Abstract
Objective:
Experiments were conducted to find the differences between post-thaw viability and chromosome aberrations in eight-cell mouse embryos at presence of dimethyl sulfoxide (DMSO) and 1, 2-propanediol (PROH) as croprotectants in different storage durations.
Materials and Methods:
In this case-control study, a total number of 720 mouse embryos from about 250 NMRI mice were vitrified with 30% PROH or DMSO; each diluted with a solution containing 30% ficol plus 0.5 M sucrose. Embryos were exposed to the solutions for 0.5 minute at 25℃ followed by cooling in liquid nitrogen, then after appropriate storage duration, they were rapidly warmed. Besides, there were 100 mouse embryos for each cryoprotectant group (totally 200 embryos) as control. Embryo survival was assessed by in vitro development, and chromosome abnormalities were analyzed by Giemsa staining.
Results:
The proportion of mitotic abnormalities in PROH/DMSO vitrified embryos was significantly higher than unfrozen control group. This was confirmed also by a reduced viability of the embryos as judged by a culture at the blastocyst stage (p<0.05 in all test groups).
Conclusion:
It can be deduced that long term cryopreservation may result in chromosomal abnormalities and/or low viability.
Keywords: Chromosome Abnormality, Cryopreservation, Mouse Embryo, Viability
Introduction
Embryo cryopreservation followed by thawing and transferring into the uterus, offers several advantages in assisted reproductive technology (ART) programs. Storing embryos in liquid nitrogen has become as a facility to transfer a limited number of embryos on consecutive occasions. This method can provide an increased pregnancy rate and reduce the risk of multiple gestations and ovarian hyperstimulation syndrome (1, 2). As multiple gestation, with its innate risk for undesirable outcome, remains a great concern in ART treatments, the single-embryo transfer (SET) strategy has become an accepted method step by step (3, 4). One consequence of SET is an increase in availability of supernumerary embryos for freezing (5), resulting in more children born after cryopreservation, and a decrease in multiple pregnancies (6). Cryopreservation will also increase the chance of pregnancy in a natural cycle without additional ovarian stimulation and oocyte retrieval (7). It is noted that vitrification is a novel cryopreservation method for mammalian blastocysts and becomes a routine procedure in infertility clinics, but any strong conclusions about the safety of these techniques have not been provided yet (8-11).
There are some reports showed freezing and thawing significantly either reduce embryo viability (12, 13) or delay embryo development (14, 15); however, the question of whether or not freeze-thawing method causing genetic damage has not been satisfactorily answered. It was suggested that depolymerization of microtubules by cryoprotectants or by cooling may prevent the normal separation of sister chromatids through which non-disjunction may lead to aneuploidy (1). If any aberrant changes are induced in the DNA of these eight-cell embryos by extraneous factors, their descendants may carry chromosome anomalies.
Although cryopreservation of embryos is part of most in vitro fertilization (IVF) programs, only limited studies on perinatal revealing the outcome of children born after replacement of the cryopreserved embryos are available, at present time (8, 16-23).
In some studies major chromosomal abnormalities such as trisomy of chromosome 13, 18 and 21, have been observed in children born from frozen embryos (17, 19, 24). On the other hand, a comparison of children conceived from frozen-thawed embryos with those born normally or from fresh IVF cycles showed a similar or even decreased incidence of congenital abnormalities after cryopreservation (16, 25, 7).
Our previous research on mouse embryos has shown that vitrification may cause some chromosomal damage (26). Also, another report has revealed increased mitotic crossing over in mouse embryos after cryopreservation (27). Based on these findings and observation of chromosome abnormalities in our previous study, we aimed to explore the link between chromosomal status and viability at presence of two different cryoprotectants in different storage durations.
Materials and Methods
Collection of eight – cell mouse embryos
About 250 female NMRI mice (Pasteur Institute, Tehran, Iran) aged 6-8 weeks were super ovulated with an intraperitoneal injection of 10 IU of pregnant mare serum gonadotropin (PMSG) (Intervet, Netherlands), followed by another intraperitoneal injection of 10 IU of human chorionic gonadotropin (hCG,Organon, Netherlands) in 48 hours later. The females were mated singly with 2 adult males from the same strain. After 66-68 hours of mating, the mice were killed by cervical dislocation and eightcell embryos were flushed from their oviducts into T6 medium. It should be mentioned that about 1000 embryos were subjected to this procedure.
Vitrification solutions
PB1 medium: Dulbecco’s phosphate-buffered saline (PBS) includes: CaCl2, 2H2O (0.132 µg/ml), KCl (200 µg/ml), KH2PO4 (200 µg/ml), MgCl2 (100 µg/ ml), NaCl (8 mg/ml), Na2HPO4 (1.15 mg/ml)], glucose (5.56 mmol/L), pyruvate (0.33 mmol/L), penicillin G (100 IU/ml), streptomycin (100 µg/ml), and bovine serum albumin (BSA) (3 mg/ml). Sucrose solution: PB1 medium contains 0.5 mol/L sucrose.
ficol-sucrose (FS) solution is prepared as follows: Ficol 70 (Mol.Wt. 70, 000) is added to 35.1 ml filter sterilized PB1medium. Leave it at room temperature until ficol disappears. Then, sucrose (8.56g) is added followed by adding 105 mg of BSA. All of the ingredients must be combined and thoroughly dissolved.
PROH FS 30% and DMSO FS 30% are prepared as follows: 30% PROH or DMSO is added to FS solution to make 30% (v/v) DMSO or PROH vitrification solution with the final concentration of 21% (w/v) ficol and 0.35M sucrose (28).
Freezing-thawing
Healthy intact eight-cell embryos in each weekly flushing were equally divided into 6 different groups based on storage durations, including: 24-hour, 1-week, 2-week, 1-month, 3-month and 6-month groups. It should be mentioned that there was 2 control groups, each contains 100 embryos: the first group was assessed for viability rate up to blastocyst stage, and the second one has been analyzed from chromosomal point of view (polyploidy or aneuploidy). PROH and DMSO were applied as the cryoprotectants for all above-mentioned test groups. Every 10 embryos were directly suspended in a vitrification solution and loaded into a 0.25ml straw, at room temperature (25℃). The configuration of the straw was described, previously (29). After exposure of the embryos to the vitrification solution for 30 seconds, the straws were plunged into liquid nitrogen. After appropriate storage durations (24 hours, 1 and 2 weeks, as well as 1, 3 and 6 months), embryos were thawed. Straws were taken out of liquid nitrogen and immediately plunged into water at 25℃. After five seconds, the straws were removed from the water, quickly wiped dry and the contents of the straw were expelled into a watch glass containing sucrose solution, by cutting two ends of the straw by scissors. The embryos were then pipetted into fresh T6 medium prepared under paraffin oil in a culture dish.
Assessment of post-thaw viability of embryos
Embryos recovered after vitrification were washed and cultured in T6 medium under paraffin oil in a culture dish in 5% CO2 incubator at 37℃. Then, the survival of embryos was assessed by their ability to develop to the blastocysts in culture dish.
Assessment of chromosome abnormalities
After four-six hours in T6 medium, the embryos were exposed to T6 medium containing colcemid for 18 hours, and then embryos were plunged in Tyrode’s solution acid for four seconds in three steps to slenderize zona pellucida. In the next step, embryos were placed in hypotonic solution (sodium citrate 1%) until swollen (three-five minutes). The swollen embryos were individually placed on a clean chilled glass microscope slide with a minimal amount of solution, and then spread using fixation solution described previously by Tarkovski (30). The slides were stained in Giemsa (3%), and examined under oil immersion microscope (×100) for numerical chromosome analysis.
Statistical analysis
Survival rates of test and control groups (PROH test group, DMSO test group and control group) were analyzed using the Chi square test (Χ2). Fisher’s exact test and chi square were used to compare chromosome abnormalities between groups. Statistically significance was defined as p<.05. Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS, Chicago).
Results
Viability
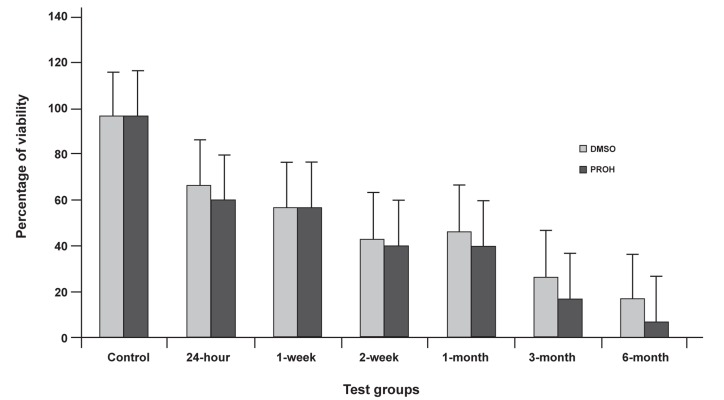
Cryopreservation impaired the in vitro development of the embryos, as demonstrated by lower rate of the blastocyst formation observed both for PROH and DMSO vitrified embryos compared with the control group (Fig 1). The viability of PROH vitrified embryos after cryopreservation was lower than DMSO (Fig 1, Table 1).
Fig 1.
Percentage of viability at the presence of DMSO and PROH as cryoprotectant for various storage durations . Error bars show SE of mean values calculated for data obtained from different samples.
Table 1.
The Results of eight-cell mouse embryos viability vitrified in DMSO/PROH solution after various storage durations
| Test groups | No. of Embryos | P value | Vitrified | Survived (%) | Degenerated | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 100 | 97(97) | 3 | <0.05 | |||||
| 24-hour | 30 | 20(66.7) | 10 | <0.05 | |||||
| 30 | 18(60) | 12 | |||||||
| 1-week | 30 | 17(56.6) | 13 | <0.05 | |||||
| 30 | 17(56.6) | 13 | |||||||
| 2-week | 30 | 13(43.3) | 17 | <0.05 | |||||
| 30 | 12(40) | 18 | |||||||
| 1-month | 30 | 14(46.7) | 16 | <0.05 | |||||
| 30 | 12(40) | 18 | |||||||
| 3-month | 30 | 8(26.6) | 22 | <0.05 | |||||
| 30 | 5(16.7) | 25 | |||||||
| 6-month | 30 | 5(16.7) | 25 | <0.05 | |||||
| 30 | 2(6.6) | 28 | |||||||
As shown in table 1 as well as figure 1, by increasing storage duration, viability rate decreased. For example, in 24-hour group vitrified by DMSO/ PROH, viability rates were 66.7% and 60%, and for 6-month group were 16.7% and 6.6%, respectively.
On the other hand, no significant difference can be seen in the viability rates of 2-week groups of both cryoprotectants (from 43.3% to 40% by DMSO/PROH, respectively), even DMSO group showed a slight increase in viability rate.
As you can see in figure 1, there is a downward trend from control group to 6-month group.
Chromosome abnormality
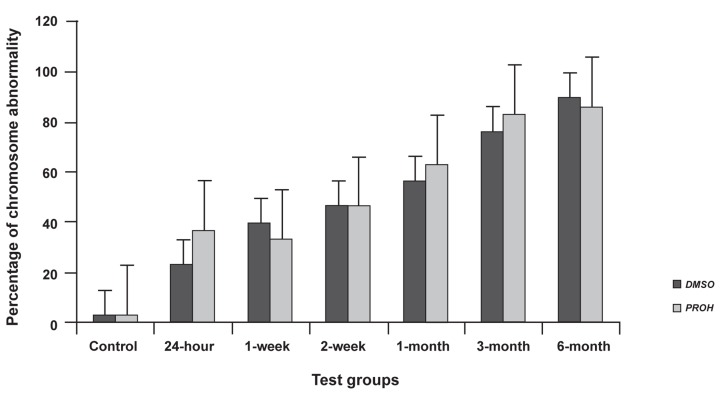
As a whole, about 360 embryos were subjected to cytological analysis. The results of cytological analysis are presented in figure 2 and table 2. Cryopreservation procedure resulted in greater than three-fold increase in the total level of mitotic abnormalities in both DMSO/PROH vitrified embryos from 24-hour group to 6-month group (24- hour group for DMSO and PROH showed 23.3% and 36.6%, respectively, but 6-month group for DMSO and PROH showed 90% and 86.6%, respectively).
In this study the total amount of chromosome abnormality, including aneuploidy and polyploidy are shown in figure 2. In this figure, there is an upward trend from control group to 6-month group, for both DMSO and PROH vitrified embryos (p values for all treatment groups were <0.05).
As indicated, increasing storage duration increased the incidence of chromosome abnormalities in all test groups compared to control group. It should be mentioned that it could be deduced from figure 2 and table 2 that DMSO was apparently a better cryoprotectant than PROH. Besides, it should be mentioned that 2-week groups of both cryoprotectants showed similar chromosome abnormalities.
Fig 2.
Percentage of chromosome abnormality at the presence of DMSO and PROH as cryoprotectant for various storage durations. Error bars show SE of mean values calculated for data obtained from different samples.
Table 2.
The results of total abnormalities of eight-cell mouse embryos vitrified in DMSO/PROH solution after various storage durations
| Test groups | No. of Embryos | P value | |||||
|---|---|---|---|---|---|---|---|
| Vitrified | Intact | Chromosome abnormal embryos | |||||
| Aneuploid * | Polyploid** | Percentage(%) | |||||
| Control | 100 | 95 | 4 | 1 | 5(3) | <0.05 | |
| 24-hour | 30 | 23 | 3 | 4 | 7 (23.3) | <0.05 | |
| 30 | 19 | 6 | 5 | 11 (36.6) | |||
| 1-week | 30 | 18 | 3 | 9 | 12(40) | <0.05 | |
| 30 | 20 | 7 | 3 | 10(33.3) | |||
| 2-week | 30 | 16 | 10 | 4 | 14 (46.6) | <0.05 | |
| 30 | 16 | 9 | 5 | 14 (46.6) | |||
| 1-month | 30 | 13 | 7 | 10 | 17 (56.6) | <0.05 | |
| 30 | 11 | 4 | 15 | 19 (63.3) | |||
| 3-month | 30 | 7 | 6 | 17 | 23(76.6) | <0.05 | |
| 30 | 5 | 9 | 16 | 25(83.3) | |||
| 6-month | 30 | 3 | 6 | 21 | 27(90) | <0.05 | |
| 30 | 4 | 3 | 23 | 26(86.6) | |||
* Aneuploid embryos; The embryos whose chromosome count was 37-43.
** Polyploid embryos; The embryos whose chromosome count was 70-84.
Discussion
Post – thaw viability
order to evaluate post-thaw viability of vitrified eight-cell mouse embryos, we examined the effect of two different cryoprotectants (DMSO, PROH) in 6 different groups based on storage durations, including: 24-hour, 1-week, 2-week, 1-month, 3-month and 6-month groups. Survival rates, assessed by the developmental potential in vitro, showed variation in rang of 6.6% to 66.7%, depending on the cryoprotectant used and storage duration. The survival rates of vitrified embryos depend on several mechanisms of cell injury, such as the chemical toxicity of the cryoprotectant, intracellular ice formation, fracture damage, and osmotic swelling during the removal of the cryoprotectant.
In this study, we considered all the embryos with any kinds of injuries as degenerated embryos to evaluate the cryoprotectants as a whole. The same exposure time, cryoprotectant percentage and temperature were implemented, but the cryoprotectants and storage durations were different.
The freezing and warming of cells protrudes a series of pressures, such as equilibration with a cryoprotectant, cooling- warming, dilution and rehydration (31). As a cryoprotectant for conventional freezing of human embryos, PROH has been widely used; although, DMSO (32) has also proven effective.
According to Figure 1, we had poor post-thaw viability in those groups with longer storage durations. So far, there is many studies reporting good results with vitrification, but they had very short storage durations (33-35). In our study, the possible of poor rates of eight-cell embryos viability may be the result of storage duration which may be harmful for zona pellucida integrity. Vitale et al. (36) has suggested that excellent quality of frozen-thawed embryos at the eight- to 16-cell stage often do not develop in vitro without full protection of an intact zona pellucida. For early stage embryos, it is thought that the zona pellucida helps to maintain cellular integrity of the blastomeres. Our results may demonstrate the possibility of zona injury besides cryoprotectant toxicity that reduces postthaw viability. On the other hand, this viability reduction can be in charge of chemical toxicity of cryoprotectants due to increase of storage duration leading to damage of intra cellular components.
However, "freezing and thawing significantly reduces embryo viability" (12). The detrimental effects of cryopreservation may also result in damages to the cell membranes and intracellular components (37 , 38 ). Ideally, the freeze-thaw procedure should not cause any loss of viability, or lead to an increased incidence of genetic aberrations, fetal malformation or losses. An almost recent study from Belgium (7) including 547 cryo- ICSI and 390 cryo-IVF children has showed that cryo-ICSI twins has significantly higher preterm birth and very low birth weight rates than twins from fresh ICSI. Furthermore, a higher rate of malformations is noticed for cryo-ICSI as compared with fresh ICSI. Besides, in a recent study that compared the viability after cryopreservation, a lower viability of the embryos after vitrification was reported (39). In addition, in another meta-analysis of cryopreservation study, vitrifying mouse embryos was undertaken to determine the treatment effect of vitrification, and they also showed that treatment by vitrification decreased embryo viability compared with controls (40)
On the contrary, two large registry studies, one from Denmark (41) and the other one from France (42) showed no difference in malformation rates between cryo-children and children born after transfer.
For the almost newly introduced technique of vitrification, very limited data have been reported on post-thaw viability outcomes. "This emphasize the urgent need for properly controlled postthaw studies, follow-up studies of these embryos, and careful assessment of evidence currently available before this technique is added to daily routines" (43).
Chromosome analysis
To the best of our knowledge, this is the first report describing the effect of storage duration on chromosomal situation of vitrified eight-cell mouse embryos at the presence of DMSO and PROH as cryoprotectant.
This study clearly demonstrated that increasing storage duration increases chromosome abnormalities. In addition, as shown in table 2, DMSO is almost a better cryoprotectant than PROH because of causing less chromosome aberrations.
In this study only the abnormalities, due to chromosome and mitotic apparatus damage have been investigated; in other word, we traced aneuploidy and polyploidy which the latter one occurred due to blastomere fusion. The lagging of whole chromosomes or their fragments are the most frequently detected mitotic abnormality due to chromosome damage. This phenomenon is due to damage of a kinetochore or loss of its function (44). Cryopreservation leads to fragmentation of chromosomes, thus increases the frequency of chromatid bridges arising in early and compacted embryos (45).
In contrast to our results, Bongso et al. (46) have demonstrated that cryopreservation of two-cell mouse embryos using DMSO or propanediol does not increase the incidence of aneuploidy or polyploidy.
It is known that the cytoskeleton of mammalian oocytes and embryos is sensitive to thermo-and chemo- stresses resulting from cryopreservation (47-49). Disorganization of the spindle after cryopreservation was observed in metaphases II oocytes and late two-cell embryos in mitosis (48). The damage of the mitotic apparatus after cryopreservation is confirmed initially by such gross disturbances as multipolar and unipolar mitosis; consequently, the unequal distribution of chromosomes between daughter cells results in aneuploidy (45). Moreover, Salumets et al. (50) has found a higher proportion of chaotic embryos after resumption of mitosis, followed by freezing and thawing of two-cell embryos. They; therefore, proposed that the freezing procedure can cause dysfunctional spindles.
In our study, polyploid embryos may be the result of blastomere fusion occurring due to possible zona injuries. Balakier et al. (51) has clearly showed that cryopreservation of early human embryos with standard propanediol technique may cause blastomere fusion in correlated with some existing membrane abnormalities that can result in fusion after freezing and thawing, leading to chromosomal aberrations.
Furthermore, in another study, Agerholm et al. (52) showed that at the time of freezing, none of the embryos had visible multinucleated blastomeres. After thawing, they found that 33% of the embryos were multinucleated. This suggests that the majority of the multinuclearity was introduced after thawing. Uncoupling of the processes that control karyokinesis and cytokinesis may result in binucleated blastomeres (53-55). Apparently, the data suggest that the freezing procedure could affect coupling of karyokinesis and cytokinesis which could; therefore, be result of the suboptimal conditions during the freezing procedure, resulting in a dysfunctional spindle (50).
From our present study, it is likely that freezing and thawing may be responsible for blastomere fusion. This observation is concordance with the obtained result of Balakier et al. (51). It may also indicate that blastomere fusion is not only because of fair and poor quality embryos, as was previously thought (1), but it can also alter embryos that are graded as "good morphology" group, as was shown by our observation (100% of affected embryos were of good quality).
Now, the question is how these vitrified embryos using in clinic can result in live and intact birth. It can also be analyzed from a different perspective: when an embryo is ready to be transferred, it must pass through certain barriers, such as cryopreservation, thawing, mitosis resumption, finally developmental obstacles; at the same time, it must be able to maintain its chromosomal status. For transferring a vitrified embryo, several other embryos may be lost due to lack of viability or chromosome abnormalities, and finally one lucky embryo passing through all of these risks is going to be transferred. More over, even after implantation, many of these embryos could be aborted because of chromosomal abnormality at the first trimester.
Conclusion
It is probably premature to draw definite conclusions concerning the reasons of chromosomally abnormal embryos among frozen-thawed embryos, and our data strongly suggest that the majority of mitotic abnormalities in eight-cell mouse embryos may be consequences of damage to the mitotic apparatus and/or zona injury that could be due to the storage duration of cryopreserved embryos.
Our results may show that long-term cryopreservation that requires a long-term exposure of embryos to cryoprotectants, can cause low viability and/or chromosomal abnormalities.
While vitrification has a clear role in ART, the researches should continue to establish optimal vitrification method which may assist in alleviating concerns over safety issues, such as storage, transport and the use of very high cryoprotectant concentrations. In addition, analysis of global gene expression following cryopreservation and even DNA apoptosis may be successfully applied on excess human embryos.
Acknowledgments
This study was supported by Royan Institute, Tehran, Iran. There is no conflict of interest in this article.
References
- 1.Trounson A. Preservation of human eggs and embryos. Fertil Steril. 1986;46(1):1–12. doi: 10.1016/s0015-0282(16)49448-3. [DOI] [PubMed] [Google Scholar]
- 2.Bergh C, Werner C, Nilsson L, Hamberger L. Cumulative birth rates following cryopreservation of all embryos in stimulated in vitro fertilization (IVF) cycles. J Assist Reprod Genet. 1995;12(3):191–194. doi: 10.1007/BF02211797. [DOI] [PubMed] [Google Scholar]
- 3.Ombelet W, De Sutter P, Van der Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction-the Belgian project. Hum Reprod Update. 2005;11(1):3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- 4.Van Landuyt L, Verheyen G, Tournaye H, Camus M, Devroey P, Van Steirteghem A. New Belgian embryo transfer policy leads to sharp decrease in multiple pregnancy rate. Reprod Biomed Online. 2006;13(6):765–771. doi: 10.1016/s1472-6483(10)61022-x. [DOI] [PubMed] [Google Scholar]
- 5.Neubourg DD, Mangelschots K, Van Royen E, Vercruyssen M, Ryckaert G, Valkenburg M, et al. Impact of patients' choice for single embryo transfer of a top quality embryo versus double embryo transfer in the first IVF/ICSI cycle. Hum Reprod. 2002;17(10):2621–2625. doi: 10.1093/humrep/17.10.2621. [DOI] [PubMed] [Google Scholar]
- 6.Gerris J, De Neubourg D, De Sutter P, Van Royen E, Mangelschots K, Vercruyssen M. Cryopreservation as a tool to reduce multiple birth. Reprod Biomed Online. 2003;7(3):286–294. doi: 10.1016/s1472-6483(10)61866-4. [DOI] [PubMed] [Google Scholar]
- 7.Belva F, Henriet S, Van den Abbeel E, Camus M, Devroey P, Van der Elst J, et al. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23(10):2227–2238. doi: 10.1093/humrep/den254. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Zhang X, Zhao L, Xia X, Wang W. Comparison of DNA apoptosis in mouse and human blastocysts after vitrification and slow freezing. Mol Reprod Dev. 2012;79(3) 3:229–236. doi: 10.1002/mrd.22018. [DOI] [PubMed] [Google Scholar]
- 9.Fathi R, Valojerdi MR, Yazdi PE, Ebrahimi B, Alipour H, Hassani F. Development of 4-cell mouse embryos after re-vitrification. Cryobiology. 2012;64(1):23–26. doi: 10.1016/j.cryobiol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Mochida K, Hasegawa A, Taguma K, Yoshiki A, Ogura A. Cryopreservation of mouse embryos by ethylene glycol-based vitrification. J Vis Exp. 2011;18:57–57. doi: 10.3791/3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WX, Lu H, Luo MJ, Xu LZ. Effects of different cryoprotectants and cryopreservation protocols on the development of 2-4 cell mouse embryos. Cryo Letters. 2011;32(3):240–247. [PubMed] [Google Scholar]
- 12.Selick CE, Hofmann GE, Albano C, Horowitz GM, Copperman AB, Garrisi GJ, et al. Embryo quality and pregnancy potential of fresh compared with frozen embryos--is freezing detrimental to high quality embryos? Hum Reprod. 1995;10(2):392–395. doi: 10.1093/oxfordjournals.humrep.a135950. [DOI] [PubMed] [Google Scholar]
- 13.Kito S, Noguchi Y, Ohta Y, Ohhata T, Abe M, Shiomi N, et al. Evaluation of developmental competence of vitrified-warmed early cleavage stage embryos and their application for chimeric mouse production. Exp Anim. 2003;52(2):179–183. doi: 10.1538/expanim.52.179. [DOI] [PubMed] [Google Scholar]
- 14.Ezzatabadypour M, Hosseini A, Baharvand H, Nematolahi SN, Heydari MH. Developmental potetial of mouse morula early and late blastocyst after vitrification. Cell J. 2002;4(1):23–32. [Google Scholar]
- 15.Ramezani M, Rezazadeh Valojerdi M, Parivar K. Comparison of the effects of different vitrification methods on development of two-cell mouse embryos. Cell J. 2004;6(2):69–74. [PubMed] [Google Scholar]
- 16.1Wada I, Macnamee MC, Wick K, Bradfield JM, Brinsden PR. Birth characteristics and perinatal outcome of babies conceived from cryopreserved embryos. Hum Reprod. 1994;9(3):543–546. doi: 10.1093/oxfordjournals.humrep.a138542. [DOI] [PubMed] [Google Scholar]
- 17.Sutcliffe AG, D’souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B. Minor congenital anomalies, major congenital malformations and development in children conceived from cryopreserved embryos. Hum Reprod. 1995;10(12):3332–3337. doi: 10.1093/oxfordjournals.humrep.a135915. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe AG, D’souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B. Outcome in children from cryopreserved embryos. Arch Dis Child. 1995;72(4):290–293. doi: 10.1136/adc.72.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wennerholm UB, Hamberger L, Nilsson L, Wennergren M, Wikland M, Bergh C. Obstetric and perinatal outcome of children conceived from cryopreserved embryos. Hum Reprod. 1997;12(8):1819–1825. doi: 10.1093/humrep/12.8.1819. [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm UB, Albertsson-Wikland K, Bergh C, Hamberger L, Niklasson A, Nilsson L, et al. Postnatal growth and health in children born after cryopreservation as embryos. Lancet. 1998;351(9109):1085–1090. doi: 10.1016/S0140-6736(97)08247-0. [DOI] [PubMed] [Google Scholar]
- 21.Wennerholm UB, Bergh C, Hamberger L, Lundin K, Nilsson L, Wikland M, et al. Incidence of congenital malformations in children born after ICSI. Hum Reprod. 2000;15(4):944–948. doi: 10.1093/humrep/15.4.944. [DOI] [PubMed] [Google Scholar]
- 22.Stanger J, Wong J, Conceicao J, Yovich J. Vitrification of human embryos previously cryostored by either slow freezing or vitrification results in high pregnancy rates. Reprod Biomed Online. 2012;24(3):314–320. doi: 10.1016/j.rbmo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Edgar DH, Gook DA. A critical appraisal of cryopreservation- slow cooling versus vitrification-of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–554. doi: 10.1093/humupd/dms016. [DOI] [PubMed] [Google Scholar]
- 24.Quiroga R, Roselló M, Martinez F, Ferrer-Bolufer I, Monfort S, Oltra S, et al. Rare chromosomal complement of trisomy 21 in a boy conceived by IVF and cryopreservation. Reprod Biomed Online. 2009;19(3):415–417. doi: 10.1016/s1472-6483(10)60177-0. [DOI] [PubMed] [Google Scholar]
- 25.Wood MJ. Embryo freezing: is it safe? Hum Reprod. 1997;12(5):32–37. [Google Scholar]
- 26.Mozdarani H, Moradi SZ. Effect of vitrification on viability and chromosome abnormalities in 8-cell mouse embryos at various storage durations. Bio Res. 2007;40(3):299–306. [PubMed] [Google Scholar]
- 27.Ishida GM, Saito H, Ohta N, Takahashi T, Ito MM, Saito T, et al. The optimal equilibration time for mouse embryos frozen by vitrification with trehalose. Hum Reprod. 1997;12(6):1259–1262. doi: 10.1093/humrep/12.6.1259. [DOI] [PubMed] [Google Scholar]
- 28.Mukaida T, Wada S, Takahashi K, Pedro PB, An TZ, Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13(10):2874–2879. doi: 10.1093/humrep/13.10.2874. [DOI] [PubMed] [Google Scholar]
- 29.Kasai M. Cryopreservation of mammalian embryos. Mol Biotechnol. 1997;7(2):173–179. doi: 10.1007/BF02761753. [DOI] [PubMed] [Google Scholar]
- 30.Tarkovski AK. An air drying method for chromosome preparation from mouse eggs. Cytogenetics. 1996;5:394–400. [Google Scholar]
- 31.Leibo SP. Techniques for preservation of mammalian germplasm. Anim Biotechnol. 1992;3:139–153. [Google Scholar]
- 32.Van der Elst J, Camus M, Van den Abbeel E, Maes R, Devroey P, Van Steirteghem AC. Prospective randomized study on the cryopreservation of human embryos with dimethylsulfoxide or 1, 2-propanediol protocols. Fert Steril. 1995;63(1):92–100. [PubMed] [Google Scholar]
- 33.Otsuka J, Takahashi A, Nagaoka M, Funabashi H. Optimal equilibration conditions for practical vitrification of two-cell mouse embryos. Comp Med. 2002;52(4):342–346. [PubMed] [Google Scholar]
- 34.Bautista JA, Takahashi Y, Kanagawa H. in vitro viability of mouse 8-cell embryos vitrified in a simple solution of ethylene glycol. Jpn J Vet Res. 1997;45(2):67–73. [PubMed] [Google Scholar]
- 35.Bautista JA, Takahashi Y, Kanagawa H. in vitro viability of mouse zygote vitrified in ethylene glycol. Japanese Journal of Veterinary Research. 1998;45:193–198. [PubMed] [Google Scholar]
- 36.Vitale NJ, Myers MW, Denniston RS, Leibo SP, Godke RA. In-vitro development of refrozen mouse embryos. Hum Reprod. 1997;12(2):310–316. doi: 10.1093/humrep/12.2.310. [DOI] [PubMed] [Google Scholar]
- 37.Dumoulin JC, Bergers-Janssen JM, Pieters MH, Enginsu ME, Geraedts JP, Evers JL. The protective effects of polymers in the cryopreservation of human and mouse zonae pellucidae and embryos. Fertil Steril. 1994;62(4):793–798. doi: 10.1016/s0015-0282(16)57006-x. [DOI] [PubMed] [Google Scholar]
- 38.Ng SC, Sathananthan AH, Wong PC, Ratnam SS, Ho J, Mok H, et al. Fine structure of early human embryos frozen with 1,2 propanediol. Gamete Res. 1988;19(3):253–263. doi: 10.1002/mrd.1120190305. [DOI] [PubMed] [Google Scholar]
- 39.AbdelHafez F, Xu J, Goldberg J, Desai N. Vitrification in open and closed carriers at different cell stages: assessment of embryo survival, development, DNA integrity and stability during vapor phase storage for transport. BMC Biotechnol. 2011;11:29–29. doi: 10.1186/1472-6750-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manno FA 3rd. Cryopreservation of mouse embryos by vitrification: a meta-analysis. Theriogenology. 2010;74(2):165–172. doi: 10.1016/j.theriogenology.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Pinborg A, Loft A, Rasmussen S. Danish national controlled cohort study on neonatal outcome of 1267 children born after transfer of cryopreserved IVF and ICSI embryos in 1995 to 2006. 24th Annual Meeting of the ESHRE. Barcelona, Spain: Hum Reprod; 2008. Jul 1-4, pp. i51–i51. [Google Scholar]
- 42.Royere D, Levy R, Mouchel T. Pregnancy issues after frozen embryo transfer analysis based on 3632 pregnancies follow-up. 22nd Annual Meeting of the ESHRE. Progue, Czech Republic: Hum Reprod; 2006. Jun 18-21, pp. i133–i133. [Google Scholar]
- 43.Van Steirteghem A. What next for assisted reproductive technology? A plea for an evidencebased approach. Hum Reprod. 2008;23(12):2615–2616. doi: 10.1093/humrep/den422. [DOI] [PubMed] [Google Scholar]
- 44.Fiskejö G. Methods in molecular biology. In: O’Hare S, Atterwill AC, editors. Allium test. Totowa, NJ: Humana Press Inc; 1995. pp. 119–127. [Google Scholar]
- 45.Khromenkova OB, Zhernoklev GV, Zhegunov GV, Grischenko VI. The incidence of mitotic abnormalities in cryopreserved eight-cell early and compacted mouse embryos. Cryo Letters. 2003;24(1):27–32. [PubMed] [Google Scholar]
- 46.Bongso A, Chye NS, Sathananthan H, Mui-Nee L, Mok H, Wong PC, et al. Chromosome analysis of two-cell mouse embryos frozen by slow and ultrarapid methods using two different cryoprotectants. Fertil Steril. 1988;49(5):908–412. doi: 10.1016/s0015-0282(16)59905-1. [DOI] [PubMed] [Google Scholar]
- 47.Pickering SJ, Johnson MH. The influence of cooling on the organization of the meiotic spindle of the mouse oocyte. Hum Reprod. 1987;2(3):207–216. doi: 10.1093/oxfordjournals.humrep.a136516. [DOI] [PubMed] [Google Scholar]
- 48.Sathananthan AH, Ng SC, Trounson AO, Bongso A, Ratnam SS, Ho J, et al. The effects of ultrarapid freezing on meiotic and mitotic spindles of mouse oocytes and embryos. Gamete Res. 1988;21(4):385–401. doi: 10.1002/mrd.1120210407. [DOI] [PubMed] [Google Scholar]
- 49.Van der Elst J, Van den Abbeel E, Jacobs R, Wisse E, Van Steirteghem A. Effect of 1, 2-propanediol and dimethylsulphoxide on the meiotic spindle of the mouse oocyte. Hum Reprod. 1988;3(8):960–967. doi: 10.1093/oxfordjournals.humrep.a136826. [DOI] [PubMed] [Google Scholar]
- 50.Salumets A, Horelli-Kuitunen N, Suikkari AM, Metspalu A, Tuuri T. Elevated incidence of chromosomally chaotic embryos among frozen-thawed preimplantation embryos. Eur J Obstet Gynecol Reprod Biol. 2004;114(1):59–63. doi: 10.1016/j.ejogrb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Balakier H, Cabaca O, Bouman D, Shewchuk AB, Laskin C, Squire JA. Spontaneous blastomere fusion after freezing and thawing of early human embryos leads to polyploidy and chromosomal mosaicism. Hum Reprod. 2000;15(11):2404–2410. doi: 10.1093/humrep/15.11.2404. [DOI] [PubMed] [Google Scholar]
- 52.Agerholm IE, Kølvraa S, Crüger DG, Berg C, Bruun-Petersen G, Ziebe S. Resumption of mitosis in frozen-thawed embryos is not related to the chromosomal constitution. Fertil Steril. 2008;90(5):1649–1655. doi: 10.1016/j.fertnstert.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Hardy K, Winston RM, Handyside AH. Binucleate blastomeres in preimplantation human embryos in vitro: failure of cytokinesis during early cleavage. J Reprod Fertil. 1993;98(2):549–558. doi: 10.1530/jrf.0.0980549. [DOI] [PubMed] [Google Scholar]
- 54.Pickering SJ, Taylor A, Johnson MH, Braude PR. An analysis of multinucleated blastomere formation in human embryos. Hum Reprod. 1995;10(7):1912–1922. doi: 10.1093/oxfordjournals.humrep.a136206. [DOI] [PubMed] [Google Scholar]
- 55.Hnida C, Engenheiro E, Ziebe S. Computer-controlled, multilevel, morphometric analysis of blastomere size as biomarker of fragmentation and multinuclearity in human embryos. Hum Reprod. 2004;19(2):288–293. doi: 10.1093/humrep/deh070. [DOI] [PubMed] [Google Scholar]