Abstract
Paramagnetic saturation transfer chemical exchange (PARACEST) complexes are exogenous contrast agents that have great potential to further extend the functional and molecular imaging capabilities of magnetic resonance. Due to the presence of a central paramagnetic lanthanide ion (Ln3+ ≠ La3+, Gd3+, Lu3+) within the chelate, the resonance frequencies of protons and water molecules bound to the PARACEST agent are shifted far away from the bulk water frequency. This large chemical shift combined with an extreme sensitivity to the chemical exchange rate make PARACEST agents ideally suited for reporting significant biological metrics such as temperature, pH, and the presence of metabolites. Also, the ability to turn PARACEST agents “off” and “on” using a frequency selective saturation pulse gives them a distinct advantage over Gd3+-based contrast agents. A current challenge for PARACEST research is translating the promising in vitro results into in vivo systems. This short review article first describes the basic theory behind PARACEST contrast agents, their benefits over other contrast agents, and their applications to magnetic resonance imaging. It then describes some of the recent PARACEST research results. Specifically, pH measurements using water molecule exchange rate modulation, T2-exchange contrast due to water molecule exchange, the use of ultra-short echo times (TE<10 μs) to overcome T2-exchange line-broadening, and the potential application of T2-exchange as a new contrast mechanism for magnetic resonance imaging.
Keywords: MRI, CEST, DIACEST, PARACEST, T2-exchange, in vivo, pH, SWIFT
1. Introduction
MR imaging agents
Magnetic resonance (MR) imaging uses a strong magnetic field and radiofrequency (RF) radiation to create an image representing the spatial distribution of water molecule protons within the subject (1,2). Since the human body is composed of mostly water (about 70 %) almost every tissue type generates an MR signal. This is one reason why MR gives such excellent anatomic detail, where the various tissue types (differentiated by their endogenous T1 and T2 relaxation times and proton densities) appear slightly brighter or darker than surrounding tissue (3). Yet, if two different tissue types have similar T1 and T2 values (and proton densities) they will appear similar in standard MR images. This can create a problem, for example, when trying to differentiate between cancerous and healthy tissue, especially at the early stages of disease. In order to highlight specific anatomic features or measure certain dynamic processes, the endogenous T1 and T2 tissue contrast can be further enhanced by modifying the echo and repetition times (TE and TR respectively) in the RF pulse sequence. If the endogenous contrast is insufficient then exogenous contrast agents can be introduced into the subject to further extend the level of contrast. Currently, the most common exogenous MR contrast agents are chelates that consist of an organic ligand and a Gd3+ ion (4). Among the lanthanide ions, Gd3+ has the largest paramagnetic relaxation enhancement of water protons and exhibits no pseudocontact (i.e., hyperfine) shift in nearby protons (5). Rapid exchange of water molecules between a single inner-sphere binding site on Gd3+ and bulk water reduces the effective T1 of bulk water protons causing regions of agent uptake to appear brighter than the surrounding tissue, allowing those regions to be easily identified. Conversely, T2* agents such as Dy3+-based chelates and superparamegnetic iron oxide nanoparticles (SPIO) reduce the effective T2 of bulk water protons by creating Bo inhomogeneities within the subject, causing regions of agent uptake to appear darker than the surrounding tissue.
A relatively new method for both endogenous and exogenous MR contrast uses chemical exchange saturation transfer (CEST). The CEST method creates negative contrast (i.e., darkening) in magnetic resonance images using a combination of spin saturation and proton exchange with bulk water (6). Protons bound to a CEST agent are frequency shifted away from the bulk water frequency with the difference defined as Δω. RF spin saturation at the exchanging proton frequency (Δω) can produce indirect partial saturation of the bulk water proton pool through chemical exchange, provided that the rate of exchange (kex) between the bound and bulk proton pools is slower than their frequency difference (i.e., kex ≤ Δω) (7). The magnitude of the bulk water signal reduction is a function of concentration of exchangeable protons associated with each CEST agent, the endogenous T1 relaxation time of tissue water protons, the RF saturation power and duration, and the proton exchange rate. For further details about CEST theory, please refer to the article by McMahon, et al., which appears in this same special issue.
Similar to the initial diamagnetic chemical exchange saturation transfer (DIACEST) agents (7), paramagnetic chemical exchange saturation transfer (PARACEST) agents also create negative contrast. The main difference between DIACEST and PARACEST agents is the latter contain a paramagnetic ion chelated by an organic multidentate ligand. Most often, the paramagnetic ion is a lanthanide ion (Ln3+ ≠ La3+, Gd3+, or Lu3+) (8), but complexes consisting of transition metal ions like Fe2+ have also been reported (9). The presence of the paramagnetic ion gives PARACEST agents two main advantages over DIACEST agents. First, the presence of a paramagnetic center typically increases the chemical shift of any ligand associated –NH and –OH proton resonance by approximately an order of magnitude or more. For example, the –NH and –OH proton chemical shifts of a typical DIACEST agent range from 1 to 5 ppm whereas the chemical shift of a similarly exchanging proton in a PARACEST agent can be 50 ppm or even larger (10). Second, the paramagnetic center often provides an additional exchange mechanism via molecular exchange of a water molecule residing in the inner coordination sphere of a lanthanide ion with other water molecules in the bulk solvent. The ability to have molecular exchange is significant because the bound water molecule chemical shift (Δω) can range from 50 ppm (for Eu3+) to as much as −720 ppm (for Dy3+) (8). Large Δω values such as these allow PARACEST agents to have faster chemical exchange rates than DIACEST agents while remaining below the intermediate exchange limit (i.e., kex ≤ Δω). A large Δω value also allows RF saturation at the bound proton frequency without direct saturation at the bulk water frequency, as well as the possibility of creating new agents having exchange frequencies (Δω) outside the tissue magnetization transfer (MT) window.
The tissue MT signal arises from dipolar exchange of protons with endogenous tissue macromolecules and typically spans from approximately +100 to −100 ppm (11). The MT signal can be much larger than the signal from a PARACEST agent at low concentrations and can severely mask the contrast produced by the agent. In most cases, water molecule exchange in a PARACEST agent is much faster than typical –NH or –OH proton exchange, therefore PARACEST agents require more saturation power (higher B1) than DIACEST agents to achieve the same level of bulk water saturation. Unfortunately, the use of higher power B1 pulses of not only increases the MT signal dramatically but also increases the chance of reaching specific absorption rate (SAR) safety limits.
As mentioned previously, the magnitude of a CEST signal depends upon the number of exchanging protons per molecule, whether it be a DIACEST molecule or a PARACEST agent. Typically there are more –NH or –OH proton exchange sites per molecule than water exchange sites in a PARACEST agent, giving proton exchange an apparent advantage over water exchange mechanisms. However, the higher water molecule exchange rates characteristic of most PARACEST agents can compensate for this concentration difference to some extent simply because one can saturate more protons per unit time during the application of a saturating RF pulse. Also, it has been demonstrated that the number of lanthanide ions per molecule of agent (and correspondingly the number of water molecule exchange sites) can be increased by a factor of 20 or more using simple polymerization procedures (12).
All together, DIACEST and PARACEST agents have shown great potential to further extend the imaging capabilities of diagnostic magnetic resonance imaging. Several of these applications are described for PARACEST agents in the next section.
PARACEST Applications
Since they were first described around a decade ago (6), numerous in vitro demonstrations of PARACEST agents in MR imaging have been reported ranging from general anatomic contrast to functional (13), cellular (14), and molecular imaging as well (15,16). This wide range of potential applications originates with the exquisite sensitivity of the CEST signal to proton and/or water molecule exchange rates. Here, the magnitude of CEST produced by a PARACEST (or DIACEST) agent is defined as the percent drop in the bulk water signal intensity when comparing the “Off” (saturation at −Δω) to the “On” (saturation at Δω) MR signal (i.e., (1−On/Off)×100) (6). If the proton or water molecule exchange rate is too fast or too slow, CEST is not observed and the agent is considered “inactive”. However, at rates intermediate between these two extremes, a reduction in the bulk water signal is observed upon RF activation at frequencies near Δω. This is best illustrated by collecting a complete CEST spectrum (a plot of bulk water intensity versus RF saturation frequency) of any potential new agent. The general shape and bandwidth of the exchange peaks in a CEST spectrum provides qualitative insights into the exchange mechanism and can provide quantitative data about the exchange rates when the spectra are fit to a proper model (17).
The sensitivity to exchange rate can be used to measure certain microenvironment biometrics surrounding the PARACEST chelate such as temperature and pH. For example, PARACEST agents have been shown to measure temperature using both a ratiometric method and a CEST exchange frequency method, where both methods were independent of agent concentration (18,19). Likewise, the sensitivity of –NH proton exchange to pH was demonstrated in the initial CEST publication on DIACEST agents (7). This was soon followed by several publications demonstrating pH measurements using the –NH proton exchange of PARACEST agents and a concentration-independent ratiometric method (10,18,20). It was recently shown that pH can also be accurately measured using the water molecule exchange of a Eu3+DOTA-based PARACEST agent and a ratiometric method (21), of which further details are described later in this article.
The proton and water exchange rates in a PARACEST agent are often found to be sensitive to nearby binding events, therefore this feature has been utilized to create metabolite-responsive agents. For example, it was shown that the PARACEST signal is not observed for Eu3+DOTA-2M-2PB in the absence of glucose because the water molecule exchange rate is too fast. Yet when bound to a single glucose molecule, the rate of water molecule exchange slows enough to turn on the PARACEST effect (22). This agent has been used to detect glucose being released from a perfused mouse liver after the addition of glucagon (23). In a second example, the PARACEST signal from a different Eu3+DOTA-based agent was deactivated upon binding to a single Zn2+ ion because, in this case, the presence of a Zn2+ ion catalyzed proton exchange from a single Eu3+-bound water molecule so effectively that the exchange rate no longer fit into the intermediate regime (24). This responsive agent could potentially be used to image the release of insulin from pancreatic beta-cells since insulin release also involves release of free Zn2+ ions into the surrounding tissue regions (13). Similar techniques have also been used to measure nitric oxide (25), enzyme activity (26), and phosphate esters (27).
Review Objectives
This review summarizes the evolution of responsive PARACEST agents, from those that show a change in CEST peak intensity in response to some physiological event, to new class of agent that switches CEST peak frequency with changes in pH. The advantage of the new class of agent is quite evident as one considers in vivo applications where the concentration of agent may not be well known. A second deterrent for in vivo applications of such sensors is the excessive line broadening contribution to the tissue water proton brought about by the simple presence of a paramagnetic complex having intermediate-to-slow water exchange kinetics. We demonstrate that this T2 exchange (T2ex) contribution to the tissue water line-width can be overcome by the use of ultra-short echo pulse sequences (e.g., UTE, SWIFT) for CEST imaging. Finally, the potential application of T2ex as a new T2 contrast mechanism, chemical approaches to optimize the sensitivity of such agents, and the challenges of translating PARACEST agents for in vivo measurements on a clinical scanner are also discussed.
2. Responsive PARACEST agents
A major focus in the development of MRI contrast agents for molecular imaging has been in the design of agents capable of responding to biologically important indices such as specific enzyme activity, pH, temperature, or tissue redox. CEST has been recognized as one of the best imaging methods available for measuring such physiological parameters for several reasons: 1) image contrast can be turned “on” and “off” at will by applying a frequency-selective radio frequency pulse; 2) CEST contrast is exquisitely sensitive to proton exchange rates (kex) and these are easily modulated by chemistry and/or physiology so responsive CEST agents are relatively easy to design; 3) it is possible to create responsive agents that provide for concentration-independent quantitative measures using ratiometric imaging methods. These features are not easily available in Gd3+-based agents.
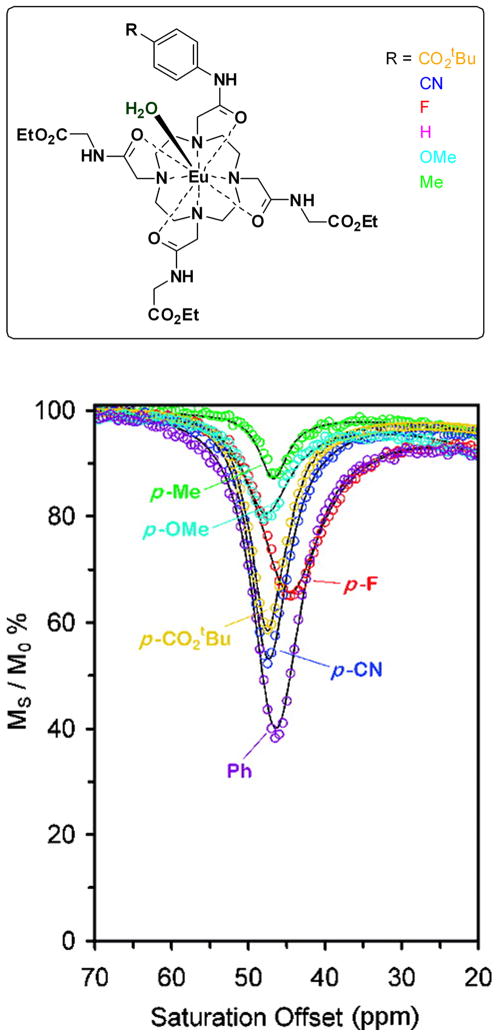
It is now well established that water exchange in Eu3+-DOTA-tetraamide complexes occurs via a dissociative mechanism (28) and this translates into an important fundamental property - water exchange can be modulated in such paramagnetic complexes by altering the electronic properties of the ligand. A general mechanism, which appears to hold for all agents reported so far, is that electron-donating substituents on a DOTA-amide ligand donate more electron density through the coordinating amide onto the central lanthanide ion and this weakens the lanthanide ion-water molecule interaction and promotes faster water exchange. Conversely, electron-withdrawing groups on the amides would tend to slow the rate of water exchange. This was demonstrated experimentally in a series of complexes in which one of the appended amide side-arms was modified to vary the electron-donating ability of the ligand (Figure 1) (29). The experimental results were consistent with the expectation that kex was slower for the complexes with an electron withdrawing group (-CO2tBu, -CN) and faster in those with an electron-donating group (-OMe). Even though the frequency of the bound water exchange CEST peak in these complexes varied somewhat, the magnitude of the CEST signal showed the largest variation with alterations in ligand electronics (Figure 1). These experimental results catalyzed the very important idea that responsive PARACEST agents could be built using the concept of variable electron donating ability. Such a platform design would be even more powerful if one could create systems where the ligand donor atoms or appended groups produce changes in CEST resonance frequency (Δω) rather than CEST intensity.
Figure 1.
Top: chemical structures of Eu(III) complexes; Bottom: CEST profiles arising from the coordinated water molecules of those complexes. The figure is reproduced from reference (29).
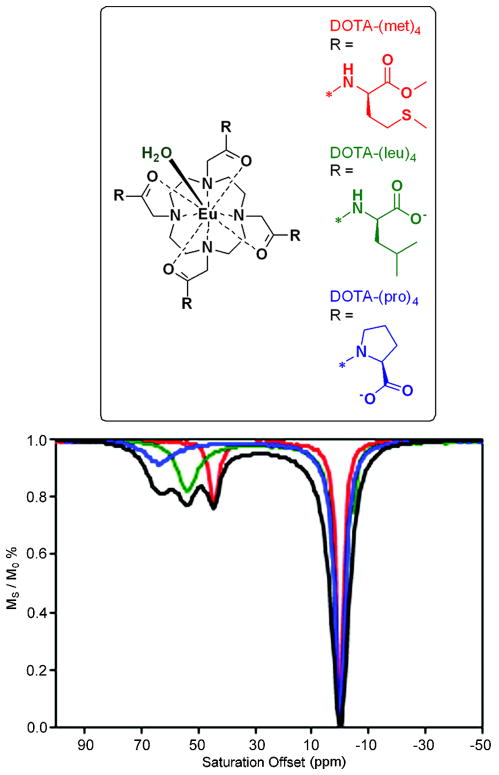
Bleaney’s theory of magnetic anisotropy (30) predicts that the second-order crystal field coefficient Bo2 determines the dipolar NMR shift of paramagnetic lanthanide complexes. This was confirmed experimentally by correlating optical and NMR spectral data for a series of macrocyclic lanthanide complexes (30). Based on that study, the relationship between ligand electronic properties, kex and Δω could be summarized as follows: ligands that produce stronger ligand fields result in complexes with larger frequency shifts (larger Δω) and faster water exchange. This relationship was nicely demonstrated in a comparison of CEST properties for various DOTA-tetraamide ligands (31) prepared from proline, leucine or methionine (Figure 2). Density functional theory (DFT) calculations indicate that the negative charge density on the amide oxygen in this ligand series increased in the order, DOTA-(met)4 < DOTA-(leu)4 < DOTA-(pro)4. More importantly and quite unexpectedly at that time, the three individual complexes showed dramatically different water exchange frequencies (Δω) and lifetimes. In this series, the water exchange peak was found at Δω = 64, 54, and 45 ppm for EuDOTA-(pro)4, EuDOTA-(leu)4, and EuDOTA-(met)4, respectively, while the bound water lifetimes (τM) varied from 55, 81 and 200 μs for this same series (Figure 2). This verified experimentally for the first time that complexes with larger water exchange frequencies (an advantage for in vivo imaging) have the shortest bound water lifetimes (a disadvantage for PARACEST activation). Nevertheless, the approximate 5 ppm differences in Δω observed for these three EuDOTA-tetraamide complexes was sufficient to allow selective imaging of each individual agent by applying a long presaturation pulse at the appropriate offset frequency. This result led to a new design principle for responsive agents whereby the triggering event (change in pH, enzyme activity, etc.) results in a change in Δω with, preferably, little to no change in τM.
Figure 2.
Top: chemical structures of Eu(III) complexes; Bottom: CEST profiles arising from the coordinated water molecules for Eu(III)-DOTA-(met)4 (red), Eu(III)-DOTA-(leu)4 (green), and Eu(III)-DOTA-(pro)4 (blue). The figure is reproduced from reference (31).
The first example of this new type of responsive PARACEST agent based on the above design principle was built by replacing one of the amide side chains in DOTA-(gly)4 with ketone-phenol unit (Figure 3) (21). This particular chemical entity was chosen because deprotonation of the phenolic proton would result in a delocalization of the excess negative charge on the phenolate oxygen through the aromatic ring and onto acetyl oxygen atom yielding a quinone-like resonance structure with negative charge on the donor oxygen atom bonded to the Eu3+. This transformation increases the overall ligand-field strength produced by the ligand that in turn results in larger hyperfine shifts in all NMR active nuclei in the complex. This is also reflected in a larger hyperfine shift of the exchanging water molecule that results in a downfield shift from 50 to 54 ppm as the phenolic proton is removed between pH 6.0 and 7.6 (Figure 3). This unique CEST property proved to be key for direct imaging of pH using ratiometric principles. For example, the ratio of bulk water intensities after presaturation of the agent at 55 ppm (high pH position) versus 49 ppm (low pH position) was found to be linearly proportional to pH and independent of agent concentration. In addition, the τM of deprotonated and protonated species were estimated at 120 and 239 μs, respectively, consistent with the theory described above.
Figure 3.
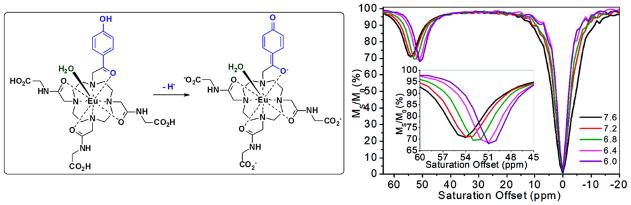
Left: and the deprotonation of the phenolic proton in the pH responsive PARACEST agent results in conjugation of the resulting quinone-like structure with the acetyl oxygen atom coordinated to the Eu(III) ion. Right: pH dependence of CEST spectra for the corresponding agent. Reproduced with permission from (21) © American Chemical Society.
3. T2 contrast from slow-to-intermediate water exchanging systems
One of the current goals in PARACEST agent detection by MR imaging is to translate these promising in vitro results to in vivo applications, specifically mouse models of human disease. Upon doping a PARACEST agent containing a small amount of free ligand with 64Cu (a β+ emitter) to follow the tissue biodistribution by positron emission tomography (PET), it was easy to demonstrate that the untargeted Eu3+DOTA-based agents clear via renal filtration and are quickly deposited in the bladder (32). For a typical clinical dose (0.1 mmol/kg), both the kidneys and bladder showed a strong PET signal with signal remaining in the kidneys for over an hour. Imaging the agent within the bladder using standard spin-echo or gradient-echo pulse sequences and simple “Off” (saturation at −Δω) minus “On” (saturation at +Δω) PARACEST techniques was straightforward and similar to in vitro imaging (6,32). Yet in the kidneys, where the tissue MT signal provides a significant background and the agent concentration is much higher, the imaging results were markedly different. In this case, the MR signal intensity in kidney images decreased by more than 60% simply due to the presence of the PARACEST agent. Notably, this decrease in signal intensity occurred even before a presaturation pulse was applied to activate the agent. This indicated that the PARACEST agent was behaving like a susceptibility or T2* contrast agent (33), which was quite surprising for such a weakly paramagnetic complex. A similar T2 effect in kidney images was recently observed using a Tm3+-based PARACEST agent (34). This effect is problematic for imaging PARACEST agents in vivo because having the regions of agent uptake appear dark in both the “Off” and “On” images means that the desired effect of the PARACEST agent itself (i.e., “Off” minus “On”) is more difficult to detect.
Since Eu3+ has little paramagnetic relaxation effect on either T1 or T2 in comparison to several other lanthanide ions (5), it was clear that the bulk water T2 line broadening originated with some chemical feature of this slow-to-intermediate exchanging system other than magnetism. It has since been shown that the same slow-to-intermediate water molecule exchange feature that enables CEST can also cause a local decrease in the bulk water T2 through the T2-exchange (T2ex) mechanism (35). Diamagnetic molecules containing exchangeable –NH and –OH protons have long been used to suppress the strong bulk water peak in high-resolution proton NMR experiments by reducing the bulk water T2 through chemical exchange (36–38). Added line broadening due toT2ex has also been reported for diamagnetic CEST agents (39) including the clinically approved x-ray contrast agent Iopamidol™ (40). Water peak suppression in high-resolution NMR experiments using water molecule exchange with paramagnetic complexes has also been previously demonstrated (41), where it was shown that an increase in Δω reduced the amount of agent required to achieve the same level of water suppression compared to diamagnetic –NH and –OH exchange by a factor of ~200 (i.e., 2.5 mM versus 500 mM).
The basic theory of T2ex is further described in detail in a recent publication (35). This publication used Eu3+ instead of Dy3+-based chelates and showed both in vitro and in vivo data. The fact that Eu3+ has one of the lowest paramagnetic relaxation enhancement values of the lanthanides (5) guaranteed that the reduction in the bulk water T2 was mainly due to water molecule exchange and not due to paramagnetism. It was shown that the transverse relaxivity due to chemical exchange (r2ex) is a function of the chemical shift (Δω) and the bound water lifetime (τB) as given below:
| (1) |
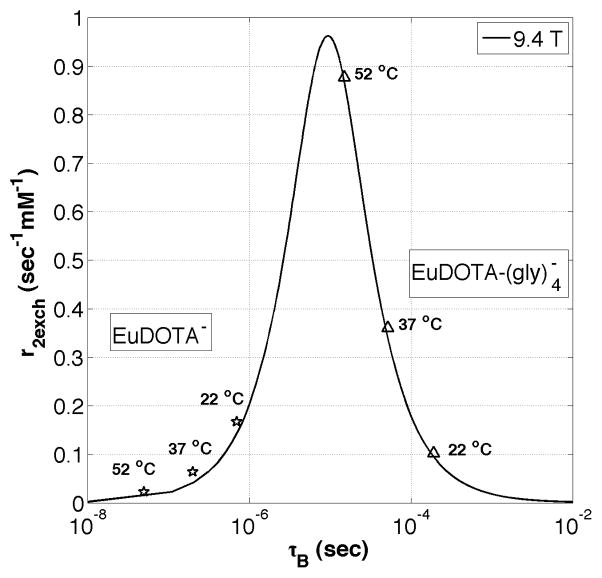
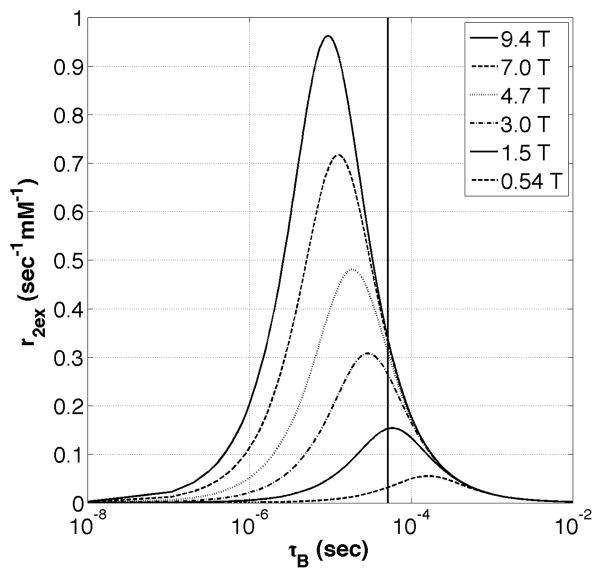
Equation (1), derived using a limiting case of Swift-Connick theory (37,42), was in exact agreement with results from Bloch-McConnell simulations of a two-pool system with chemical exchange (43). Equation (1) was used to create a “Swift-Connick” plot of r2ex (s−1 mM−1) versus τB (s) for Eu3+ complexes at 9.4 T where the chemical shift of the exchanging water molecule (Δω) was fixed at 50 ppm (see Figure 4). It can been seen from Figure 4 that r2ex is minimized at fast and slow water exchange rates, but reaches a well-defined maximum (0.96 s−1 mM−1) at a single intermediate exchange rate (τB = Δω−1, or 9.4 μs). The value of r2ex is highly dependent on the water molecule exchange rate as demonstrated by the experimental data points for EuDOTA (fast exchange) and EuDOTA-(gly)4 (slow to intermediate exchange) measured at three different temperatures (22, 37, and 52 °C) (35). It can be seen that the bound water lifetime for each compound varies by an order of magnitude over the 30°C change in temperature. Furthermore, the bound water lifetimes predicted by these fits are in agreement with recent exchange rate data for similar EuDOTA-based compounds (44). These T2ex data nicely explain why images of mouse kidneys appear dark after injection of EuDOTA-(gly)4 (intermediate exchange) but remain bright after injection of EuDOTA (fast exchange) or EuTETA (no exchange) in images collected with conventional spin-echo or gradient-echo MR sequences at 9.4 T (35).
Figure 4.
A Swift-Connick plot showing the relation between exchange transverse relaxivity (r2ex) and bound water lifetime (τB) for water molecule exchange with Eu3+ at 9.4 T. The markers show the total r2 values of each Eu3+ compound (measured at 9.4 T) fitted to the Swift-Connick plot.
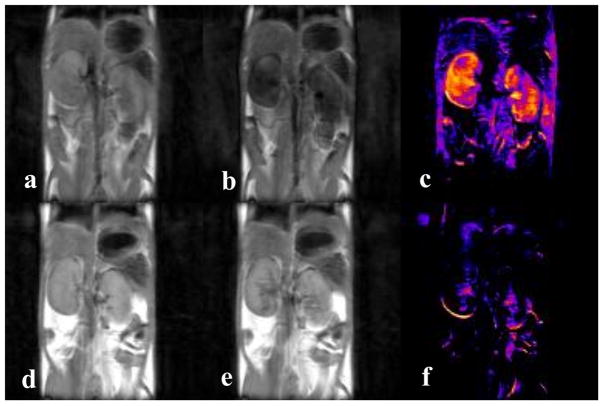
The sensitivity of T2ex to the rate of water molecule exchange can also be demonstrated in vivo by simply changing the internal temperature of the animal. Figure 5 shows images of healthy mouse kidneys collected at two different temperatures, 37 °C (top row) and 25 °C (bottom row). These images were collected on the same mouse but on different days after an intravenous injection of 0.5 mmol/kg EuDOTA-(gly)4. From the difference images, it can be seen that the level of T2ex contrast in the kidneys is much lower at 25 °C than 37 °C, in agreement with the Swift-Connick plot (Figure 4) for EuDOTA-(gly)4.
Figure 5.
(a–c) Fast spin-echo images of healthy mouse kidneys showing T2ex contrast due to a 0.5 mmol/kg intravenous dose of EuDOTA-(gly)4 at 37 °C; (a) pre injection; (b) 15 minutes post injection; (c) pre minus post. (d–f) Fast spin-echo images of healthy mouse kidneys showing T2ex contrast due to a 0.5 mmol/kg intravenous dose of EuDOTA-(gly)4 at 25 °C; (d) pre injection; (e) 15 minutes post injection; (f) pre minus post. The same mouse was used for each experiment, but on different days. These results agree with the EuDOTA-(gly)4 data from Figure 4.
4. Overcoming T2ex with SWIFT-CEST
As discussed previously, both the PARACEST and T2ex effects are highly dependent upon the water molecule exchange rate. Fortunately, these two contrast mechanisms appear to reach a maximum at different exchange rates for a given lanthanide ion chelate (see Discussion section). This means that one could select an exchange rate where the PARACEST signal is maximized while the T2ex is minimized. Also, since T2ex contrast is highly dependent on Δω (and therefore Bo), one might choose to image PARACEST agents having intermediate-to-slow water exchange rates at lower magnetic fields where the T2ex effects are less prominent (see Figure 6). Another option to overcome T2ex is to use imaging sequences designed to detect short T2 components by using very short echo times (TE). The standard spin-echo and gradient-echo pulse sequences used previously had minimum TE times of about 10 ms and 1 ms, respectively, which were not short enough to capture the MR signal from regions of reduced T2 (e.g., the mouse kidneys) caused by the presence of the PARACEST agent. By using the sweep imaging with Fourier transform (SWIFT) pulse sequence, one can acquire MR images with essentially zero TE times (<10 μs) (45). It was recently shown that the ultra-short TE times used in SWIFT can indeed overcome bulk water T2 shortening caused by T2ex with PARACEST agents (46). SWIFT-CEST enables fast and straightforward imaging of PARACEST agents using simple “Off” minus “On” image techniques. Along with fast and silent acquisition of 3D data, SWIFT also has the advantage of being insensitive to both motion and flow, as well as Bo inhomogeneities due to changes in susceptibility.
Figure 6.
A Swift-Connick plot of exchange transverse relaxivity (r2ex) versus bound water lifetime (τB) for water molecule exchange with Eu3+ at several different Bo strengths. As Bo increases, the maximum r2ex value increases proportionally and moves towards faster exchange. The vertical line represents the water molecule exchange rate of EuDOTA-(gly)4 at 37 °C (52 μsec).
Although SWIFT can be used to overcome T2ex, the MT effect is still present in tissues like the kidneys. In order to overcome the MT signal, the dose of PARACEST agent used in the SWIFT-CEST experiment was increased to 1.0 mmol/kg (~10-fold higher than a “standard” clinical dose of Gd3+). Unfortunately, higher doses not only increase the contribution from T2ex but also move us away from the goal of improving MR sensitivity in functional and molecular imaging at lower doses. An alternative way to improve the MR sensitivity of PARACEST agents is to use a lanthanide ion with a bound water frequency (Δω) far outside the MT frequency window (+100 to −100 ppm). An increase in Δω will also increase T2ex proportionally. Also, unlike Eu3+, other lanthanide ions will have paramagnetic contributions that will further reduce the T2 of the bulk water. Therefore, SWIFT-CEST or other ultra-short echo time sequences will likely be needed to image PARACEST agents having large Δω values. Detection of a Tb3+DOTA-based agent (Δω = −600 ppm) without interference from MT was recently demonstrated in vivo using SWIFT-CEST at 9.4 T (47).
5. Embracing T2ex for T2 Contrast
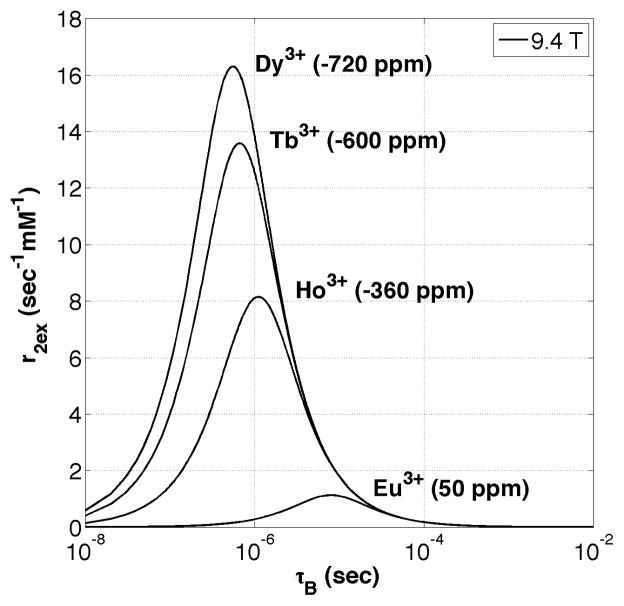
Given that typical water exchange-based PARACEST agents also serve as T2ex agents, there may be useful in vivo applications where this imaging feature could be used to advantage. Since T2ex is proportional to the bound water chemical shift, choosing a lanthanide ion complex with a large Δω value and working at high magnetic fields (Bo) would maximize the effect. Figure 6 illustrates Swift-Connick plots for Eu3+ (Δω = +50 ppm) at several different Bo strengths. It can be seen that as Bo increases, the maximum r2ex increases proportionally with the curve maximum shifting toward faster exchange at higher fields. Note that for EuDOTA-(gly)4 at 37 °C, which is represented by the vertical line at τB = 52 μs, there is very little change in r2ex once Bo is greater than 4.7 T. Yet there is significant reduction in r2ex at fields lower than 4.7 T. This suggests that for certain PARACEST complexes, imaging at lower Bo field strengths might be advantageous because r2ex will be minimized. Figure 7 illustrates Swift-Connick plots for several different lanthanide ions at 9.4 T. It can be seen that Dy3+ (Δω = −720 ppm) gives the highest value for exchange-based transverse relaxivity (r2ex) simply because Δω is largest for this lanthanide ion. A Dy3+-based PARACEST chelate with a bound water lifetime of around 500 ns would have a transverse exchange relaxivity of about 16 s−1 mM−1. It is important to note that data shown in Figure 7 is for r2 due to water molecule exchange only (r2ex) and does not include the paramagnetic contribution to the total r2. Unlike Eu3+, lanthanide ions such as Dy3+ will also have a significant paramagnetic component added to the total r2, which would simply shift the Swift-Connick peaks in the positive vertical direction and increase the overall r2 for a given bound water lifetime. Although the total r2 for a Dy3+ PARACEST chelate is lower than that of typical iron oxide (T2*) contrast agents such as superparamagnetic ion oxide (SPIO) nanoparticles (48), it is important to note that the origin of T2 contrast for the two species are completely different. By creating relatively low molecular weight polymers of PARACEST chelates (12), the total r2 (per molecule) could easily be enhanced by a factor of 100 or more. Using this approach, a highly sensitive T2 contrast agent with a r2 comparable to (or even greater than) superparamagnetic ion oxide nanoparticles might be possible while retaining the advantages of a low molecular weight molecule. This principle was recently demonstrated by synthesizing a polymerized version of the Eu3+DOTA-(gly)4 chelate (12) and performing in vivo imaging in mice (49). Through a modest 20-fold degree of polymerization the effective r2ex was increased from approximately 0.4 s−1 mM−1 to 8 s−1 mM−1 at 9.4 T. Figure 8 shows the effect of the Eu3+DOTA-(gly)4 polymer in the mouse kidneys and surrounding tissue after an intramuscular injection of 70 μmol/kg of polymer (1.4 mmol/kg of Eu3+). Note, a 50% decrease in total signal intensity was observed in images of the kidneys. Similarly, since T2ex is proportional to Δω, by using Tb3+ (Δω = −600 ppm) instead of Eu3+ (Δω = 50 ppm) the same level of contrast in the kidneys (50%) can be achieved at a much lower dose of lanthanide (0.18 mmol/kg versus 1.40 mmol/kg) as shown in Figure 9 (47). Subsequent polymerization of the Tb3+-based T2ex agent could then reduce the required molecular dose into the low μmol/kg or even nmol/kg range. Such a platform may prove useful for imaging the movement of stem cells or for detecting agents targeted to specific biological structures in vivo.
Figure 7.
A Swift-Connick plot of exchange transverse relaxivity (r2ex) versus bound water lifetime (τB) for water molecule exchange with several different Ln3+ ions at 9.4 T. As Δω increases, the maximum r2ex increases proportionally and moves towards faster exchange. Reproduced with permission from John Wiley & Sons Ltd. (35).
Figure 8.
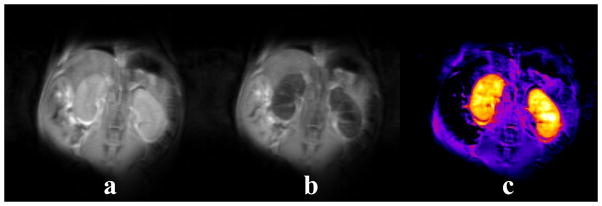
In vivo T2ex contrast demonstrated by 2 mm thick fast spin-echo coronal slices of healthy mouse kidneys (a) before and (b) 2 hours after a 70 μmol/kg intramuscular dose of Eu3+DOTA-(gly)4 polymer (1.4 mmol/kg of Eu3+). A 50% reduction in kidney signal can be seen in the post-injection image. (c) The pre-injection image minus the post-injection image revealing the agent uptake in the kidneys and surrounding tissue. Note that there is no uptake within the stomach.
Figure 9.
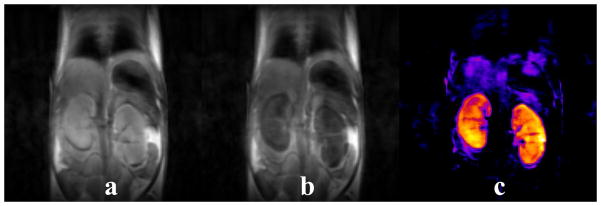
In vivo T2ex contrast demonstrated by 2 mm thick fast spin-echo coronal slices of healthy mouse kidneys (a) before and (b) 10 minutes after a 0.18 mmol/kg intravenous dose of Tb3+DOTA-4AmP-4Bu monomer. A 50% reduction in kidney signal can be seen in the post-injection image. (c) The pre-injection image minus the post-injection image revealing the agent uptake in the kidneys.
6. Discussion
The wide range of PARACEST applications stem mainly from their exquisite sensitivity to proton and water molecule exchange rates. If exchange is too slow, a PARACEST signal is not observed but as the exchange rate increases into the intermediate-exchange region, a decrease in tissue water signal intensity at Δω can be detected in a CEST spectrum. As the exchange rate increases further, the CEST intensity at Δω reaches a maximum then begins to broaden and decrease while the exchange peak shifts towards the bulk water frequency (0 ppm) (17). CEST then disappears completely when the exchange rate becomes too fast (kex > Δω) and no differentiation between the bound and bulk protons can be made. These resonance-like characteristics of the “CEST peak” in the CEST spectrum can be observed by simply modulating the exchange rate using temperature (44).
In order to improve the MR sensitivity of these agents, it is important to identify which slow-to-intermediate exchange rate gives the maximum PARACEST effect. NMR Bloch theory predicts (43) that CEST will be maximal for the experimental condition:
| (2) |
so targeting an appropriate B1 for a particular in vivo application allows one to predict the optimal exchange rate that one should target for the agent. However, as shown in Figure 7, T2ex also reaches a maximum value at a predictable exchange rate. Fortunately, it appears that the optimal exchange rate for CEST (assuming some appropriate B1 of 100–200 Hz) and T2ex differ to some extent. For water molecule exchange with Eu3+ at 9.4 T, the PARACEST signal would be maximal at an exchange rate of ~10 kHz (τb = 1×10−4 s) assuming a B1 of 10 μT (17), while the maximal contribution from T2ex (independent of B1) would occur at an exchange rate at ~100 kHz (τb = 1×10−5 s), an order of magnitude faster (35). Therefore it may become important, especially at higher Bo fields, to pick an appropriate chemical system that has exchange rate that maximizes the PARACEST signal and minimizes T2ex. This consideration along with the use of ultra-short echo time sequences like SWIFT or UTE (46) will help insure detection of PARACEST signals at as low a concentration as possible in vivo.
Even at the ideal proton or water molecule exchange rate, the sensitivity of PARACEST contrast agents is quite low compared to nuclear imaging modalities such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Nuclear medical imaging methods like PET and SPECT detect and image the radiation emitted from the nucleus of a single unstable isotope (e.g., the 140 keV gamma ray from 99mTc). This sensitivity, combined with the low background radioactivity in the human body, means that doses as low as nM to pM can be used for imaging. Conversely, MR depends on an ensemble of thousands of hydrogen nuclei to generate signal adequate for imaging so the typical dose of an MR contrast agent is in the mM range. In order to truly extend the functional and molecular imaging capabilities of MR, the sensitivity of PARACEST contrast agents must be increased substantially. One can of course improve the sensitivity of PARACEST agents by simply increasing the number of water molecule exchange sites per molecular unit by forming polymers of the agent (102 increase) (12), coating the surface of perfluorocarbon-filled nanoparticles with agent (105 increase) (50), and even liposomal encapsulation of the agent (1010 increase) (51,52). While a polymerized form of a PARACEST agent yielded an enhancement of only about 102 so far, this might be improved upon substantially while retaining the advantages of relatively low molecular weight species for improved biological uptake.
An alternative way to improve the sensitivity of PARACEST agents for molecular imaging is to use a paramagnetic chelate that has a bound water molecule frequency (Δω) outside of the tissue MT window (100 to −100 ppm). This would allow activation of the PARACEST agent without co-activation of the MT signal and potentially provide more CEST contrast at lower B1 power, thereby also reducing SAR. It was recently shown that a Tb3+DOTA-based PARACEST agent (Δω = −600 ppm) can be imaged in vivo using the ultra-short echo times of SWIFT to overcome any T2ex and paramagnetic reduction of the bulk water T2 (47).
7. Conclusions
PARACEST contrast agents show great promise for extending the functional and molecular imaging capabilities of MR. The sensitivity of the PARACEST signal to proton or water molecule exchange rates make these agents well-suited to image and measure relevant biomarkers such as temperature, pH, and specific metabolites. Translating the encouraging early in vitro results to in vivo applications and eventually the clinic promises to be challenging, but will have high rewards for the study and diagnoses of human disease such as cancer and diabetes.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA-115531, CA-126608, RR-02584 and EB-004582) and the Robert A. Welch Foundation (AT-584). The authors also acknowledge support from the Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant 1P30 CA142543 for partial support of the animal imaging experiments.
Abbreviations used
- CEST
chemical exchange saturation transfer
- DIACEST
diamagnetic chemical exchange saturation transfer
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MT
magnetization transfer
- NMR
nuclear magnetic resonance
- PARACEST
paramagnetic chemical exchange saturation transfer
- PET
positron emission tomography
- RF
radiofrequency
- SAR
specific absorption rate
- SPECT
single photon emission computed tomography
- SPIO
superparamagnetic iron oxide nanoparticles
- SWIFT
sweep imaging with Fourier transform
- TE
echo time
- TR
repetition time
- UTE
ultra-short echo time
References
- 1.Lauterbur PC. Image Formation by InducedLocal Interactions -Examples Employing Nuclear Magnetic-Resonance. Nature. 1973;242(5394):190–191. [PubMed] [Google Scholar]
- 2.Damadian R, Goldsmith M, Minkoff L. Nmr in Cancer .16. Fonar Image of Live Human-Body. Physiol Chem Phys M. 1977;9(1):97. -&. [PubMed] [Google Scholar]
- 3.Damadian R. Tumor Detectionby Nuclear Magnetic Resonance. Science. 1971;171(3976):1151. doi: 10.1126/science.171.3976.1151. -&. [DOI] [PubMed] [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99(9):2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem Rev. 2010;110(5):2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Winter P, Wu K, Sherry AD. A novel europium(III)-based MRI contrast agent. J Am Chem Soc. 2001;123(7):1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 7.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SR, Sherry AD. Physical characteristics of lanthanide complexes that act as magnetization transfer (MT) contrast agents. J Solid State Chem. 2003;171(1–2):38–43. [Google Scholar]
- 9.Dorazio SJ, Tsitovich PB, Siters KE, Spernyak JA, Morrow JR. Iron(II) PARACEST MRI Contrast Agents. Journal of the American Chemical Society. 2011;133(36):14154–14156. doi: 10.1021/ja204297z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aime S, Barge A, Castelli DD, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magnet Reson Med. 2002;47(4):639–648. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]
- 11.Wolff SD, Balaban RS. Magnetization Transfer Contrast (Mtc) and Tissue Water Proton Relaxation Invivo. Magnet Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Zhou Y, Ouari O, Woods M, Zhao P, Soesbe TC, Kiefer GE, Sherry AD. Polymeric PARACEST agents for enhancing MRI contrast sensitivity. J Am Chem Soc. 2008;130(42):13854–13855. doi: 10.1021/ja805775u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods M, Zhang S, Sherry AD. Toward the Design of MR Agents for Imaging beta-Cell Function. Curr Med Chem Immunol Endocr Metab Agents. 2004;4(4):349–369. doi: 10.2174/1568013043357338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aime S, Carrera C, Castelli DD, Crich SG, Terreno E. Tunable imaging of cells labeled with MRI-PARACEST agents. Angew Chem Int Edit. 2005;44(12):1813–1815. doi: 10.1002/anie.200462566. [DOI] [PubMed] [Google Scholar]
- 15.Aime S, Delli Castelli D, Fedeli F, Terreno E. A paramagnetic MRI-CEST agent responsive to lactate concentration. Journal of the American Chemical Society. 2002;124(32):9364–9365. doi: 10.1021/ja0264044. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Trokowski R, Sherry AD. A paramagnetic CEST agent for imaging glucose by MRI. J Am Chem Soc. 2003;125(50):15288–15289. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- 17.Woods M, Woessner DW, Sherry AD. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem Soc Rev. 2006;35(6):500–511. doi: 10.1039/b509907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terreno E, Castelli DD, Cravotto G, Milone L, Aime S. Ln(III)-DOTAMGIY complexes: A versatile series to assess the determinants of the efficacy of paramagnetic chemical exchange saturation transfer agents for magnetic resonance imaging applications. Invest Radiol. 2004;39(4):235–243. doi: 10.1097/01.rli.0000116607.26372.d0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Malloy CR, Sherry AD. MRI thermometry based on PARACEST agents. J Am Chem Soc. 2005;127(50):17572–17573. doi: 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aime S, Delli Castelli D, Terreno E. Novel pH-reporter MRI contrast agents. Angew Chem Int Edit. 2002;41(22):4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Soesbe TC, Kiefer GE, Zhao P, Sherry AD. A responsive europium(III) chelate that provides a direct readout of pH by MRI. J Am Chem Soc. 2010;132(40):14002–14003. doi: 10.1021/ja106018n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trokowski R, Zhang SR, Sherry AD. Cyclen-based phenylboronate ligands and their Eu(3+) complexes for sensing glucose by MRI. Bioconjugate Chem. 2004;15(6):1431–1440. doi: 10.1021/bc0498976. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Trokowski R, Zhang S, Malloy CR, Sherry AD. Imaging the tissue distribution of glucose in livers using a PARACEST sensor. Magn Reson Med. 2008;60(5):1047–1055. doi: 10.1002/mrm.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trokowski R, Ren JM, Kalman FK, Sherry AD. Selective sensing of zinc ions with a PARACEST contrast agent. Angew Chem Int Edit. 2005;44(42):6920–6923. doi: 10.1002/anie.200502173. [DOI] [PubMed] [Google Scholar]
- 25.Liu GS, Li YG, Pagel MD. Design and characterization of a new irreversible responsive PARACEST MRI contrast agent that detects nitric oxide. Magnet Reson Med. 2007;58(6):1249–1256. doi: 10.1002/mrm.21428. [DOI] [PubMed] [Google Scholar]
- 26.Yoo B, Pagel MD. A Paracest Mri Contrast Agent to Detect Enzyme Activity. Journal of the American Chemical Society. 2006;128(43):14032–14033. doi: 10.1021/ja063874f. [DOI] [PubMed] [Google Scholar]
- 27.Huang CH, Morrow JR. A PARACEST Agent Responsive to Inner-And Outer-Sphere Phosphate Ester Interactions for MRI Applications. Journal of the American Chemical Society. 2009;131(12):4206-+. doi: 10.1021/ja900290z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunand FA, Dickins RS, Parker D, Merbach AE. Towards rational design of fast water-exchanging Gd(dota-like) contrast agents? Importance of the M/m ratio. Chem-Eur J. 2001;7(23):5160–5167. doi: 10.1002/1521-3765(20011203)7:23<5160::aid-chem5160>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Ratnakar SJ, Woods M, Lubag AJM, Kovacs Z, Sherry AD. Modulation of water exchange in europium(III) DOTA-tetraamide complexes via electronic substituent effects. Journal of the American Chemical Society. 2008;130(1):6-+. doi: 10.1021/ja076325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickins RS, Parker D, Bruce JI, Tozer DJ. Correlation of optical and NMR spectral information with coordination variation for axially symmetric macrocyclic Eu(III) and Yb(III) complexes: axial donor polarisability determines ligand field and cation donor preference. Dalton T. 2003;7(1264):1271. [Google Scholar]
- 31.Viswanathan S, Ratnakar SJ, Green KN, Kovacs Z, De Leon-Rodriguez LM, Sherry AD. Multi-Frequency PARACEST Agents Based on Europium(III)-DOTA-Tetraamide Ligands. Angew Chem Int Edit. 2009;48(49):9330–9333. doi: 10.1002/anie.200904649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soesbe TC, Wu Y, Hao G, Sun X, Sherry AD. In vivo MR and PET imaging of a highly sensitive polymeric PARACEST contrast agent. ISMRM Annual Meeting; Honolulu, HI. 2009. [Google Scholar]
- 33.Soesbe TC, Rojas-Quijano FA, Merritt ME, Sherry AD. Eu3+-based PARACEST agents with intermediate water exchange rates also act as T2 exchange (T2exch) contrastagents. ISMRM Annual Meeting; Stockholm, Sweeden. 2010. [Google Scholar]
- 34.Jones CK, Li AX, Suchy M, Hudson RHE, Menon RS, Bartha R. In Vivo Detection of PARACEST Agents With Relaxation Correction. Magnet Reson Med. 2010;63(5):1184–1192. doi: 10.1002/mrm.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soesbe TC, Merritt ME, Green KN, Rojas-Quijano FA, Sherry AD. T(2) Exchange Agents: a New Class of Paramagnetic MRI Contrast Agent That Shortens Water T(2) by Chemical Exchange Rather Than Relaxation. Magnet Reson Med. 2011;66(6):1697–1703. doi: 10.1002/mrm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leigh JS. Relaxation Times in Systems with Chemical Exchange -Some Exact Solutions. J Magn Reson. 1971;4(3):308–311. [Google Scholar]
- 37.Granot J, Fiat D. Effect of Chemical Exchange on Transverse Relaxation Rate of Nuclei in Solution Containing Paramagnetic-Ions. J Magn Reson. 1974;15(3):540–548. [Google Scholar]
- 38.Rabenstein DL, Fan S, Nakashima TT. Attenuation of the Water Resonance in Fourier-Transform H-1-Nmr Spectra of Aqueous-Solutions by Spin Spin Relaxation. J Magn Reson. 1985;64(3):541–546. [Google Scholar]
- 39.Aime S, Nano R, Grandi M. A New Class of Contrast Agents for Magnetic-Resonance Imaging Based on Selective Reduction of Water-T2 by Chemical-Exchange. Invest Radiol. 1988;23:S267–S270. doi: 10.1097/00004424-198809001-00058. [DOI] [PubMed] [Google Scholar]
- 40.Aime S, Calabi L, Biondi L, De Miranda M, Ghelli S, Paleari L, Rebaudengo C, Terreno E. Iopamidol: Exploring the potential use of a well-established X-ray contrast agent for MRI. Magnet Reson Med. 2005;53(4):830–834. doi: 10.1002/mrm.20441. [DOI] [PubMed] [Google Scholar]
- 41.Aime S, Botta M, Barbero L, Uggeri F, Fedeli F. Water Signal Suppression by T2-Relaxation Enhancement Promoted by Dy(Iii) Complexes. Magn Reson Chem. 1991;29:S85–S88. [Google Scholar]
- 42.Swift TJ, Connick RE. NMR-relaxation mechanisms of O17 in aqueous solutions of paramagnetic cations and the lifetime of water molecules in the first coordination sphere. J Chem Phys. 1962;37(2):307–321. [Google Scholar]
- 43.Woessner DE, Zhang SR, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magnet Reson Med. 2005;53(4):790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 44.Dixon WT, Ren JM, Lubag AJM, Ratnakar J, Vinogradov E, Hancu I, Lenkinski RE, Sherry AD. A Concentration-Independent Method to Measure Exchange Rates in PARACEST Agents. Magnet Reson Med. 2010;63(3):625–632. doi: 10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idiyatullin D, Corum C, Park JY, Garwood M. Fast and quiet MRI using a swept radiofrequency. J Magn Reson. 2006;181(2):342–349. doi: 10.1016/j.jmr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Soesbe TC, Togao O, Takahashi M, Sherry AD. SWIFT-CEST: A new MRI method to overcome T(2) shortening caused by PARACEST contrast agents. Magn Reson Med. 2011 doi: 10.1002/mrm.23302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soesbe TC, Rojas-Quijano FA, Sherry AD. In vivo magnetic resonance imaging beyond the MT window using SWIFT-CEST and a Tb-based PARACEST contrast agent. ISMRM Annual Meeting, Abstract 1661; Melbourne, Austalia. 2012. [Google Scholar]
- 48.Tsai Z-T, Wang J-F, Kuo H-Y, Shen C-R, Wang J-J, Yen T-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating withchitosan: A potential MRI contrast agent useful for cell tracking. Journal of Magnetism and Magnetic Materials. 2010;322:208–213. [Google Scholar]
- 49.Soesbe TC, Merritt ME, Togao O, Green KN, Rojas-Quijano FA, Takahashi M, Sherry AD. T2 exchange: A new, unintended contrast mechanism from PARACEST agents. Advanced Imaging Research Center annual Symposium and Training XIX; UT Southwestern Medical Center; Dallas, Texas. 2011. [Google Scholar]
- 50.Schmieder AH, Winter PM, Caruthers SD, Harris TD, Williams TA, Allen JS, Lacy EK, Zhang HY, Scott MJ, Hu G, Robertson JD, Wickline SA, Lanza GM. Molecular MR imaging of melanoma angiogenesis with alpha(nu)beta(3)-targeted paramagnetic nanoparticles. Magnet Reson Med. 2005;53(3):621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 51.Aime S, Castelli DD, Terreno E. Highly sensitive MRI chemical exchange saturation transfer agents using liposomes. Angew Chem Int Edit. 2005;44(34):5513–5515. doi: 10.1002/anie.200501473. [DOI] [PubMed] [Google Scholar]
- 52.Aime S, Castelli DD, Lawson D, Terreno E. Gd-loaded liposomes as T1, susceptibility, and CEST agents, all in one. Journal of the American Chemical Society. 2007;129(9):2430–2431. doi: 10.1021/ja0677867. [DOI] [PubMed] [Google Scholar]