Abstract
Objective:
Endothelial progenitor cells (EPCs) have a potential application for cell therapy, however, their biological nature is not well-understood. EPCs also possess some stemness features, such as their clonogenicity and differentiation capacity. The main aim of this study was to evaluate the expression of certain transcription factors regulating selfrenewal property of stem cells.
Materials and Methods:
In this experimental study, peripheral blood mononuclear cells were isolated from fresh human blood of several volunteers and were cultured in fibronectin- coated plates. EPCs were identified based on their morphology and growth characteristic. Then, the expression of some markers implicated in self-renewal capacity was assessed in the isolated cells using reverse transcription-polymerase chain reaction (RTPCR) and immunocytochemistry.
Results:
Expression of the cell surface markers, CD31 and CD34, was determined by RT-PCR and immunocytochemistry. Furthermore, these cells had the ability for Di-ACLDL incorporation as well as attachment to lectin I. EPCs did not express the main stem cell markers, like OCT4-A, Nanog, and Sox2; nevertheless, they expressed the weaker pluripotent markers, including OCT4B and OCT4-B1 spliced variants, such as Nucleostemin and ZFX. Furthermore, the expression of Nucleostemin and ZFX genes revealed a decreasing pattern from days 4th to 11th.
Conclusion:
The main regulators of stem cell self-renewal genes, including OCT4-A, Nanog, and Sox2 are not expressed in EPCs. Forced expression of these genes can elevate the stemness property and clinical application of EPCs.
Keywords: Endothelial, Progenitor Cell, Marker, Stem Cells, Peripheral Blood
Introduction
Vasculogenesis was initially thought to be as the generation of new blood vessels by stem or progenitor cells, taking place only during embryonic development (1). The belief is later revised after Asahara et al. identified the endothelial progenitor cells (EPCs) in adult human peripheral blood (2). EPCs, a heterogeneous subpopulation of bone marrow mononuclear cells, are mobilized in response to ischemia and are able to differentiate into endothelial cells in ischemic organs (3). Conventionally, EPCs are recognized by the expression of both hematopoietic stem cell and endothelial cell markers (4). However their molecular criteria are not well-understood.
EPCs play an essential role in wound healing process as well as pathogenic conditions, such as tumor growth (5) and metastases (6). Dysfunction of EPCs occurs by various risk factors of coronary artery disease, such as aging, diabetes, hypercholesterolemia, and hypertension (7). Researchers have now begun to estimate the potential therapeutic use of EPCs in treatment of ischemic cardiovascular disease. Results indicate that EPCs are a safe and powerful tool in cell therapy of cardiovascular disease (8).
EPCs seem to have some features of stem cells, such as their clonogenicity and differentiation capacity (4). Similar to stem cells, they have the capacity of differentiation into several types of cells, including: cardiomyocytes, smooth muscle cells and endothelial cells (9). An important characteristic of stem cells is their self- renewal property, controlled by certain transcription factors which are exclusively expressed in stem cells (10). The main stem cell self-renewal regulatory genes are OCT4, Nanog, and Sox2, as well as Nucleostemin and ZFX. Evaluating the expression of these genes is one of the first steps in assigning the stemness property to stem cells.
While EPCs seem to be an important cell source for cell therapy, many aspects of their identity and characteristic still remain unclear. For example, a potential expression of selfrenewal genes in EPCs has not been comprehensively examined. In this study, we isolated endothelial progenitor cells from human peripheral blood and characterized the cellular and molecular identity of isolated cells as putative stem/progenitor cells.
Materials and Methods
Cell isolation and culture
In this experimental study, peripheral blood (100 ml) was obtained from some healthy volunteers. Mononuclear cells were separated from other components of the peripheral blood by density-gradient centrifugation on Histopaque 1077 (Sigma Aldrich, Belgium). Isolated cells were resuspended in EGM-2-MV Bullet Kit (Clonetics, USA) medium consisting of endothelial basal medium, 5% fetal bovine serum human epidermal growth factor (hEGF), vascular epidermal growth factor (VEGF), human growth factor-basic recombinant (hFGF-B), insulin-like growth factor 1 (IGF-1), ascorbic acid, and heparin. The peripheral blood mononuclear cells (PBMNCs) (1 × 107 per well) were then cultured in the coated (2.5µg per cm2 fibronectin; Sigma/Aldrich, Japan) six-well plates, and incubated in a 5% CO2 incubator at 37℃. After 4 days of culture, the medium containing non-adherent cells were collected, centrifuged, washed with phosphate-buffered saline (PBS), resuspended in fresh medium, and returned to the cell culture until 7th day. The attached cells were cultured for 3 weeks. Culture medium was changed every 3 day. The experimental procedure was approved by the Ethical Board of Tarbiat Modares University.
RNA extraction and RT-PCR
Total RNA was extracted on days 4th, 7th and 11th after seeding of mononuclear cells (MNCs) using the RNX Plus kit according to the manufacturer’s instruction (CinnaGen, Iran). The quality of RNA was estimated by gel electrophoresis as well as by optical density at 260 nm and 280 nm, respectively.
Therefore, 1 µg of the extracted RNA was treated with RNase-free DNase (Fermentas, Germany) and used for cDNA synthesis using 0.2 µg random hexamer and MMuLV Reverse Transcriptase (Fermentas, Germany) in a 20 µg reaction according to the manufacturer’s instructions. For each sample, a negative control, without addition of MMuLV, was also employed. PCR was performed using 2µg of synthesized cDNA with 1U of Taq polymerase (CinnaGen, Iran), 2.5 µl of 10x PCR buffer, 0.5 µl of 20 mM deoxy nucleotide tri-phosphates (dNTPs), 0.5 µl of each primer and deionized distilled water in a 25µl PCR reaction. The PCR amplification was carried out for 35 cycles for all genes, except for the internal control gene GAPDH, where the number of cycles was 30. The cycling conditions were as follows: 94℃ for 30 seconds, 55-64℃ (depending on primers used, Table 1) for 30 seconds, 72℃ for 45 seconds, and a final extension of 72℃ for 5 minutes. Each experiment was repeated at least three times.
Table 1.
The sequences, annealing temperatures, and product sizes of the primers used to amplify genes of interest
| Genes | Product size (bp) | Annealing temperature (˚C) | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|---|
| Oct4-A | 496 | 63.5 | CTTCTCGCCCCCTCCAGGT | AAATAGAACCCCCAGGGTGAGC |
| Oct4-B | 267 | 64 | AGACTATTCCTTGGGGCCACAC | CTCAAAGCGGCAGATGGTCG |
| Oct4-B1 | 492 | 64 | AGACTATTCCTTGGGGCCACAC | CTCAAAGCGGCAGATGGTCG |
| Sox2 | 426 | 60 | ATGGGTTCGGTGGTCAAGTC | GTGGATGGGATTGGTGTTCTC |
| Nanog | 470 | 59 | ACCTATGCCTGTGATTTGTGG | AAGAGTAGAGGCTGGGGTAGG |
| Nucleostemin | 747 | 60 | CAGAGATCCTCTTGGTTGCAG | AATGAGGCACCTGTCCACTC |
| ZFX | 465 | 60 | TGATTCCAGGCAGTACCAAAC | TGACGAAAACCCTTACCACAC |
| CD31 | 578 | 59.5 | AGGACATCCATGTTCCGAGA | TGAACCGTGTCTTCAGGTTG |
| CD34 | 219 | 56 | TGAGCCTCTCACCTGTACTC | AGGAGCTGATCTGGGCTATG |
| KDR | 180 | 61 | CCCTGCCGTGTTGAAGAGTT | GGACAGGGGGAAGAACAAAA |
| VCAM1 | 375 | 58 | AGAAATGCCCATCTATGTCC | CGGCATCTTTACAAAACCTG |
| Tie1 | 173 | 57 | GCAAACTCTGCTGTCTAACC | GGATGCCCAGGATAGCTATG |
| GAPDH (1) | 139 | 64 | GCCCCAGCAAGAGCACAAGA | TAGGCCCCTCCCCTCTTCAA |
| GAPDH (2) | 224 | 60 | AGGGTCTCTCTCTTCCTCTTGTGCTC | CCAGGTGGTCTCCTCTGACTTCAACA |
Immunocytochemistry
Immunocytochemistry was performed on EPCs at 7th day of seeding. Briefly, the cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature. Cells were then washed with PBS containing 1% bovine serum albumin (BSA), 0.02% Triton X-100 and 10% rabbit serum while incubated with blocking solution for 30 minutes at room temperature. The cells were then incubated with primary polyclonal antibodies (anti-CD31, 1:40; anti-CD34, 1:50 from Dako, USA and anti-OCT4, 1:50 from Santa Cruz Biotechnology, USA) for 2 hours at 37℃. After washing with PBS, the cells were incubated with the secondary antibodies (dilution 1:100) as follows: anti-mouse IgG conjugated with cy3 (Cmicon, CA, USA), anti-goat IgG conjugated with FITC (Jackson Immuno Research Labs, USA), and anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC) (Sigma-Aldrich, USA), respectively, for attachment to anti-CD31, anti-CD34 and anti-OCT4 for 45 minutes at 37℃ followed by washing further three times with PBS at room temperature.
Finally, the immunoreactivity was analyzed with a fluorescent microscope (Nikon TE300, Japan).In a similar approach, the negative control were prepared, except for the omission of primary antibodies.
Results
Isolation of endothelial progenitor cells
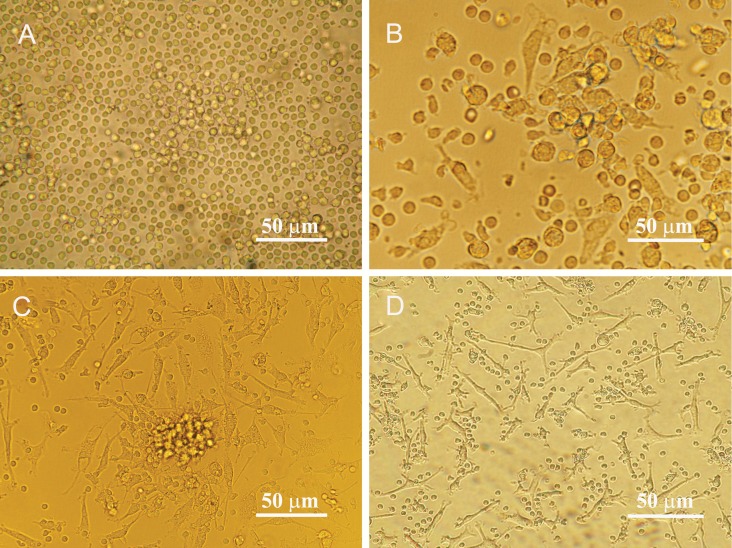
On the 7th day of MNCs culture, ex vivo expanded EPCs showed two kinds of morphology, including spindle-shape cells and round cells, yet some EPC colonies were observable. With continued culture, the number of round cells decreased and spindleshape cells become dominant cell type (Fig 1).
Fig 1.
Morphological features of isolated endothelial progenitor cells before and after differentiation. A. Mononuclear cells isolated from human peripheral blood appeared small and round. B. Most cells were attached to the plates coated with human fibronectin (day 4). The attached cells gradually exhibited a spindle-shaped, endothelial cell-like morphology on day 7th C. and day14th D. after seeding.
By the 7th day, the identity of EPCs was confirmed through double staining for Di-AC-LDL and lectin binding (Fig 2). The obtained data was further validated by immunocytochemistry results revealing that the isolated cells express CD31 and CD34, cell surface markers (Fig 3). Moreover, further characterization of EPCs was carried out by studying the expression of CD34, KDR, VCAM-1, CD31 and Tie-1 by RTPCR, where all of these markers were expressed in isolated cells (Fig 4). All together, our data confirmed the genuine nature of the isolated cells as EPCs.
Fig 2.
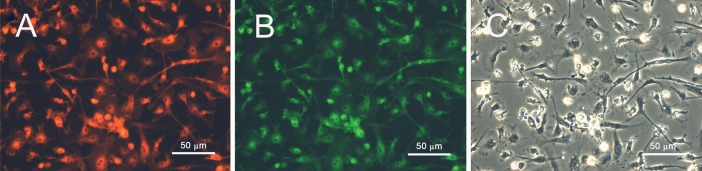
Fluorescence images of isolated endothelial progenitor cells on day 7th after seeding showing positive staining for: A. acetylated LDL (DiI-acLDL) and B. lectin. C. The phase contrast counterpart of A and B.
Fig 3.
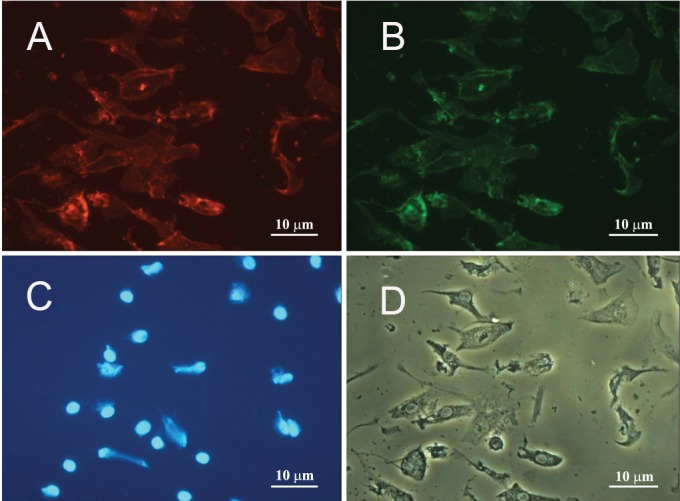
A. Immunocytochemistry of EPCs double-stained with anti-CD31, and B. anti-CD34 antibodies. C. Cells' nuclei were visualized with DAPI . D. The same phase contrast view of the cells in A-C.
Fig 4.
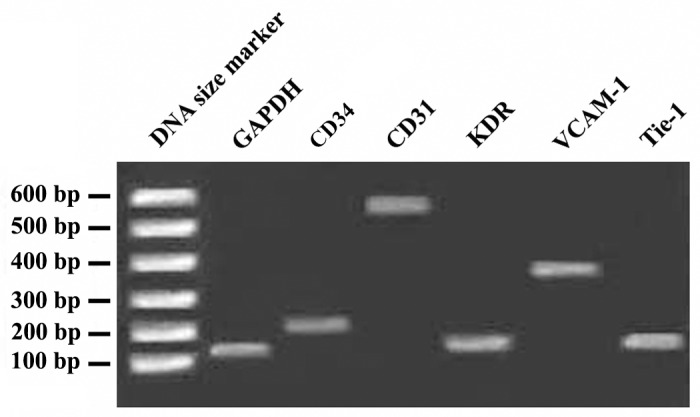
ART-PCR analysis of the expression of endothelial markers in endothelial progenitor cells (EPCs) at day 7th after seeding. As it is evident, EPCs express CD34, CD31, KDR, VECAM-1 and Tie-1 genes. GAPDH was used as an internal control.
Evaluating the expression of stem cell marker genes in EPCs
RT-PCR analysis of EPCs on 7th day of culture revealed that the main regulators of self-renewal property in embryonic stem cells, namely OCT4A; Nanog; and Sox2 are not expressed in EPCs (Fig 5). However, the adult stem cell markers, Nucleostemin and ZFX, and to a lesser extent the other spliced variants of OCT4, like OCT4B and OCT4B1 were expressed in the cells (Fig 5). The expression of genes was compared to that of NT2, a human carcinoma cell line used as a positive control. Moreover, the expression of GAPDH, a housekeeping gene, was monitored in all experiments as an internal control. Also, a no-reverse transcription (no- RT) control was accompanying all PCR experiments, as a negative control.
Fig 5.
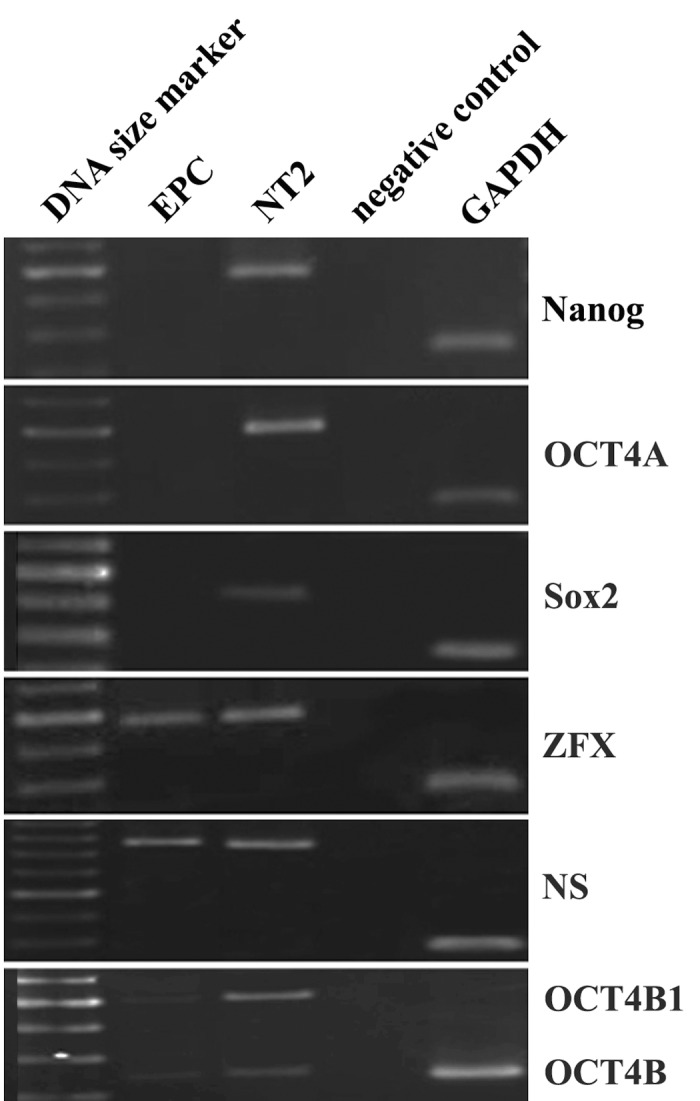
RT-PCR analysis of the expression of some selfrenewal genes in EPCs on day 7th after seeding. Note that EPCs do not express OCT4A variant, Nanog, and Sox2, but express Nucleostemin (NS), ZFX, OCT4B, and OCT4B1 variants. The embryonic carcinoma cell line NT2 was used as a positive control for pluripotency. GAPDH was used as an internal control.
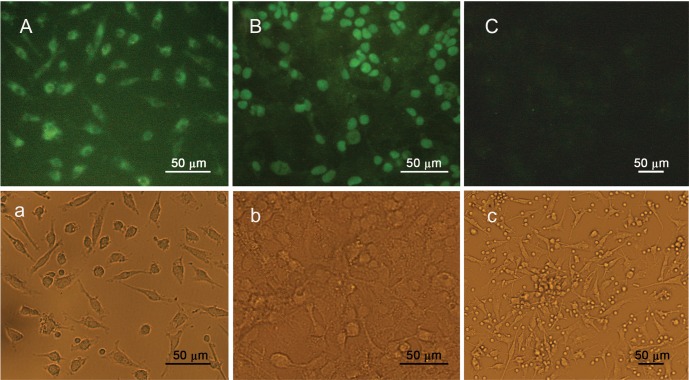
Immunocytochemistry analysis of the subcellular distribution of OCT4 demonstrated a cytoplasmic signal in EPCs, with no immunoreactivity presence within the nuclei of the cells. The observations further confirmed the expression of OCT4B and/or OCT4B1 variants which are localized within the cytoplasm of the cells. On contrary, the OCT4 immunoreactivity was primarily visualized within the nuclei of the pluripotent cell line NT2, which is consistent with the expression of OCT4A variant within these cells. As expected, no staining signal was detected in the negative control, in which all of the conditions were kept the same, except for the omission of the primary antibody (Fig 6).
Fig 6.
Sub-cellular localization of OCT4 in EPCs was stained with a polyclonal antibody against C-terminal domain of OCT4. A. Immunocytochemistry revealed a cytoplasmic immunoreactivity for EPCs. B. NT2 cells were used as a positive control and demonstrated a primarily nuclear immunoreactivity. C. Negative control slide, all the conditions were the same as A and B, except for the elimination of the primary antibodies. a, b, and c depict the phase-contrast counterparts of A, B and C, respectively.
The expression levels of the Nucleostemin and ZFX genes seem to be down-regulated from days 4th to 11th. For Nucleostemin and ZFX genes, an apparent decrease in the expression level was observed on days 4th and 7th of the seeding (Fig 7).
Fig 7.
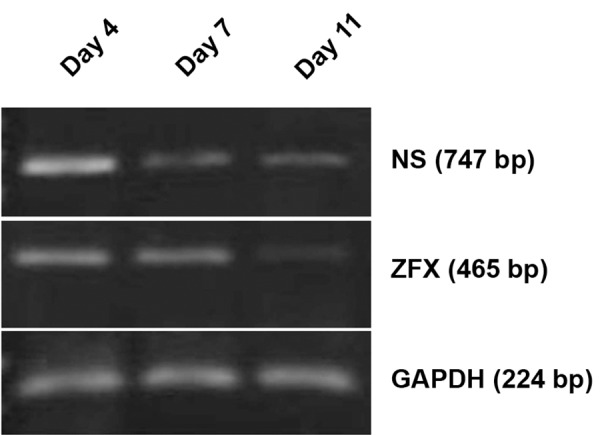
Changes in NS and ZFX genes expression during differentiation of EPCs. As it is evident, there is a decline in the expression of NS and ZFX in the course of cell differentiation from the day 4th to day 11th of cell seeding.
Discussion
EPCs in the human peripheral blood are a potential source of stem cells to promote angiogenesis in ischemic tissues (3). However, the cells have not yet been well characterized and many questions remain to be answered about their nature and molecular characteristics.
In the present study, the isolation of EPCs was carried out by an ex vivo expansion of a heterogeneous PBMC fraction. In this method of cell isolation, the number of isolated cells is higher than other methods (11). In this regard, using magnetic beads for EPCs evaluation may influence the viability of the cells. It can also preferentially exclude some cells with endothelial potential, thereby decrees the number of cells within an isolation population (12). Moreover, it seems that cultivated EPCs in this method are more heterogeneous. After 7 days of culture, the attached cells have the ability of Dil-AC-LDL incorporation, lectin banding, as well as the expression of CD34, CD31, VCAM-1, Tie-1 and KDR genes. All of the mentioned properties indicate an endothelial identity for the expanded cells.
After isolation and expansion of EPCs, the expression of self-renewal genes, like OCT4, Nanog, and Sox2 were evaluated by RT-PCR .These genes form the core of main regulatory network that induce the expression of genes maintaining the pluripotency of embryonic stem cells (ESCs), and also suppress some genes inducing differentiation (10). Our results revealed that none of these genes is expressed in EPCs; therefore, we suggest that EPCs do not have self-renewal properties similar to ES cells. This finding is in accordance with some recent reports that suggest OCT4A is exclusively expressed in pluripotent ES/EC cells (13). The absence of OCT4A expression in already reported in some adult stem cells, such as peripheral blood mononuclear cells (14) and rat bone marrow stromal stem cells (15).
However, our data is in contrast with Romagnani et al. who have previously reported the expression of OCT4 and Nanog in a subpopulation of EPCs, CD14 + CD34 LOW (16). Interestingly, all the previous publications on OCT4A expression in different cell types is doubted with the presence of several expressed pseudogenes of OCT4, as well as with the presence of two other splicing variants of the gene (OCT4B and OCT4B1). The very high sequence conservation between OCT4A, its pseudogenes, and splicing variants could be likely a source of misinterpretation of RT-PCR and immunostaining experiments, as genuine OCT4A expression. Therefore, here we designed specific primers to avoid pseudogenes amplification, and also to discriminate OCT4 alternatively spliced variants (13). Our data revealed no expression of OCT4A, but expression of OCT4B and OCT4B1 spliced variants in endothelial progenitor cells. While OCT4A has a well-known critical role in maintaining the pluripotency of embryonic stem cells, little is known about the functional role of OCT4B and OCT4B1 variants in pluripotent and non-pluripotent cells. However, it has been reported recently that OCT4B and OCT4B1 are also expressed in non-pluripotent cells, where their proteins are localized within the cytoplasm of the cells (13, 17, 18).
In addition, we also detected for the first time the expression of ZFX, a newly identified self-renewal regulatory gene, in EPCs. ZFX has been shown to be expressed in both embryonic and adult (hematopoietic) stem cells, likely an anti-apoptotic role in these cells (19, 20). Hematopoietic stem cells are one of the major sources of EPCs in peripheral blood. Therefore, it is concluded that the expression of ZFX in this pathway continues until completion of the differentiation of EPCs which it likely seems to have the same role in self-renewal of EPCs. Furthermore, our results demonstrated that EPCs express Nucleostemin (NS) gene and that its expression was down-regulated from days 4th to 11th. NS is highly expressed in bone marrow stromal cells, and the expression is suddenly turned off with the induction of neural differentiation in the cells (15). Based on recent reports, NS was hypothesized to be a proliferating factor in stem cells, where it regulates cell cycle proceeding (21).
Conclusion
Our data revealed that EPCs express endothelial markers, an indication that they are in a late stage of cell differentiation. The data also revealed that none of the main ES-specific regulatory genes OCT4A, Sox2 and Nanog is expressed in EPCs; a finding that indicates these cells have a limited self-renewal capacity compared to embryonic stem cells. The latter finding suggests that the cells could be considered as a safer source compared to ES cells for cell therapy, in terms of the likelihood of teratoma generation.
Acknowledgments
This study was supported by a joint research grant from the Tarbiat Modares University and the Kurdistan University of Medical Sciences. The authors have no conflict of interest in this paper.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 4.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 5.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113(20):2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 6.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 7.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 9.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107(7):1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 10.Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001;26(3):137–148. doi: 10.1247/csf.26.137. [DOI] [PubMed] [Google Scholar]
- 11.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez DA, Townsend LE, Uzieblo MR, Haan ME, Callahan RE, Bendick PJ, et al. Human endothelial cell cultures from progenitor cells obtained by leukapheresis. Am Surg. 2000;66(4):355–359. [PubMed] [Google Scholar]
- 13.Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26(12):3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 14.Kotoula V, Papamichos SI, Lambropoulos AF. Revisiting OCT4 expression in peripheral blood mononuclear cells. Stem Cells. 2008;26(1):290–291. doi: 10.1634/stemcells.2007-0726. [DOI] [PubMed] [Google Scholar]
- 15.Yaghoobi MM, Mowla SJ, Tiraihi T. Nucleostemin, a coordinator of self-renewal, is expressed in rat marrow stromal cells and turns off after induction of neural differentiation. Neurosci Lett. 2005;390(2):81–86. doi: 10.1016/j.neulet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, et al. CD14 + CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97(4):314–322. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 17.Farashahi Yazd E, Rafiee MR, Soleimani M, Tavallaei M, Salmani MK, Mowla SJ. OCT4B1, a novel spliced variant of OCT4, generates a stable truncated protein with a potential role in stress response. Cancer Lett. 2011;309(2):170–175. doi: 10.1016/j.canlet.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT- 4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281(44):33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 19.Galan-Caridad JM, Harel S, Arenzana TL, Hou ZE, Doetsch FK, Mirny LA, et al. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007;129(2):345–357. doi: 10.1016/j.cell.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cellot S, Sauvageau G. Zfx: at the crossroads of survival and self-renewal. Cell. 2007;129(2):239–241. doi: 10.1016/j.cell.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24(4):1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]