Abstract
Parkinson disease and dementia with Lewy bodies are featured with the formation of Lewy bodies composed mostly of α-synuclein (α-Syn) in the brain. Although evidence indicates that the large oligomeric or protofibril forms of α-Syn are neurotoxic agents, the detailed mechanisms of the toxic functions of the oligomers remain unclear. Here, we show that large α-Syn oligomers efficiently inhibit neuronal SNARE-mediated vesicle lipid mixing. Large α-Syn oligomers preferentially bind to the N-terminal domain of a vesicular SNARE protein, synaptobrevin-2, which blocks SNARE-mediated lipid mixing by preventing SNARE complex formation. In sharp contrast, the α-Syn monomer has a negligible effect on lipid mixing even with a 30-fold excess compared with the case of large α-Syn oligomers. Thus, the results suggest that large α-Syn oligomers function as inhibitors of dopamine release, which thus provides a clue, at the molecular level, to their neurotoxicity.
Keywords: exocytosis, neuroscience, vesicle fusion, alternating-laser excitation, single-vesicle
Alpha-Synuclein (α-Syn) is a 140-residue peripheral membrane-binding protein predominantly expressed in central neurons and localized in presynaptic terminals (1, 2). It is a major component of the amyloid fibrils known as Lewy bodies found in the brains of patients with Parkinson disease (PD) and those with dementia with Lewy bodies (3, 4). In addition to the general sporadic form of PD, the point mutants of α-Syn and duplication or triplication of the α-Syn gene (PARK1) are associated with autosomal dominant familial PD (5–9). Although the deposition of the fibril forms of α-Syn in Lewy bodies is a common hallmark of PD, currently the hypothesis that large soluble oligomeric or protofibril intermediates of α-Syn are toxic agents is widely accepted (10, 11). Another distinctive hallmark of PD is the preferential destruction of dopaminergic neurons in the early stage of PD (12–14). This observation led to the conjecture that dopamine, particularly its oxidant forms generated by oxidative stress, may play an important role in PD (11, 15, 16). Indeed, Conway et al. (17) have shown that dopamine accelerates formation of large α-Syn oligomers while suppressing fibril formation, demonstrating the connection between dopamine and large α-Syn oligomers (18–21). However, the molecular origin of the toxicity of dopamine-induced large α-Syn oligomers remains unclear (15, 22).
Because α-Syn is localized at the presynaptic terminal, its physiological functions frequently have been linked to synaptic transmission and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, the core fusion machinery for vesicle fusion (23–26). For example, overexpression of α-Syn resulted in reduction of dopamine release by interfering with a step after vesicle docking in exocytosis (27, 28) or by inhibiting the reclustering of synaptic vesicles at the active zone after endocytosis (29). When the α-Syn level was reduced, the number of vesicles in the reserved pool decreased (30), but more vesicles were found in the readily releasable pool (31). In contrast, Südhof and coworkers (25) recently found that α-Syn promotes SNARE complex formation through its binding to a vesicular SNARE protein, synaptobrevin-2. However, it does not promote the release, indicating that it might play a maintenance role by helping stabilize the spent cis-SNARE complex on the plasma membrane before recycling (25, 26). Therefore, although it is highly likely that α-Syn oligomers might interfere with the fusion machinery, the nature and the molecular mechanism of this interference are completely unknown.
Here, we show that dopamine-induced large α-Syn oligomers efficiently inhibit neuronal SNARE-mediated lipid mixing. Using single-vesicle and bulk in vitro lipid-mixing assays, we find that large α-Syn oligomers preferentially bind to the N-terminal domain of synaptobrevin-2, which blocks the docking between vesicles. In contrast, the α-Syn monomer presents no appreciable effect on bulk lipid mixing, although it promotes SNARE complex formation, consistent with the results by Südhof and coworkers (25). Also, we show that exocytosis in PC12 cells is reduced dramatically by large α-Syn oligomers whereas the monomer has a negligible effect on it, confirming the inhibitory role of large α-Syn oligomers in exocytosis. These results suggest that large α-Syn oligomers inhibit exocytosis, which may explain the neurotoxicity of large α-Syn oligomers at a molecular level.
Results
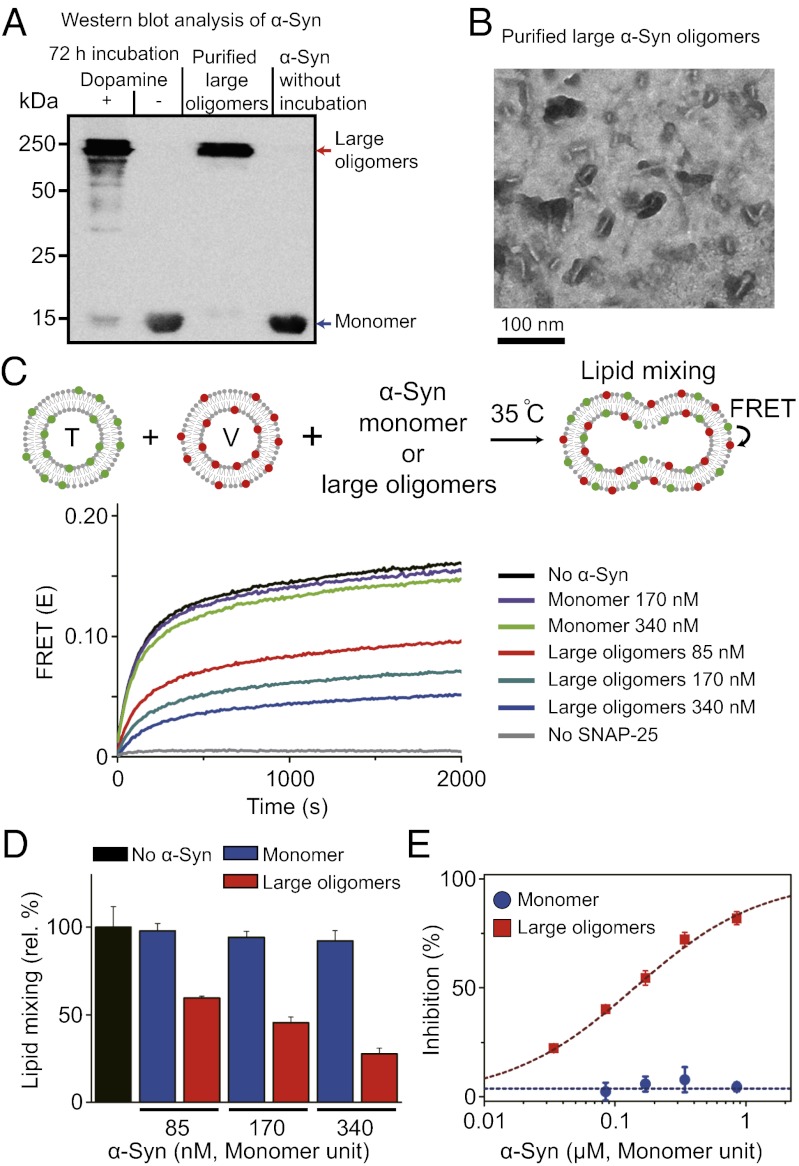
Soluble SDS-resistant large α-Syn oligomers were generated by incubating α-Syn (10 μM) in the presence of 10-fold molar excess amounts of dopamine (100 μM) at 37 °C for 72 h (17–19). The pattern of the oligomeric bands obtained from SDS/PAGE was consistent with previous work (Fig. 1A) (18, 19), in which dimer, trimer, and higher molecular weight oligomers were observed. In contrast, without dopamine, α-Syn produced no oligomeric species (Fig. 1A). We purified higher molecular weight oligomers using size exclusion chromatography (Fig. S1), which resulted in a band at around 250 kDa (Fig. 1A). We observed a short nonfibrillar rod-shaped structure of large oligomers using transmission electron microscopy (Fig. 1B). The generation of the rod-shaped large α-Syn oligomers was reproducible in multiple independent experimental runs. Typically, α-Syn fibrils measure a few micrometers in length and around 10 nm in width (18). In contrast, the average length and width of the large oligomers in Fig. 1B were estimated to be ∼37 nm and 5 nm, respectively (Fig. S2).
Fig. 1.
Dopamine-induced large α-Syn oligomers and their inhibitory effect on SNARE-mediated lipid mixing. (A) Western blot of the α-Syn monomers and oligomers. First lane, α-Syn oligomers generated by incubating 10 μM α-Syn with 100 μM dopamine at 37 °C for 72 h. Second lane, a control without dopamine. Third lane, purified large α-Syn oligomers (Fig. S1). Fourth lane, α-Syn monomers. (B) Typical electron micrograph of purified large α-Syn oligomers. Rod-shaped large α-Syn oligomers are dominant. The average length and width are estimated to be 37 nm and 5 nm, respectively (Fig. S2). (C) The effect of large α-Syn oligomers on an in vitro lipid-mixing assay. When t-vesicles doped with donor dyes [reconstituted with t-SNAREs [T]) and v-vesicles doped with acceptor dyes [reconstituted synaptobrevin-2 (V)] are hemifused or fused together, lipid mixing between two vesicles occurs and increases the FRET signal. No α-Syn: T and V (20 μM in lipid concentration) were mixed together at 35 °C without α-Syn. No SNAP-25: a lipid mixing control without SNAP-25 in T. When the α-Syn monomers were added to the T–V mixture, no inhibition of lipid mixing was observed (170 nM, violet line; 340 nM, light green line). When large α-Syn oligomers were added to the T–V mixture, a significant reduction was observed in lipid mixing (85 nM, red line; 170 nM, dark green line; 340 nM, blue line; all concentrations in monomer units). (D) Relative percentages of lipid mixing at 1,800 s from C. (E) Percentages of lipid-mixing inhibition at various α-Syn concentrations. Blue ●, monomers; red ▪, large oligomers. Error bars were obtained from three independent experiments.
Large α-Syn Oligomers Inhibit SNARE-Mediated Lipid Mixing.
Using the purified large α-Syn oligomers, we first tested whether large α-Syn oligomers inhibited neuronal SNARE-mediated lipid mixing (32, 33) with an in vitro lipid-mixing assay using proteoliposomes (34). Lipid mixing in such a bulk assay may result from hemifusion as well as fusion (35–37), but in any case requires trans-SNARE complex formation. Furthermore, a bulk lipid-mixing assay cannot distinguish between effects caused by differences in docking, hemifusion, or complete fusion. Our single-vesicle alternating-laser excitation (ALEX) lipid-mixing method (discussed below) can distinguish between docking and hemifusion/fusion (38), although it still cannot distinguish between hemifusion and complete fusion (35–37).
In this bulk lipid-mixing assay, t-SNAREs [syntaxin Habc-truncated (HT) and synaptosomal-associated protein-25 (SNAP-25)] and the v-SNARE synaptobrevin-2 are reconstituted separately into two populations of proteoliposomes (t- and v-vesicles, respectively) (34). Membrane fusion may occur when t- and v-vesicles are mixed (Fig. 1C). Lipid mixing between t- and v-vesicles is monitored by the increase of fluorescence resonance energy transfer (FRET) signal (Fig. 1C). When we applied purified large α-Syn oligomers to the reaction mixture, the extent of lipid mixing was reduced significantly compared with the control (Fig. 1C). The inhibition was as much as 25% when the concentration of large α-Syn oligomers was 30 nM (in monomer units). In sharp contrast, the same amount of α-Syn monomer did not show any inhibitory effect on lipid mixing (Fig. 1D). In fact, the α-Syn monomer showed no effect even with a 30-fold excess (0.9 μM) of large oligomers (Fig. 1E). When we examined the potential effects of the oxidized dopamine and probe dyes incorporated into vesicles on the inhibition of lipid mixing, we found no inhibitory effect (Figs. S3 and S4). These results show that large α-Syn oligomers inhibit SNARE-mediated lipid mixing.
Large α-Syn Oligomers Preferentially Bind to Synaptobrevin-2.
We then investigated the mechanism by which large α-Syn oligomers inhibit lipid mixing at a molecular level. For both α-Syn monomers and aggregates, there are three known modes of interactions between α-Syn and SNARE-reconstituted proteoliposomes: (i) binding to a negatively charged membrane surface (39–41), (ii) permeabilization of lipid membranes (42), and (iii) binding to v-SNARE synaptobrevin-2 (25). We questioned whether these modes of interaction are relevant for large α-Syn oligomers.
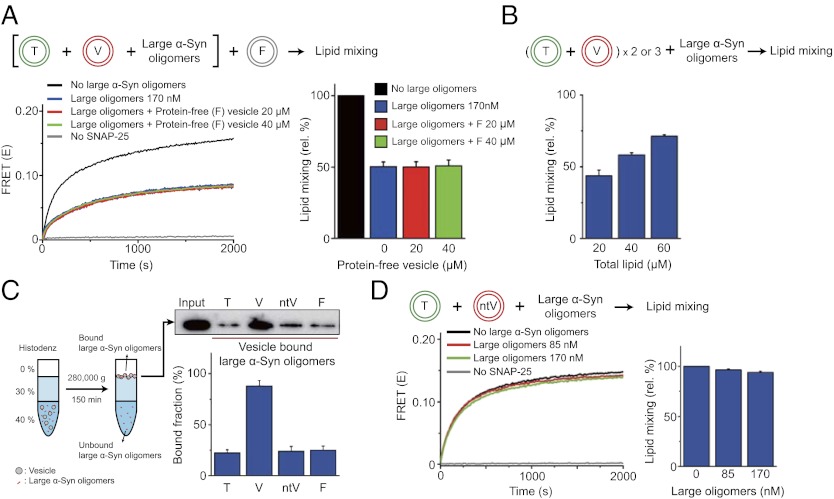
We tested scenario i by adding protein-free liposomes to the reaction mixture in the presence of large α-Syn oligomers (Fig. 2A). If large α-Syn oligomers had interacted with phospholipids in vesicles, the oligomers would bind to protein-free liposomes, which should have reduced the number of free large α-Syn oligomers compared with the case without protein-free liposomes: The addition of protein-free liposomes would have dampened the inhibitory effect of large α-Syn oligomers on lipid mixing. However, we observed no change in the inhibitory effect, even with a threefold excess of protein-free liposomes (Fig. 2A). The addition of large α-Syn oligomers to the reaction mixture showed 50% inhibition of lipid mixing (Fig. 2A, blue bar). When we added large α-Syn oligomers to the reaction mixture in the presence of protein-free liposomes, the same 50% inhibition of lipid mixing was observed (Fig. 2A, red and green bars), indicating that large α-Syn oligomers do not bind to protein-free liposomes or phospholipids. In contrast, when we increased the concentration of SNARE-reconstituted proteoliposomes while keeping the concentration of large α-Syn oligomers the same, the extent of inhibition was reduced significantly (Fig. 2B). These results imply that the binding of large α-Syn oligomers to pure phospholipids is relatively weak compared with their interactions with SNARE-reconstituted proteoliposomes, and the phospholipid binding may not contribute much to the inhibitory function of large α-Syn oligomers. The weak lipid binding by large α-Syn oligomers was confirmed again by a coflotation assay (Fig. 2C).
Fig. 2.
Large α-Syn oligomers inhibit lipid mixing by binding to the N-terminal domain of synaptobrevin-2. (A) Test of large α-Syn oligomer binding to phospholipids. Protein-free vesicles (F) (50 nm in diameter) were added to the 20 μM T–V mixture in the presence of 170 nM large α-Syn oligomers (blue line, no F; red line, 20 μM F; and green line, 40 μM F, in lipid concentrations). Addition of F has no effect on lipid-mixing inhibition. Results are summarized in the bar graph. (B) Large α-Syn oligomers binding to SNARE-carrying proteoliposomes. While keeping the concentration of large α-Syn oligomers the same, the amount of T–V mixture was varied from 20 μM to 60 μM (in lipid concentration) (± SD, n = 3). (C) Coflotation assay for large α-Syn oligomer binding to vesicles. (Left) Schematic description of the coflotation assay. T, V, ntV, or F was incubated with large α-Syn oligomers, respectively, and then vesicle-bound large α-Syn oligomers were separated from unbound large α-Syn oligomers. Here, ntV denotes v-vesicles reconstituted with N-terminal truncated synaptobrevin-2 (nt-synaptobrevin-2, amino acids 29–116). (Right) The amounts of vesicle-bound large α-Syn oligomers were quantified by Western blot (± SD, n = 3). (D) The effect of large α-Syn oligomers on lipid mixing reconstituted with nt-synaptobrevin-2. Bar graphs were obtained from three independent measurements.
We then tested scenario ii by using proteoliposomes encapsulating sulforhodamine B (Fig. S5). We would expect to observe the leakage-induced dequenching of the fluorescence signal had there been permeabilization of the membrane. However, we did not observe such leakage. This result is consistent with previous work on membrane permeabilization by α-Syn aggregates (42, 43). In that work, membrane permeabilization occurred when vesicles were composed of 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) or 1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (DOPG) (42, 43). However, when 1,2-dioleoyl-sn-glycero-3-(phospho-L-serine) (DOPS) was used as a negatively charged lipid or the composition of neutral lipids was over 50 mol%, no permeabilization by α-Syn aggregates was observed (43). In this work we used 7% DOPS as negatively charged lipid in liposomes. Thus, the possibility of membrane permeabilization by large α-Syn oligomers in our work was low.
Next, we investigated whether large α-Syn oligomers interacted with SNARE proteins (scenario iii). We used a coflotation assay (Fig. 2C) similar to the one Südhof and coworkers (25) used to identify the interaction between the α-Syn monomer and synaptobrevin-2. We collected large α-Syn oligomers bound to vesicles and analyzed the quantities by Western blot. We found that large α-Syn oligomers preferentially bound to v-vesicles compared with the binding to t-vesicles and protein-free liposomes (Fig. 2C). This result shows that, like monomers, large oligomers have preferential binding to v-SNARE synaptobrevin-2.
Large α-Syn Oligomers Inhibit Lipid Mixing by Interacting with the N-Terminal Domain of Synaptobrevin-2.
To confirm that the specific binding of large α-Syn oligomers to synaptobrevin-2 indeed was responsible for inhibiting lipid mixing, we prepared a truncation mutant of synaptobrevin-2 (nt-synaptobrevin-2, amino acids 29–116) lacking the N-terminal region. It was shown previously that the α-Syn monomer binds to the N-terminal domain of synaptobrevin-2 (25). Similarly, if large α-Syn oligomers bind synaptobrevin-2 through its N-terminal region, nt-synaptobrevin-2 would lose its interaction with large oligomers and, consequently, the inhibitory effect of large oligomers would be annihilated. As expected, the coflotation assay revealed that large α-Syn oligomers have a much lower binding affinity to v-vesicles reconstituted with nt-synaptobrevin-2 than to vesicles carrying full-length synaptobrevin-2 (Fig. 2C, Western blot), supporting that large oligomers interact with the N-terminal domain of synaptobrevin-2. Next, we tested whether large α-Syn oligomers have a similar inhibitory effect on lipid mixing with nt-synaptobrevin-2 (Fig. 2D). Again, as expected, large oligomers showed no inhibitory effect on lipid mixing when nt-synaptobrevin-2 was used. These results clearly demonstrate that large α-Syn oligomers inhibit lipid mixing by interacting with the N-terminal region of synaptobrevin-2.
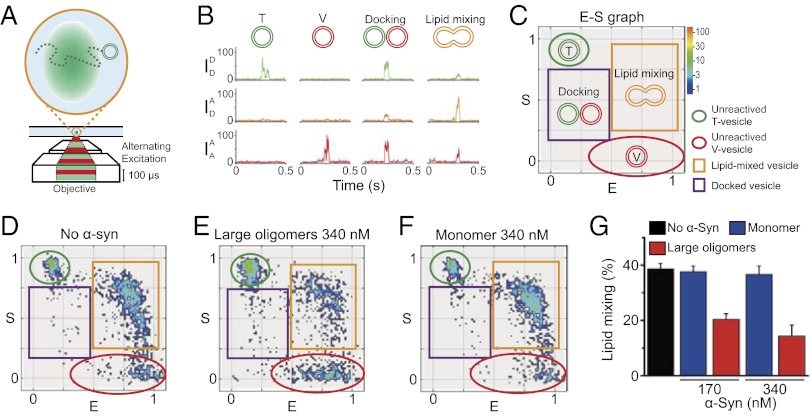
Large α-Syn Oligomers Inhibit the Vesicle-Docking Step.
How does the large α-Syn oligomers’ interaction with synaptobrevin-2 inhibit lipid mixing? The most likely scenario is the inhibition of SNARE complex formation by large α-Syn oligomers, thereby reducing vesicle docking (44). To verify this scenario, we used ALEX, a single-vesicle assay that can discriminate between docking and lipid-mixing steps (Fig. 3) (38). ALEX observes one vesicle at a time, diffusing in solution (Fig. 3A), of which fluorescence intensity readouts report its status, i.e., unreacted, docked, and lipid mixed (Fig. 3B). The result of ALEX is represented by the E (FRET efficiency) vs. S (sorting number) graph (Fig. 3C), in which green and red ellipsoids denote the locations of unreacted t- and v-vesicles, respectively; the purple square represents docked vesicles; and the orange square represents lipid-mixed vesicles. After incubating the reaction mixture for 30 min at 35 °C, we measured the subpopulations of the mixture (Fig. 3 D–F). We observed that large α-Syn oligomers reduced the subpopulation of lipid-mixed vesicles significantly (Fig. 3E), whereas the α-Syn monomer had no effect on the subpopulation of lipid-mixed vesicles, consistent with our bulk lipid-mixing experiments (Fig. 3F). Here, we note that the subpopulation of docked vesicles (purple squares) did not increase in Fig. 3E. If the lipid-mixing step were inhibited, the docked population must have accumulated in the solution as much as the reduced subpopulation of lipid-mixed vesicles (38). The fact that the docked population did not increase implies that large α-Syn oligomers inhibited the docking step, i.e., the initial complex assembly between t-SNARE and synaptobrevin-2. Thus, these results suggest that large α-Syn oligomers’ binding to synaptobrevin-2 limits the accessibility of synaptobrevin-2 to t-SNARE for SNARE complex formation.
Fig. 3.
Single-vesicle assay for inhibition of lipid mixing by large α-Syn oligomers. (A) Schematic description of single-vesicle lipid-mixing assay by ALEX. Sufficient dilution to 100 pM vesicle concentration ensures that only one vesicle passes through the excitation volume at a given time. The detected fluorescent emissions result in fluorescence time traces (SI Text). (B) Typical fluorescence time traces of vesicles obtained by ALEX. ALEX generates three types of fluorescence emissions for a vesicle: 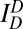 , emission of donor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)] excited by donor-excitation laser (green line);
, emission of donor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI)] excited by donor-excitation laser (green line); 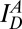 , emission of acceptor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD)] excited by donor-excitation laser, which is FRET signal (orange line); and
, emission of acceptor dye [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD)] excited by donor-excitation laser, which is FRET signal (orange line); and 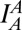 , emission of acceptor dye excited by acceptor-excitation laser (red line). Depending on the reaction status, unreacted T and V, docked, and lipid-mixed vesicles have different sets of three fluorescence intensities. (C) Schematic description of the 2D E (FRET efficiency)–S (sorting number) graph. Three fluorescent intensities of a vesicle from the time traces in B are used to calculate E and S (SI Text). Unreacted, docked, and lipid-mixed vesicles locate at different areas in the E–S graph: T (green oblique), V (red oblique), docked vesicle (purple box), and lipid-mixed vesicle (orange box). (D–F) 2D E–S graphs obtained by ALEX. Each dot denotes a vesicle. (D) Without α-Syn. (E) In the presence of 340 nM of large α-Syn oligomers. (F) In the presence of 340 nM α-Syn monomer. (G) Fractions of lipid-mixed vesicles measured from ALEX (± SD, n = 3).
, emission of acceptor dye excited by acceptor-excitation laser (red line). Depending on the reaction status, unreacted T and V, docked, and lipid-mixed vesicles have different sets of three fluorescence intensities. (C) Schematic description of the 2D E (FRET efficiency)–S (sorting number) graph. Three fluorescent intensities of a vesicle from the time traces in B are used to calculate E and S (SI Text). Unreacted, docked, and lipid-mixed vesicles locate at different areas in the E–S graph: T (green oblique), V (red oblique), docked vesicle (purple box), and lipid-mixed vesicle (orange box). (D–F) 2D E–S graphs obtained by ALEX. Each dot denotes a vesicle. (D) Without α-Syn. (E) In the presence of 340 nM of large α-Syn oligomers. (F) In the presence of 340 nM α-Syn monomer. (G) Fractions of lipid-mixed vesicles measured from ALEX (± SD, n = 3).
Large α-Syn Oligomers Transduced into PC12 Cells Reduce Exocytosis.
Next, we tested whether large α-Syn oligomers block SNARE-mediated exocytosis at the cellular level using PC12 cells. To accomplish this, we delivered large α-Syn oligomers directly into the cytoplasm of PC12 cells using the protein transfection method (Fig. 4A) (45). We then determined the level of exocytosis by measuring the amount of [14C]-acetylcholine release induced by depolarization of docked vesicles with a solution of high K+ concentration (Fig. 4B). We observed that large α-Syn oligomers reduced exocytosis in PC12 cells significantly (Fig. 4B). In contrast, α-Syn monomers did not affect the high K+-induced acetylcholine release. The delivery of large α-Syn oligomers and monomers into PC12 cells was confirmed by Western blot (Fig. 4A). Without treatment with the transfection reagents, neither large oligomers nor monomers were transfected into the cytoplasm of PC12 cells (Fig. 4A) and no reduction of exocytosis was observed (Fig. 4C). In all cases, the cytotoxicity of large oligomers and monomers was monitored by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which showed no toxicity for any of them (Fig. 4 B and C). Thus, the results show that large α-Syn oligomers delivered into PC12 cells inhibit exocytosis, consistent with the outcomes of the in vitro measurements.
Fig. 4.
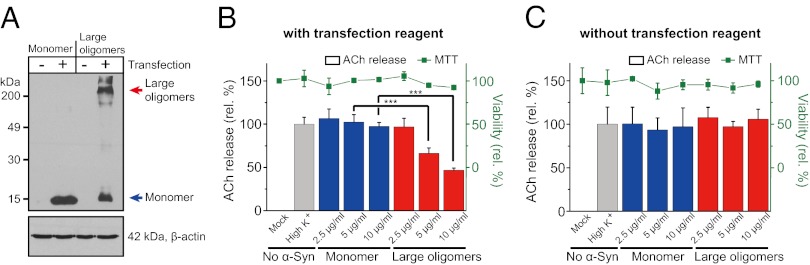
Large α-Syn oligomers transduced into PC12 cells reduced exocytosis. (A) The delivery of α-Syn monomers and large oligomers into PC12 cells was confirmed by Western blot. Cell lysates, 50 μg, were applied to the Western blot analysis and probed with anti–α-Syn antibody (Upper). Actin levels were measured to confirm the equal amount of protein loading (Lower). (B) The effect of large α-Syn oligomers on exocytosis in PC12 cells. After transfecting α-Syn into the cells, the amount of [14C]-acetylcholine released by the depolarization of docked vesicles with a solution of high-K+ concentration was measured. Gray bar, without α-Syn; blue and red bars, transduced with α-Syn monomer and large oligomers, respectively (***P < 0.005). Cell viability after transfecting α-Syn was measured by the MTT method (green ▪). (C) Controls without transfection reagents. PC12 cells were incubated with the α-Syn monomers or large oligomers without the treatment of transfecting reagents. All error bars were obtained from five independent measurements.
Discussion
In the present work, we studied the effect of large α-Syn oligomers on neuronal SNARE-mediated lipid mixing. The rod-shaped large α-Syn oligomers produced by dopamine oxidation had an inhibitory effect on vesicle lipid mixing, whereas α-Syn monomer showed no such effect. The large oligomers did not permeabilize the vesicles, nor did they bind directly to phospholipids. We found that the large oligomers bound preferentially to synaptobrevin-2, a SNARE protein in synaptic vesicles, which results in inhibition of docking between donor and acceptor vesicles. We also tested whether such inhibitory effect occurs in the cellular level by transfecting large α-Syn oligomers directly into PC12 cells, which resulted in a marked reduction of exocytosis.
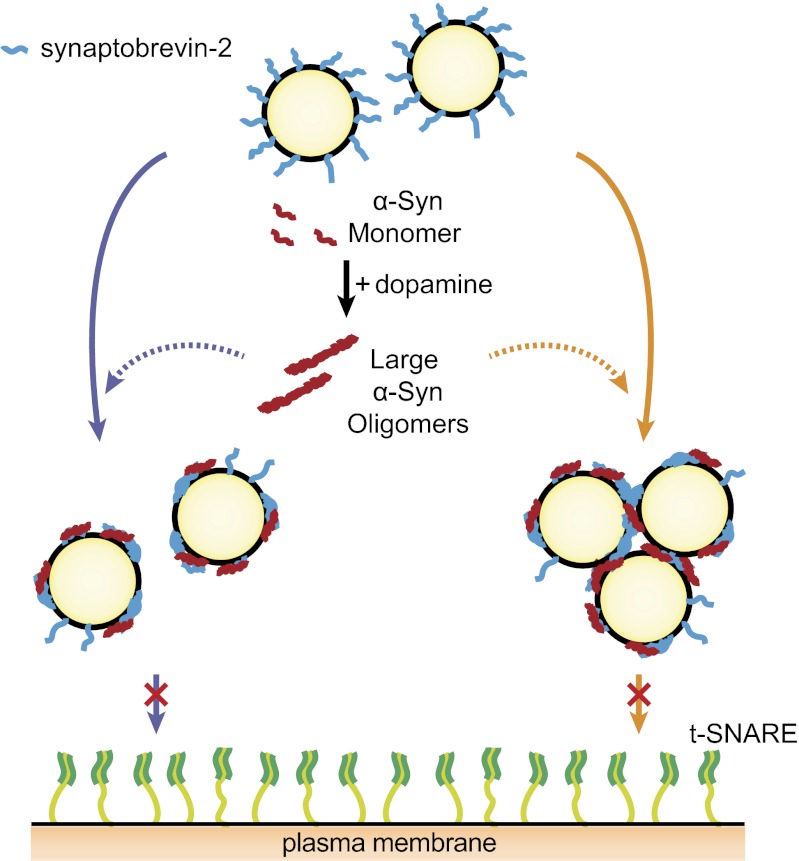
Using a single-vesicle lipid-mixing assay, we showed that large α-Syn oligomers block vesicle docking, which is the initial complex formation step between synaptobrevin-2 and t-SNARE. Because large α-Syn oligomers have multiple binding sites for synaptobrevin-2 on the v-vesicle, they might sequester most of the synaptobrevin-2 on the vesicle to make less synaptobrevin-2 available for SNARE complex formation (Fig. 5). Alternatively, it is possible that multiple synaptobrevin-2 binding sites on a large α-Syn oligomer bind synaptobrevin-2 proteins from several vesicles to induce vesicle clustering (Fig. S6), thereby limiting the number of v-vesicles available for docking. Recently, it was suggested that synaptobrevin-2 is not required for the initial docking of synaptic vesicles to the plasma membrane in vivo (46, 47). Our results show that large α-Syn oligomers specifically inhibit SNARE complex formation between synaptobrevin-2 and t-SNAREs after initial docking by other synaptic factors, such as synaptotagmin-1, in the neuron. We note that this work is limited to α-Syn’s effect on SNARE-induced lipid mixing; α-Syn’s effect on Ca2+-triggered membrane fusion involving synaptotagmin-1 (36) has not been investigated.
Fig. 5.
A model for large α-Syn oligomers’ inhibition effect on exocytosis. Oxidative stress induces oxidants of dopamine that accelerate the formation of large α-Syn oligomers. Large α-Syn oligomers bind to the N-terminal domain of synaptobrevin-2, which blocks SNARE complex formation. Large α-Syn oligomers may sequester most of the synaptobrevin-2 using multiple binding sites for synaptobrevin-2 on vesicles; thus, less synaptobrevin-2 is available for SNARE complex formation. Alternatively, multiple synaptobrevin-2 binding sites on a large α-Syn oligomer bind synaptobrevin-2 proteins from several vesicles to induce vesicle clustering, which limits the number of v-vesicles available for docking.
Another distinct feature of large α-Syn oligomers is that the large α-Syn oligomers’ interaction with synaptobrevin-2 is stronger than that with phospholipids (Fig. 2 A and C). Thus, large α-Syn oligomers might bypass phospholipid binding in cytosol. Because large α-Syn oligomers are resistant even to degradation (22), the accumulation of a small amount of large α-Syn oligomers in cytosol might be sufficient to hinder neurotransmitter release.
It is remarkable that the α-Syn monomer and large oligomers have opposite effects on SNARE complex formation despite their using the same interaction mode. The monomer promotes SNARE complex formation in vitro (Fig. S7), consistent with the results by Südhof and coworkers (25), whereas the large oligomers inhibit SNARE complex formation. It is particularly interesting that the α-Syn monomer does not enhance SNARE-dependent lipid mixing, despite its activity in promoting SNARE complex formation. Consistently, Südhof and coworkers (25) showed that α-Syn monomer did not enhance exocytosis in neurons. Even αβγ-synuclein triple-knockout mice did not show a significant difference in synaptic strength, indicating that the α-Syn monomer has no immediate impact on vesicle fusion under normal conditions. Collectively, these results suggest that the α-Syn monomer plays a maintenance role in keeping spent cis-SNARE complex on the plasma membrane stable and properly recycled, rather than directly regulating trans-SNARE complex formation relevant to vesicle fusion.
It should be noted that A9 dopaminergic neurons, which contain a high level of α-Syn (48, 49) and increased pigmentation by neuromelanin in the early stage of PD before producing Lewy bodies (12, 50, 51), exhibit early vulnerability. Analysis of brain samples from PD patients showed that the optical density of neuromelanin increased together with α-Syn accumulation around the neuromelanin granules in A9 neurons (51), and α-Syn was found to be a major proteic component, cross-linked to neuromelanin from brains of PD patients (52). These observations showed that the enhanced dopamine oxidation and the high level of α-Syn are specific features of A9 neurons, which provide favorable environments for the generation of large α-Syn oligomers (17). The increased level of large α-Syn oligomers would inhibit dopamine release, which potentially leads to the preferential destruction of A9 neurons.
In summary, this work shows that large α-Syn oligomers induced by dopamine oxidants function as inhibitors of SNARE-mediated vesicle docking. For the inhibitory effect, large α-Syn oligomers use their interactions with synaptobrevin-2. This specific inhibition might create havoc in all trafficking pathways involving synaptobrevin-2. Thus, our work suggests a potential mechanism of cytotoxicity of large α-Syn oligomers on dopaminergic neurons in PD.
Methods
Preparation of α-Syn Monomer and Large Oligomers.
The gene of recombinant α-Syn fused with GST was inserted into pGEX-KG, which was transformed into Escherichia coli Rosetta (DE3) pLysS (Novagene). The cells were grown at 37 °C in LB medium with 100 μg/mL ampicillin until the absorbance at 600 nm reached 0.6–0.8. The cells were grown further overnight after addition of Isopropyl β-D-1-thiogalactopyranoside (0.5 mM final concentration) at 16 °C. The cell pellets were harvested and resuspended in lysis buffer (1% sarcosine and 2 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride in 1× PBS). Cells then were broken by sonication. The supernatant of the lysate was transferred to a column containing glutathione-agarose beads, which was incubated for 1 h at 4 °C and washed several times with 1× PBS buffer. The thrombin reaction was conducted to cleave the α-Syn protein from the resin. To prepare large α-Syn oligomers, 10 μM α-Syn was mixed with 100 μM dopamine in 20 mM sodium phosphate buffer (pH 7) at 37 °C for 72 h. After incubation, the mixture was centrifuged to remove large aggregates at 162,000 × g for 5 min at 4 °C. Then, the supernatant was concentrated using Ultracel 10K membrane (Millipore). Large oligomers were purified by size exclusion chromatography using Superdex 200 10/300 GL (GE Healthcare) and concentrated again using Ultracel 10k-membrane.
Preparation of SNARE Proteins, Proteoliposome Reconstitution, in Vitro Lipid-Mixing Assay, and the Coflotation Assay.
The procedures of protein purification, reconstitution, in vitro lipid-mixing assay, and coflotation assay are described in detail in SI Text.
Single-Vesicle Lipid-Mixing Assay by ALEX.
The instrumental setup and data analysis of ALEX (Fig. S8) were described previously (38, 53), and the modifications for this work are described in SI Text.
PC12 Cell Culture and Determination of [14C]-Acetylcholine Release.
PC12 cells were purchased from the Korean Cell Line Bank. The PC12 cells were plated on poly-D-lysine–coated culture dishes and maintained in Roswell Park Memorial Institute (RPMI) medium 1640 containing 100 μg/mL streptomycin, 100 U/mL penicillin, 2 mM L-glutamine, and 10% (vol/vol) heat-inactivated FBS at 37 °C in 5% CO2 incubator. After PC12 cells were grown in 24-well plates at a density of 2 × 105 cells per dish, large α-Syn oligomers or monomers were transduced into the PC12 cells at 80% confluence by Pro-Ject Protein Transfection Reagent (Pierce) according to the manufacturer’s instructions. Briefly, PC12 cells were predepolarized with high-K+ solution (115 mM NaCl, 50 mM KCl, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, 11 mM glucose, and 15 mM Hepes-Tris at pH 7.4) to release already-loaded neurotransmitters of vesicles for exocytosis (the readily releasable pool). After 15 min, mixtures of 2.5 µl of the transfection reagent with various concentrations of α-Syn were added and incubated for 3 h at 37 °C. Then, [14C]-acetylcholine (1 μCi/mL) was applied for 60 min in low-K+ solution (140 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, 11 mM glucose, and 15 mM Hepes-Tris at pH 7.4). The cells were washed four times to remove unincorporated radiolabeled neurotransmitters, then depolarized with a high-K+ solution for 15 min to assess the stimulated release (54). Extracellular medium was transferred to scintillation vials, and the quantity of released neurotransmitter was measured by liquid scintillation counting. The test of cell viability using a modified MTT assay is described in SI Text.
Supplementary Material
Acknowledgments
We thank I. Mook-Jung for critically reading the manuscript. This work was supported by the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology [2011-0016059 (to N.K.L.)], the Basic Science Research Program [2009-0083540 (to D.-H.K.) and 2011-0013849 to (M.-G.C.)], and the fund from Korean Institute of Science and Technology and National Institutes of Health Grant R01 GM051290 (to Y.-K.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218424110/-/DCSupplemental.
References
- 1.Iwai A, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 2.Clayton DF, George JM. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 4.Irizarry MC, et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57(4):334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Krüger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 8.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 9.Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 10.Bucciantini M, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416(6880):507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 11.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334(6180):345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 13.Halliday GM, et al. Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J Clin Neurosci. 1996;3(1):52–60. doi: 10.1016/s0967-5868(96)90083-1. [DOI] [PubMed] [Google Scholar]
- 14.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 15.Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33(12):559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294(5545):1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 18.Cappai R, et al. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19(10):1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 19.Rekas A, et al. The structure of dopamine induced alpha-synuclein oligomers. Eur Biophys J. 2010;39(10):1407–1419. doi: 10.1007/s00249-010-0595-x. [DOI] [PubMed] [Google Scholar]
- 20.Mazzulli JR, et al. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26(39):10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakawa K, et al. Dopamine facilitates alpha-synuclein oligomerization in human neuroblastoma SH-SY5Y cells. Biochem Biophys Res Commun. 2010;391(1):129–134. doi: 10.1016/j.bbrc.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Vicente M, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118(2):777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper AA, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabin DE, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burré J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Larsen KE, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26(46):11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gitler AD, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105(1):145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20(9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yavich L, Tanila H, Vepsäläinen S, Jäkälä P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24(49):11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 34.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92(6):759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 35.Diao J, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife. 2012;1:e00109. doi: 10.7554/eLife.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyoung M, et al. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc Natl Acad Sci USA. 2011;108(29):E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyoung M, Zhang Y, Diao J, Chu S, Brunger AT. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat Protoc. 2013;8(1):1–16. doi: 10.1038/nprot.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JY, et al. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 2012;31(9):2144–2155. doi: 10.1038/emboj.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273(16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 40.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275(44):34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 41.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307(4):1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 42.Volles MJ, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: Implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40(26):7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 43.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41(14):4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 44.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 45.Jiang J, et al. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenase-2-transfected PC12 cells. J Neurochem. 2004;90(5):1036–1049. doi: 10.1111/j.1471-4159.2004.02577.x. [DOI] [PubMed] [Google Scholar]
- 46.de Wit H, et al. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138(5):935–946. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Gerber SH, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321(5895):1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JY, Henning Jensen P, Dahlström A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113(2):463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 49.Andringa G, Du F, Chase TN, Bennett MC. Mapping of rat brain using the Synuclein-1 monoclonal antibody reveals somatodendritic expression of alpha-synuclein in populations of neurons homologous to those vulnerable to Lewy body formation in human synucleopathies. J Neuropathol Exp Neurol. 2003;62(10):1060–1075. doi: 10.1093/jnen/62.10.1060. [DOI] [PubMed] [Google Scholar]
- 50.Kastner A, et al. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson’s disease related to their neuromelanin content? J Neurochem. 1992;59(3):1080–1089. doi: 10.1111/j.1471-4159.1992.tb08350.x. [DOI] [PubMed] [Google Scholar]
- 51.Halliday GM, et al. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain. 2005;128(Pt 11):2654–2664. doi: 10.1093/brain/awh584. [DOI] [PubMed] [Google Scholar]
- 52.Fasano M, Giraudo S, Coha S, Bergamasco B, Lopiano L. Residual substantia nigra neuromelanin in Parkinson’s disease is cross-linked to alpha-synuclein. Neurochem Int. 2003;42(7):603–606. doi: 10.1016/s0197-0186(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 53.Kim C, Kim JY, Kim SH, Lee BI, Lee NK. Direct characterization of protein oligomers and their quaternary structures by single-molecule FRET. Chem Commun (Camb) 2012;48(8):1138–1140. doi: 10.1039/c2cc16528g. [DOI] [PubMed] [Google Scholar]
- 54.Ray P, Berman JD, Middleton W, Brendle J. Botulinum toxin inhibits arachidonic acid release associated with acetylcholine release from PC12 cells. J Biol Chem. 1993;268(15):11057–11064. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.