Abstract
Although maternal cigarette smoking during pregnancy is a well-documented risk factor for a variety of adverse pregnancy outcomes, how prenatal cigarette smoke exposure affects postnatal neurobehavioral/cognitive development remains poorly defined. In order to investigate the cause of an altered behavioral phenotype, mice developmentally exposed to a paradigm of ‘active’ maternal cigarette smoke is needed. Accordingly, cigarette smoke exposed (CSE) and air-exposed C57BL/6J mice were treated for 6 h per day in paired inhalation chambers throughout gestation and lactation and were tested for neurobehavioral effects while controlling for litter effects. CSE mice exhibited less than normal anxiety in the elevated zero maze, transient hypoactivity during a 1 h locomotor activity test, had longer latencies on the last day of cued Morris water maze testing, impaired hidden platform learning in the Morris water maze during acquisition, reversal, and shift trials, and impaired retention for platform location on probe trials after reversal but not after acquisition or shift. CSE mice also showed a sexually dimorphic response in central zone locomotion to a methamphetamine challenge (males under-responded and females over-responded), and showed reduced anxiety in the light-dark test by spending more time on the light side. No differences on tests of marble burying, acoustic startle response with prepulse inhibition, Cincinnati water maze, matching-to-sample Morris water maze, conditioned fear, forced swim, or MK-801-induced locomotor activation were found. Collectively, the data indicate that developmental cigarette smoke exposure induces subnormal anxiety in a novel environment, impairs spatial learning and reference memory while sparing other behaviors (route-based learning, fear conditioning, and forced swim immobility). The findings add support to mounting evidence that developmental cigarette smoke exposure has long-term adverse effects on brain function.
Keywords: behavior, cigarette smoke, tobacco, inhalation exposure, pregnancy, prenatal
1. Introduction
In the United States alone, approximately one million infants are exposed prenatally to cigarette smoke each year (Centers for Disease Control and Prevention 2004;Centers for Disease Control and Prevention 2006;Mathews 2001). Despite extensive national publicity regarding the detrimental intrauterine effects of tobacco use, cigarette smoking continues in approximately 16.3% of all pregnancies in the U.S. (SAMSA 2011). Nationwide, this percentage escalates to nearly 50% for women in lower socioeconomic populations (Cnattingius 2004;Cornelius et al. 1999). Moreover, compared with alcohol, marijuana, and other illicit drug use, tobacco use is less likely to decline as pregnancy progresses (Cornelius et al. 1999;Substance Abuse and Mental Health Services Administration 2000). Maternal cigarette smoking during pregnancy has been associated with a myriad of adverse pregnancy outcomes including a higher incidence of spontaneous abortions, placental abruption, reduced birth weight, perinatal lethality, and sporadic congenital malformations (Abbott and Winzer-Serhan 2012;Abel 1980;Centers for Disease Control and Prevention 2004;Mitchell et al. 2002;Zdravkovic et al. 2005). Additional abnormal developmental outcomes evident in infancy and childhood include Sudden Infant Death Syndrome (SIDS), and an increased incidence of acute upper respiratory infections, middle ear problems, bronchitis, pneumonia, asthma, and neurobehavioral changes (Burke et al. 2012;Centers for Disease Control and Prevention 2004;Centers for Disease Control and Prevention 2006;Cooke 1998;Joad 2000;Ostfeld et al. 2010).
While the above-noted risks of smoking during pregnancy have been well documented, the long-term effects of prenatal cigarette smoke exposure on neurodevelopmental and behavioral outcomes are still emerging and in this area animal models may be especially helpful. Human epidemiological studies and clinical case reports suggest that offspring of women who actively smoked during pregnancy display a variety of behavioral abnormalities and cognitive deficits (DiFranza et al. 2004;Dwyer et al. 2008;Ernst et al. 2001;Olds 1997;Olds et al. 1994;Pauly and Slotkin 2008;Winzer-Serhan 2008). Such studies have reported associations between maternal cigarette smoking during pregnancy and altered behavioral/cognitive function in their offspring including attention deficits, hyperactivity and impulsivity (Heath and Picciotto 2009;Law et al. 2003;Leech et al. 1999;Milberger et al. 1996); perceptual deficits (Cornelius et al. 2001;Fried and Watkinson 2000;McCartney et al. 1994;Peck et al. 2010); altered learning and memory (Anderko et al. 2010;Kafouri et al. 2009;Knopik 2009;O’Callaghan et al. 2010); lowered IQ and impaired intellectual development (Batty et al. 2006;Braun et al. 2009;Olds 1997;Rahu et al. 2010), and an increased propensity for conduct, behavioral, or psychological disorders (Batstra et al. 2003;Cornelius et al. 2012;Day et al. 2000;Ernst et al. 2001;Wakschlag et al. 2002). However, limitations in study design, including small sample sizes, uncontrolled variables, and interpretational difficulties associated with retrospective data have resulted in ambiguities regarding these linkages. In fact, while the U.S. Surgeon General has identified pregnant women as an especially vulnerable “at risk” population with regard to the adverse effects of smoke exposure on their unborn (Centers for Disease Control and Prevention 2004), the Surgeon General’s 2004 and 2006 Reports on the health consequences of ‘active’ smoking and involuntary smoke exposure determined that the evidence to date, although compelling, was inadequate to infer a causal relationship between maternal smoking and neurobehavioral or cognitive deficits in infants and children (Centers for Disease Control and Prevention 2004;Centers for Disease Control and Prevention 2006).
Animal studies focused on the toxicity of cigarette smoke, including examination of effects on neurodevelopment, behavior and cognition, are numerous, however the vast preponderance of these have examined individual components of cigarette smoke such as carbon monoxide and/or nicotine. As a result, animal studies examining the effects of actual inhalation exposure of cigarette smoke on neurodevelopmental endpoints are generally lacking. As cigarette smoke contains a complex mixture of over 4,000 identifiable compounds and approximately 600 additives some of which are neurotoxic (nicotine, arsenic, lead) (California Environmental Protection Agency 1999), the interpretation of animal studies focusing on individual components of cigarette smoke in terms of the neurodevelopmental risks of human maternal cigarette smoking during pregnancy is constrained. Nevertheless, such investigations have highlighted some of the cellular and molecular underpinnings of cigarette smoke’s toxicity. These studies, primarily focusing on the developmental effects of nicotine in animal models have tested a variety of doses, routes of administration, species, times of exposure, and the developmental outcomes (cellular, molecular, electrophysiological or behavioral). The preponderance of data on mammalian animal models indicates that, when administered during embryofetal development, nicotine is a potent developmental neurotoxin with a range of effects on the central nervous system (Abbott and Winzer-Serhan 2012;Dwyer et al. 2008;Ernst et al. 2001;Levin and Slotkin 1998;Slikker, Jr. et al. 2005). Nicotine has been documented to elicit alterations in catecholaminergic, serotonergic, and GABAergic signaling systems, as well as alterations in nicotinic cholinergic receptor development in prenatally exposed offspring (Li et al. 2002;Slotkin 2004;Slotkin et al. 2007;van de Kamp and Collins 1994;Xu et al. 2001;Xu et al. 2002). The ramifications of such alterations extend beyond localized neurochemical changes, since alterations in the precise spatio-temporal profiles of neurotransmitter/receptor expression during pre- and postnatal development, have been suggested to disrupt the neurotrophic actions of these neurotransmitters, with resultant perturbations in the neuroanatomical and cytoarchitectural development of the brain. Indeed, studies examining the cellular and molecular mechanisms underlying adverse neurodevelopmental outcomes of prenatal nicotine exposure in animal models have noted effects on neurogenesis, neuronal migration, cytoarchitecture, and the physiology of neuronal circuitry in defined regions of the developing brain (Ernst et al. 2001;Muhammad et al. 2012;Santiago and Huffman 2012). Prenatal exposures to nicotine have been shown to perturb brain morphogenesis through excessive neuroepithelial cell apoptosis (Roy et al. 1998;Zhao and Reece 2005); elicit shortfalls in neuronal cell numbers through decreased cell proliferation or enhanced apoptosis (Jang et al. 2002;Navarro et al. 1989;Slotkin et al. 1986;Slotkin et al. 1997); alter cell size, packing density, and cortical thickness (Gospe, Jr. et al. 1996;Roy et al. 2002); promote abnormal gliosis at the expense of neurogenesis (Roy et al. 2002); and interfere with the development of neural circuitry (Abbott and Winzer-Serhan 2012;Slawecki and Ehlers 2002;Slawecki et al. 2000) — all in the developing brain.
Several animal studies, also have investigated neurobehavioral effects of maternal and offspring exposure to nicotine. Methods have included maternally implanted osmotic minipumps (Eppolito et al. 2010;Parameshwaran et al. 2012), direct nicotine injection to pups (MacPhail et al. 2007), or gavage to mother or pups (Lesage et al. 2006;Mitchell et al. 2012). These studies have shown that offspring express increased anxiety (Eppolito et al. 2010), changes in acoustic startle response (ASR) and open-field locomotor activity (Gaworski et al. 2004;MacPhail et al. 2007), greater immobility time in the forced swim test (Parameshwaran et al. 2012), and other effects. However, another study found no effects on open-field, ASR, or passive avoidance when each was tested at different ages (Gaworski et al. 2004).
While developmental nicotine experiments have been valuable, they do not capture the range of chemicals found in cigarette smoke. Moreover, animal paradigms using cigarette smoke more accurately model human exposure — whether it be developmental (Esposito et al. 2008;Gaworski et al. 2004) or adult (Harris et al. 2010). To investigate this issue, a mouse model simulating ‘active’ maternal smoking during pregnancy was developed (Esposito et al. 2008). Using a computerized cigarette smoke inhalation system, the paradigm exposes mice to a combination of mainstream and side-stream smoke, creating a more complete model of exposure to nicotine and the many other components of cigarette smoke (Esposito et al. 2008). The combination of side-stream and mainstream smoke is potentially important, since they do not have identical chemical profiles (Schick and Glantz 2005). The model induces lower birth weight and decreased fetal crown-rump length (Esposito et al. 2008;Horn et al. 2008;Mukhopadhyay et al. 2010). Previous studies with this model have also identified windows of developmental sensitivity (Esposito et al. 2008) and changes in gene expression in the hippocampus, including altered pathways for neurogenesis, serotonergic innervation, and proliferation/cell survival (Mukhopadhyay et al. 2010).
In light of previous research showing behavioral deficiencies in models of nicotine exposure (primarily in rats), the present study investigated the behavioral phenotype of a cigarette smoke inhalation exposure model in mice. Cigarette smoke-exposed (CSE) and air-exposed sham/Control mice were generated at the Birth Defects Center at the University of Louisville and transported to Cincinnati Children’s Research Foundation for neurobehavioral testing. For this experiment, mice were exposed from embryonic day (E) 0 to postnatal day (P) 21 to ensure that all phases of brain development — comparable to in utero brain development in humans — were included (Bayer et al. 1993;Clancy et al. 2001;Clancy et al. 2007a;Clancy et al. 2007b;Rice and Barone, Jr. 2000).
2. Materials and Methods
2.1 Animals
C57BL/6J mice were obtained from Jackson Labs (Bar Harbor, ME). All animals were housed in ventilated racks and were maintained at the University of Louisville’s Research Resources Center until transfer to Cincinnati Children’s for behavioral testing. Animals were retained in rooms with a 12-hour light/dark cycle at 20 ± 1°C and 50 ± 10% humidity with ad libitum access to food and water in both AAALAC-accredited vivaria. Treatment and testing procedures were approved by the Institutional Animal Use and Care Committees of the University of Louisville and Cincinnati Children’s Research Foundation. Handling and testing of mice conformed to the NIH Guide for the Care and Use of Laboratory Animals. Timed pregnancies were established by overnight mating of a single mature male with two nulliparous females. The presence of a vaginal plug was considered evidence of mating and the time considered E0.
2.2 Cigarette Smoke Inhalation Exposure Paradigm
The Teague TE-10C Smoke Inhalation Exposure System (Teague Enterprises; Davis, CA) is as previously described (Teague et al. 1994). It contains a microprocessor controlled cigarette-smoking instrument that delivers aged and diluted cigarette smoke of desired concentrations or ambient air to paired enclosed inhalation chambers. The instrument produces isolated side-stream smoke, or a combination of mainstream and side-stream smoke to simulate ‘passive’ or ‘active’ tobacco smoke exposures. For the present study, a mixture of aged and diluted cigarette smoke, simulating ‘active’ tobacco smoke exposure, was generated using Marlboro Red Class 20 A cigarettes (the brand most widely used by young adults in the United States) (SAMSA 2008). Each cigarette contains approximately 15 mg of tar and 1.1 mg of nicotine. The cigarettes were stored at 4°C until 48 h prior to use when they were placed in a closed chamber at 23°C and brought to a relative humidity of 60%. Cigarettes were smoked using the standard Federal Trade Commission method: a 2 s, 35 cubic cm puff, once per min for a total of nine puffs (Shopland 1997;Teague et al. 1994). Side-stream smoke generated from the smoldering end of the cigarette between puffs and mainstream smoke drawn from the puff port were drawn into a conditioning chamber, diluted with air, aged, and passed into the exposure chamber where mice were placed during the 6 h treatment period.
For all exposure cohorts, pregnant mice (E0) were weighed and randomly assigned to two treatment groups: cigarette smoke exposed (CSE) or ambient air exposed (sham/Control). Animals were exposed from E0, throughout the entirety of gestation, and following parturition were exposed with offspring until P21. Aged, diluted side- and mainstream smoke was delivered to the CSE chamber under conditions providing total suspended particulates (TSPs) in the range of 20-30 mg/m3 – an exposure that elevates dam/pup plasma cotinine levels (Koren et al. 1992;Koren et al. 1998) to those resembling levels in pregnant women who are ‘active’ smokers (i.e., plasma cotinine greater than 12 ng/ml (Jarvis et al. 2008)). TSP levels represent the “dose” of cigarette smoke to which the animal is exposed, while plasma cotinine (primary metabolite of nicotine) serves as a biomarker of the internal dose. Control animals were sham exposed to ambient air in whole body inhalation chambers under identical conditions (temperature, humidity, flow rate) to CSE mice. As noted, because of species differences in the timing of brain development, the smoke exposure period was extended into the postnatal period (P21) in order to simulate human tobacco smoke exposure during all stages equivalent to human in utero brain development. Up to the first three weeks of postpartum development in rodents correspond to the second and third trimesters of human gestation (Bayer et al. 1993;Clancy et al. 2001;Clancy et al. 2007a;Clancy et al. 2007b).
The paired exposure chambers (one receiving a mixture of main- and side-stream cigarette smoke and one receiving ambient air (sham) were characterized three times daily for TSP, temperature, carbon monoxide levels, and humidity. TSPs were measured using 25 mm Teflon-coated filters (TX40H120-WW, Pallflex Products Co.; Putnum, CT). Chamber temperature and humidity were monitored by a digital hygrometer/thermometer (Control Company; Friendsworth, TX) and carbon monoxide levels were measured using a digital carbon monoxide detector (KIDDE; Mebane, NC). Cumulative inhalation exposure chamber conditions for all animal cohorts are noted in Table 1. Although a fuller characterization of the instrument-generated cigarette smoke in our animal model, and its comparison to that generated by normal smoking patterns in the human population, is beyond the scope of the present study, such comparisons between machine-generated smoke using the FTC method and actual human exposures have been published and reviewed elsewhere (Borgerding and Klus 2005).
TABLE 1.
| Inhalation Exposure Chamber Conditions for Cigarette Smoke- and Sham-Exposed Dams/Offspring1 | ||
|---|---|---|
| Exposure Period: Embryonic Day 1 through Postnatal Day 21 | ||
| Condition | Sham Chamber2 | CSE3 Chamber2 |
| Carbon Monoxide (ppm) | ND4 | 139.8 ± 1.7 |
| Humidity (% RH) | 38.1 ± 0.4 | 54.5 ± 0.55 |
| Temperature (°C) | 22.0 ± 0.1 | 22.0 ± 0.6 |
| Total Suspended Particulates (mg/m3) | 0.2 ± 0.1 | 25.5 ± 0.5 |
Chamber measurements were monitored on a daily basis (spanning 146 days) immediately prior to, and then twice during, the daily exposure period.
Data for chamber conditions are reported as mean ± S.E.M.
CSE = Cigarette Smoke Exposure
ND = Not Detected
Cigarettes were smoked according to an Federal Trade Commission-mandated protocol which includes preconditioning of cigarettes in an environment with a relative humidity of 60%. As a result, the average relative humidity in the smoke exposure chamber is elevated compared with that in the sham exposure chamber which receives filtered ambient air.
The actual dose of cigarette smoke that the animals (dams and pups) received during the pre- and postnatal treatment period was monitored at regular intervals by determining plasma cotinine levels. Blood samples were collected at the end of the daily smoking session from representative dams on gestational days 3, 12, and 18 and from pups on P7, 14, and 21 for determination of cotinine levels using a Cotinine Direct Elisa Kit (Immunalysis; Pomona, CA) according to the manufacturer’s protocol. Plasma cotinine levels for CSE dams and pups in all cohorts were (mean ± SEM): 82.74 ± 10.34 ng/ml, which is above the accepted minimum criterion of plasma cotinine levels associated with ‘active’ smoke exposure (12 ng/ml) (Jarvis et al. 2008). The equivalent values for Controls were: 0.51 ± 0.24 ng/ml.
Throughout the exposure period, dams and pups were monitored for signs of toxicity: weight loss, morbidity, mortality, coat condition, movement, inappetence, and chromodacryorrhea (Esposito et al., 2008). In order to prevent isolation stress, mice were group-housed throughout the exposure period. Litters were culled to a maximum size of eight, balancing for sex when possible.
2.3 Behavioral Testing
Offspring were weaned on P25 and transported to Cincinnati by air-conditioned overnight trucks. Mice were quarantined upon arrival for 30 days and sentinel mice tested for pathogens prior to vivarium entry. Three cohorts of mice were transferred to Cincinnati several months apart and were balanced for treatment groups on all occasions.
2.3.1 Behavioral Procedures
One male and one female per litter were tested in one of two sequences starting at P90: Arm-A: elevated zero maze (EZM), locomotor activity, object burying, acoustic startle response (ASR) with prepulse inhibition (PPI), Morris water maze-cued version (MWM-cued), Cincinnati water maze (CWM), MWM-matching-to-sample (MTS), conditioned fear, forced swim test (FST), and locomotor activity with drug challenge. Arm-B: Light-Dark exploration, MWM-cued, MWM-hidden (three phases: acquisition, reversal, and shift), and locomotor activity with drug challenge.
2.3.1.1 Arm-A Tests
Elevated zero maze
The apparatus is a circular runway (105-cm diameter), 72 cm above the floor with a 10-cm path divided in equal quadrants. Two opposite quadrants have 28-cm walls and two have 1.3-cm clear acrylic curbs. Mice were videotaped for 5 min. Time-in-open, head-dips, latency to first open zone entry, and zone-crossings were scored (Braun et al. 2011;Shepherd et al. 1994).
Locomotor activity
Arenas measured approximately 40 × 40 cm (16 LED-photocells in x- and y-planes spaced 2.5 cm apart and positioned 2 cm above the floor); mice were tested for 1 h (Accuscan Instruments; Columbus, OH) and data were analyzed in 5 min intervals.
Marble burying
Testing was performed in standard mouse cages (16 × 27 cm) with 5 cm deep clean woodchip bedding. On the surface 15 blue marbles (1.5 cm in diameter) were spaced 4.5 cm apart in 3 rows of 5 each. Mice were placed in the cage for 20 min and at the end of the session the number of marbles ≥ two-thirds buried was recorded.
Acoustic Startle Response with Prepulse Inhibition (ASR-PPI)
An SR-LAB apparatus (San Diego Instruments; San Diego, CA) was used with 5-min acclimation, followed by a 4 × 4 Latin square of four trial types: no-stimulus, startle signal (SS), 70-dB prepulse + SS, or 76-dB prepulse + SS. Inter-trial interval was 8 s; inter-stimulus interval was 70 ms. The Latin square was balanced for 16 trials and the Latin square was repeated three times for a total of 48 trials. Signal was a mixed-frequency white noise burst (110 dB SPL, 20 ms). Peak response amplitudes (VMAX) were analyzed.
Morris water maze (MWM)
The tank was 122-cm diameter. Mice in both test arms received MWM-Cued testing. Those in Arm-A subsequently received the working memory version, i.e., MWM-Matching to Sample (MTS), whereas those in Arm-B received the reference memory version, i.e., fixed platform with random starts. The latter consisted of three phases, acquisition, reversal, and shift (each phase had the platform in a different location (see below)).
MWM-Cued
Curtains were closed around the maze to remove as many distal visual cues as possible. The stainless steel tank was painted flat white inside and had no discernible features that could be used as local cues. A 10 cm diameter clear acrylic platform was positioned in one quadrant halfway between the center and the side and was submerged 1 cm below the surface with a small brass rod mounted vertically protruding 10 cm above the water with an orange plastic ball mounted at the top as a visual cue. Day-1: six trials with the start and platform in a fixed location (start was in position W and the platform in position E [not actual compass positions but rather relative to the room with S being where the experimenter was positioned). The purpose of these trials was to teach the mouse to swim to the platform, climb and remain on it until removed. Days 2-6: two trials/day with random start and random goal positions.
Cincinnati water maze (CMW)
The CWM has been described previously (Vorhees 1987;Vorhees et al. 2011;Vorhees et al. 1991) and is a 9-unit multiple-T maze. Mice were given up to 5 min/trial and 2 trials/day for 15 days but few mice searched for the escape, therefore, no usable data could be collected.
MWM-MTS
The MTS procedure uses the same apparatus as above in the MWM-cued test but with the visual cue removed. Mice were given 2 trials/day, both trials with the platform and start in the same position, but both the start and platform were changed to new positions each day for 5 consecutive days. Mice had up to 2 min/trial to reach the platform.
Conditioned fear
Mice in Arm-A received conditioned fear testing. The test chambers were scaled for mice and had a grid floor connected to a scrambled foot shock device. Each chamber was mounted inside a ventilated sound-attenuated chamber and had a house light and speaker mounted in the ceiling and a video camera in the top of the test chamber (Coulbourn Instruments, Allentown, PA). The test was conducted over three days. Day-1: mice were placed in the apparatus for 10 min, at the end of which they received three tone-footshock pairings (tone: 82 dB, 2 kHz, 30 s duration; shock 1 s, 0.3 mA). Each pairing was spaced 180 s apart. Day-2: mice were returned to the apparatus for 6 min with no tone present. Day-3: the grid floor was replaced with a wire floor of a different type and mice were placed back in the apparatus for 3 min with no tone present followed by 3 min of tone. Freeze Frame software (Coulbourn Instruments, Allentown, PA) was used to score immobility, defined as no movement for at least 4 s per episode. Freezing responses on Day-2 (contextual fear) are an index of hippocampally-mediated conditioning; freezing responses on Day-3 (post-tone relative to pre-tone) are an index of amygdala-mediated cued conditioning.
Forced swim test (FST)
Mice in Arm-A received the FST for 6 min. Each mouse was placed in a clear acrylic cylindrical apparatus 25 cm tall and 20 cm in diameter filled with 16 cm of room temperature water. Mice were scored min-by-min for active swimming vs. immobility (not counting minor limb movements to maintain an upright orientation). After testing, each mouse was towel dried.
Locomotor activity with drug challenge
Since Arm-A mice had already been exposed to the locomotor test apparatus, this test began with a 30 min re-acclimation period in the apparatus. After re-habituation, each mouse was briefly removed, given a subcutaneous injection of (+)-methamphetamine HCl (1.0 mg/kg expressed as the free base, NIDA >95% pure) and returned to the test arena for an additional 180 min.
2.3.1.2 Arm-B Test Order
Light-Dark test
The apparatus was the locomotor test described above except with a black acrylic insert that divided the arena in half so that one side was light (20 × 40 cm) and the other dark (20 × 40 cm) with a 7 × 7 cm opening between them. Mice were placed in one corner of the light side and given 10 min to explore. Number of photobeam interruptions on each side was recorded as was the number of crossings and latency to first entry into the dark compartment.
MWM-Cued
The procedure for Arm-B mice was identical to that for Arm-A mice described above.
MWM Fixed platform
Mice received three phases of hidden fixed platform testing, 4 trials/day for 6 days with a 30-s probe trial on the seventh day (24 h after the last training trial of each phase as a test of reference memory). Each phase used a progressively smaller platform than the one before (10, 7, 5 cm in diameter). Phase-1 was acquisition with the platform in the SW quadrant, Phase-2 was reversal with the platform in the NE quadrant, and Phase-3 was shift with the platform in the NW quadrant. To minimize the start positions near the goal (such as S or W when the platform was in the SW position) we used distal start positions (a mixture of distal cardinal and ordinal starts) as described previously (Vorhees and Williams 2006). When the platform was in the SW quadrant, start positions were NW, N, E, and SE with the restriction that one of each start positions was used once/day in a quasi-random order with a different order each day. The animal’s performance was monitored with an overhead video camera connected to a computer with AnyMaze tracking software (Stoelting Instruments, Wood Dale, IL). On platform (training) trials, dependent variables analyzed were latency to the platform, cumulative distance from the platform, path length to the platform, and swim speed. On probe trials with the platform removed, dependent measures were average distance to the platform site, number of site crossovers, and percent time and distance in the target quadrant.
Locomotor activity with drug challenge
Arm-B mice were exposed to the locomotor apparatus previously used for the light-dark test, therefore, this procedure began with a 30 min re-acclimation period without presence of the divider. After re-habituation, each mouse was briefly removed, given a subcutaneous injection of the NMDA receptor antagonist MK-801 (0.2 mg/kg, Sigma-Aldrich, St. Louis, MO expressed as the free base) and returned to the test arena for an additional 180 min.
2.4 Statistical analyses
Behavioral data were analyzed using mixed-linear ANOVA with repeated-measures or by ANCOVA (SAS version 9.2, SAS Institute; Cary, NC) where appropriate and used the Kenward-Roger method of adjusted degrees of freedom. Where there were significant interactions involving a repeated measure, follow-up analyses were performed using slice-effect ANOVAs (i.e., tests of simple main effects on each level of the repeated measure). Since there were only two groups, pairwise group comparisons were compared by two-tailed t-test for independent samples. Results were considered statistically significant when P ≤ 0.05.
3. Results
3.1. Body weight
CSE mice were weighed at the end of smoke exposure and at the end of behavioral testing to determine whether pre- and postnatal tobacco smoke exposure had long-term effects on body weight. At the end of treatment on P21 the mean ± SEM body weights were: Control = 9.8 ± 0.1 g, CSE = 8.7 ± 0.1 g (P<0.02, t-test). At the end of the behavioral testing, there was a significant effect of treatment on body weight (F(1,116) = 10.95, P<0.002) but no treatment × sex interaction. The mean ± SEM body weight combined for males and females in both Arm-A and B were: Control = 27.1 ± 0.2, CSE = 26.0 ± 0.2 g (a 4% reduction in the CSE group).
Arm-A
3.2 Elevated zero maze
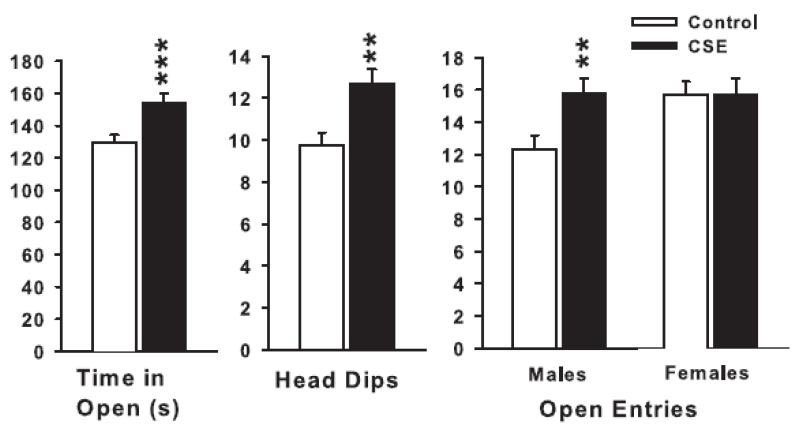
There was a significant treatment main effect for time-in-open areas (F(1,57) = 11.9, P<0.001); sex was also significant (P<0.02) (females spent more time in the open than males), but there was no treatment × sex interaction. There was a similar main effect of treatment on the number of open zone entries (F1,57) = 4.25, P<0.05) and head-dips (F(1,57) = 9.56, P<0.01), but not on latency to first open zone entry. For open zone entries there was also a treatment × sex interaction (F(1,57) = 4.1, P<0.05) with male CSE mice entering the open more than male Control mice and no difference between females. The significant treatment-related effects are illustrated in Fig. 1. The data indicate that CSE mice were less anxious since they spent more time in the open, investigated more by looking over edges, and CSE males entered more open zones than Control males whereas there were no differences among females.
Figure 1.
Elevated zero maze: Left, time-in-open quadrants; Middle, number of head-dips; Right, number of open quadrant entries. In all figures, data are shown as least square means ± SEM. **P <0.01, ***P <0.001, CSE vs. Control. Group sizes: total (males/females): Control = 34 (14/19), CSE = 27 (14/13).
3.3 Locomotor activity
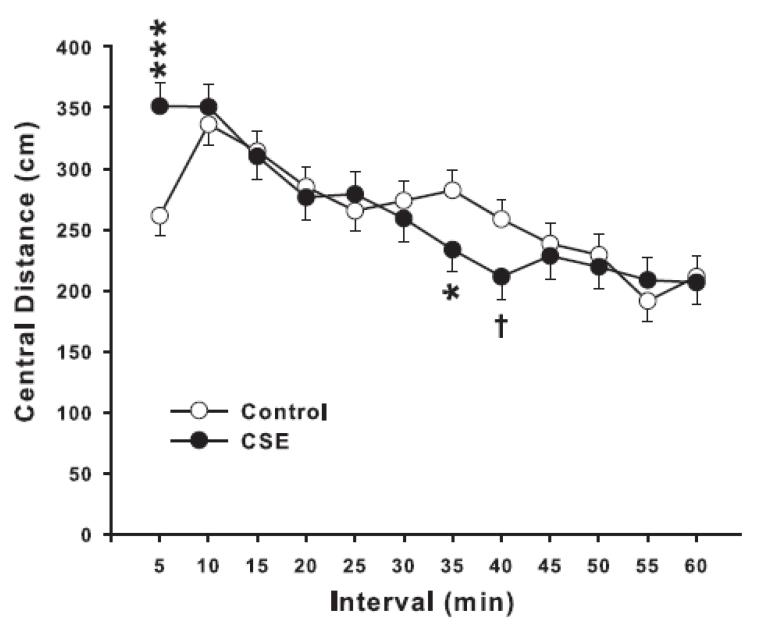
For the 1 h locomotor test, there were no significant effects of treatment on total photobeam interruptions. Sex was significant (P<0.01; females were more active than males), and there was a treatment × interval interaction (F(11,512) = 2.88, P<0.001) and a treatment × sex × interval interaction (F(11,512) = 1.80, P<0.05). In order to understand this effect, total photobeam interruptions were further analyzed by pattern, i.e., sequential vs. repetitive beam interruptions. Sequential beam interruptions are converted by the software to total distance (cm) moved. We analyzed distance by region as central vs. peripheral distance. There were no treatment-related effects on peripheral distance, but there was a treatment × interval interaction on central distance (F(11,523) = 3.70, P<0.0001). As can be seen in Fig. 2, CSE mice were less active in the central region than Control mice during some later intervals. The figure also shows that during the first 5 min the CSE mice were more active; however, in this instance it appears the Controls were unusual since they did not show a typical exploratory phase.
Figure 2.
Locomotor activity—central distance (cm): Mice were tested for 60 min and data analyzed in 5 min intervals. *P <0.05, ***P<0.001, †P <0.10, CSE vs Control. Group sizes: total (males/females): Control = 34 (14/19), CSE = 27 (15/12).
Repetitive beam breaks are a reflection of fine motor movements, such as grooming, where the animal is stationary but moving enough to repeatedly interrupt the same infrared beam. There was no significant treatment or sex effect, but there were treatment × interval (F(11,516) = 2.83, P<0.01) and treatment × sex × interval interactions (F(11,516) = 1.83, P<0.05). Slice-effect ANOVAs on each sex at each interval showed one effect: during the first 5 min, Control mice showed less repetitive movements than CSE mice. Each quarter turn (90°) that the animals made was also analyzed. No treatment or sex main effects were seen, but there was a treatment × interval interaction (F(11,525) = 2.19, P<0.02). Slice-effect ANOVA again showed only one interval to have a significant effect and it was, as above, in the first interval. As above, Control mice turned fewer quarter turns than CSE mice. Overall, the locomotor activity data show that CSE mice were slightly less active in the center region at 35-40 min, but during the first 5 min Controls were unusually less active. The data provide little evidence of a change in exploration, anxiety (central zone activity), habituation to a novel environment, or baseline activity change in CSE mice compared with Control mice.
3.4 Marble burying
There were no significant effects of CSE on number of marbles buried and no interaction between burying and sex (Table 2).
Table 2.
| Control | CSE | |||||
|---|---|---|---|---|---|---|
| Function | Test | Variable | M | F | M | F |
| Defensive Anxiety |
Number of Animals |
15 | 18 | 14 | 13 | |
| Marble Burying |
No. 2/3rd buried |
13.9 ± 0.4 | 14.1 ± 0.3 | 13.6 ± 0.4 | 13.8 ± 0.4 | |
| Startle | Number of Animals |
11 | 17 | 11 | 10 | |
| ASR | Peak Amplitude |
99.0 ± 12.0 | 128.0 ± 9.6 | 97.8 ± 12.0 | 143.0 ± 12.6 | |
| PPI | % Inhibition | PP70 | 42.5 ± 5.8 | 52.9 ± 4.7 | 48.2 ± 5.8 | 60.0 ± 6.1 |
| PP76 | 51.5 ± 5.8 | 61.7 ± 4.7 | 59.4 ± 5.8 | 70.9 ± 6.1 | ||
| PP80 | 51.8 ± 6.6 | 40.2 ± 6.5 | 40.5 ± 9.2 | 15.3 ± 9.2 | ||
| Working Memory |
Number of Animals |
15 | 18 | 13 | 13 | |
| MWM-MTS | T1-T2 (s) | −3.8 ± 3.3 | 3.0 ± 3.0 | 0.7 ± 3.6 | 6.6 ± 3.6 | |
| Emotional Learning |
Cond. Fear | Number of Animals |
M+F = 33a | M+F = 27a | ||
| % Freezing | Pre-tone | 0.4 ± 0.2 | 0.0 ± 0.2 | |||
| Post-tone | 52.0 ± 5.5 | 50.8 ± 6.1 | ||||
| Depression | Number of Animals |
15 | 18 | 14 | 13 | |
| FST | Immobility time (s) |
169.3 ± 15.0 | 169.1 ± 15.0 | 170.8 ± 15.5 | 153.3 ± 16.1 | |
| Spatial Learning |
Swim Speed | Number of Animals |
20 | 19 | 10 | 10 |
| Acquisition | cm/s | 17.4 ± 0.6 | 17.5 ± 0.6 | 16.2 ± 0.6 | 16.2 ± 0.7 | |
| Acq-probe | 18.4 ± 1.0 | 16.3 ± 1.0 | 15.4 ± 1.1 | 16.7 ± 1.1 | ||
| Reversal | 16.1 ± 0.8 | 16.6 ± 0.9 | 15.2 ± 0.9 | 15.7 ± 1.0 | ||
| Rev-probe | 14.5 ± 1.5 | 14.6 ± 1.7 | 11.8 ± 1.8 | 13.4 ± 1.8 | ||
| Shift | 14.2 ± 1.1 | 14.2 ± 1.1 | 12.4 ± 1.2 | 13.5 ± 1.2 | ||
| Shift-probe | 11.9 ± 1.7 | 11.3 ± 1.7 | 10.0 ± 1.9 | 10.7 ± 1.9 | ||
Imbalance in the number of males (M) versus females (F) on this test were such that one cell did not have sufficient numbers to be used statistically, therefore, for this test sexes were combined.
3.5 ASR/PPI
There were no significant effects of treatment or interaction of treatment with sex or prepulse intensity on ASR amplitude with or without a prepulse present. There was the typical inhibitory effect of the prepulses that was intensity-dependent but no other effects (Table 2).
3.6 Morris water maze (MWM), cued platform
Day-1 involved six trials with the same start and goal positions. No significant treatment or treatment × trial interaction was obtained.
Days 2-6 had two trials per day with the start and goal randomly positioned. There were no significant treatment or sex effects. There was a significant treatment × day interaction (F(4,335) = 2.96, P<0.02) but no interaction between these factors and sex. Slice-effect ANOVAs on each day showed an effect on day-6 in which CSE mice took longer to reach the platform than Controls (Fig. 3), but no differences on the other days.
Figure 3.
MWM (cued). Latency to reach goal (s). Mice were given 2 trials/day on days 2-6 with the platform and goal repositioned randomly on each trial with the platform clearly marked with an orange ball mounted on a pole affixed to the center of the platform. **P<0.01, CSE vs Control. Group sizes: total (males/females): Control = 67 (33/34), CSE = 53 (27/26).
3.7 CWM
An analysis of this procedure could not be completed because a large number of mice failed to search for the escape platform (data not shown).
3.8 MWM-MTS
The working memory or MTS procedure is a trial-dependent method where a new platform location is learned and remembered by the animal. Hence, what was learned the previous day is of no value the next day. If the animal can use short-term memory for the new position, it should find the platform in less time and in a shorter distance traveled on the second trial of the day compared with the first. There were no treatment differences on the first trial of each day. The data of primary interest were treatment-related differences on the short-term memory trial-2. Analyses of latency, path length, cumulative distance, and initial heading error showed no significant effects of treatment or interaction with other factors on the time savings between trial-1 and trial-2 (Table 2). On average heading error, there was a treatment × sex effect (F(1,55) = 6.05, P<0.02). Neither slice-effect ANOVAs on males (P<0.10) or females (P<0.07) was significant; therefore, further analyses were not justified.
3.9 Conditioned fear
As noted, conditioning to the chamber and the tone occurred simultaneously on day-1. Day-2 assessed immobility in response to the chamber without the tone. A trend was observed in the treatment main effect (F(1,56) = 3.18, P<0.08). Not all mice showed conditioning, therefore, we analyzed the proportion of mice in each group that showed immobility. No significant group differences were found. Day-3 was cued conditioning. On this day, no significant treatment or treatment-related interactions were obtained when the tone was presented (Table 2).
3.10 Forced Swim Test (Fst)
Analyses of total immobility time and latency to immobility showed no significant treatment or treatment × sex interactions (Table 2).
3.11 Locomotor activity with methamphetamine challenge
An analysis of the pre-challenge locomotor activity for total and repetitive beam interruptions showed no significant treatment or treatment-related effects. Sequential beam interruptions (cm) showed no treatment effects for peripheral distance, but a significant treatment main effect for central distance (F(1,55.8) = 4.59, P<0.04).
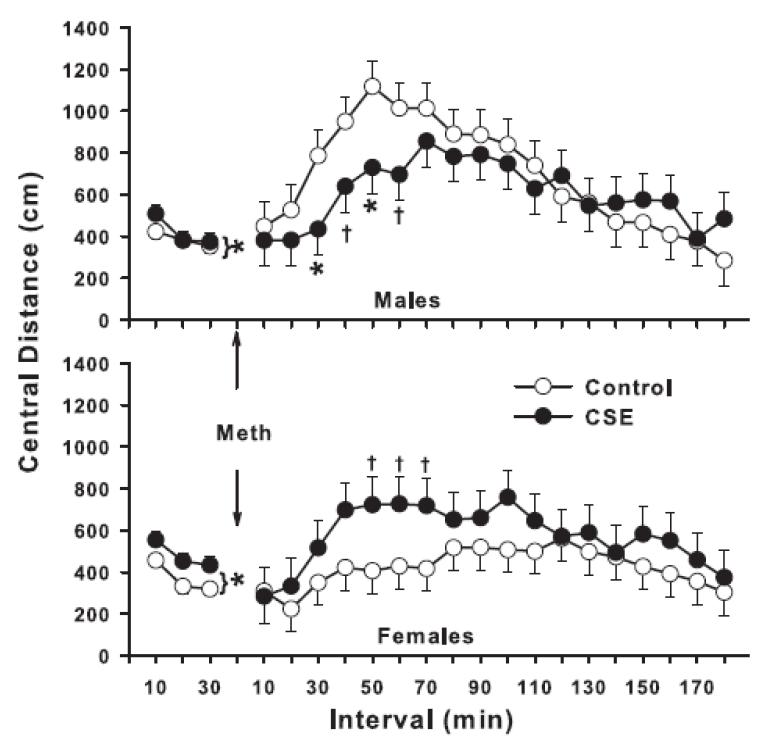
Post-challenge activity using the pre-challenge data as a covariate was analyzed by ANCOVA. For total beam breaks post-challenge, there were no treatment or treatment-related effects, nor was there any effect on peripheral sequential beam breaks (cm). However, there was a treatment × sex × interval effect on central distance (F(17,833) = 1.88, P<0.02). A similar effect was found on repetitive beam breaks, i.e., a treatment × sex × interval interaction (F(17,849) = 1.83, P<0.03). As can be seen for central distance in Fig. 4, the interaction was the product of CSE males under-responding to the activity-inducing effect of methamphetamine, while CSE females over-responded. This pattern was identical for repetitive beam interruptions (data not shown). An analysis of quarter turns showed a similar pattern but was only a trend (P<0.06).
Figure 4.
Locomotor activity with methamphetamine challenge—central distance (cm). Mice were re-acclimated to the test for 30 min prior to methamphetamine administration (1.0 mg/kg s.c.). After treatment with methamphetamine they were placed back in the test apparatus for an additional 180 min. Data are least square mean ± SEM per 5 min interval. *P<0.05, †P <0.10, CSE vs. Control. Note *P<0.05 with bracket indicates significant difference averaged across intervals for the pre-challenge test session. Post-challenge data were analyzed using the pre-challenge data as a covariate to control for differences in baseline prior to methamphetamine treatment. Group sizes: total (males/females): Control = 33 (15/18), CSE = 27 (14/13).
Arm-B
3.12 Light/Dark test
No significant treatment effects were obtained on number of dark compartment entries or latency to first dark compartment entry. There was a significant treatment main effect on time in the light compartment (F(1,56) = 7.91, P<0.01). CSE mice spent significantly more time in the light, suggesting reduced anxiety compared with Control mice (Mean ± SE: Control = 362.6 ± 21.7 vs. CSE = 453.8 ± 24.0).
3.13 MWM hidden platform
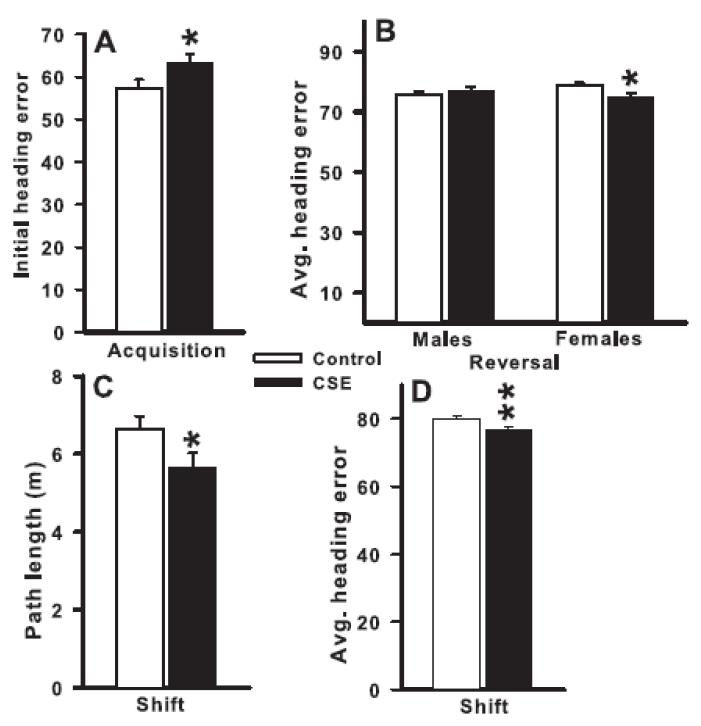
There were no treatment effects or interactions with treatment on most of the indices of learning during the acquisition phase (latency, path length, cumulative distance, or average heading error) to find the platform and no differences in swim speed. There was a treatment main effect for initial heading error (F(1,55.2) = 4.11, P<0.05; Fig. 5A). The CSE mice had a larger initial heading error than the Control mice. No differences in swim speed were seen (Table 2).
Figure 5.
MWM Fixed hidden platform. Mice were tested in three phases (acquisition, reversal, shift) each consisting of 6 training days (4 trials/day) followed 24 h later by a single probe trial for reference memory with the platform removed. Differences were found on acquisition for initial heading error (panel A), on reversal average heading error for females (panel B), and on shift for path length (panel C) and average heading error (panel D). Heading error is expressed in degrees. Data are averaged across days to reflect the main effect of treatment or treatment and sex (mean ± SEM). *P <0.05, **P <0.01, CSE vs Control. Group sizes: total (males/females) = Control = 33 (15/18), CSE = 27 (14/13).
During the reversal phase, there were again no treatment effects or interactions with treatment on most of the indices of learning (latency, path length, cumulative distance, or initial heading error), but there was a treatment × sex interaction on average heading error (F(1,57.6) = 5.12, P<0.03). The CSE female mice had smaller errors than the Control female mice (Fig. 5B). No differences in swim speed were seen (Table 2).
During the shift phase, there were no significant treatment effects or interactions with treatment on latency, cumulative distance, or initial heading error, but there were significant treatment main effects on path length (F(1,56.1) = 4.01, P=0.05; Fig. 5C) and average heading error (F(1,55.9) = 6.70, P<0.02; Fig. 5D). The CSE mice had shorter path lengths and smaller average heading errors than the Control mice. No differences in swim speed were found (Table 2)
3.14 MWM Probe trials
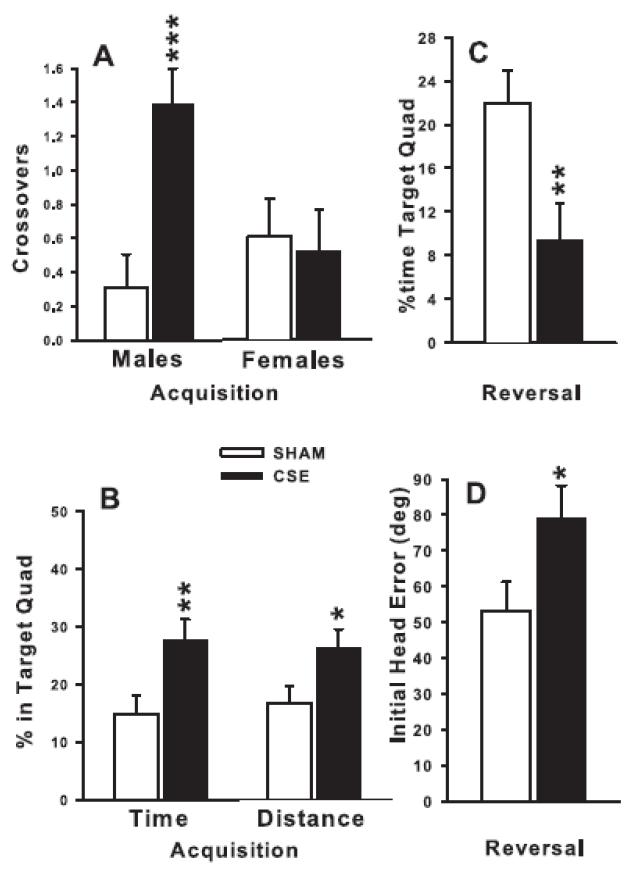
24 h after the last trial of each platform phase, the platform was removed and animals were tested for 30 s starting from a novel position that had not been used during that phase. On the acquisition probe trial, there were no treatment effects on average distance to the platform site, swim speed, or average or initial heading error, but CSE mice had more crossovers (F(1,53) = 5.95, P<0.02; Fig. 6A), greater percent time in the target quadrant (F(1,53) = 7.02, P<0.01; Fig. 6B, left), and greater percent distance in the target quadrant (F(1,53) = 4.57, P<0.05; Fig. 6B, right) compared with Control mice. Moreover, for acquisition probe crossovers there was also a treatment × sex interaction (F1,53) = 7.78, P<0.01; Fig. 6A) showing that the effect was limited to the male CSE mice. On the reversal probe trial, similar main effects were seen for percent time and percent distance in the target quadrant (F(1,51) = 7.50 and 10.23, respectively, both P<0.01, Fig. 6C and not shown, respectively), and for initial heading error (F1,51) = 4.65, P<0.05, Fig. 6D. Unlike acquisition, the CSE mice spent less time in the target quadrant and had larger initial heading errors than Control mice. However, no treatment effects were obtained on average distance, crossovers, or swim speed. On the shift probe trial, no treatment effects were obtained on any of the indices. All probe trial swim speed data are summarized in Table 2.
Figure 6.
MWM probe trials. Data are least square mean ± SEM on probe trials for acquisition-probe site crossovers (panel A), for acquisition-probe percent time and percent distance in the target quadrant (panel B), for reversal-probe for percent time in the target quadrant (panel C), and on reversal-probe initial heading error (in degrees; panel D). Probe trials were given 24 h after the last platform trial of each phase. *P <0.05, **P <0.01, ***P <0.001, CSE vs Control. Group sizes: total (males/females) = Control = 33 (15/18), CSE = 27 (14/13).
3.15 Locomotor activity with MK-801 challenge
During the pre-challenge acclimation period, there were no treatment main effects or interactions with treatment on total, central, peripheral, or repetitive beam breaks. Post-challenge, MK-801 induced the typical pattern of hyperactivity in both groups. ANCOVA of the post-challenge data showed no treatment or interactions with treatment on total or peripheral activity. A treatment trend was seen on central locomotion (F(1,100) = 2.94, P < 0.09) but no interactions with treatment were obtained. No treatment effects or interactions with treatment were found on repetitive beam breaks (data not shown).
4. Discussion
Most animal models have focused on administration of nicotine rather than cigarette smoke (reviewed in (Slikker, Jr. et al. 2005;Slotkin 2008)). Here, we employed a model of developmental cigarette smoke exposure previously shown to cause intrauterine growth retardation (Esposito et al. 2008) and low birth weight (unpublished data). Mice were exposed prenatally throughout the entirety of gestation and postnatally up to P21 in order to span the period of brain development in mice that is homologous to human in utero brain development (Bayer et al. 1993;Clancy et al. 2001;Clancy et al. 2007a;Clancy et al. 2007b;Herlenius and Lagercrantz 2004;Rakhade and Jensen 2009;Rice and Barone, Jr. 2000). The focus of the present study was on long-term effects of developmental cigarette smoke exposure on brain function. In order to address this, we used a test battery to screen a wide range of functions as per frequent recommendation in order to obtain a comprehensive characterization of the entire phenotype (Crawley 2000;Crawley 2007;Crawley 2008;Vorhees 1996;Vorhees 1997). The results show a complex pattern of effects that are selective, with some behaviors affected by CSE and others not. Among the affected functions were changes in anxiety-related behavior. These included increased time in open zones and more head-dips in the EZM, more time spent in the light side of the light-dark test, and more central zone activity in the open-field during the first five minutes of exploration compared with Controls. CSE male mice also showed more open arm visits in the EZM, although females did not. In the open-field, CSE mice were also less active during later intervals.
In the MWM, CSE mice were more off-course (initial heading error) at the beginning of each trial during acquisition than Controls, but during reversal CSE females were less off-course (average heading error) than female Controls. Nonetheless, no differences in path length, latency, and cumulative distance were observed. During the shift phase, CSE mice were less off-course (average heading error) and had shorter path lengths than Control mice. Twenty-four hours after each learning phase, mice were given a single probe trial to test for reference memory. On the acquisition probe, CSE males performed better than Controls in terms of average number of platform site crossovers and CSE mice of both sexes spent proportionately more time and swam longer distances in the target quadrant. But the situation was complex, in that while the CSE mice showed evidence of better performance on acquisition probe, this pattern was inverted on reversal probe. On this memory trial, the CSE mice spent significantly less time in the target quadrant and were more off-course in their initial heading than Controls. No differences on average distance to the target site were found on acquisition or reversal probe trials which is surprising given that this is one of the best validated indices of memory retention in mice in the MWM (Maei et al. 2009). No differences were seen on the shift probe trial. At no point during learning or probe trials did CSE mice show swim speed differences. This was confirmed by no differences in swim latency during straight channel trials. There were also no latency differences on day-1 of MWM cued testing during which mice were given 6 trials to swim from a fixed start to a fixed visible platform. The single exception was on MWM random cued trials. While there were no differences on the first 4 days of cued random trials, there was a difference on the last day (day-5) during which CSE mice took longer to reach the goal than Controls. This isolated effect is not consistent with any of the other swim speed measures and therefore does not appear reliable.
The opposite pattern of learning versus memory effects in the CSE mice on MWM acquisition and reversal may suggest that CSE mice have a harder time orienting to the goal but later adjust and show no differences. The slightly better than Control acquisition probe performance may reflect increased motivation to escape. High motivation may have contributed to their difficulty with reversal probe as well. Perhaps CSE mice persisted in searching for the platform where it was previously located thereby interfering with adapting to the new position when the platform was moved. Reversal impairments are common in animals with hippocampal dysfunction. Although the effect in CSE mice was modest, future studies might profitably focus on the hippocampus and other hippocampally-dependent behaviors. Interestingly, the CSE reversal probe effect did not carry over to the shift phase because CSE mice performed better than Controls in terms of average heading error and path length and showed no differences in shift probe performance.
Another study that used a pharmacological challenge (Lesage et al. 2006) following acute in utero nicotine exposure, found no differential effects of the prenatal nicotine treatment after an acute nicotine exposure. Several studies found no change in radial-arm acquisition but a reduced number of arm entries to the first repeated arm choice (reduced accuracy) after prenatal nicotine (2 or 4 mg/kg/day E4-20 by osmotic minipump) when tested in an identical maze in a different room and in response to drug challenges (Cutler et al. 1996;Levin et al. 1993;Levin et al. 1996). In our studies, we used an indirect dopaminergic agonist to induce drive animals into hyperactivity. Both CSE and Control mice of both sexes showed methamphetamine-induced locomotor stimulation that lasted ~2 h. However, CSE males significantly under-responded while CSE females over-responded to the drug. This suggests that neostriatal circuits which play a dominant role in mediating locomotor activation, are affected by CSE exposure in a sexually dimorphic manner. The fact that males responded differently than females, may suggest that cigarette smoke blunts the normal differences between males and females, although further investigation is required to test this prediction.
In one study, 2.5 or 5 mg/kg/day of nicotine was given to pregnant rats by osmotic minipumps from E8 to birth (Sobrian et al. 2003). The 5 mg/kg nicotine-exposed offspring showed below normal anxiety in an elevated plus maze, similar to what we found in the EZM in this study. Also, the 5 mg/kg nicotine group was more active during the first 5 min in the open-field, as seen in our data. Another study (Vaglenova et al. 2004) reported that 28-day osmotic minipump administration of 6 mg/kg/day nicotine beginning on E3 to rats resulted in offspring that were more active during 3 min open-field tests; but in contrast to our data and those of Sobrian et al. (2003), they found reduced time in open arms in the elevated plus maze. They also reported reduced hole-board nose-poke exploration and shuttle-box avoidance learning. A study in rats (Romero and Chen 2004) used 21-day release nicotine pellets implanted from E8 (4.4-6.2 mg/kg/day) and found no locomotor activity differences in males and reduced activity in females only on day-3 of testing. Intravenous nicotine exposure of 0.03 mg/kg given to rats every 14 min for 16 h/day from E4 to parturition (Lesage et al. 2006) produced no nicotine-related locomotor differences on P19-21, but on P19, nicotine exposed offspring had reduced same-beam interruptions. When analyzed by interval, nicotine-exposed offspring were less active during the first 5 min. Another study used rats implanted on E3 with 28-day osmotic minipumps that provided 6 mg/kg/day of nicotine (Vaglenova et al. 2008). Exposed offspring showed delayed surface righting and inclined plane rotation (P5-9), and males spent less time in open arms of the elevated plus maze than Controls on P40 and P60 but not on P180; females showed the same pattern on P60. Nicotine-exposed offspring also showed reduced acquisition and retention in shuttle-box avoidance. A similar study (Eppolito et al. 2010) used rats implanted with 28-day release osmotic minipumps on E4 that delivered 1 or 2 mg/kg/day. At P32, the 2 mg/kg nicotine group showed reduced time in open arms in the elevated plus maze, as did 1 mg/kg females. Arm entries were also reduced in males while head dips were increased in the 1 mg/kg nicotine group. No differences were found in contextual or cued fear conditioning, but extinction was slower in the nicotine groups during 2 out of 19 min of the test. Nicotine exposed rats showed increased mRNA expression of the β2 subunit of nAChR in prelimbic cortex and hippocampus at P30 but not at P75 with no change in the α4 subunit. In addition, reductions in the α4 and β2 subunits were seen in the ventral hippocampus at P75 but not at P30. No changes in the α7 subunit at either age were found. An earlier study also found that prenatal nicotine exposure (2 or 6 mg/kg/day on E4-20 in rats by minipump) reduced hemicholinium binding to AChRs in the offspring in striatum and hippocampus (Zahalka et al. 1992). In addition, a study found that prenatal nicotine exposure of rats — which had no initial effect on offspring nicotine self-administration — after an interruption resulted in prenatally nicotine exposed offspring increasing their self-administration during reinstatement more than controls (Levin et al. 2006). In terms of learning effects, radial-arm acquisition deficits in rats were found after prenatal exposure to nicotine in drinking water (Sorenson et al. 1991). This study is noteworthy because they used a 15 s delay between trials — which is important in this test for determining effects on working memory — and found large deficits in the nicotine-exposed group.
Although no studies to date have examined the neurobehavioral outcomes associated with prenatal cigarette smoke inhalation exposure in mice, one study examined such outcomes in rats (Gaworski et al. 2004). Pregnant rats were exposed to cigarette smoke containing 150, 300, or 600 TPM/m3 for 2 h/day in restraining devices for 4 weeks prior to breeding and throughout gestation and lactation (except on P0-4) and tested for locomotor activity, ASR, and shuttle-box active avoidance prior to weaning and as adults, air-righting on P30-32, and physical landmark ontogeny. There were effects on pinna detachment, incisor eruption, eye opening, vaginal patency, and preputial separation. No effects were found on air righting, open-field activity, or active avoidance. The 150 and 600 TPM groups showed reduced ASR at P22 at 80 dB but not at 120 dB, and the 300 and 600 TPM groups showed reduced ASR at P61 at 80 dB but not at 120 dB.
A study in which rats were exposed to cigarette smoke from E2-22 examined brainstem respiratory control nuclei on P2 by immunoblot for PKCα, β, γ and δ, eNOS, and nNOS (Hasan et al. 2001). Reductions in immunoreactivity were found for PKCγ, PKCδ, and nNOS, but not for eNOS, PKCα, or PKCβ. In a study examining the pedunculopontine nucleus of the reticular activating system (RAS) in offspring of CSE pregnant rats exposed to CSE for 15 min × 3/day from E14 to parturition (Garcia-Rill et al. 2007), auditory evoked potentials using the P-3 waveforms and showed increased excitability in the CSE group compared with controls in three exposed litters but reduced excitability in one exposed litter resulting in a mixed set of outcomes. In addition, E14 to birth CSE in rats with offspring brain slices of pedunculopontine nucleus assessed found more excitable cellular responses than controls (Garcia-Rill et al. 2007). A developmental study in mice using CSE from E0-9, or from E0-17 found reduced embryonic weight and delayed ossification but no increase in malformations (Bnait and Seller 1995).
Collectively, the studies outlined above demonstrate considerable variability in outcomes. Some find reduced, and others increased, anxiety after developmental exposure to nicotine or CSE. Some find increased early open-field exploration and others find it reduced. One found reduced ASR but we did not. Two found reduced active avoidance acquisition, while one did not. One (Eppolito et al. 2010) found a fear extinction deficit in smoke- and nicotine-exposed offspring. We found complex spatial learning and reference memory changes in the MWM. Several studies report radial-arm deficits, but only one did so in the absence of a drug challenge suggesting that changes are subtle and require unmaking using supplemental stimulation. Several studies found effects on active avoidance, but they are difficult to interpret because many of the subjects failed to perform the and had to be excluded raising concerns about selection bias. Moreover, in these studies, shock sensitivity and/or reactivity were not determined and hence could not be ruled out as contributing factors. Moreover, unlike nicotine exposure by osmotic minipump, smoke inhalation is an irritant and is inescapable during the exposure sessions which may be stressful to the dam and to the pups, and developmental stress is known to have long-term neurobehavioral effects. Hence, some of the effects observed here may be secondary to smoke-induced stress either in the mother, the pups, or both.
Collectively, the data presented in the current study indicate that developmental CSE induces subnormal anxiety in when placed in new environments, impairs aspects of spatial learning and reference memory while sparing others and not affecting fear conditioning or forced swim behavior. The findings add support to evidence that developmental CSE has long-term adverse effects on central nervous system (CNS) function. While the cumulative experimental evidence is sufficient to demonstrate that prenatal nicotine or CSE has lasting effects on brain development and behavior, this area of investigation requires: (1) more studies using a consistent exposure of the proximate agent, cigarette smoke, and (2) a broader range of outcome measures, including more tests of higher CNS functions. The existing data suggest that higher CNS functions are more vulnerable to such exposures than reflexes, locomotor patterns, or other basic functions. This inference is consistent with findings from human prospective, longitudinal studies of neurocognitive outcomes of children whose mothers smoked cigarettes during pregnancy that show reductions in complex components of IQ and in auditory memory (Fried et al. 2003). Future studies using cigarette smoke inhalation exposures should concentrate, therefore, on learning, memory, attention, anxiety, and executive functions such as anticipation of reward and punishment, response inhibition, set shifting, and other cognitive capacities.
Research Highlights.
Prenatal cigarette smoke exposure (CSE) affects development
The experiment tested the effects of CSE on neurobehavioral development
C57BL mice were exposed prenatally and postnatally to CSE or air daily
CSE offspring showed abnormally low anxiety
CSE offspring showed mixed spatial learning and reference memory changes
Acknowledgements
We thank Mary Moran for statistical support. Financial support for this research was provided in part by NIH training grant T32 ES007051 stipend support (RMAK), and NIH research grants R01 DA021394 (CVV), R01 HD053509 (MMP and RMG) and P20 RR017702 from the COBRE program of the NIGMS (MMP and RMG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest regarding the research reported herein.
Reference List
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev. Toxicol. 2012;42:279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Abel EL. Smoking during pregnancy: a review of effects on growth and development of offspring. Hum. Biol. 1980;52:593–625. [PubMed] [Google Scholar]
- Anderko L, Braun J, Auinger P. Contribution of tobacco smoke exposure to learning disabilities. J. Obstet. Gynecol. Neonatal Nurs. 2010;39:111–117. doi: 10.1111/j.1552-6909.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum. Dev. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring’s cognitive ability: empirical evidence for complete confounding in the US national longitudinal survey of youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bnait KS, Seller MJ. Ultrastructural changes in 9-day old mouse embryos following maternal tobacco smoke inhalation. Exp. Toxicol. Pathol. 1995;47:453–461. doi: 10.1016/S0940-2993(11)80327-1. [DOI] [PubMed] [Google Scholar]
- Borgerding M, Klus H. Analysis of complex mixtures--cigarette smoke. Exp. Toxicol. Pathol. 2005;57(Suppl 1):43–73. doi: 10.1016/j.etp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol. Biochem. Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Kalkbrenner A, Zimmerman J, Nicholas JS. The effect of maternal smoking during pregnancy on intellectual disabilities among 8-year-old children. Paediatr. Perinat. Epidemiol. 2009;23:482–491. doi: 10.1111/j.1365-3016.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency Health Effects of Exposure to Environmental Tobacco Smoke. NCI Smoking and Tobacco Control Monograph. 1999;10:1–464. [Google Scholar]
- Centers for Disease Control and Prevention . Women and Smoking: A report of the Surgeon Genereal. U.S. Department of Health and Human Services; Atlanta, GA: 2004. [Google Scholar]
- Centers for Disease Control and Prevention . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A report of the Surgeon General. U.S. Department of Helath and Human Servicesl, Office of Smoking and Health; Atlanta, GA: 2006. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine. Tob. Res. 2004;6(Suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Cooke RW. Smoking, intra-uterine growth retardation and sudden infant death syndrome. Int. J. Epidemiol. 1998;27:238–241. doi: 10.1093/ije/27.2.238. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Day N, Richardson G, Taylor P. Epidemiology of substance abuse druing prenancy. In: Ott P, Tarter R, Ammerman R, editors. Sourcebook on Substance Abuse: Etiology, Epidemiology, Assessment and Treatment. Allyn and Bacon Publisher; Needham Heights, MA: 1999. pp. 1–13. [Google Scholar]
- Cornelius MD, Goldschmidt L, De Genna NM, Larkby C. Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14-year-old offspring of teenage mothers. Matern. Child Health J. 2012;16:694–705. doi: 10.1007/s10995-011-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J. Dev. Behav. Pediatr. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York: 2000. [Google Scholar]
- Crawley JN. What’s Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice. John Wiley & Sons; Hoboken, NJ: 2007. [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: Preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol. Teratol. 1996;18:635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Cornelius MD. Effects of prenatal tobacco exposure on preschoolers’ behavior. J. Dev. Behav. Pediatr. 2000;21:180–188. [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res. C. Embryo. 2008;84:30–44. doi: 10.1002/bdrc.20118. Today. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Bachus SE, McDonald CG, Meador-Woodruff JH, Smith RF. Late emerging effects of prenatal and early postnatal nicotine exposure on the cholinergic system and anxiety-like behavior. Neurotoxicol. Teratol. 2010;32:336–345. doi: 10.1016/j.ntt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Esposito ER, Horn KH, Greene RM, Pisano MM. An animal model of cigarette smoke-induced in utero growth retardation. Toxicology. 2008;246:193–202. doi: 10.1016/j.tox.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 2000;22:11–20. doi: 10.1016/s0892-0362(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Buchanan R, McKeon K, Skinner RD, Wallace T. Smoking during pregnancy: postnatal effects on arousal and attentional brain systems. Neurotoxicology. 2007;28:915–923. doi: 10.1016/j.neuro.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol. Sci. 2004;79:157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Gospe SM, Jr., Zhou SS, Pinkerton KE. Effects of environmental tobacco smoke exposure in utero and/or postnatally on brain development. Pediatr. Res. 1996;39:494–498. doi: 10.1203/00006450-199603000-00018. [DOI] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol. Biochem. Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SU, Simakajornboon N, MacKinnon Y, Gozal D. Prenatal cigarette smoke exposure selectively alters protein kinase C and nitric oxide synthase expression within the neonatal rat brainstem. Neurosci. Lett. 2001;301:135–138. doi: 10.1016/s0304-3940(01)01624-x. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp. Neurol. 2004;190:S8–S21. doi: 10.1016/j.expneurol.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Horn KH, Esposito ER, Greene RM, Pisano MM. The effect of cigarette smoke exposure on developing folate binding protein-2 null mice. Reprod. Toxicol. 2008;26:203–209. doi: 10.1016/j.reprotox.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, Kim EH, Kim CJ. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13:1509–1513. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008;103:1553–1561. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- Joad JP. Smoking and pediatric respiratory health. Clin. Chest Med. 2000;21:37–viii. doi: 10.1016/s0272-5231(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Kafouri S, Leonard G, Perron M, Richer L, Seguin JR, Veillette S, Pausova Z, Paus T. Maternal cigarette smoking during pregnancy and cognitive performance in adolescence. Int. J. Epidemiol. 2009;38:158–172. doi: 10.1093/ije/dyn250. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev. Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G, Klein J, Forman R, Graham K, Phan MK. Biological markers of intrauterine exposure to cocaine and cigarette smoking. Dev. Pharmacol. Ther. 1992;18:228–236. [PubMed] [Google Scholar]
- Koren G, Pastuszak A, Ito S, Drugs in, pregnancy N. Engl. J. Med. 1998;338:1128–1137. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: Effects on attention and impulsivity of 6 year olds. Neurotoxicol. Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Lesage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol. Biochem. Behav. 2006;85:575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol. Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol. Biochem. Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. In: Slikker W, Chang LW, editors. Handbook of Developmental Neurotoxicology. Academic Press; San Diego: 1998. pp. 587–615. [Google Scholar]
- Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res. Dev. Brain Res. 1996;97:207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- Li SP, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking exposure decreases GABA(B1) receptor expression in the rat hippocampus. Neurosci. Lett. 2002;334:135–139. doi: 10.1016/s0304-3940(02)01065-0. [DOI] [PubMed] [Google Scholar]
- MacPhail RC, Farmer JD, Jarema KA. Sensitization and tolerance with episodic (weekly) nicotine on motor activity in rats. Neurotoxicol. Teratol. 2007;29:341–347. doi: 10.1016/j.ntt.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the Most Sensitive Measure of Water Maze Probe Test Performance? Front Integr. Neurosci. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ. Smoking during pregnancy in the 1990s. Natl. Vit. Stat. Rep. 2001;49:1–15. [PubMed] [Google Scholar]
- McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol. Teratol. 1994;16:269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am. J. Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Thompson JM, Robinson E, Wild CJ, Becroft DM, Clark PM, Glavish N, Pattison NS, Pryor JE. Smoking, nicotine and tar and risk of small for gestational age babies. Acta Paediatr. 2002;91:323–328. doi: 10.1080/08035250252834003. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision-making in adulthood. Behav. Pharmacol. 2012;23:34–42. doi: 10.1097/FBP.0b013e32834eb04a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Mychasiuk R, Nakahashi A, Hossain S, Gibb R, Kolb B. Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse. 2012 doi: 10.1002/syn.21589. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Horn KH, Greene RM, Michele PM. Prenatal exposure to environmental tobacco smoke alters gene expression in the developing murine hippocampus. Reprod. Toxicol. 2010;29:164–175. doi: 10.1016/j.reprotox.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J. Pharmacol. Exp. Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- O’Callaghan FV, Al MA, O’Callaghan M, Alati R, Williams GM, Najman JM. Is smoking in pregnancy an independent predictor of academic difficulties at 14years of age? A birth cohort study. Early Hum. Dev. 2010;86:71–76. doi: 10.1016/j.earlhumdev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Olds D. Tobacco exposure and impaired development: A review of the evidence. Ment. Retard. Dev. Dis. Res. Rev. 1997;3:257–269. [Google Scholar]
- Olds DL, Handerson CR, Jr., Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- Ostfeld BM, Esposito L, Perl H, Hegyi T. Concurrent risks in sudden infant death syndrome. Pediatrics. 2010;125:447–453. doi: 10.1542/peds.2009-0038. [DOI] [PubMed] [Google Scholar]
- Parameshwaran K, Buabeid MA, Karuppagounder SS, Uthayathas S, Thiruchelvam K, Shonesy B, Dityatev A, Escobar MC, Dhanasekaran M, Suppiramaniam V. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cell Mol. Life Sci. 2012;69:829–841. doi: 10.1007/s00018-011-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Peck JD, Neas B, Robledo C, Saffer E, Beebe L, Wild RA. Intrauterine tobacco exposure may alter auditory brainstem responses in newborns. Acta Obstet. Gynecol. Scand. 2010;89:592–596. doi: 10.3109/00016340903511068. [DOI] [PubMed] [Google Scholar]
- Rahu K, Rahu M, Pullmann H, Allik J. Effect of birth weight, maternal education and prenatal smoking on offspring intelligence at school age. Early Hum. Dev. 2010;86:493–497. doi: 10.1016/j.earlhumdev.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Hlth. Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol. Biochem. Behav. 2004;78:675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J. Pharmacol. Exp. Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J. Pharmacol. Exp. Ther. 2002;300:124–133. doi: 10.1124/jpet.300.1.124. [DOI] [PubMed] [Google Scholar]
- SAMSA . Results from the 2007 National Survey on Drug Use and Health: National Findings. U.S. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2008. [Google Scholar]
- SAMSA . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- Santiago SE, Huffman KJ. Postnatal effects of prenatal nicotine exposure on body weight, brain size and cortical connectivity in mice. Neurosci. Res. 2012;73:282–291. doi: 10.1016/j.neures.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob. Control. 2005;14:396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Shopland DL. Smoking and Tobacco Control Monograph 7: The FTC Cigarette Test Method for Determining Tar, Nicotine and Carbon Monoxide Yields of Cigarettes. Smoking and Tobacco Control. 1997;7:1–275. [Google Scholar]
- Slawecki CJ, Ehlers CL. Lasting effects of adolescent nicotine exposure on the electroencephalogram, event related potentials, and locomotor activity in the rat. Brain Res. Dev. Brain Res. 2002;138:15–25. doi: 10.1016/s0165-3806(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. Neonatal nicotine exposure alters hippocampal EEG and event-related potentials (ERPs) in rats. Pharmacol. Biochem. Behav. 2000;65:711–718. doi: 10.1016/s0091-3057(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr., Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit Rev. Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Greer N, Faust J, Cho H, Seidler FJ. Effects of maternal nicotine injections on brain development in the rat: ornithine decarboxylase activity, nucleic acids and proteins in discrete brain regions. Brain Res. Bull. 1986;17:41–50. doi: 10.1016/0361-9230(86)90159-0. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, McCook EC, Seidler FJ. Cryptic brain cell injury caused by fetal nicotine exposure is associated with persistent elevations of c-fos protooncogene expression. Brain Res. 1997;750:180–188. doi: 10.1016/s0006-8993(96)01345-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res. Bull. 2007;74:91–103. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:501–518. doi: 10.1016/S0278-5846(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol. Biochem. Behav. 1991;40:991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . National Household Survey on Drug Abuse. U.S. Deptarment of Helath and Human Services, Office of Applied Studies; Rockville, MD: 2000. [Google Scholar]
- Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhalabtion Technology. Taylor and Francis; New York: 1994. pp. 1–20. [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav. Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol. Learn. Mem. 2008;90:527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- van de Kamp JL, Collins AC. Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacol. Biochem. Behav. 1994;47:889–900. doi: 10.1016/0091-3057(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol. Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Design considerations in the use of behavioral test batteries for the detection of CNS dysfunction in laboratory animals. Ment. Retard. Dev. Dis. Res. Rev. 1996;2:227–233. [Google Scholar]
- Vorhees CV. Methods for detecting long-term CNS dysfunction after prenatal exposure to neurotoxins. Drug Chem. Toxicol. 1997;20:387–399. doi: 10.3109/01480549709003895. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/--methylenedioxymethamphetamine, (+)-amphetamine and +/--fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol. Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am. J. Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr., Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Cousins MM, Slikker W, Jr., Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- Zahalka EA, Seidler FJ, Lappi SE, McCook EC, Yanai J, Slotkin TA. Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: differential effects on choline acetyltransferase activity and [3H]hemicholinium-3 binding. Neurotoxicol. Teratol. 1992;14:375–382. doi: 10.1016/0892-0362(92)90047-e. [DOI] [PubMed] [Google Scholar]
- Zdravkovic T, Genbacev O, McMaster MT, Fisher SJ. The adverse effects of maternal smoking on the human placenta: a review. Placenta. 2005;26(Suppl A):S81–S86. doi: 10.1016/j.placenta.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Reece EA. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Res. B Dev. Reprod. Toxicol. 2005;74:383–391. doi: 10.1002/bdrb.20052. [DOI] [PubMed] [Google Scholar]