Abstract
Background
While bipolar disorder (BD) is a leading cause of disability, and an important contributor to disability in BD is cognitive impairment, there is little systematic research on the longitudinal course of cognitive function and instrumental activities of daily living (IADLs) in late-life. In this report, we characterize the 2-year course of cognitive function and IADLs in older adults with BD.
Method
We recruited non-demented individuals 50 years and older with BD I or BD II (n=47) from out-patient clinics or treatment studies at the University of Pittsburgh. Comparator subjects (`controls') were 22 individuals of comparable age and education with no psychiatric or neurologic history, but similar levels of cardiovascular disease. We assessed cognitive function and IADLs at baseline, 1- and 2-year time-points. The neuropsychological evaluation comprised 21 well-established and validated tests assessing multiple cognitive domains. We assessed IADLs using a criterion-referenced, performance-based instrument. We employed repeated-measures mixed-effects linear models to examine trajectory of cognitive function. We employed non-parametric tests for analysis of IADLs.
Results
The BD group displayed worse cognitive function in all domains and worse IADL performance than the comparator group at baseline and over follow-up. Global cognitive function and IADLs were correlated at all time-points. The BD group did not exhibit accelerated cognitive decline over 2 years.
Conclusions
Over 2 years, cognitive impairment and associated functional disability of older adults with BD appear to be due to long-standing neuroprogressive processes compounded by normal cognitive aging rather than accelerated cognitive loss in old age.
Keywords: Aged adults, bipolar disorder, cognition, disease progression
Introduction
Bipolar disorder (BD) is a leading cause of disability worldwide (Murray & Lopez, 1996). The disability associated with BD exceeds that of major depressive disorder (on an individual level) and approaches that of schizophrenia, with which it shares roughly the same prevalence in the US population (Kessler et al. 2006). An important contributor to disability in BD is cognitive dysfunction (Bowie et al. 2010; Bearden et al. 2011).
Over 75 studies and five reviews have established an association between BD and cognitive dysfunction (Bearden et al. 2001; Robinson et al. 2006; Torres et al. 2007; Arts et al. 2008; Bora et al. 2010). Dysfunction is found in executive function, verbal memory and processing speed. The reports, mostly cross-sectional, show that dysfunctions are not solely related to residual mood effects, drug effects or other confounding factors. Dysfunction appears related to illness severity, and deficits are apparent in first-degree relatives (Robinson et al. 2006; Bearden et al. 2011).
Whether cognitive dysfunction in BD is due to a neurodevelopmental process (i.e. a `fixed' deficit), a neurodegenerative process (i.e. a `progressive' deficit), or both has been an area of recent interest and discussion. While it is now well-recognized that cognitive impairment is a core feature of BD across mood states and early on in the disease (Malhi et al. 2007; Adida et al. 2011; Mann-Wrobel et al. 2011), the etiology of this impairment has not been established (Savitz et al. 2005). Several possible pathways have been hypothesized, involving dysregulated dopaminergic and glutamatergic systems, mitochondrial dysfunction and oxidative stress, and inflammation (Berk et al. 2010). These different mechanisms suggest different approaches to targeting interventions for cognitive dysfunction and/or deterioration. If cognitive impairment associated with BD is due to a neurodegenerative process, interventions might prevent, halt, or even reverse cognitive decline.
While some published data support a neurodegenerative model in older adults with BD, the data are mixed and not conclusive. In a preliminary study of global cognitive function assessed with the Dementia Rating Scale (Mattis, 1988), our research group reported that older adults with BD (n=33; mean age 69.7 years; s.d.=7.9) not only had worse performance than mentally healthy individuals of similar age and education, but also appeared to have a faster decline over 1–3 years of follow-up (Gildengers et al. 2009). This report concurred with the findings of Dhingra & Rabins (1991), who found accelerated cognitive decline in a group of elders (n=25; age 60 years and older; mean and s.d. of their sample not provided) hospitalized for mania after 5–7 years of follow-up (Dhingra & Rabins, 1991). However, two other groups have not found accelerated cognitive decline in older adults with BD (Depp et al. 2008; Delaloye et al. 2011).
Depp et al. (2008) examined cognitive function in 35 community-dwelling out-patients with BD (mean age 58 years, s.d.=10.0) with a battery of neurocognitive tests repeated 1–3 years after baseline. They compared the cognitive performance of the BD individuals with mentally healthy comparators and patients with schizophrenia. They found that the trajectory of global cognitive function in older adults with BD and schizophrenia did not differ from normal controls (i.e. no faster than expected cognitive deterioration). However, they did find that older adults with BD had greater variability in cognitive function in comparison with normal controls and patients with schizophrenia. Further, at baseline, cognitive function in the BD group was impaired, with performance approaching that of the patients with schizophrenia.
Delaloye et al. (2011) examined cognitive function and structural brain abnormalities in 15 older adults with BD (mean age 67.9 years, s.d.=5.18) and a comparison group of normal controls. They found that while patients with BD displayed significantly lower performances in processing speed and episodic memory (not working memory and executive function), they did not exhibit a faster trajectory of decline than controls. Further, longitudinal gray matter and white matter changes did not differ between BD patients and controls.
Cognitive dysfunction related to BD needs to be distinguished from aging-related cognitive decline. Normal aging effects have been well studied and are broadly grouped into the effects on `fluid' abilities versus `crystallized' abilities (Salthouse, 2010; Glorioso & Sibille, 2011). `Fluid' abilities comprise processing speed, problem-solving, inhibitory function, working memory, long-term memory and spatial abilities. Fluid abilities decline with age. Mechanisms associated with normal aging decline are thought to include shrinkage of dendritic arbor and cell bodies, decrease in synaptic density, loss of glial cells, reduction of myelination, and potentially decreases in vascularization (Salthouse, 2011). In contrast, `crystallized' abilities relate to knowledge or expertise, such as vocabulary, world knowledge, general knowledge, implicit memory, etc. Crystallized abilities do not decline over time and even show some improvement. Understanding aging effects on the brain can help distinguish normal versus pathologic decline. For example, loss of semantic knowledge, which is considered crystallized information, occurring in Alzheimer's disease, is not consistent with normal aging-related cognitive decline (Chertkow et al. 2008; Salmon, 2012). Further, comparing BD subjects with a group of mentally healthy comparators can help distinguish pathological changes due to BD versus normal aging-related cognitive decline.
In this report, we extend our preliminary analysis and provide a finer-grained description of the trajectory of cognitive function across individual cognitive domains in a larger sample of individuals with BD. An examination of distinct cognitive domains may help to clarify areas of pathology and point to interventions that would be helpful for specific targets. For example, declines in executive function suggest pathology involving the pre-frontal cortex or underlying subcortical white matter, while memory impairment suggests involvement of the hippocampus and related circuitry. Based on our preliminary data and the existing literature, our main hypothesis was that, compared with a group of mentally healthy individuals of similar age and education, older adults with BD would exhibit faster cognitive decline over 2 years in the domain of information processing speed and executive function, but not in other domains. To clarify the relationship between cognitive function and disability, we also explored the relationship between cognitive function and observed instrumental activities of daily living (IADLs) over time. We expected greater declines in information processing speed and executive function compared with other domains because of our suspicion that BD is not a dementing illness, but rather accelerates existing aging (`wear and tear') effects on top of potentially early developmental abnormalities. Since the goal of the study was to identify whether longer-term cognitive effects are secondary to lifelong BD rather than acute effects due to impaired performance due to mania or depression, we assessed all subjects when they were stably euthymic at baseline and follow-up time-points.
Method
Study subjects
As previously described, we enrolled individuals with BD I or BD II from out-patient clinics or treatment studies carried out at the University of Pittsburgh (Gildengers et al. 2005, 2008). Comparator subjects (`controls') were individuals with no psychiatric or neurologic history selected to make the groups similar in age, education and cardiovascular burden. We recruited these comparator subjects through health fairs, advertisements in local papers, and ongoing projects studying the relationship between late-life mood disorders and cognitive function (Butters et al. 2004; Bhalla et al. 2009). Special efforts were directed at recruiting comparator subjects with general medical burden from primary care practices (Reynolds et al. 2011). All subjects provided written informed consent, as required by the Institutional Review Board at the University of Pittsburgh.
Inclusion criteria
The inclusion criteria were: age 50 years or older; clinical euthymia for 4 weeks preceding neuropsychological (NP) assessment with scores of 10 or less on the 17-item Hamilton Rating Scale for Depression (Hamilton, 1967) and 10 or less on the Young Mania Rating Scale (Young et al. 1978) at the time of baseline and follow-up assessment; ability to comprehend and speak English fluently; and corrected visual ability to read newspaper headlines and hearing capacity adequate to respond to a raised conversational voice.
Exclusion criteria
The exclusion criteria were: pre-existing history of dementia or neurologic disorder affecting the central nervous system (for example, Parkinson's disease, traumatic brain injury or multiple sclerosis); electro-convulsive therapy within the past 6 months; and substance abuse or dependence within the past 12 months. Follow-up testing was anchored to the nearest yearly interval. Subjects who were not euthymic at the time of their scheduled assessment had testing delayed for up to 4 months to re-establish euthymia. Subjects who did not re-establish euthymia within 4 months were not tested that year. Extending the window beyond 4 months may have enhanced the practice effects from one assessment to another.
Diagnosis and treatment
Diagnosis was established by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV) administered by trained clinicians. The majority of subjects received treatment in our university-based clinics. In these clinics, the goals of the pharmacotherapy intervention for BD have been to maximize the appropriate use of lithium or divalproex, either singly or in combination, to achieve remission of acute mood episodes, maintain euthymia, and limit adjunctive anti-psychotic or antidepressant medication.
Recruitment
From 16 May 2005 to 10 June 2008, 151 individuals with BD and 40 mentally healthy individuals were screened for study participation. Of these, 83 individuals with BD and 24 mentally healthy comparators consented to study participation; 36 individuals with BD and two comparators were excluded or did not complete the baseline assessments. Thus, 47 individuals with BD and 22 comparators completed at least the baseline assessment and were included in the analyses. The high rate of drop-out among the BD individuals between consenting for study participation versus enrolling was primarily related to recruitment of individuals while they were hospitalized for in-patient psychiatric care. Subjects were approached and enrolled in the study during an acute mood episode, but did not undergo NP evaluation until stably euthymic (often several months from the time of consent). During this interval between signing consent and baseline assessment, many individuals reconsidered their study participation or were lost to follow-up (n=15). The remaining subjects withdrew consent due to a variety of reasons including concurrent medical problems, moving away, or inability to establish sustained euthymia.
Measures
We employed a broad-based assessment of cognitive function and IADLs. The NP evaluation encompassed 21 well-established and validated individual tests measuring multiple cognitive domains (see Table 1 for the individual tests) (Lezak, 2004). As previously described (Butters et al. 2004; Gildengers et al. 2007), we transformed raw scores for all individual tests into Z scores using the baseline distribution of the mentally healthy comparators (i.e. comparators' performance on any test has a mean of 0 and s.d. of 1). Based on factor analyses, we developed four composite factor scores reflecting four distinct cognitive domains: delayed memory; information processing speed/executive function; language; and visuomotor (Gildengers et al. 2012). Some components of executive function were present in the delayed memory, language and visuomotor domains. For each subject, Z scores for the individual tests comprising each factor were averaged to produce four domain factor scores. Five tests that did not group within particular domains were excluded from the distinct cognitive domains, but were included in the global score calculated by averaging all Z scores. Cronbach's α for the four domain scores and the global score are presented in Table 1. Within each domain, Cronbach's α's ranged from 0.70 to 0.77, reflecting the heterogeneity of the component instruments.
Table 1.
Individual tests comprising neuropsychological assessment battery organized by domain
| Cognitive domain | Tests | Cronbach's α (n=69) |
|---|---|---|
| Delayed memory | Logical memory (WMS-III), Rey–Osterrieth Complex Figure Recall, California Verbal Learning Test, Wisconsin Card Sorting Test | 0.77 |
| Information processing speed/executive function | Trails A, Stroop, Executive Interview, Animal Fluency, Digit Symbol Substitution Test | 0.76 |
| Language | Spot the Word, Letter Fluency, Silly Sentences | 0.70 |
| Visuomotor ability | Rey–Osterreith Complex Figure Copy, Simple Drawings, Block Design, Trails B/Trails A | 0.70 |
| Global score | Grooved Pegboard, Digit span, Boston Naming Test, Clock, Finger Tapping (in addition to tests listed above) | 0.88 |
WMS-III, Wechsler Memory Scale, third edition.
We assessed IADLs using a criterion-referenced, performance-based instrument, the Performance Assessment of Self-Care Skills (PASS; Rogers & Holm, 2000). An occupational therapist performed an in-home assessment of functional abilities, using 10 PASS items (three items for money management: shopping, bill paying by check, and checkbook balancing; one for medication management; two for current events: obtaining critical information from auditory and visual media; one for home maintenance: small repairs; one for environmental awareness: home safety; and two for meal preparation: stovetop use and use of sharp utensils). The occupational therapist assigned a score from 0 (complete independence; requires no assistance for task initiation, continuation, or completion) to 9 (complete dependence; requires total assistance) such that higher scores indicate worse performance.
We assessed medical illness co-morbidity with the Cumulative Illness Ratings Scale for geriatrics (CIRS-G; Miller et al. 1992). The CIRS-G organizes medical burden across different systems (heart, vascular, etc.), rating burden within each system from `0' (`no problem') to `4' (`extremely severe'). A cardiovascular subscale was composed by adding CIRS-G items no. 1 (heart) and no. 2 (vascular) (Gildengers et al. 2012). For example, for item no. 1, angina treated with medications as needed would be rated as a `1' (`current mild problem or past significant problem'). For item no. 2, hypertension requiring a daily anti-hypertensive would be rated as `2 ' (`moderate disability or morbidity – requires “first-line ” treatment'). Adding these two items would yield a subscale score of `3'.
Statistical analysis
Prior to statistical testing, the data were examined for normality and transformations were used where necessary. If transformation did not normalize the distribution, a non-parametric test was used. Missing data due to cognitive impairment were imputed with the lowest score of the group for that test: Trails B, Spot the Word and Silly Sentences for one BD subject; Stroop for two separate BD subjects. Descriptive statistics were generated to characterize the BD and comparator samples on basic demographics and clinical variables. To test for group differences on the continuous variables, t tests were used; Fisher exact tests were used to test categorical variables. For the cognitive domains, we fit repeated-measures mixed-effects linear models across time including group, time and group × time interactions in the model as well as age and education as covariates (Brown & Prescott, 2006). Given the distribution of IADL scores non-parametric Spearman correlation coefficients were used to examine the relationship between IADLs and global cognitive function among BD subjects. Based on the sample size at year 2, we determined having 80 % power to detect a medium effect size for the repeated-measures within-between interaction: Cohen's f ranges 0.15–0.20 for correlation ranges of 0.4–0.7 (Cohen's f: small= 0.10, medium=0.25, large=0.40).
Results
Table 2 summarizes the baseline demographic and clinical characteristics of the BD subjects and mentally healthy comparators. Both groups were similar in age, education and cardiovascular burden; however, among the BD subjects, total medical burden was higher and there was a trend to a higher percentage of Caucasians. Of the 47 BD subjects, 39 (83%) had BD I and eight (17%) had BD II. Table 3 summarizes psychotropic usage among the BD subjects at the three time-points of assessment. There was a high rate of antidepressant use, especially at the 2-year follow-up.
Table 2.
Baseline demographics and medical burden among BD subjects and mentally healthy comparators
| BD (n=47) | Comparators (n=22) | Statistics | |
|---|---|---|---|
| Mean age, years (s.d.; range) | 68.0 (9.3; 50.5–86.1) | 66.3 (7.3; 52.6–80.5) | t(67)=0.73, p=0.47 |
| Female, % (n) | 70.2 (33) | 59.1 (13) | Fisher exact p=0.42 |
| Caucasian, % (n) | 91.5 (43) | 72.7 (16) | Fisher exact p=0.06 |
| Mean education, years (s.d.) | 15.3 (3.0) | 15.1 (2.9) | t(67)=0.18, p=0.86 |
| Mean Cumulative Illness Rating Scale-Geriatric, total score (s.d.) | 9.5 (4.6) | 7.1 (2.9) | t(60.3)a=2.58, p=0.01 |
| Mean count of number of organ systems affected (s.d.) | 6.0 (2.6) | 4.5 (1.6) | t(60.7)a=2.99, p=0.004 |
| Mean heart and vascular burdenb (s.d.) | 2.0 (1.5) | 2.0 (1.3) | t(67)=0.18, p=0.86 |
BD, Bipolar disorder; s.d., standard deviation.
Satterthwaite method used due to unequal variance.
Items no. 1 and no. 2.
Table 3.
Psychotropic medications in subjects with bipolar disorder
| Medication | Baseline (n=47) | 1 year (n=37) | 2 year (n=30) |
|---|---|---|---|
| Antidepressants (SSRIs, SNRIs, etc.) | 31 (66.0) | 25 (67.6) | 24 (80.0) |
| Antipsychotics (novel or conventional) | 12 (25.5) | 13 (35.1) | 10 (33.3) |
| Anticonvulsants (divalproex, carbamazepine or lamotrigine) | 20 (42.6) | 16 (43.2) | 17 (56.7) |
| Benzodiazepines (lorazepam, clonazepam, etc.) | 16 (34.0) | 12 (32.4) | 13 (43.3) |
| Cognitive enhancer (cholinesterase inhibitor or memantine) | 4 (8.5) | 6 (16.2) | 4 (13.3) |
| Lithium | 12 (25.5) | 10 (27.0) | 6 (20.0) |
| Sedative-hypnotics (trazodone, zaleplon or zolpidem) | 8 (17.0) | 5 (13.5) | 5 (16.7) |
| Stimulants (dexedrine, methylphenidate or modafinil) | 2 (4.3) | 3 (8.1) | 3 (10.0) |
Data are given as number of subjects (%).
SSRIs, Selective serotonin reuptake inhibitors; SNRIs, serotonin–norepinephrine reuptake inhibitors.
Baseline demographic and clinical variables did not differ between the 31 BD subjects who completed 2 years of follow-up and the 16 subjects who did not. Similarly, completers and non-completers did not differ in their NP performance or IADLs at baseline or 1-year follow-up. A total of 10 subjects did not complete the 1-year NP assessment due to unstable mood (n=4), poor health (n=3), withdrawal of consent (n=1) or reasons unrelated to the study (n=2). A total of 17 subjects did not complete the 1-year NP assessment due to poor health (n=7), unstable mood (n=3), death (n=3), withdrawal of consent (n=2) or reasons unrelated to the study (n=2). In addition, one subject did not complete the IADL assessment at 1 and 2 years because she moved out of state and had constraints on her time during annual assessment. Another subject was unable to schedule the 2-year IADL assessment within the 4-week window required by the study. Causes of death in the three subjects who died in the course of study participation were heart failure (n=2) and cancer (n=1). Antidepressant use was not related to drop-out or missing data.
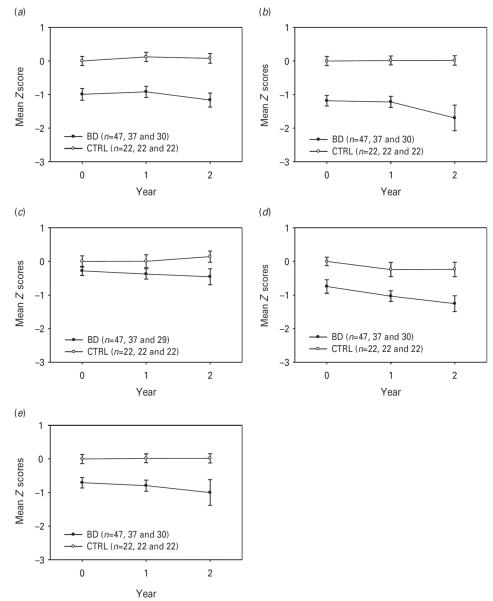
Fig. 1 displays the course of cognitive function over 2 years for the four cognitive domains and globally. While there were statistically significant group effects for all domains and globally, only the language domain showed group×time interaction (see Table 4). Hence, as a group, BD subjects performed worse than mentally healthy comparators, but over 2 years they did not display accelerated decline either globally or in any specific cognitive domain, except for language. Language showed improvement in healthy comparators (probably related to practice effects) and decline in BD subjects over time. As expected, age and education were significant covariates for most cognitive domains, with higher age and lower education associated with worse performance. However, age was not significantly related to visuomotor ability or language performance. CIRS-G total score, CIRS-G number of organ systems affected, and cardiovascular burden, as measured with CIRS-G items no. 1 and no. 2, were not significant covariates in any of the models and thus were excluded in the final models. Table 5 presents the least square means and effects sizes for the NP performance of the BD subjects versus mentally healthy comparators.
Fig. 1.
Two-year trajectories of cognitive function among bipolar disorder (BD) subjects and mentally healthy comparators (CTRL). (a) Delayed memory; (b) information processing speed/executive function; (c) language; (d) visuomotor ability; (e) global score. Values are means, with standard deviations represented by vertical bars. See Table 4 for test statistics.
Table 4.
Mixed-effects model of neuropsychological performance over 2 years
| Effect | F statistica | P |
|---|---|---|
| Delayed memory | ||
| Age | 14.77 | <0.001 |
| Education | 9.82 | 0.003 |
| Time | 2.03 | 0.14 |
| Group | 25.92 | <0.001 |
| Group × time | 0.89 | 0.42 |
| Information processing speed/executive function | ||
| Age | 12.26 | <0.001 |
| Education | 8.31 | 0.005 |
| Time | 0.27 | 0.77 |
| Group | 24.93 | <0.001 |
| Group × time | 0.21 | 0.81 |
| Language | ||
| Age | 0.58 | 0.45 |
| Education | 23.03 | <0.001 |
| Time | 0.31 | 0.73 |
| Group | 4.61 | 0.036 |
| Group × time | 3.57 | 0.034 |
| Visuomotor ability | ||
| Age | 2.93 | 0.09 |
| Education | 10.75 | 0.002 |
| Time | 5.19 | 0.008 |
| Group | 11.84 | 0.001 |
| Group × time | 0.33 | 0.72 |
| Global score | ||
| Age | 7.65 | 0.007 |
| Education | 14.81 | <0.001 |
| Time | 1.70 | 0.19 |
| Group | 22.83 | <0.001 |
| Group × time | 0.64 | 0.53 |
Degrees of freedom=1,65 for age, education and group and 2,65 for time and group × time.
Table 5.
Scores and effect sizes for neuropsychological performancea over 2 years, BD subjects versus mentally healthy comparators
| Cognitive domain | Time, year | BD (n=47) | Comparators (n=22) | Cohen's d | (95% CI) |
|---|---|---|---|---|---|
| Delayed memory | 0 | −0.97 (0.14) | −0.04 (0.21) | 0.98 | (0.44–1.52) |
| 1 | −0.86 (0.12) | 0.09 (0.17) | 1.17 | (0.62–1.73) | |
| 2 | −1.12 (0.13) | 0.04 (0.17) | 1.41 | (0.84–1.98) | |
| Information processing speed/ | 0 | −1.16 (0.13) | −0.03 (0.18) | 1.33 | (0.76–1.89) |
| executive function | 1 | −1.12 (0.12) | −0.01 (0.17) | 1.41 | (0.84–1.98) |
| 2 | −1.32 (0.26) | −0.01 (0.34) | 0.81 | (0.28–1.34) | |
| Language | 0 | −0.27 (0.11) | 0.01 (0.16) | 0.39 | (−0.13 to 0.90) |
| 1 | −0.37 (0.13) | 0.02 (0.18) | 0.46 | (−0.06 to 0.98) | |
| 2 | −0.50 (0.15) | 0.16 (0.20) | 0.68 | (0.15–1.21) | |
| Visuomotor ability | 0 | −0.73 (0.16) | −0.01 (0.23) | 0.67 | (0.15–1.20) |
| 1 | −1.06 (0.15) | −0.25 (0.20) | 0.86 | (0.32–1.39) | |
| 2 | −1.16 (0.18) | −0.24 (0.23) | 0.84 | (0.30–1.37) | |
| Global score | 0 | −0.68 (0.09) | −0.01 (0.14) | 1.07 | (0.53–1.62) |
| 1 | −0.77 (0.08) | −0.03 (0.11) | 1.41 | (0.84–1.98) | |
| 2 | −0.91 (0.13) | −0.07 (0.17) | 1.03 | (0.48–1.57) |
Data are given as least squares mean (standard error) and as effect size (95% CI).
BD, Bipolar disorder; CI, confidence interval.
Controlling for age and education.
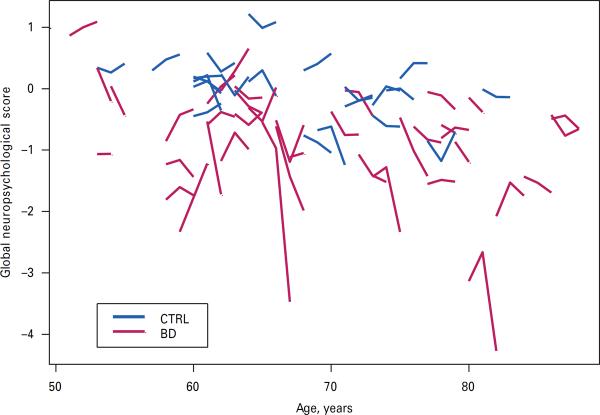
While we did not have sufficient power to statistically examine three-way interaction effects (age×time×group), we did examine age×time×group graphically with a `matchstick' plot (see Fig. 2). In the matchstick plot, we observed greater variability in the BD versus comparator subjects, overall worse performance, and overall decline over time.
Fig. 2.
`Matchstick' plot of depicting age×time×group interactions in global neuropsychological score among bipolar disorder (BD) subjects and mentally healthy comparators (CTRL).
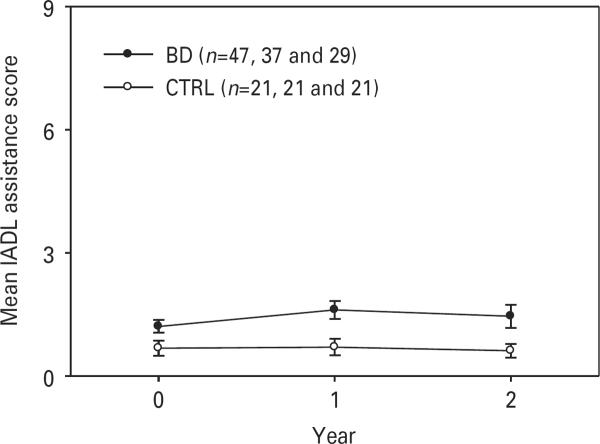
Fig. 1 displays the course of IADL levels of assistance over 2 years. Among the BD subjects, baseline IADLs were correlated with global cognitive function (n=47, rs=−0.42, p=0.003) as well as at 1-year (n=36, rs=−0.63, p<0.001) and 2-year time-points (n=28, rs=−0.76, p<0.001). We examined the relationship between baseline cognitive performance and change in IADL for the whole group and by BD and mentally health comparators. We found a significant correlation in the whole group in the baseline information processing speed/executive function domain and change in IADLs at year 2 (rs=−0.31927, p=0.0238, n=50): higher baseline cognitive function was related to larger decrease in the level of support needed for IADLs. Global (NP) score and examining relationships in the BD and mentally healthy comparators separately did not reveal statistically signifi-cant correlations.
During regular review of the NP data at 1- and 2-year follow-up assessments; we identified eight (17%) BD and no comparator subjects to be significantly cognitively impaired (i.e. testing in the dementia range, meaning 1.5 s.d. below or more on all tests within a domain or several tests in multiple domains) requiring discussion of the test results with the subject and/or family members. The research team met with the subject and family members to discuss these results, their implications, and the next steps in clinical management, including follow-up with the subject's primary care physician (PCP) or psychiatrist. Ignoring drop-outs, the higher rate of significant cognitive impairment in the BD group versus the mentally healthy comparators (eight subjects versus zero subjects) was statistically significant (Fisher's exact=0.048).
Discussion
Older adults with BD exhibited worse cognitive and IADL performance than mentally healthy comparators of similar age, education and cardiovascular burden at baseline and over 2 years of follow-up. However, they did not exhibit faster cognitive decline. Our findings do not support a process of neurodegeneration in the very short term in older adults with BD. This is congruent with two other recent reports on the longitudinal course of cognition in BD elders consistent with the notion that in older adults with BD cognitive decline is primarily a result of normal cognitive aging (Depp et al. 2008; Delaloye et al. 2011). Thus, the cognitive impairment and the associated functional disability of older adults with BD may be due to longstanding neuroprogressive processes compounded by normal cognitive aging rather than accelerated cognitive loss in old age. In older age, the neuroprogressive processes in BD continue, but seem to have a lesser impact compared with cognitive aging. As in our prior reports, we found that IADL performance was significantly correlated with cognitive function (Gildengers et al. 2007, 2012). To our knowledge, our report is based on the largest number of patients with BD followed longitudinally using a comprehensive NP battery administered when patients were stably euthymic.
Congruent with prior cross-sectional reports, the cognitive domain most impaired among BD subjects was information processing speed/executive function (Depp et al. 2007; Gildengers et al. 2007). The domain least affected was language. The language domain is more consistent with `crystallized' abilities, less affected by normal age-related cognitive decline than `fluid' abilities of information processing speed/executive function. We offer two explanations for this finding: (1) since language is a crystallized ability, it tends to improve over time – in the case of individuals with BD, they do not improve to the same extent as mentally healthy comparators; and (2) patients with BD have worse memory function compared with mentally healthy comparators – consequently, they do not benefit to the same extent from practice effects.
One caveat to consider is that the patients with BD in this study were treated in a state-of-the-field manner at an academic medical center that may not reflect BD patients treated in the community. In the community, patients might be treated in a more anti-psychotic-heavy manner, resulting in more negative cognitive side-effects from medications. In addition, this study has both strengths and limitations that need to be considered. While this is a longitudinal study of BD in older adults using a sensitive NP test battery to date, follow-up over 2 years may be insufficient to detect longer-term longitudinal differences in trajectories of cognitive function. Since our study was powered to detect a medium to large effect over 2 years, this study rules out a medium to large effect in older age within a 2-year time-frame rather than ruling out accelerated aging or neuroprogression over the longer term. Consequently, we continue to follow subjects in this report in anticipation of a follow-up report examining the course of cognitive function over 3–5 years. Longer-term follow-up would also enable the use of statistical methods (e.g. reliable change index) to assess pathological change. Further, the number of missing observations increased over time, raising concerns of drop-out bias. However, a comparison of the baseline characteristics and cognitive performance of the subjects who dropped out did not suggest a drop-out bias. Even though baseline characteristics between completers and drop-outs did not differ, this does not rule out that subjects who dropped out were not those who experienced the greatest decline over 2 years. In our ongoing follow-up assessments, we have shortened the NP assessment battery to decrease subject burden and decrease likelihood of drop-out. A related concern is having BD subjects entered into the study during in-patient treatment. Having patients tested after an acute episode may have biased BD subjects to those with worse baseline performance at baseline, thus making it more difficult to find changes over 2–3 years of follow-up. However, we mitigated against this possibility by making sure that subjects were tested when stably euthymic. Another limitation is that the measure of cardiovascular burden is a proxy measure and may not directly measure the extent of vascular disease in the brain. While our report did not detect a significant relationship between vascular burden and cognitive performance, this may reflect a limitation of measuring vascular disease indirectly rather than assessing vascular disease in the CNS directly through neuro-imaging. Finally, the numbers of mood episodes in the BD subjects were too few to examine their impact on the trajectory of cognitive function.
While our findings suggest that older adults with BD do not experience accelerated cognitive decline in old age, they do suggest that older adults with BD are at greater risk for dementia by virtue of having decreased cognitive reserve (Stern, 2002, 2006). Individuals who have lower cognitive function earlier will cross the threshold for clinically significant cognitive impairment sooner than individuals with higher cognitive function despite following the same trajectory of normal aging-related cognitive decline (Jack, 2012). Hence, interventions targeting cognitive function need to be focused early on to prevent cognitive deterioration. Although some data have shown that cholinesterase inhibitors may slow cognitive decline in older adults with unipolar depression and mild cognitive impairment (Reynolds et al. 2011), the risk–benefit ratio of these medications has not been established in patients with BD. Similarly, promising data suggest that lithium and divalproex appear to have neuroprotective effects (Schloesser et al. 2008), but the longer-term clinical effects on cognitive function have yet to be confirmed in a large controlled study in this population of patients. Thus, at this time, efforts at cognitive protection in BD should focus on prevention of mood episodes (i.e. ensuring clinical stability), healthy life-style, and addressing treatable physical illnesses, such as hypertension, hypercholesterolemia, and overweight/obesity (Barnes & Yaffe, 2011). At the same time, conducting research directed at understanding the pathophysiology of BD and consequent cognitive dysfunction is critical and may identify novel interventions that may address both mood stability and longer-term cognitive protection (Berk et al. 2010).
Fig. 3.
Two-year trajectories of instrumental activities of daily living (IADLs) levels of assistance among bipolar disorder (BD) subjects and mentally healthy comparators (CTRL) (higher scores indicate worse performance). Values are means, with standard deviations represented by vertical bars.
Acknowledgements
The authors thank Ms Michelle Zmuda for the recruitment of control subjects and coordination of all neuropsychological assessments.
This work was supported in part by Public Health Service grants no. K23 MH 073772 (to A.G.G.), R01 MH 084921 (to A.G.G.), R01 MH072947 (to M.A.B.), P30 MH071944 (to C.F.R.), P30 MH68446 (to C.F.R.) K24 MH069430 (to B.H.M.), U01 MH68846 (to B.H.M.), and the UPMC (University of Pittsburgh Medical Center) Endowment in Geriatric Psychiatry (to C.F.R.) and John A. Hartford Center of Excellence in Geriatric Psychiatry (to C.F.R.). The sponsors of this research had no role in the design and conduct of the study (aside from approving the study design and human subjects concerns), collection, management, analysis and interpretation of the data, and preparation, review or approval of the manuscript. A.G.G. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
M.A.B. has received payment from Northstar Neuroscience and Medtronic for providing neuropsychological assessment services to clinical trials and from Fox Learning Systems [via National Institutes of Health (NIH)-funded small business innovation research (SBIR)] for computerized test development. A.G.G. has received research support from GlaxoSmithKline for an investigator-initiated study. B.H.M. has received research support in the form of pharmaceutical supplies for his NIH-sponsored research from Bristol-Myers Squibb, Eli Lilly and Company, Pfizer and Wyeth. C.F.R. has received research support in the form of pharmaceutical supplies for his NIH-sponsored research from Bristol-Myers Squibb, Eli Lilly and Company, Forest and Pfizer.
Footnotes
Declaration of Interest The following disclosures within the past 5 years are reported in connection with the paper.
References
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biological Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, Van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological Medicine. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurology. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder : a critical review. Bipolar Disorders. 2001;3:106–153. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Shih VH, Green MF, Gitlin M, Sokolski KN, Levander E, Marusak S, Hammen C, Sugar CA, Altshuler LL. The impact of neurocognitive impairment on occupational recovery of clinically stable patients with bipolar disorder : a prospective study. Bipolar Disorders. 2011;13:323–333. doi: 10.1111/j.1399-5618.2011.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Conus P, Kapczinski F, Andreazza AC, Yücel M, Wood SJ, Pantelis C, Malhi GS, Dodd S, Bechdolf A, Amminger GP, Hickie IB, Mcgorry PD. From neuroprogression to neuroprotection : implications for clinical care. Medical Journal of Australia. 2010;193:S36–S40. [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, Aizenstein HJ, Raina KD, Dekosky ST, Reynolds CF., III Patterns of mild cognitive impairment after treatment of depression in the elderly. American Journal of Geriatric Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Cognitive impairment in affective psychoses : a meta-analysis. Schizophrenia Bulletin. 2010;36:112–125. doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, Mcgrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders : a comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley and Sons Ltd; Chichester: 2006. [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, III, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Whatmough C, Saumier D, Duong A. Cognitive neuroscience studies of semantic memory in Alzheimer's disease. In Essence of Memory. In: Sossin WS, Lacaille J-C, Castellucci VF, Belleville S, editors. Progress in Brain Research. vol. 169. Elsevier; Amsterdam; 2008. pp. 393–407. Chapter 25. [DOI] [PubMed] [Google Scholar]
- Delaloye C, Moy G, de Bilbao F, Weber K, Baudois S, Haller S, Xekardaki A, Canuto A, Giardini U, Lövblad KO, Gold G, Giannakopoulos P. Longitudinal analysis of cognitive performances and structural brain changes in late-life bipolar disorder. International Journal of Geriatric Psychiatry. 2011;26:1309–1318. doi: 10.1002/gps.2683. [DOI] [PubMed] [Google Scholar]
- Depp CA, Moore DJ, Sitzer D, Palmer BW, Eyler LT, Roesch S, Lebowitz BD, Jeste DV. Neurocognitive impairment in middle-aged and older adults with bipolar disorder : comparison to schizophrenia and normal comparison subjects. Journal of Affective Disorders. 2007;101:201–209. doi: 10.1016/j.jad.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Savla GN, Moore DJ, Palmer BW, Stricker JL, Lebowitz BD, Jeste DV. Short-term course of neuropsychological abilities in middle-aged and older adults with bipolar disorder. Bipolar Disorders. 2008;10:684–690. doi: 10.1111/j.1399-5618.2008.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra U, Rabins PV. Mania in the elderly : a 5–7 year follow-up. Journal of the American Geriatrics Society. 1991;39:581–583. doi: 10.1111/j.1532-5415.1991.tb03597.x. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Chisholm D, Anderson SJ, Begley A, Holm M, Rogers JC, Reynolds CF, 3rd, Mulsant BH. Cognition in older adults with bipolar disorder versus major depressive disorder. Bipolar Disorders. 2012;14:198–205. doi: 10.1111/j.1399-5618.2012.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Chisholm D, Rogers JC, Holm MB, Bhalla RK, Seligman K, Dew MA, Reynolds CF, 3rd, Kupfer DJ, Mulsant BH. Cognitive functioning and instrumental activities of daily living in late-life bipolar disorder. American Journal of Geriatric Psychiatry. 2007;15:174–179. doi: 10.1097/JGP.0b013e31802dd367. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Mulsant BH, Begley A, Mazumdar S, Hyams AV, Reynolds CF, III, Kupfer DJ, Butters MA. The longitudinal course of cognition in older adults with bipolar disorder. Bipolar Disorders. 2009;11:744–752. doi: 10.1111/j.1399-5618.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Mulsant BH, Begley AE, McShea M, Stack JA, Miller MD, Fagiolini A, Kupfer DJ, Young RC, Reynolds CF., III A pilot study of standardized treatment in geriatric bipolar disorder. American Journal of Geriatric Psychiatry. 2005;13:319–323. doi: 10.1176/appi.ajgp.13.4.319. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Whyte EM, Drayer RA, Soreca I, Fagiolini A, Kilbourne AM, Houck PR, Reynolds CF, 3rd, Frank E, Kupfer DJ, Mulsant BH. Medical burden in late-life bipolar and major depressive disorders. American Journal of Geriatric Psychiatry. 2008;16:194–200. doi: 10.1097/JGP.0b013e318157c5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso C, Sibille E. Between destiny and disease : genetics and molecular pathways of human central nervous system aging. Progress in Neurobiology. 2011;93:165–181. doi: 10.1016/j.pneurobio.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Jack CR., Jr. Alzheimer disease : new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263:344–361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, Jin R, Merikangas KR, Simon GE, Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. American Journal of Psychiatry. 2006;163:1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press; Oxford and New York: 2004. [Google Scholar]
- Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disorders. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder : an update and investigation of moderator variables. Bipolar Disorders. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research : application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The Global Burden of Disease : A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- Reynolds CF, 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, Lenze EJ, Holm M, Rogers JC, Mazumdar S, Houck PR, Begley A, Anderson S, Karp JF, Miller MD, Whyte EM, Stack J, Gildengers A, Szanto K, Bensasi S, Kaufer DI, Kamboh MI, Dekosky ST. Maintenance treatment of depression in old age : a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Archives of General Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rogers JC, Holm MB. Daily living skills and habits of older women with depression. Occupational Therapy Journal of Research. 2000;20:68S–85S. [Google Scholar]
- Salmon DP. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Current Topics in Behavioral Neurosciences. 2012;10:187–212. doi: 10.1007/7854_2011_171. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of International Neuropsychological Society. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder : a critical opinion. Bipolar Disorders. 2005;7:216–235. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve ? Theory and research application of the reserve concept. Journal of the International Neuropsychology Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(Suppl. 2):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatrica Scandinavica Supplementum. 2007;434:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]