D.R., Matsumoto, K., and Bowerman, B. 1999. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399: 793–797. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Yanagisawa, M., Arakawa, H., Kimura, N., Hisatsune, T., Kawabata, M., Miyazono, K., and Taga, T. 1999. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284: 479–482. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R., Matsumoto, Y., Matsuda, T., Ariizumi, T., Heike, T., Asashima, M., and Yokota, T. 1999. Activation of Stat3 by cytokine receptor gp130 ventralizes Xenopus embryos independent of BMP-4. Dev. Biol. 216: 481–490. [DOI] [PubMed] [Google Scholar]
- O'Shea J.J., Gadina, M., and Schreiber, R.D. 2002. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell 109: S121–S131. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yamaguchi, K., Shirakabe, K., Tonegawa, A., Gotoh, Y., Ueno, N., Irie, K., Nishida, E., and Matsumoto, K. 1996. TAB1: An activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 272: 1179–1182. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Iwata, H., Masuyama, N., Gotoh, Y., Yamaguchi, K., Irie, K., Matsumoto, K., Nishida, E., and Ueno, N. 1998. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 17: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C. 1995. Mesoderm-inducing factors and mesodermal patterning. Curr. Opin. Cell. Biol. 7: 856–861. [DOI] [PubMed] [Google Scholar]
- Smith J.C., Conlon, F.L., Saka, Y., and Tada, M. 2000. Xwnt11 and the regulation of gastrulation in Xenopus. Philos. Trans. R Soc. Lond. B 355: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes K., Yordan, C., and Mercola, M. 1994. Morphological differences in Xenopus embryonic mesodermal cells are specified as an early response to distinct threshold concentrations of activin. Development 120: 2339–2346. [DOI] [PubMed] [Google Scholar]
- Thomsen G.H. and Melton, D.A. 1993. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell 74: 433–441. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Fraser, S.E., and Harland, R.M. 2002. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev. Cell 2: 695–706. [DOI] [PubMed] [Google Scholar]
- Whitman M. 1998. Smads and early developmental signaling by the TGFβ superfamily. Genes & Dev. 12: 2445–2462. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ohkawara, B., Ichimura, N., Hyodo-Miura, J., Urushiyama, S., Shirakabe, K., and Shibuya, H. 2003. Negative regulation of Wnt signalling by HMG2L1, a novel NLK-binding protein. Genes Cells 8: 677–684. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Shirakabe, K., Shibuya, H., Irie, K., Oishi, I., Ueno, N., Taniguchi, T., Nishida, E., and Matsumoto, K. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270: 2008–2011. [DOI] [PubMed] [Google Scholar]