Abstract
Background
Following administration of an antibiotic, the concentration in blood changes over time and is dependent on the type of antibiotic, the route, and species of the individual. The most relevant pharmacodynamic property of a bacteriostatic antibiotic such as doxycycline is the minimum inhibitory concentration (MIC), whereas pharmacokinetics may include rates of absorption and elimination from blood.
Methods
We determined serum concentrations of doxycycline following administration of 5 mg/kg in two macaques.
Results
The area under the concentration-time curve over 24 hours (AUC0-24) following 2 doses was extrapolated from the curve over 12 hours following a single dose, with the purpose of calculating the AUC0-24:MIC.
Conclusion
Other than a somewhat faster rate of elimination, the PK-PD values for doxycycline in macaques appears similar to those determined for humans. This information will be valuable for treating disease in macaques and for research in bacterial infection models that use macaques.
Keywords: vibramycin, pharmacodynamic, AUC, nonhuman primate, Borrelia
Introduction
The tetracycline class of antibiotics are broad-spectrum antimicrobial compounds used to treat a variety of infections [12], including susceptible intracellular and/or zoonotic pathogens. Determination of the probable efficacy of an antibiotic is based on measurement of the blood level of that antibiotic over time, and association of those pharmacokinetic/pharmacodynamic (PK-PD) parameters with clearance of infection. Much of this work is based on murine models of infection, but has nonetheless led to a significant knowledge base regarding recommended doses and treatment regimens for human infection [3]. Of importance is the PK-PD measure that best predicts efficacy, such as the importance of time versus concentration of antibiotic.
One such pathogen for which the second-generation tetracycline antibiotic doxycycline is commonly used is Borrelia burgdorferi, the Lyme disease agent. The nonhuman primate exhibits the hallmark signs of Lyme disease [13] and has been tested by us for indications of antimicrobial efficacy against B. burgdorferi [7]. Interestingly, available data on the PK-PD properties of oral doxycycline using dose/weight controlled experiments in rhesus macaques is lacking [9]. The PK-PD measure thought to be the best predictor of the time-dependent antibiotic is the ratio of the area under the concentration time-curve over 24 h to the MIC (AUC0-24:MIC). As such, we conducted a time-course assessment of serum doxycycline levels over 24 hours using a 5 mg/kg dose at 0 and 12 hr (bis in die regimen).
Material and Methods
Ethics statement
Practices in the housing and care of animals conformed to the regulations and standards of the PHS Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. The Tulane National Primate Research Center is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care-International. The Institutional Animal Care and Use Committee (IACUC) of the Tulane National Primate Research Center approved all animal-related protocols, including anesthesia, oral gavage and blood collection. All animal procedures were overseen by veterinarians and trained staff.
Doxycycline administration and blood collection
Two male Indian rhesus macaques aged 2.27 and 2.55 years and weighing 3.49 and 4.42 kg, respectively, were used in this study. Animals were anesthetized and fed 5 mg/kg liquid doxycycline monohydrate (Vibramycin) oral suspension (Pfizer), 5 mg/mL, twice, at 0 hr and 12 hr. Animals were anesthetized with 8 mg/kg Telazol for the first hour and then ≤5 mg/kg ketamine as needed for subsequent collections. For administration of the drug, animals were held upright while an 8 French feeding tube was passed through the oral cavity to the gastric lumen. The appropriate volume of doxycycline was administered via a syringe attached to the feeding tube. Blood samples were drawn at the following intervals between 30 min-24 hours: 0 hr, 30 min, 1 hr, 2 hr, 4 hr, 6 hr, 8 hr, 12 hr, 24 hr. In order to minimize blood collection volumes, yet obtain accurate curves following a single dose, the 12-24 curve was extrapolated from the 0-12 hr values.
Determination of serum doxycycline concentrations
A suspension of bacteria (Bacillus subtilis, ATCC #6633), grown in trypticase soy broth (BD, Franklin Lakes, NJ), was streaked onto Mueller-Hinton agar plates (BD) such that a lawn of bacteria would grow after 18-24 hours of incubation at 37 °C. To determine antibiotic concentration in serum, 75 μL of serum from treated animals was applied to 6-mm diameter plain paper discs (BD) 25 μL at a time and allowed to dry for 30 minutes. Standards were made by dissolving doxycycline hyclate (Sigma-Aldrich) into normal monkey serum; these were applied to the discs in the same manner. Standard doxycycline concentrations used were 0, 0.1, 0.25, 0.5, 1, 5, 10, and 25 μg/mL. The test discs were placed onto the prepared agar plates and incubated overnight at 37°C. The zones of inhibition for duplicate test discs were measured three times in radial mm and averaged. A standard curve was generated using the measurements of the control/standards. The test measurements were compared to the standard curve giving an approximate doxycycline concentration from the test serum.
Calculation of the AUC0-24:MIC
Zones of inhibition (mm) were converted to concentration by linear regression of the standard curves. We next derived constants k1, k2, k3 that best fit a curve of the form C(t) = k1(e−k2t – e−k3t), where C(t) is concentration and t is time, to the data using the method of least squares. The constants k2 and k3 rate represent the elimination (kel) and the absorption rate (ka), respectively. Estimates for kel and ka were derived as described [10]. For ka a linear regression was performed for the changes in concentration over time for the upslope (absorption) between 0.5 hr-2hr, a segment in the linear range. The change in concentration over time was 1.209869/1.5 hr, so ka =0.80658μg/hr. For kel, a linear fit for the decline in concentration over time from peak to trough (2-12 hr) was determined. The serum concentrations from 1.72 μg/mL-0.86μg/mL were in the linear range of decline and from that line the half-life (t1/2) was calculated to be 6.76 hr. So, kel= 0.693/6.76=0.1025. Subsequently, we compared the constants k2, k3 derived by the method of least squares for curve fit to Kel and ka for closeness, finding them to be within 10% of one another (k2 = 0.119, k3 = 0.818)Using these values, the area under the curve C(t) was determined from t = 0 to t = 12 and compared to the value obtained by the Trapezoidal rule applied to the data for reasonableness. To logically estimate the AUC from 0-24 hours, the area under the curve C(t) from t = 0.5 to t = 12.5 was extrapolated and added to the AUC for 0-12 h, as shown in Figure 2. The theoretical parameters were chosen because the value at 24 h is not 0, yet closer to the 0.5 h value. The predicted values are actually lower than the real values, indicating that our extrapolation is an underestimate.
Figure 2.
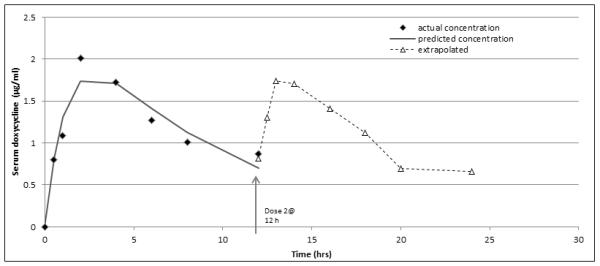
Results
Doxycycline efficacy is time-dependent rather than concentration-dependent, meaning that bactericidal activity is predicated on the amount of time bacteria are exposed to a concentration above the minimum inhibitory concentration (MIC). We therefore aimed to determine the AUC0-24:MIC. This required us to measure serum concentrations at intervals from 0-24 hr. We administered doxycycline to the macaques at a dose of 5 mg/kg. This antibiotic dose was chosen because it is higher than the standard (2 mg/kg) recommended to achieve appropriate bloods levels in humans [17], but less than the dose used in our previous study for treatment of early disseminated infection [3].
Peak and trough values after a single dose
We used a modification of the Kirby-Bauer disc diffusion assay to quantify the concentration of doxycycline at 0.5, 1, 2, 4, 6, 8, 12 and 24 h following an oral dose of 5 mg/kg at the 0 and 12 h time points. The absorption rate was calculated at 0.8066 (μg/mL/hour) and is evident by a rapid increase in the serum concentration within 2 hours for both animals (Figure 1). The peak value, found at 2 h post-ingestion, was close to 2 μg/mL (Table 1). The trough values were found between 8-12 hours, and did not drop below 0.8 μg/mL (Table 1). The 12 h value for animal 2 was very close (1.089, Figure 1).
Figure 1.
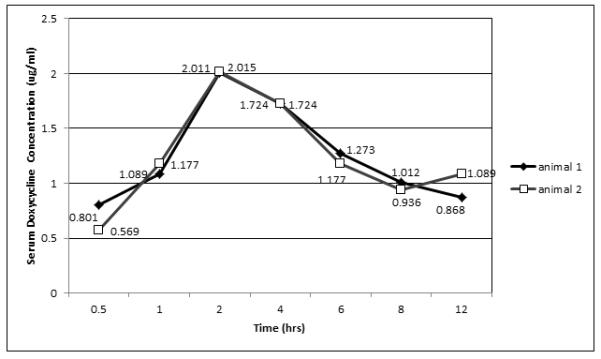
Table 1.
Pharmacokinetic parameters of a single 5 mg/kg dose of oral doxycycline monohydrate.
| Animal | Peak value (time) | Trough value (time) |
|---|---|---|
| 1 | 2.01 μg/mL (2 h) | 0.868 μg/mL (12 h) |
| 2 | 2.02 μg/mL (2 h) | 0.936 μg/mL (8 h) |
Determination of efficacy by extrapolation of the AUC0-24:MIC
The absorption and elimination rates were calculated from the actual 0-12 h data and then compared to the constants derived by the method of least squares for curve fit (Table 2). Each of these was reasonably close to the derived values. We used the values from 0-12 hours to establish an actual curve, incorporated those data to establish a curve of best fit with the equation C(t) = k1(e−k2t – e−k3t) and extrapolated the 12-24 hour curve using those values (Figure 2). As stated the actual values are higher than the predicted values, indicating that our results are an underestimate of the real AUC from 0-24 h (Fig. 2). The areas under each curve were determined and added together to derive the AUC from 0-24 h (Table 2). The AUC0-24:MIC was also calculated for MIC values of 0.25 and 0.5 μg/mL (Table 2).
Table 2.
Relation of serum concentration over time to the efficacy standard.
| kel | k2 | Value difference (k2/3-kel/a/k2/3) |
|||
|---|---|---|---|---|---|
| 0.1025 | 0.119 | 0.138 | |||
| ka | k3 | ||||
| 0.8066 | 0.818 | 0.014 | |||
| Animal | AUC0-12 (μg·h/mL) |
AUC12-24 (μg·h/mL) |
AUC0-24 (μg·h/mL) |
AUC0-24:MIC (MIC of 0.25 μg/mL) |
AUC0-24:MIC (MIC of 0.5 μg/mL) |
| 1 | 15.11 | 15.23 | 30.34 | 121.36 | 60.68 |
| 2 | 15.22 | 15.39 | 30.61 | 122.44 | 61.22 |
Discussion
The doxycycline MIC for the B31 strain of B. burgdorferi has been reported to be between 0.125-1 μg/mL [5, 6], with a value of 4 μg/mL reported for one strain [5]. In our studies with the B. burgdorferi strain B31.5A19, the value of the MIC is consistently 0.25 μg/mL (data not shown). However, published data indicate that significant variability in this property exists, depending on the strain of B. burgdorferi sensu lato [14]. Compilation of data from several studies indicate that the doxycycline MIC for the majority of B. burgdorferi strains tested is between 0.125-0.5 μg/mL [6, 8, 14]. Using the dose of 5 mg/kg, we found that the levels peaked around 2 h at ~2 μg/mL and that even at the trough, the serum concentrations did not drop below 0.8 μg/mL. This level of antibiotic was still above the B. burgdorferi MIC for most strains, indicating that by peak and trough indicators, this dose should be efficacious.
In comparison to human data, the pharmacokinetics of oral doxycycline in rhesus macaques appears to be quite similar. In our study, the values were: (1) for the rate of absorption (Ka)=0.8066; (2) for the rate of elimination (Kel)=0.1025, and (3) for the elimination half-life (t1/2{elim})=6.76 h. In a study of doxycycline pharmacokinetics in humans, comparing fasted to non-fasted individuals (our animals were fasted, due to anesthesia), the ranges for each pharmacokinetic parameter were as follows: (1) for the rate of absorption (Ka)=0.26-1.03; (2) for the rate of elimination (Kel)=0.54-0.082, and (3) for the elimination half-life (t1/2{elim})=8.8-14.2 h [16]. Thus, the rate of absorption (Ka) in macaques was found to be close to what has been determined in humans, but the rate of elimination (Kel) was somewhat faster [16] further emphasizing the need to determine the area under the time-concentration curve in evaluation of efficacy. Importantly, the rate of elimination in macaques is much closer to that of humans than what has been observed with multiple antibiotics in mice [4].
Given that the bactericidal activity of doxycycline is more time-dependent than concentration-dependent, the pharmacokinetic-pharmacodynamic measure that has been proposed to be most closely correlated with efficacy is the AUC0-24:MIC [2]. Due to limitations on the frequency and amount of blood that could be collected from our macaques, we were able to collect values over 12 h to generate a curve, but had to extrapolate from that curve for the subsequent 12 h. Given that the starting point for 12-24 h was not a concentration of zero, our extrapolation is more likely an underestimate than an overestimate. In our study, dosing was based on weight in two macaques of similar age and weight. While we have little rhesus macaque data for comparison, one study looked at peak-trough levels following a 25 mg dose of doxycycline b.i.d. for 2 days. The body weights of these animals varied significantly, but averaged 7.7 kg, which would be 3.24 mg/kg per dose. For day 1, average peak and trough levels were 0.54 and 0.435 μg/mL but rose to 1.89 and 0.685 on day 2 [9], suggesting that concentrations would increase somewhat with each subsequent dose.
With regard to B. burgdorferi infection and susceptibility, an exact value for the AUC0-24:MIC associated with clearance of infection has not been established. However, based on available human and murine data, pharmacokinetic modeling has been used to establish a value for efficacy for a preventive dose of doxycycline [11]. Here, the amount of free drug in serum following a single dose, divided by the MIC (fAUC0-∞:MIC) was used as the efficacy parameter. In mice, a value of 84 for the fAUC0-∞:MIC was associated with 100% efficacy, whereas a value of 36 was associated with 87% efficacy for humans. Specific values to establish efficacy for the AUC0-24:MIC using doxycycline and B. burgdorferi in an animal model has not been published. However, in mouse models of S. aureus and S. pneumonia infection, the mean AUC0-24:MIC associated with an efficacious bacteriostatic effect of Linezolid was 83 for S. aureus and 48 for pneumococci [5]. In addition, the minocycline-derived antibiotic Tigecycline (GAR-936) was deemed efficacious in a neutropenic mouse model of infection (multiple bacterial species) when serum levels were above the MIC for >50% of the time [15]. We found the value for AUC0-24 to be approximately 30 μg·h/mL with our 2 macaques. Studies in humans using oral doses of 100 mg have resulted in AUC0-24 values of 13-40 μg·h/mL [1]. Given that our dose of 5 mg/kg was above the MIC beginning at 0.5 h through 24 h, and that the AUC0-24:MIC was well over 100 for the standard MIC (0.25 μg/mL) for B. burgdorferi, the 5 mg/kg dose should be sufficient for efficacy. This calculation assumes that spirochetes are uniformly exposed to serum doxycycline concentrations, as measured.
Acknowledgments
Funding provided by NIH/NCRR Grant8 P20 GM103458-09 (MEE)
References
- 1.Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. Journal of Antimicrobial Chemotherapy. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose PG, Bhavnani SB, Rubino CM, Louie A, Gumbo T, Forrest A. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clinical Infectious Diseases. 2007:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 3.Andes D, Anon J, Jacobs MR, Craig WA. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of respiratory tract infections. Clin Lab Med. 2004;24:477–502. doi: 10.1016/j.cll.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents. 2002;19:261–268. doi: 10.1016/s0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 5.Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid) Antimicrobial Agents & Chemotherapy. 2002;46:3484–3489. doi: 10.1128/AAC.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baradaran-Dilmaghani R, Stanek G. In vitro susceptibility of thirty Borrelia strains from various sources against eight antimicrobial chemotherapeutics. Infection. 1996;24:60–63. doi: 10.1007/BF01780660. [DOI] [PubMed] [Google Scholar]
- 7.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. Persistence of Borrelia burgdorferi in Rhesus Macaques following Antibiotic Treatment of Disseminated Infection. PLoS ONE. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrobial Agents & Chemotherapy. 2005;49:1294–1301. doi: 10.1128/AAC.49.4.1294-1301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly DJ, Chulay JD, Mikesell P, Friedlander AM. Serum concentrations of penicillin, doxycycline, and ciprofloxacin during prolonged therapy in rhesus monkeys. Journal of Infectious Diseases. 1992;166:1184–1187. doi: 10.1093/infdis/166.5.1184. [DOI] [PubMed] [Google Scholar]
- 10.Korth-Bradley JM. Clinical Pharmacokinetics Handbook. The Annals of Pharmacotherapy 40:Chpt. 2: CLINICAL PHARMACOKINETIC EQUATIONS AND CALCULATIONS; 2006. [Google Scholar]
- 11.Lee J, Wormser GP. Pharmacodynamics of doxycycline for chemoprophylaxis of Lyme disease: preliminary findings and possible implications for other antimicrobials. Int J Antimicrob Agents. 2008;31:235–239. doi: 10.1016/j.ijantimicag.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Nelson ML, Levy SB. The history of the tetracyclines. Annals of the New York Academy of Sciences. 2011;1241:17–32. doi: 10.1111/j.1749-6632.2011.06354.x. [DOI] [PubMed] [Google Scholar]
- 13.Philipp MT, Johnson BJ. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends in Microbiology. 1994;2:431–437. doi: 10.1016/0966-842x(94)90800-1. [DOI] [PubMed] [Google Scholar]
- 14.Sicklinger M, Wienecke R, Neubert U. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. Journal of Clinical Microbiology. 2003;41:1791–1793. doi: 10.1128/JCM.41.4.1791-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Ogtrop ML, Andes D, Stamstad TJ, Conklin B, Weiss WJ, Craig WA, Vesga O. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrobial Agents & Chemotherapy. 2000;44:943–949. doi: 10.1128/aac.44.4.943-949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welling PG, Koch PA, Lau CC, Craig WA. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrobial Agents & Chemotherapy. 1977;11:462–469. doi: 10.1128/aac.11.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wormser Gary P., Dattwyler Raymond J., Shapiro Eugene D., Halperin John J., Steere A, Klempner Mark S., Krause P, Bakken J, Strle F, Stanek G, Bockenstedt L, Fish D, Stephen Dumler J, Nadelman Robert B. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]


