Abstract
Drug addiction is a psychiatric disease state, wherein a drug is impulsively and compulsively self-administered despite negative consequences. This repeated administration results in permanent changes to nervous system physiology and architecture. The molecular pathways affected by addictive drugs are complex and inter-dependent on each other. Recently, various new proteins and protein families have been discovered to play a role in drug abuse. Emerging players in this phenomenon include TRP (Transient Receptor Potential) family channels, which are primarily known to function in sensory systems. Several TRP family channels identified in both vertebrates and invertebrates are involved in psychostimulant-induced plasticity, suggesting their involvement in drug dependence. This review summarizes various observations, both from studies in humans and other organisms, which support a role for these channels in the development of drug-related behaviors.
Keywords: Drug, Abuse, Addiction, Nicotine, Cocaine, Ethanol, Behavior, TRP, Channel, Nucleus Accumbens, Prefrontal Cortex, C. elegans
Introduction
In humans, drugs of abuse target different neurotransmitter systems, but they all converge on midbrain dopamine (DA) neurons in the ventral tegmental area or in the projections of these neurons to forebrain structures, such as the amygdala, striatum, especially the nucleus accumbens, and prefrontal cortex (Lammel, et al. 2008). Some drugs have a straightforward action on DA signaling, such as cocaine and amphetamine, which act as indirect monoamine agonists by blocking the clearance of DA from the parenchyma, thereby prolonging the activity of the transmitter at its cognate receptors (Porter-Stransky, et al. 2011,Stuber, et al. 2005). The action of other drugs, such as nicotine and ethanol, seems to be more complex. These drugs mainly interact with G protein-coupled receptors, monoamine transporters, or alter the function of ion channels to modulate DA levels in appetitive motivation (Luscher and Ungless 2006), learning (Jones, et al. 2010), and executive control circuits in the brain (Koob and Volkow 2010). An increasing number of studies suggest that transient receptor potential (TRP) channels are important targets of second messengers in these mammalian neural circuits that become compromised in addiction.
TRP channels are perhaps best known for their role as one of the prominent protein superfamilies modulating sensory signaling pathways (Montell 2001,Montell 2005,Nilius and Owsianik 2011). The members of the TRP channel superfamily have six transmembrane domains that form homo- or heterotetrameric cation channels, with strong homology to its founding member, the Drosophila protein, TRP. The TRP superfamily includes seven subfamilies: canonical (TRPC), vanilloid (TRPV), ankyrin (TRPA), melastatin (TRPM), polycystin (TRPP), MucoLupin (TRPML) and NompC-like (TRPN). These functionally divergent, non-selective cation channels are conserved from nematodes to vertebrates and are considered to be coincidence detectors and convergent signal integrators (Kang, et al. 2010,Xiao and Xu 2009). The diverse activation mechanisms and biophysical properties of different TRP family members allow these proteins to modulate complex behaviors, especially behaviors related to drug-seeking and drug-taking. (Cavalie 2007,Gulbransen, et al. 2008,Oliveira-Maia, et al. 2009). Here, we outline the emerging role for TRP channels in drug dependence.
Canonical TRP (TRPC) channels in drug dependence
Of the TRP channel superfamily, TRPC channels are most closely homologous to the Drosophila TRP, the founding member of the TRP channel superfamily (Montell and Rubin 1989). These channels are mainly activated in a phospholipase C (PLC)-dependent manner (Venkatachalam and Montell 2007). In humans, there are six TRPC channels that form homo- and heterotetramers (Venkatachalam and Montell 2007). These are multi-functional channels implicated in the regulation of diverse physiological functions, such as kidney filtration, acrosomal reaction, vascular tone and pheromone recognition (Nilius and Owsianik 2011). Specific to drug dependence, genome-wide association (GWA) studies between smoker and non-smoker cohorts implicate TRPC channels in nicotine addiction. These studies particularly identify the TRPC7 channel among other novel genes that were previously not associated with addiction (Bierut, et al. 2007,Lessov-Schlaggar, et al. 2008). TRPC7 is enriched in brain tissue, especially in striatal regions where it impinges on neurons imperative for behavioral responses to drugs of abuse (Numaga, et al. 2007). Interestingly, another GWA study implicates TRPC4 in drug dependence, based on comparisons between European-American and African-American polysubstance abusers or non-abusing controls (Uhl, et al. 2008). TRPC4 is important for the vasorelaxation of arteries and neurotransmitter release from thalamic dendrites (Cavalie 2007).
While direct evidence demonstrating a role for mammalian TRPC channels in drug addiction is still lacking, rodent fear-learning studies reveal a clear role for TRPC5 in forming associations between an unconditioned stimulus (US) and a conditioned stimulus (CS) in the amygdala (Riccio, et al. 2009). The amygdala is critical for learning associations between the CS and US (Schafe, et al. 2005), and human drug users show event-related potentials (ERP) viewing drug-related paraphernalia similar to the ERPs they show when viewing positive emotional stimuli (Dunning, et al. 2011). In a functional MRI study, the amygdala showed decreased focal signal in response to an unpredicted cocaine administration (Breiter, et al. 1997).
Cocaine modulates intrinsic plasticity of accumbens neurons (Kourrich, et al. 2007) and affects metabotropic glutamate receptor (mGluR)-dependent synaptic plasticity in the nucleus accumbens (Huang, et al. 2011) and prefrontal cortex (Huang, et al. 2007). TRPC1 is an mGluR target in cerebellar Purkinjie cells (Kim, et al. 2003), while both TRPC3 and TRPC7 are known targets of mGluR activity in striatal cholinergic interneurons (Berg, et al. 2007). Moreover, TRPC5 mRNA is located within the shell subregion of the nucleus accumbens (Fowler, et al. 2007), which is preferentially activated by cocaine (Aragona, et al. 2008) and is particularly responsive to the unconditioned aspects of stimuli (Wheeler, et al. 2011). It will be interesting to test whether TRPC channels have a role in the motivational, learning and executive control circuits drugs of abuse undermine when recreational drug users succumb to addiction.
The most direct evidence supporting a role for TRPC channels in drug-related behaviors comes from the nematode Caenorhabditis elegans. C. elegans requires the TRPC homologues TRP-1 and TRP-2 for nicotine-dependent behaviors (Feng, et al. 2006). The C. elegans genome encodes members of all the seven TRP channel subfamilies (Xiao and Xu 2011). Most of these members are involved in various chemosensory or mechanosensory pathways, either as primary sensors or as signal transducers or amplifiers (Xiao and Xu 2011). There are three TRPC subfamily members in C. elegans: TRP-1, TRP-2 and TRP-3. While TRP-3 is enriched in sperm, the neuronally-expressed TRP-1 and TRP-2 modulate nicotine-dependent behavior in C. elegans (Feng, et al. 2006,Xu and Sternberg 2003).
C. elegans exhibits a variety of behavioral responses to nicotine, including acute response, adaptation, withdrawal and sensitization. Specifically, acute nicotine treatment stimulates locomotion (Feng, et al. 2006), an innate behavior that forms the foundation of most, if not all behaviors (Piggott, et al. 2011). Repeated intermittent administration of nicotine sensitizes C. elegans to nicotine, and long-term nicotine treatment elicits tolerance to the drug (Feng, et al. 2006). Nicotine-adapted worms exhibit hyperlocomotion when placed in a nicotine-free environment, a withdrawal response to nicotine (Feng, et al. 2006). These nicotine dependent behaviors require the C. elegans nicotinic acetylcholine receptor (nAChR) genes acr-15 and acr-16 (Feng, et al. 2006). Both genes function in neurons to modulate nicotine responses in worms. Notably, trp-1 and trp-2 mutant animals are severely defective in nicotine dependent behaviors (Feng, et al. 2006). Interestingly, TRP-1 and TRP-2 appear to act downstream of the nAChRs ACR-15 and ACR-16 in a PLC-dependent manner (Feng, et al. 2006). This work further demonstrates that neuronal expression of ACR-15 and ACR-16 as well as TRP-1 and TRP-2 are required for nicotine-induced behaviors in C. elegans (Feng, et al. 2006). Moreover, neuronal Ca2+ influx is greatly diminished in response to nicotine exposure in trp-1 or trp-2 null mutant worms, suggesting that these TRPC channels functionally regulate neuronal nicotine responses (Benowitz 2010,Feng, et al. 2006). Interestingly, the mouse α4β2 nAChR, which is known to be essential for nicotine-associated behaviors, can rescue nicotine behavioral defects in acr-15 null mutant animals; similarly, the human TRPC3 channel functionally substitutes for worm TRP-2 in nicotine responses (Feng, et al. 2006), suggesting that the role of TRPC channels and nAChRs in nicotine responses may be evolutionarily conserved.
In addition to this functional interaction with nAChR, TRPC channels interact with both CREB and Homer proteins, which are important for gene transcription related to drug dependence and drug-related changes in neural plasticity (Pandey, et al. 2005,Ron and Jurd 2005,Talavera, et al. 2008). Both TRPC3 and TRPC6 overexpression potentiate phosphorylation of CREB which stimulates both early and late CREB-dependent gene transcription (Jia, et al. 2007). The role of this CREB-dependent transcription in drug-induced neural plasticity is well documented (Kumar, et al. 2011,Philpot, et al. 2012). Homer proteins are a group of EVH1 domain-containing scaffolding proteins involved in coupling metabotropic glutamate receptors (mGluR1) and inositol-1,4,5-triphosphate receptors (IP3R) with TRPC channels (Mast, et al. 2010,Yuan, et al. 2003). Homer-IP3R interactions regulate trafficking of TRPC3 to the plasma membrane, while coupling of mGluR and IP3R with TRPC channels results in mGluR-mediated neuronal conductance, which may have a role in drug-related behavioral plasticity (Kim, et al. 2006). Together, these data make a case for more in-depth studies of mammalian TRPC channels in relation to drugs of abuse.
Vanilloid TRP (TRPV) channels in drug dependence
TRPV channels share homology with the founding member of the subfamily, TRPV1, which was identified through its response to the vanilloid capsaicin. These channels respond to a range of stimuli, such as heat, mechanical stimulation, and pro-inflammatory agents as well as other chemical stimuli (Kauer and Gibson 2009,Venkatachalam and Montell 2007). Mammalian neurons expressing TRPV1 show a decrease in the amplitude of capsaicin-induced action potentials after acute nicotine treatment. Moreover, repeated and intermittent nicotine treatment sensitizes capsaicin-induced currents in these cells (Liu, et al. 2004). Moreover, TRPV1 is also known to interact with many nAChRs and is associated with anxiogenic behavioral responses, indicating that this channel might be responsible for the anxiety and ‘nervousness’ associated with nicotine withdrawal responses (Casarotto, et al. 2012). Besides, the TRPV1 activity is potentiated by ethanol, and Trpv1 null mutants show higher preference to ethanol and higher consumption in two-bottle choice assays as compared to wild-type mice (Blednov and Harris 2009). These findings suggest the role for TRPV1 channels in specific behaviors associated to ethanol dependence.
In invertebrates, the Drosophila TRPV homologue inactive (iav) mediates behavioral sensitization to cocaine (McClung and Hirsh 1998). In this model, stereotypical behavioral responses to cocaine include intense grooming at low doses, with moderate doses affecting rapid rotations and sideways or backward movements. High doses, in turn, result in tremors and paralysis. With repeated cocaine administration, these behaviors become more vigorous in response to decreased cocaine concentrations. This behavioral sensitization, however, is not present in iav null mutants despite a wild-type response to acute cocaine exposure (McClung and Hirsh 1998). This sensitization deficit appears to result from decreased levels of the monoamines tyramine and octopamine, implicating TRPV proteins in the regulation of monoamine neurotransmitter systems (McClung and Hirsh 1999). It should be noted, however, that this behavioral sensitization phenotype has not been rescued transgenically in iav mutants, which allows the possibility that the phenotype may be due to some unidentified background mutation in this line. Regardless, these invertebrate studies and the findings in rodents suggest TRPV proteins as targets for understanding the action that drugs of abuse have on the brain.
In mammals, the endocannabinoid anandamide (AEA) activates not only the CB1 and CB2 GPCRs but also TRPV1. A recent study demonstrates that TRPV1 is critical for long-term depression (LTD) of medium spiny neurons (MSN) in the rodent nucleus accumbens and cocaine administration disrupts this phenomenon (Grueter, et al. 2010). TRPV1 channels are also critical for coupling ACh signals with the endocannabinoid 2-archidonylglycerol (2AG) in the striatum. This coupling is vital for both LTD and long-term potentiation (LTP) at corticostriatal synapses (Musella, et al. 2010). Furthermore, the TRPV1 agonist capsaicin induces LTP in the amygdala (Zschenderlein, et al. 2011). In addition, repeated methamphetamine exposure increases TRPV1 mRNA within the prefrontal cortex (Tian, et al. 2010), a brain region responsible for inhibiting unwanted actions whose dysfunction can lead to hyperactivity and compulsive behaviors such as drug-taking (Koob 2009). Collectively, these studies suggest that TRPV1 channels may play a role in usurping natural motivational, learning and executive control circuits to effect addiction.
Other TRP channel subfamilies in drug dependence
Besides TRPC and TRPV subfamilies, other TRPs (mainly TRPA and TRPM) are involved either in primary sensing of addictive drugs or in their long-term effects. In vertebrates, nicotine activates both TRPM5-dependent and independent gustatory pathways. The TRPM5-dependent mechanism affects a general taste pathway and is required for nicotine-specific behavioral and gustatory cortex circuit responses. It has also been shown to be involved in peripheral sensing of nicotine in the nasal cavity (Gulbransen, et al. 2008,Oliveira-Maia, et al. 2009).
TRPA1, meanwhile, is involved in nicotine-induced irritation and facilitates the mouse airway constriction reflex to nasal administration of nicotine (Talavera, et al. 2009). This channel is also known to be responsible for the airway neurological inflammation caused by α,β-unsaturated aldehydes, one of the main caustic agents in cigarette smoke (Andre, et al. 2008). These facts make TRPA1 a potential nicotine target for developing smoking cessation therapeutics with milder side effects. While TRPA1 acts as an irritant-sensing channel in cigarette smoke, the menthol receptor TRPM8 acts as a counterirritant channel in menthol-flavored cigarettes (Willis, et al. 2011). Activation of TRPM8 by menthol suppresses the irritant sensation caused by TRPA1 during smoking, thus masking the caustic irritants and promoting smoking behavior. These differential actions of TRP channels in the periphery might be important in the preliminary stages of nicotine dependence. In addition, ethanol inhibits TRPM8, while potentiating the activity of TRPV1 (Benedikt, et al. 2007). Further evidence of the complicated role TRP channels play in drug use is seen with ‘hangover pain’, a pathological symptom after ethanol consumption, which is mediated by TRPA1 (Bang, et al. 2007).
Conclusion
The effects of addictive drugs on primary targets, such as their cognate receptors, and secondary targets, such as kinases and lipases that those receptors modulate, are well known. However, the role of those gene families with less obvious involvement in drug addiction, such as TRP family channels, remains unclear. Interestingly, there is growing evidence implicating TRP channels in drug dependence. TRPC channels, in particular TRPC4/7 were identified in two GWA studies. Similarly, two C. elegans TRPC homologues (TRP-1 and TRP-2) are essential for nicotine dependent behaviors, and their mammalian counterparts can functionally substitute for them, suggesting a functional conservation among species.
On the other hand, TRPV channels are implicated in the control of extracellular monoamine levels, as well as in anxiety-related behaviors, suggesting that these channels might be responsible for the neural changes that lead to the adverse effects of withdrawal and behavioral sensitization following repeated drug use. This TRP channel subfamily is not only implicated in behavioral responses to several drugs of abuse, but also performs conserved roles in motivational, learning and executive control circuits usurped by drugs of abuse to elicit addiction.
Beyond the TRPC and TRPV families, it is important to note that many more members of the TRP superfamily are implicated in responses to drugs of abuse. TRP superfamily proteins are involved both at the primary sensing level (TRPA1 and TRPM8) and in maintaining long-term neural changes (TRPM5). These properties, with the ever-growing evidence related to their association with drugs of abuse, support a role for TRP channels in the development of drug dependence.
Nevertheless, we are only beginning to appreciate the role of TRP channels in drug dependence, and many unanswered questions remain. For example, despite the mounting data in invertebrates, genetic and behavioral studies showing a role for TRP channels in mammalian addiction-related behaviors are limited. While mice lacking functional TRPC, TRPV, TRPA1, and TRPM5 channels exist, the performance of these null mutants in standard paradigms to test drug-taking or drug-seeking behaviors has not been examined. Moreover, there persists a lack of understanding as to how these channels function to influence terminal release of monoamines related to addiction as well as to how they alter the firing rates of cells within brain regions known to have an impact in addiction-related behaviors. Future studies, particularly, genetic, behavioral and pharmacological studies in rodents promise exciting insights into the possible interactions among these TRP channels and the classical neurotransmitter systems canonically associated with drugs of abuse in mammals.
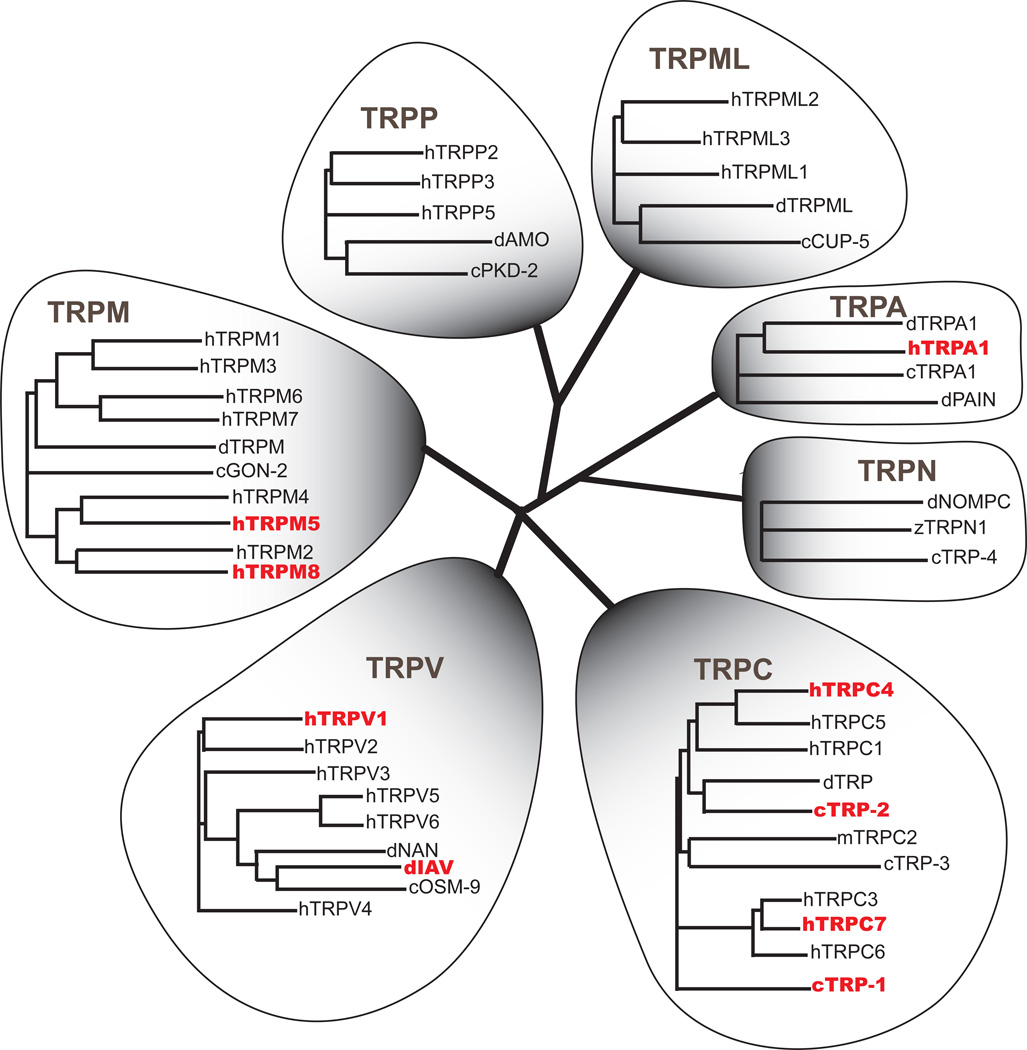
Figure 1.
Phylogenetic tree of all the human TRP channel familes along with representative members from other species. Protein marked in red are involved in drug addiction. Different species are represented by prefixes: c. C. elegans, d. Drosophila melanogaster, z. Danio rerio, m. Mus musculus, h. Homo sapiens.
Table 1.
Putative Roles for Mammalian TRP Channels in Drug Abuse
| Mammalian Channel |
Brain Region/Pathway |
Putative Role(s) |
References |
|---|---|---|---|
| TRPA1 | Nociceptive Pathways | Withdrawal Pain Nicotine Dependence |
Bang et al., 2007 Talavera et al., 2009 |
| TRPC1 | Nucleus Accumbens Prefrontal Cortex |
Synaptic Plasticity | Kim et al., 2003 |
| TRPC3 | Striatum | Synaptic Plasticity Nicotine Dependence |
Berg et al., 2007 Feng et al., 2006 Jia et al., 2007 |
| TRPC4 | Thalamus | Dendritic Neurotransmission |
Cavalie, 2007 Uhl et al., 2008 |
| TRPC5 | Nucleus Accumbens Amygdala |
Appetitive Processing CS-US Associations |
Riccio et al., 2009 Schafe et al., 2005 |
| TRPC7 | Striatum | Synaptic Plasticity |
Beirut et al., 2007 Berg et al., 2007 Lessov-Schlagger et al., 2008 Numaga et al., 2007 |
| TRPM5 | Gustatory Pathways | Peripheral CS Processing |
Gulbransen et al., 2008 Oliveira-Maia et al., 2009 |
| TRPM8 | Nociceptive Pathways | Peripheral CS Processing Ethanol Dependence |
Benedickt et al., 2007 Willis et al., 2011 |
| TRPV1 | Prefrontal Cortex Striatum Amygdala |
Synaptic Plasticity Ethanol Dependence |
Benedickt et al., 2007 Blednov & Harris, 2009 Grueter et al., 2010 Kauer & Gibson, 2009 Liu et al., 2004 McClung & Hirsch, 1998 McClung & Hirsch, 1999 Tian et al., 2010 Venkatachalam & Montell, 2007 |
Acknowledgements
The authors thank NIDCD, NIDA and NIGMS for funding.
References
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. The Journal of clinical investigation. 2008;118(7):2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28(35):8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. The European journal of neuroscience. 2007;26(9):2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- Benedikt J, Teisinger J, Vyklicky L, Vlachova V. Ethanol inhibits cold-menthol receptor TRPM8 by modulating its interaction with membrane phosphatidylinositol 4,5-bisphosphate. Journal of neurochemistry. 2007;100(1):211–224. doi: 10.1111/j.1471-4159.2006.04192.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. The New England journal of medicine. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27(33):8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human molecular genetics. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology. 2009;56(4):814–820. doi: 10.1016/j.neuropharm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Terzian AL, Aguiar DC, Zangrossi H, Guimaraes FS, Wotjak CT, Moreira FA. Opposing roles for cannabinoid receptor type-1 (CB) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(2):478–486. doi: 10.1038/npp.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalie A. Ionic channels formed by TRPC4. Handbook of experimental pharmacology. 2007;(179):93–108. doi: 10.1007/978-3-540-34891-7_5. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users--an ERP study. Eur J Neurosci. 2011;33(9):1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127(3):621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One. 2007;2(6):e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13(12):1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. Journal of neurophysiology. 2008;99(6):2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27(11):2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31(11):4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nature neuroscience. 2007;10(5):559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67(8):737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67(3):381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends in neurosciences. 2009;32(4):215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. The Journal of biological chemistry. 2006;281(43):32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426(6964):285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Deb I, Chakraborty J, Mukhopadhyay S, Das S. A polymorphism of the CREB binding protein (CREBBP) gene is a risk factor for addiction. Brain research. 2011;1406:59–64. doi: 10.1016/j.brainres.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Pergadia ML, Khroyan TV, Swan GE. Genetics of nicotine dependence and pharmacotherapy. Biochemical pharmacology. 2008;75(1):178–195. doi: 10.1016/j.bcp.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhu W, Zhang ZS, Yang T, Grant A, Oxford G, Simon SA. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. Journal of neurophysiology. 2004;91(4):1482–1491. doi: 10.1152/jn.00922.2003. [DOI] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS medicine. 2006;3(11):e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast TG, Brann JH, Fadool DA. The TRPC2 channel forms protein-protein interactions with Homer and RTP in the rat vomeronasal organ. BMC neuroscience. 2010;11:61. doi: 10.1186/1471-2202-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Current biology : CB. 1998;8(2):109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Current biology : CB. 1999;9(16):853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Science's STKE : signal transduction knowledge environment. 2001;2001(90):re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Science's STKE : signal transduction knowledge environment. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2(4):1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Musella A, De Chiara V, Rossi S, Cavasinni F, Castelli M, Cantarella C, Mataluni G, Bernardi G, Centonze D. Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience. 2010;167(3):864–871. doi: 10.1016/j.neuroscience.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome biology. 2011;12(3):218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaga T, Wakamori M, Mori Y. Trpc7. Handbook of experimental pharmacology. 2007;(179):143–151. doi: 10.1007/978-3-540-34891-7_8. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, Desimone JA, Nicolelis MA, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(5):1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcoholism, clinical and experimental research. 2005;29(2):176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Engberg ME, Wecker L. Effects of nicotine exposure on locomotor activity and pCREB levels in the ventral striatum of adolescent rats. Behavioural brain research. 2012 doi: 10.1016/j.bbr.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott BJ, Liu J, Feng Z, Wescott SA, Xu XZ. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell. 2011;147(4):922–933. doi: 10.1016/j.cell.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Wescott SA, Hershman M, Badrinarayan A, Vander Weele CM, Lovic V, Aragona BJ. Cocaine must enter the brain to evoke unconditioned dopamine release within the nucleus accumbens shell. Neurosci Lett. 2011;504(1):13–17. doi: 10.1016/j.neulet.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van't Veer A, Meloni EG, Carlezon WA, Jr, Bolshakov VY, Clapham DE. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137(4):761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jurd R. The "ups and downs" of signaling cascades in addiction. Science's STKE : signal transduction knowledge environment. 2005;2005(309):re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Doyere V, LeDoux JE. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25(43):10010–10014. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30(5):853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nature neuroscience. 2009;12(10):1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Talavera K, Nilius B, Voets T. Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends in neurosciences. 2008;31(6):287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tian YH, Lee SY, Kim HC, Jang CG. Repeated methamphetamine treatment increases expression of TRPV1 mRNA in the frontal cortex but not in the striatum or hippocampus of mice. Neurosci Lett. 2010;472(1):61–64. doi: 10.1016/j.neulet.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, Li CY, Buck K, Crabbe J. "Higher order" addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochemical pharmacology. 2008;75(1):98–111. doi: 10.1016/j.bcp.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annual review of biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69(11):1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(12):4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZ. Function and regulation of TRP family channels in C. elegans. Pflugers Arch. 2009;458(5):851–860. doi: 10.1007/s00424-009-0678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZ. C. elegans TRP channels. Advances in experimental medicine and biology. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Sternberg PW. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114(3):285–297. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114(6):777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zschenderlein C, Gebhardt C, von Bohlen Und Halbach O, Kulisch C, Albrecht D. Capsaicin-induced changes in LTP in the lateral amygdala are mediated by TRPV1. PLoS One. 2011;6(1):e16116. doi: 10.1371/journal.pone.0016116. [DOI] [PMC free article] [PubMed] [Google Scholar]