Abstract
Background
The greatest burden of Group A streptococcal (GAS) disease worldwide is due to acute rheumatic fever (ARF) and rheumatic heart disease (RHD). Safe, effective and affordable vaccines designed to prevent GAS infections that trigger ARF could reduce the overall global morbidity and mortality from RHD. The current study evaluated the potential coverage of a new 30-valent M protein-based vaccine using GAS isolates from school children in Bamako, Mali, a population at high risk for the development of RHD.
Methods
The bactericidal activity of rabbit antisera against the 30-valent vaccine was assessed using a collection of GAS isolates recovered during a study of the epidemiology of pharyngitis in Bamako.
Results
Single isolates representing 42 of 67 emm-types, accounting for 85% of the GAS infections during the study, were evaluated. All (14/14) of the vaccine emm-types in the collection were opsonized (bactericidal killing >50%) and 26/28 non-vaccine types were opsonized. Bactericidal activity was observed against 60% of the total emm-types recovered in Bamako, which accounted for 81% of all infections.
Conclusions
Multivalent vaccines comprised of N-terminal M peptides elicit bactericidal antibodies against a broad range of GAS serotypes, indicating that their efficacy may extend beyond the emm-types included in the vaccine.
Keywords: group A streptococcal vaccine, M protein, bactericidal activity, vaccine coverage
1. Introduction
Group A streptococci (GAS) cause a broad spectrum of diseases worldwide that ranges from uncomplicated pharyngitis and skin infections to life-threatening invasive infections that include pneumonia, bacteremia, necrotizing fasciitis, streptococcal toxic shock syndrome, as well as the immune-mediated nonsuppurative sequelae of acute rheumatic fever (ARF), rheumatic heart disease (RHD), and glomerulonephritis. The greatest burden of GAS disease, as defined by morbidity and mortality, is due to RHD in low- and middle-income countries. Minimum estimates suggest that RHD affects 15 million individuals resulting in ~230,000 deaths each year [1]. However, as more data become available from low-income countries [2-4], the true burden of RHD is becoming clearer and it appears that the disease burden outlined previously is considerably underestimated.
Current strategies for the prevention of ARF and RHD rely on antibiotic treatment of symptomatic pharyngitis (primary prevention) or continuous administration of antibiotics to individuals with documented ARF (secondary prevention). These prevention strategies are costly and, for the most part, are ineffective in resource-poor countries where 90% of the disease burden exists [5]. The development of a safe, effective and affordable vaccine designed to prevent the GAS infections that trigger ARF/RHD has long been considered an attractive approach. Vaccine development has faced obstacles related to the complex epidemiology of GAS infections, especially in developing countries [6], and safety concerns based on the theoretical possibility that the vaccines may elicit autoimmune responses that could mimic ARF or other sequelae of GAS infections [7].
Significant progress has been made recently in the design and clinical development of complex multivalent M protein-based vaccines [8-10]. The surface M protein is a major virulence determinant of GAS that contains opsonic epitopes [11]. M protein antibodies that are produced following natural infection [12] or vaccination [9] are bactericidal in human blood. Bactericidal antibodies have been associated with protection against infection with the homologous serotype of GAS [13]. A number of structure-function studies have revealed that the N-terminal peptides of M proteins contain epitopes that evoke antibodies with the greatest bactericidal activity and are least likely to cross-react with human tissue antigens [7]. This observation has been the basis for the design of complex recombinant hybrid vaccine proteins containing opsonic epitopes representing M proteins from multiple serotypes of GAS [8, 10].
We have previously shown that a 26-valent vaccine containing M peptides from serotypes of GAS representing the vast majority of infections in the USA and Canada [14] was safe and immunogenic in adult volunteers [9]. In pre-clinical and clinical studies, the vaccine evoked bactericidal antibodies against all of the vaccine serotypes of GAS [9]. Recently, we constructed a 30-valent vaccine whose composition was based on more extensive surveillance data from the USA and Europe [15-17]. In animal studies, the 30-valent vaccine evoked bactericidal antibodies against all of the vaccine serotypes of GAS [10]. In addition, significant bactericidal activity was observed against a number of laboratory strains representing non-vaccine serotypes of GAS, suggesting that the potential efficacy of this vaccine may extend beyond those serotypes represented in the vaccine [10]. This observation is potentially significant because the predicted coverage of the previous 26-valent vaccine in resource-poor countries is low compared to economically developed countries [6].
In the current study, we have evaluated the bactericidal antibody activity of the 30-valent vaccine antisera raised in rabbits using a collection of pharyngeal isolates of GAS obtained from symptomatic school children in Bamako, Mali [18]. The overall goal was to assess the potential coverage of the new vaccine in a sub-Saharan African population at high risk for developing ARF and RHD and where the epidemiology and emm-type prevalence of GAS infections is very different from that observed in the USA, Canada or Europe [15-17].
2. Materials and Methods
Immunization of rabbits with the 30-valent vaccine
Three New Zealand white rabbits were immunized with 800μg doses of the 30-valent vaccine proteins adsorbed to alum at time 0, 4 weeks and 8 weeks, as previously reported [10]. Serum was obtained prior to the first injection and at 2 weeks following the last injection. The antibody titers against the M peptides contained in the vaccine and functional bactericidal activity of the antisera against all of the vaccine serotypes of GAS have previously been published [10]. Animal protocols were approved by the Institutional Animal Care and Use Committees of the University of Tennessee Health Science Center and the Veterans Affairs Medical Center, Memphis, TN.
Clinical isolates of GAS from school children in Bamako
The clinical isolates of GAS used in this report were obtained during a comprehensive assessment of the molecular epidemiology of symptomatic pharyngitis in school children in Bamako, Mali conducted during 2006-2009 [18]. Briefly, children ranging from five to 16 years of age attending any of four participating schools in Djikoroni-para and Sebenikoro urban neighborhoods in Commune 4 of Bamako were eligible.
Students who presented to the school infirmary or school-associated health center complaining of sore throat were enrolled. Upon presentation, study personnel confirmed that the student was experiencing throat pain, then symptoms of pharyngitis were obtained and a brief focal examination was performed. Throat culture swabs were placed in Amies charcoal media (CultureSwab™, Becton Dickinson) and transported to the microbiology laboratory at the Center for Vaccine Development-Mali at ambient temperature within six hours. Upon arrival in the laboratory, swabs were streaked onto 5% sheep’s blood agar, a bacitracin disk (0.04 U) was placed, and the plate was incubated at 35-37°C. GAS were beta-hemolytic, catalase-negative, bacitracin-sensitive, Gram-positive cocci in pairs and chains that tested positive for group A streptococcal antigen on agglutination test. Bacterial isolates were stored at −80°C in trypticase soy broth with 15% glycerol. All human subjects protocols were approved by the Ethics Committee of the Faculte de Medecine, Pharmacie et Odontostomatologie in Bamako, Mali, and the respective Institutional Review Boards of the University of Maryland School of Medicine, the University of Tennessee Health Science Center, and the Memphis Veterans Affairs Medical Center.
Emm-typing
DNA from the GAS isolates was extracted and the 5′ portion of the emm gene was amplified using standard methodology developed at the Centers for Disease Control, Atlanta, GA, USA [19]. The amplified DNA product was sent to the University of Maryland Biopolymer Laboratory for sequencing. The nucleotide sequence was then compared using a standard FASTA alignment algorithm against known emm gene sequences on the CDC website and an emm-type and sub-type were assigned.
Indirect bactericidal tests
Bactericidal assays were performed as previously described [20]. Briefly, 0.05 ml of Todd-Hewitt broth containing bacteria (~50-200 CFU) was added to 0.1 ml of preimmune or immune serum and 0.35 ml of lightly heparinized non-immune human blood and the mixture was rotated for three hours at 37!C. Then 0·1 ml of this mixture was diluted 10- and 100-fold and 0.1 ml aliquots were added to melted sheep’s blood agar. Pour plates were prepared and viable organisms (CFU) were counted after overnight incubation at 37!C. The results were expressed as percent killing, which was calculated using the following formula: [(CFU after three hours growth with preimmune serum – CFU after three hours growth with immune serum) ! CFU after three hours growth with preimmune serum] × 100. For each assay, the CFU added to the test mixtures was determined and only those assays that resulted in growth of the test strain to at least five generations in the presence of preimmune serum were used to express percent killing in the presence of immune serum.
Emm sequence analyses
Primary structural homologies among emm sequences contained in the 30-valent vaccine and the emm sequences of non-vaccine types were identified using the Swiss Institute of Bioinformatics SIM alignment program [21]. The translated sequences corresponding to the N-terminal 50 amino acid residues of the mature M proteins [22] were individually aligned with the complete sequences of the 30-valent vaccine proteins using the comparison matrix PAM40, a gap open penalty of 12 and a gap extension penalty of 4. The alignments resulting in the top three scores were analyzed for their position in the 30-valent vaccine proteins and percent identity was recorded.
3. Results
During the epidemiologic study in Bamako, a total of 372 GAS were recovered from throat cultures of enrolled subjects, of which 305 were available for analysis in this report. A complete description of the incidence of GAS pharyngitis, the molecular epidemiology of the infections and the prevalence of specific emm-types and sub-types recovered is published elsewhere [18]. The goal of this study was to determine the potential efficacy of a new 30-valent M protein-based vaccine by assessing the functional activity of vaccine antiserum using isolates of GAS recovered from infected children in Mali. The GAS isolates analyzed in this study contained a total of 67 emm-types, 18 of which were emm-types included in the vaccine (VTs) and 49 that were non-vaccine emm-types (NVTs) (Table 1). Single clinical isolates of GAS representing 42 of the most common emm-types recovered (14 VTs and 28 NVTs) were obtained from the collection in Bamako to use in bactericidal assays.
Table 1.
Summary of GAS emm-types and functional bactericidal assays
| Variable | No. of emm- types (%) |
No. of Isolates (%)a |
|---|---|---|
| Total emm-types and isolates in the collection | 67 (100) | 305(100) |
| Vaccine emm-types in the collection | 18 (27) | 113 (37) |
| Non-vaccine emm-types in the collection | 49 (73) | 192 (63) |
| Vaccine emm-types tested | 14 (21) | 102 (33) |
| Vaccine emm-types killed >50% | 14 (21) | 102(33) |
| Non-vaccine emm-types tested | 28 (42) | 159(52) |
| Non-vaccine emm-types killed >50% | 26 (39) | 144 (47) |
| Total emm-types killed >50% | 40 (60) | 246 (81) |
| Total emm-types killed + vaccine types not testedb | 44 (66) | 257(84) |
| Non-vaccine emm-types killed among non-vaccine types tested | 26/28 (93) | |
| Total emm-types killed among total emm types tested | 40/42 (95) |
The number isolates in the collection represented by the various emm-types. The number and percent killed have been extrapolated based on results from the emm-types tested.
The four vaccine emm-types present in the collection that were not tested were previously shown to be opsonized by the 30-valent vaccine antisera [10].
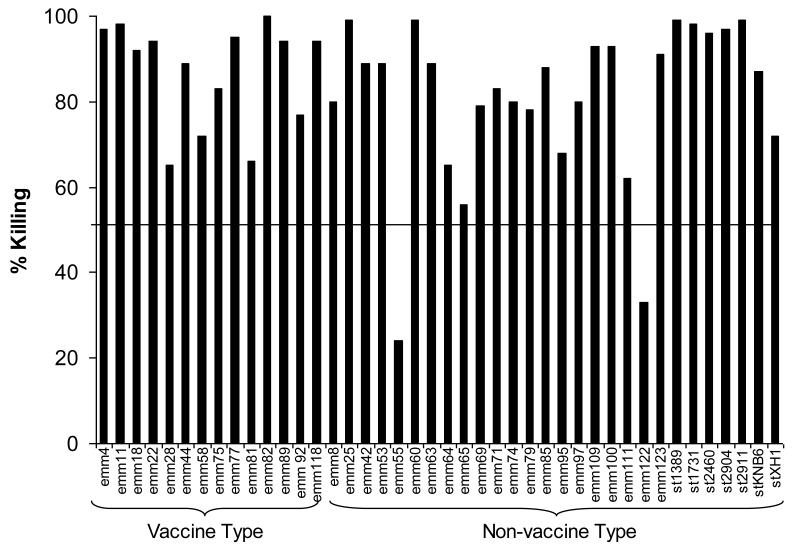
Indirect bactericidal assays were performed using a single rabbit antiserum raised against the 30-valent vaccine [10]. Bactericidal activity was demonstrated against all14 VTs tested (mean, 87%; range 65-100%) (Fig. 1). Of the NVTs tested, 26 of 28 (93%) were killed at a level >50% (overall mean 81%, range 24-99%). The total number of emm-types killed among the total number tested was 40/42 (95%) (Table 1). Among the 42 emm-types present in the isolates studied in bactericidal assays, 10 were represented by 2 emm alleles (subtypes) and 1 was represented by 3 alleles. The remainder contained emm genes of the same allele (data not shown). Of the 14 VTs tested, 7 of the isolates contained emm genes that differed in sequence from the subtype represented in the vaccine and all were opsonized by the vaccine antisera (Fig. 1).
Figure 1.
Indirect bactericidal activity of 30-valent GAS vaccine rabbit antiserum against selected isolates of GAS recovered from symptomatic school children in Bamako, Mali. Indirect bactericidal tests were performed using non-immune human blood mixed with the test isolate and either pre-immune serum or 30-valent vaccine antiserum. Bactericidal killing was calculated as described in the text.
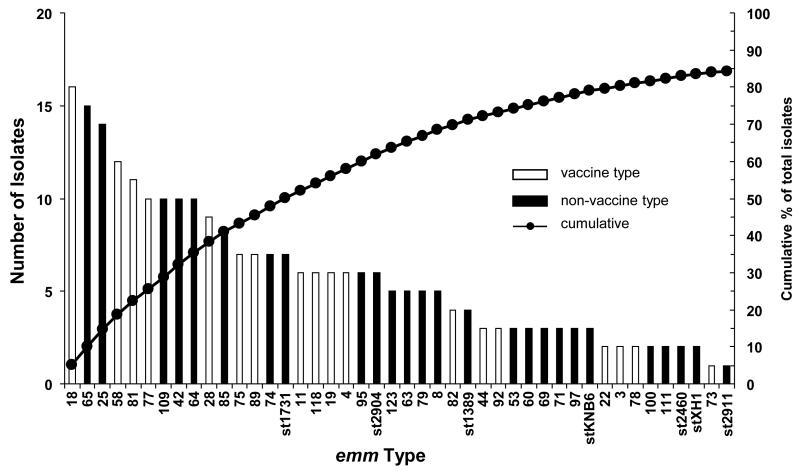
The potential (theoretical) efficacy of the 30-valent vaccine in a specific population can be estimated using the prevalence of specific emm-types within a relatively large sample of GAS isolated from symptomatic children in combination with the results of functional in vitro assays that have been associated with protection in animal models [23] or humans [13]. Therefore, we analyzed the results of bactericidal assays in relation to the emm-types present in the collection of 305 GAS isolates (Fig. 2). The vaccine antisera resulted in >50% bactericidal killing of emm-types accounting for 84% of the infections in this cohort of children. Included in this assessment are all 18 of the VTs, four of which were not available from the Mali collection but were tested previously [10], and the 26 NVTs that were opsonized by the antisera. These results, which are based on bactericidal killing of NVTs, is in distinct contrast to the potential of preventing 37% of infections caused by only those emm-types represented in the vaccine (Table 1).
Figure 2.
Summary of GAS emm-types that showed >50% bactericidal killing in the presence of 30-valent vaccine antisera. Non-vaccine emm-types that were cross-opsonized by the rabbit antisera (solid bars) accounted for 47% of the total GAS in the collection. Together with vaccine emm-types in the collection (open bars), potential coverage of the vaccine could reach 84% of the total GAS recovered (solid circles).
One explanation for the degree of bactericidal activity observed with the 30-vaccine antisera against NVTs is the potential presence of shared opsonic epitopes among M proteins of GAS from different emm-types. To assess this possibility, we performed sequence alignments of the translated sequences of the four proteins contained in the 30-valent vaccine and the N-terminal 50 amino acids of the mature M proteins from each NVT that was cross-opsonized (Table 2). In many cases there appeared to be significant sequence identity with one or more of the vaccine M peptide sequences, which could account for the cross-reactive bactericidal activity observed against some NVTs. However, in some cases the sequence identity was lower than might be predicted to result in cross-reactive antibody binding to M epitopes.
Table 2.
Protein sequence identities between the 26 non-vaccine emm-types that were opsonized and 30-valent vaccine proteins.a
| Non- vaccine emm-type |
30-Valent Vaccine Sequence Identity |
% Identity |
30-Valent Vaccine Sequence Identity |
% Identity |
30-Valent Vaccine Sequence Identity |
% Identity |
|---|---|---|---|---|---|---|
| 25.1 | Emm58.0 (2-37)b | 50 | Emm87.0 (1-33) | 52.9 | Emm82.0 (1-38) | 41 |
| 65.0 | Emm44.0 (4-40) | 75.7 | Emm11.0 (16-50) | 54.1 | Emm118.0 (17-48) | 43.8 |
| 109.1 | Emm28.0 (10-50) | 72.7 | Emm22.0 (19-50) | 90.6 | Emm4.0 (13-50) | 50 |
| 42.0 | Emm11.0 (20-50) | 71.9 | Emm44.0 (11-46) | 56.8 | Emm81.0 (12-35) | 58.3 |
| 64.4 | Emm83.1 (2-48) | 55.3 | Emm24.0 (23-33) | 54.5 | ||
| 74.0 | Emm14.3 (3-49) | 55.3 | Emm29.2 (3-43) | 39 | Emm19.0 (38-50) | 46.2 |
| 85.1 | Emm11.0 (6-50) | 87.5 | Emm44.0 (10-46) | 59.5 | Emm81.0 (12-44) | 48.5 |
| 95.0 | Emm82.0 (22-29) | 75 | ||||
| 123.0 | Emm83.1 (30-48) | 73.7 | Emm4.0 (10-32) | 56.5 | Emm29.2 (23-40) | 55.6 |
| st1731.1 | Emm78.0 (5-50) | 68.8 | Emm22.0 (1-50) | 51.1 | Emm4.0 (19-50) | 54.5 |
| st2904.2 | Emm118.0 (24-50) | 76.9 | Emm49.0 (29-50) | 73.9 | Emm44.0 (14-41) | 46.4 |
| 8.0 | Emm78.0 (1-50) | 66 | Emm4.0 (42-49) | 87.5 | Emm78.0 (15-27) | 53.8 |
| 63.0 | Emm4.0 (18-37) | 57.9 | Emm44.0 (16-47) | 45.2 | Emm11.0 (21-50) | 45.2 |
| 79.0 | Emm87.0 (4-50) | 70.2 | Emm58.0 (3-40) | 57.9 | Emm82.0 (4-41) | 42.1 |
| 97.1 | Emm3. 1 (41-50) | 70 | Emm114.0 (28-34) | 71.4 | ||
| stKNB.6 | Emm49.0 (8-50) | 97.7 | Emm118.0 (8-50) | 78.7 | Emm89.0 (15-41) | 55.6 |
| st1389.0 | Emm22.0 (31-37) | 62.5 | Emm58.0 (10-17) | 62.5 | Emm3. 1 (45-48) | 100 |
| 69.1 | Emm44.0 (3-39) | 75.7 | Emm11.0 (16-50) | 54.1 | Emm118.0 (17-48) | 43.8 |
| 71.1 | Emm5.14 (1-25) | 53.8 | Emm18.0 (28-50) | 59.1 | ||
| 53.4 | Emm83.1 (30-50) | 76.2 | Emm18.0 (31-42) | 66.7 | Emm29.2 (23-39) | 47.1 |
| 60.0 | Emm78.0 (5-38) | 58.8 | Emm22.0 (1-31) | 68.8 | Emm89.0 (25-37) | 69.2 |
| 100.2 | Emm18.0 (4-46) | 44.2 | Emm24.0 (1-50) | 40.8 | Emm18.0 (30-50) | 56.5 |
| 111.0 | Emm83.1 (32-41) | 50 | Emm22.0 (24-34) | 54.5 | Emm19.0 (36-45) | 60 |
| st2460.0 | Emm58.0 (27-50) | 75 | Emm83.1 (31-40) | 80 | Emm58.0 (29-50) | 40.9 |
| stXH1.0 | Emm11.0 (24-37) | 92.9 | Emm92.0 (10-46) | 51.4 | Emm44.0 (9-26) | 66.7 |
| st2911.0 | Emm18.0 (40-49) | 60 | Emm83.1 (18-40) | 43.5 | Emm4.0 (21-28) | 75 |
Sequence identities for regions of vaccine peptides of fewer than seven consecutive amino acids were not tabulated, thus some fields are blank.
Emm designates the peptide fragment within the 30-valent protein that shares identity with the N-terminal 50 amino acid sequence of the M protein from the non-vaccine emm-type. The numbers in parentheses indicate the amino acid sequence locations within the peptides that exhibit the sequence identities.
4. Discussion
GAS diseases, especially ARF and RHD, cause significant morbidity and mortality throughout the world [1]. Vaccine prevention of the infections that trigger rheumatic fever would represent a new strategy that would not require the additional community health infrastructure necessary to implement and maintain programs using antibiotic prevention. Although there are several GAS vaccine candidates in various stages of clinical and pre-clinical development [24], the best evidence developed over the longest period of time supports the use of M protein peptides to elicit protective immunity [11]. The major practical obstacle in the development of a single vaccine that could be deployed to all geographic regions has been the complexity of the epidemiology of GAS infections in low- and moderate-income countries [6]. Using straightforward analyses of potential vaccine coverage based only on the M serotypes included in the vaccines compared to the serotypes of GAS reported from various geographic locations, the multivalent vaccines have been declared sub-optimal in many parts of the world [6]. In the present study we have demonstrated that the 30-valent vaccine evoked bactericidal antibodies against a significant number of NVTs of GAS. If vaccine serotypes only were used to predict the utility of the 30-valent vaccine in Bamako, the coverage would be approximately 37% of all pharyngeal infections. However, when functional bactericidal activity was included in the analysis, the potential coverage increased to 84% of infections and 66% of all emm-types isolated. Of note is that not all isolates from Bamako were assessed in functional assays because we selected those that were recovered most frequently for this study. Of the NVTs that were evaluated, bactericidal killing of >50% was observed with 93% of the isolates.
The degree of bactericidal activity against NVTs of GAS observed in this study was not totally unanticipated considering sequence similarities in the N-terminal regions of the >200 emm-types, which excludes additional unverified sequence types, now listed by the Streptococcal Laboratory of the Centers for Disease Control [22]. The N-terminal regions of the M proteins have historically been shown to contain “type-specific” epitopes, as originally defined by Lancefield [11]. M typing antisera were used for decades in immunoprecipitation reactions to define the serotype of the M protein expressed by a particular isolate of GAS. The typing antisera were absorbed with various heterologous serotypes of GAS to ensure type-specific precipitation of the unknown M protein. The current practice of assigning an emm-type based on the 5′ sequence of the emm gene effectively differentiates one isolate from another in epidemiologic studies but none of the new emm-types has been studied to differentiate them based on serologic similarities or differences. We believe that the structural similarities among M proteins, especially the newer emm-types, may at least partially account for the range of cross-reactive bactericidal antibodies observed in this study.
5. Conclusion
Taken together with our previous report [10], the 30-valent vaccine evoked antibodies with bactericidal activity against a total of 72 emm-types of GAS, which includes the 30 VTs and 42 NVTs. These results suggest that vaccine coverage against pharyngitis isolates of GAS could extend well beyond the emm-types contained in the vaccine. Future studies to determine the overall potential coverage of the vaccine will require functional assays using a comprehensive collection of GAS from diverse regions of the world. The true efficacy of any GAS vaccine will ultimately be defined by carefully designed clinical trials in different geographic locations. The results of such trials will indicate whether a multivalent M protein-based vaccine can be designed for high-income, as well as low- and moderate-income countries that will have a significant impact on the overall incidence of GAS infections and their sequelae.
Highlights.
-
!!
Multivalent group A streptococcal vaccine
-
!!
Extended vaccine coverage to include non-vaccine serotypes of group A streptococci
-
!!
Potential protection in a population at high risk for RHD
-
!!
Potential for vaccine prevention of GAS infections that may trigger acute rheumatic fever
Acknowledgements
This work was supported by USPHS NIH grants U01AI060592 (J.B.D.) and RO1AI10085 (J.B.D.). The authors thank Deborah Bueltemann for preparation of the manuscript and figures and Drs. Harry Courtney and David Hasty for their critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Authors Contributions
J.B.D. was the primary author of the manuscript, supervised the laboratory-based studies at the University of Tennessee and was responsible for the overall content of the manuscript. T.A.P. performed and supervised the performance of the bactericidal tests, performed and analyzed the emm sequence designations, and contributed to the writing and editing of the manuscript.
B.T. was responsible for the isolation and identification of GAS isolates obtained from subjects enrolled in the clinical study in Bamako and the isolation of genomic DNA and preparation of PCR fragments for sequencing.
S.O.S., M.T. and K.L.K. were responsible for the conduct of the clinical study in Bamako and contributed to the writing of the manuscript.
J.P.N. was responsible for emm sequencing and initial assignments of emm-types in the collection.
Disclosure Statement
Conflicts of Interests
J.B.D. is the inventor of certain technologies related to the development of GAS vaccines. The University of Tennessee Research Corporation has licensed the technology to Vaxent, LLC.
J.B.D. serves as the Chief Scientific Officer of Vaxent.
All other authors disclose no financial or personal potential conflicts.
References
- [1].Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. The Lancet infectious diseases. 2005;5(11):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- [2].Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357(5):470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- [3].Paar JA, Berrios NM, Rose JD, Caceres M, Pena R, Perez W, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Card. 2010;105(12):1809–14. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 2012;125(25):3127–32. doi: 10.1161/CIRCULATIONAHA.112.092312. [DOI] [PubMed] [Google Scholar]
- [5].Jackson SJ, Steer AC, Campbell H. Systematic Review: Estimation of global burden of non-suppurative sequelae of upper respiratory tract infection: rheumatic fever and post-streptococcal glomerulonephritis. Trop Med Int Health. 2011;16(1):2–11. doi: 10.1111/j.1365-3156.2010.02670.x. [DOI] [PubMed] [Google Scholar]
- [6].Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm-type distribution of group A streptococci: systematic review and implications for vaccine development. The Lancet infectious diseases. 2009;9(10):611–6. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- [7].Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- [8].Hu M, Walls M, Stroop S, Reddish M, Beall B, Dale J. Immunogenicity of a 26-Valent Group A Streptococcal Vaccine. Infect Immun. 2002;70:2171–7. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, et al. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41(8):1114–22. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- [10].Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29(46):8175–8. doi: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lancefield RC. Current knowledge of the type specific M antigens of group A streptococci. J Immunol. 1962;89:307–13. [PubMed] [Google Scholar]
- [12].Lancefield RC. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959;110:271–92. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wannamaker LW. The epidemiology of streptococcal infection. Columbia University Press; New York: 1954. [Google Scholar]
- [14].Shulman ST, Tanz RR, Kabat W, Kabat K, Cederlund E, Patel D, et al. Group A streptococcal pharyngitis serotype surveillance in North America, 2000-2002. Clin Infect Dis. 2004;39(3):325–32. doi: 10.1086/421949. [DOI] [PubMed] [Google Scholar]
- [15].Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, et al. Seven-year surveillance of north american pediatric group a streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49(1):78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]
- [16].O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853–62. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- [17].Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47(4):1155–65. doi: 10.1128/JCM.02155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tapia M, Sow SO, Tamboura B, Nataro JP, Penfound TA, Dale JB, et al. Molecular epidemiology of streptococcal pharyngitis in Bamako, Mali. Manuscript submitted. 2012 [Google Scholar]
- [19].Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34(4):953–8. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lancefield RC. Differentiation of group A streptococci with a common R antigen into three serologic types with special reference to the bactericidal test. J Exp Med. 1957;106:525–44. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Swiss Institute of Bioinformatics SIM Alignment Program. 2012 Aug 23; cited. Available from: http://web.expasy.org/sim.
- [22].CDC Streptococcal Reference Page. 2012 Aug 23; cited. Available from: ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tstransl/
- [23].Penfound T, Chiang E, Ahmed E, Dale J. Protective efficacy of group A streptococcal vaccines containing type-specific and conserved M protein epitopes. Vaccine. 2010;28(31):5017–22. doi: 10.1016/j.vaccine.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dale JB, Fischetti VA, Carapetis JR, Steer AC, Sow SO, Kumar R, et al. Group A streptococcal vaccines: paving a path for accelerated development. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.09.045. In-press. [DOI] [PubMed] [Google Scholar]