Introduction
Langerhans cells (LC) are the sole dendritic cell (DC) in the epidermis, the outermost layer of the skin. They have been a focus of intense study since their identification as an antigen-presenting cell in the mid 1970’s and were considered the proto-typical tissue dendritic cell on which the “dendritic cell paradigm” was based [1–3]. Their location at a barrier surface provides them with early access to skin pathogens, commensal organisms, foreign chemicals (a.k.a. contact sensitizers) as well as to epidermal self-antigens. A considerable body of work suggests that LC transport these antigens to regional lymph nodes where they present to naïve T cells thereby initiating adaptive cutaneous immune responses. Despite this, their function remains stubbornly enigmatic. Functional studies in mice suggest that many of the properties initially ascribed to LC are performed by CD103+ dermal DC and that LC have the capacity to both initiate and suppress cutaneous immune responses. This debate has been extensively examined elsewhere [4,5] and will not be covered in this review. Rather, we will focus on the question of how does the interaction between LC and T cells affect the development of antigen-specific responses.
LC and Cross Presentation
Cross presentation is the capacity of an antigen-presenting cell to acquire exogenous antigen and present the processed peptides in the context of MHC-I. This function is critically important for the development of self-tolerance as well as for CTL responses against most tumors and viruses that do not infect antigen presenting cells. Not all DC have the capacity for cross presentation and targeting antigen to those DC subsets that can induce a CTL response has been a focus of vaccine development for many years[6,7]
Experiments designed to test LC cross-presentation fall into three broad categories: 1) in vitro generation or isolation of LC from tissue which are then loaded with whole antigen and incubated with transgenic CD8 T cells in vitro; 2) exposure of LC to antigen in vivo followed by ex vivo incubation with CD8 T cells; and 3) exposure of LC to antigen in vivo followed by analysis of the CD8 response in vivo.
The strongest evidence supporting LC cross presentation employs the first two approaches. Human LC that were either grown in vitro from CD34+ cells or isolated from human skin explants efficiently promoted CD8 proliferation in vitro and were much more effective than dermal DC [8•]. Similarly, mouse LC isolated by allowing them to crawl-out of epidermal sheets in vitro also efficiently stimulated OT-I cells (transgenic CD8 T cells) in vitro. This was true both when LC were loaded with antigen in vitro and when they were isolated from mice in which the antigen was expressed by keratinocytes [9].
It has been more difficult to demonstrate LC cross-presentation in vivo. Many studies have used the adoptive transfer of ovalbumin specific OT-I CD8 T cells into mice in which ovalbumin expression is restricted to skin under control of keratin promoters to model LC cross presentation of self- antigen (i.e. cross-tolerance) in vivo. LC were found to cross-tolerize in some cases [10] but were redundant in others [11]. These discrepancies may be due, at least in part, to the ability of stromal cells in the lymph node to express self-antigen and promote tolerance [12]. In early studies before the identification of CD103+ Langerin+ dDC, the requirement of Langerin+ DC for clearance of B16-Ova melanoma in response to epicutaneously applied OVA and the ability of anti-Langerin-OVA conjugates to induce strong OT-I proliferation were taken as evidence of cross presentation by LC [13,14]. In contrast, when LC were selectively ablated, OT-I proliferation in response to epicutaneously applied OVA remained intact. Thus, cross presentation seemed to depend on CD103+ Langerin+ dDC in both the steady-state and in the presence of an adjuvant [15–17].
Strong evidence that cross presentation of cutaneous antigen depends on CD103+ Langerin+ dDC and not LC comes from a series of ex vivo murine experiments comparing DC isolated from mice after viral skin infection. Initially, cross-presentation of antigen following a skin herpes simplex virus (HSV) infection was shown to depend on LN-resident CD8+ DC and not migratory DC [18]. This was later thought to be due to the highly cytopathic nature of HSV infection [19,20]. When DC subsets isolated from the draining LN of mice during the viral recrudescence phase of HSV were incubated ex vivo with HSV-specific CD8 T cells, CD103+ Langerin+ dDC most efficiently promoted proliferation [21••]. Similarly, only CD103+ Langerin+ dDC isolated from the LN of K5-Ova mice induced proliferation of OT-I cells [21••,22•]. Finally, in the skin, CD103+ Langerin+ dDC expressed genes such as XCR1 and CLEC9a that are associated with a family of DC that efficiently cross present [23].
The importance of CD103+ Langerin+ dDC for cross presentation of skin antigen was confirmed in vivo using adoptively transferred OT-I cells in the setting of a skin infection with recombinant Candida albicans-Ag (recombinant C. albicans expressing several antigens recognized by CD4 and CD8 TCR transgenic mice including OT-I). Mice with a selective ablation of CD103+ Langerin+ dDC showed a severe defect in OT-I proliferation in contrast to mice with a LC-specific ablation in which OT-I proliferation was unaffected [24••]. In addition, using an anti-Langerin-OVA targeting technique that delivered antigen selectively to LC and not CD103+ Langerin+ dDC, OT-I proliferation was not observed even in the presence of a strong adjuvant [24••]. These data cannot exclude the possibility that antigen acquired by Langerin does not enter the cross-presentation pathway[25].
Recent studies examining human skin DC in vitro appear to confirm these studies. Langerin expressed by LC is an uptake receptor for the Measles virus. Despite acquiring antigen and promoting CD4+ T cell proliferation, LC were unable to cross-present exogenous UV-inactivated Measles virus or virus-infected apoptotic cells [26]. In addition, a comprehensive analysis of DC resident in human skin demonstrated that CD141hi DC were able to cross present much more efficiently than LC [27••]. Transcriptome analysis of CD141hi DC confirmed their identity as the human equivalent of the CD103+ dDC. Thus, these data are similar to the murine data and are consistent with a model in which a small subset of dermal DC (CD103+ in the mouse and CD141hi in the human) are the only skin resident DC subset able to cross-prime CD8 T cells.
These data obtained now from multiple human and mouse systems are at odds with the earlier reports showing efficient LC cross-presentation in vitro by both human [8] and mouse [9] LC. The source of LC may be an important variable. Cross-presentation was observed by human LC derived from CD34+ precursors but not in primary LC obtained either by skin digestion or by epidermal crawl-out[26,27••]. Similarly, LC obtained directly from mouse skin [9] but not from skin-draining LN[21•,22•] cross-present antigen. Assuming that preparations of cross-presenting LC do not contain even small amounts of contaminating CD103/CD141+ dDC, this suggests that LC may acquire the potential to cross-present antigen in at least certain circumstances—perhaps only when they reside in the epidermis where they function to activate skin-resident effector memory CD8 T cells [28].
Antigen presentation to CD4 cells
The capacity of LC to promote CD4 T cell proliferation has been well established[29–33]. It is less clear, though, how antigen-presentation by LC affects the development of Th phenotypes, tolerance, and/or the establishment of memory. There is a rich literature exploring the role of LC in a wide variety of biological processes (e.g. CHS and skin infection) that has been recently reviewed[4,5]. We will focus on those experiments in which responses to specific peptide antigens have been monitored.
Infection of mouse flank skin with recombinant Candida albicans-Ag produces a self-limited local infection that generates both Th1 and Th17 CD4 T cell responses. The Th1 response depended on CD103+ Langerin+ dDC, since it is greatly reduced in mice lacking these cells[24••]. In contrast, when mice lacking LC are infected, total CD4 proliferation and Th1 differentiation was largely unaffected but the number of Th17 cells was greatly reduced. A similar result was obtained with mice in which MHC-II was selectively deleted from LC indicating that Th17 differentiation depended on direct cognate interaction between LC and CD4 T cells. It is likely that LC-derived cytokines are required for Th17 development in this system since mice with a selective ablation of MyD88 in LC also showed reduced Th17 differentiation and MyD88-deficient LC had reduced expression of IL-1β, IL-6 and IL-23[34]. Finally, a robust Th17 response could be generated in response to both C. albicans or Staphylococcus aureus infection even if antigen presentation occurred exclusively on LC[24•]. Thus, in the setting of skin infection with extracellular pathogens, antigen presentation by LC is both necessary and sufficient for the differentiation of Th17 cells.
Unlike the infectious setting, epicutaneous patching with ovalbumin drove OT-II transgenic CD4 T cells towards Th2 differentiation in vivo[35•]. This was associated with increased antigen-specific IgG1 and IgE. Interestingly, Th2 differentiation was dependent on the direct effect of TSLP on LC thereby suggesting that secretion of this cytokine presumably by keratinocytes affects Th-differentiation through the modulation of LC.
These skin infection experiments focused primarily on early time points. Experiments to determine the effect of presentation by LC over many days relied on clever bone-marrow chimera experiments[36]. Since LC remain of host origin, chimeras were generated such that the MHC-II protein I-E was expressed by LC and not by cells of hematopoietic origin. Transgenic CD4 T cells that recognize antigen only in the context of I-E were transferred and the mice were given subcutaneous immunizations with antigen and CFA (complete Freund’s adjuvant). Interestingly, although CD4 T cells proliferated, they did not secrete IFNγ or IL17. Moreover, they failed to form effector/memory cells and ultimately became unresponsive to antigen through a Treg-independent mechanism. These data suggest that an important function of LC is the formation of peripheral tolerance which occurs both in the steady-state and in the presence of strong adjuvant.
Antigen presentation by human LC derived from CD34+ to naïve CD4 T cells in vitro produced a robust proliferation and high levels of IL-13, a Th2-type cytokine, in addition to IL-2, IL-10 and IFNγ[8]. Primary LC also induced an expansion of IFNγ secreting CD4 T cell clones, but other cytokines were not examined[26]. In contrast, primary LC incubated with autologous skin-resident memory T cells isolated from the same skin section resulted in the activation and proliferation of Treg cells[37•]. This was dependent on cognate interaction since addition of MHC class II blocking antibodies inhibited proliferation. Interestingly, when heat killed Candida albicans was added to the culture, Treg proliferation was inhibited and Th17 proliferation increased in a dose-dependent manner. Finally, LC and Treg are found in close proximity in the human epidermis leading to the hypothesis that a major function of LC is the activation of regulatory skin resident CD4 T cells in the steady-state and the activation of effector cells when an inflammatory stimulus is present.
Summary
There has been considerable progress understanding the function of LC in the past few years. As is often the case, there are conflicting data and multiple experimental approaches that must be reconciled. It is now clear that in the skindraining LN, CD103+ dDC are responsible for cross-priming naïve T cells and LC promote Th17 responses to extracelluar pathogens, though exclusive stimulation of CD4 T cells by LC may ultimately lead to tolerance/anergy. The possibility that LC activate skin-resident memory CD4 and CD8 T cells is an attractive, though still unproven hypothesis. It is also becoming increasingly evident that LC have a function quite distinct from dermal DC but it may vary depending on the context. The presence or absence of adjuvant and the particular adjuvant encountered likely contribute to the ultimate outcome of the T cell:LC interaction. One challenge for the future is to decipher those cues and the mechanistic underpinnings that result in such variable T cell responses.
Figure 1.


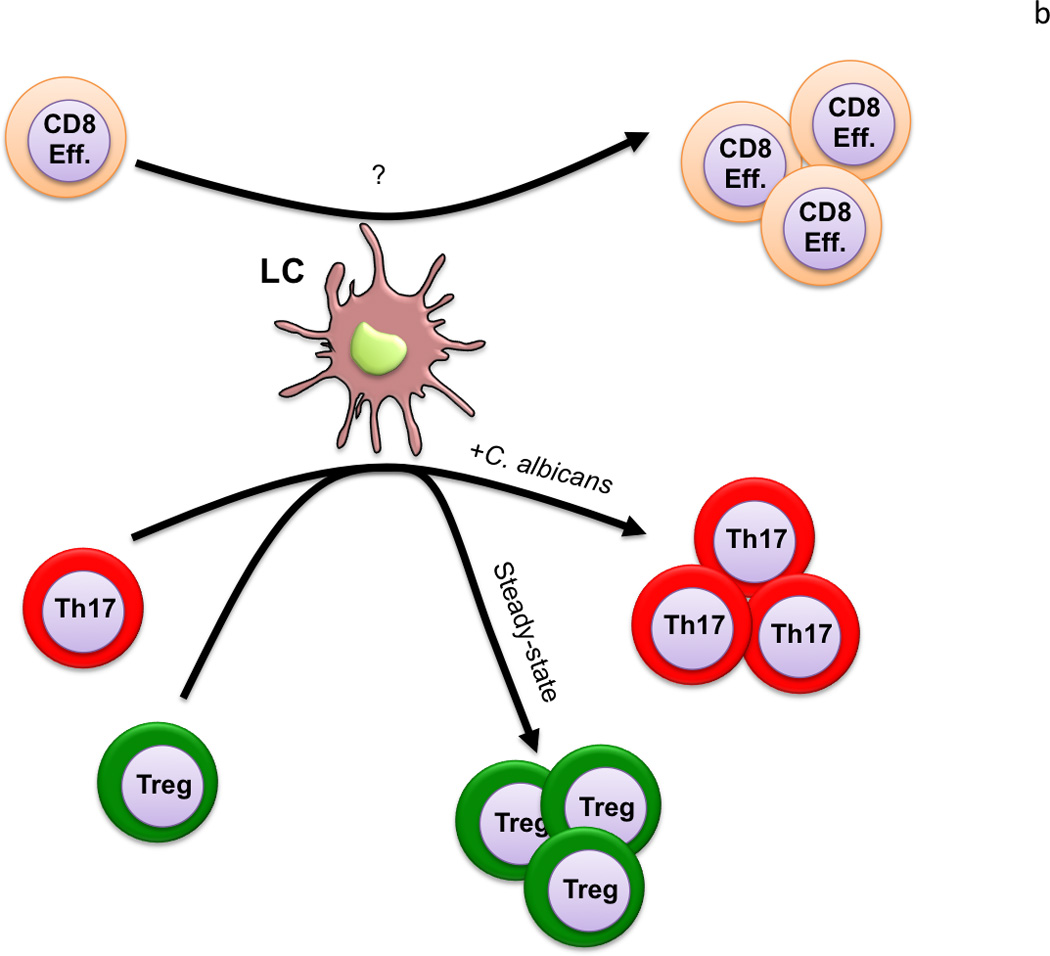
a) Skin draining LN: Antigen presentation by LC to naïve CD4 T cells results in their differentiation into Th17 cells in the setting of infection with an extracellular pathogen. The presence of TLSP drives Th2 differentiation. Presentation by LC also promotes the development of tolerance/anergy under different conditions. CD103+ Langerin+ dDC are the only skin-migratory DC able to cross-prime CD8 T cells in the LN. They also promote Th1 differentiation in the setting of skin infection. B) Skin: Antigen presentation by LC isolated directly from skin drive proliferation of skin-resident CD4+ Treg in the steady-state and CD4+ Th17 memory cells in an inflammatory context. LC may also have the capacity to induce proliferation of skin-resident CD8 memory T cells.
Highlights.
CD103+ dDC, not LC are responsible for cross-priming
LC promote Th17 in response to extracellular pathogens
LC promote Th2 in response to epicutaneous immunization
Presentation by LC may result in T cell anergy/deletion
Acknowledgments
This work was supported by a grant from the NIH AR056632 and the American Skin Association. BZI was supported by a grant from the Dermatology Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 3.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more. langerinexpressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 8. Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013.. This study systematically compared the capacity of LC as well as CD1a+ and CD14+ dermal DC to cross-present antigen as well as drive Th-phenotype differentiation. This was one of the first studies to carefully compare the function of human skin-resident DC subsets and found that DC subsets in the skin can promote distinct T cell phenotypes.
- 9.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcmann M, Stoitzner P, Drobits B, Luehrs P, Stingl G, Romani N, Maurer D, Sibilia M. Skin inflammation is not sufficient to break tolerance induced against a novel antigen. J Immunol. 2009;183:1133–1143. doi: 10.4049/jimmunol.0713351. [DOI] [PubMed] [Google Scholar]
- 11.Bursch LS, Rich BE, Hogquist KA. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol. 2009;182:4657–4664. doi: 10.4049/jimmunol.0803656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 13.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 14.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, Kissenpfennig A, Hermans IF, Ronchese F. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 15.Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olvera-Gomez I, Hamilton SE, Xiao Z, Guimaraes CP, Ploegh HL, Hogquist KA, Wang L, Jameson SC. Cholera toxin activates nonconventional adjuvant pathways that induce protective CD8 T-cell responses after epicutaneous vaccination. Proc Natl Acad Sci U S A. 2012;109:2072–2077. doi: 10.1073/pnas.1105771109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 18.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 19.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 20.He Y, Zhang J, Donahue C, Falo LD., Jr. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al . Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724.. This elegant study examined the abilty of skin-resident DC to cross-prime naïve CD8 T cells during HSV infection. Cross-priming of HSV antigen was largely restricted to CD103+ dDC. CD103+ dDC were also the primary DC able to cross-present keratinocyte derived self antigen.
- 22. Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964.. This paper exhaustively phenotypically characterized mouse skin-resident DC. CD103+ dDC were found to most efficiently cross-present self antigen.
- 23.Crozat K, Tamoutounour S, Vu Manh TP, Fossum E, Luche H, Ardouin L, Guilliams M, Azukizawa H, Bogen B, Malissen B, et al . Cutting edge. expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8alpha+ type. J Immunol. 2011;187:4411–4415. doi: 10.4049/jimmunol.1101717. [DOI] [PubMed] [Google Scholar]
- 24. Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al . Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005.. Using a Candida albicans skin infection model, the authors provided in vivo evidence that Langerhans cells are necessary and sufficient for induction of Th17 response, and that CD103+ dermal dendritic cells are required for induction of Th1 cells and CTL formation
- 25.Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, Koch F, Park CG, Steinman RM, Idoyaga J, et al . Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Vlist M, de Witte L, de Vries RD, Litjens M, de Jong MA, Fluitsma D, de Swart RL, Geijtenbeek TB. Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4(+) T cells but are incapable of cross-presentation. Eur J Immunol. 2011;41:2619–2631. doi: 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- 27. Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al . Human Tissues Contain CD141(hi) Cross- Presenting Dendritic Cells with Functional Homology to Mouse CD103(+) Nonlymphoid Dendritic Cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012.. This paper to identified CD141+ dDC as the human homolog of mouse CD103+ dDC. In additional, transcriptome analysis of individual human vs. mouse DC subsets found them to be highly similar
- 28.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, Inaba K, Steinman RM. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 31.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stingl G, Gazze-Stingl LA, Aberer W, Wolff K. Antigen presentation by murine epidermal langerhans cells and its alteration by ultraviolet B light. J Immunol. 1981;127:1707–1713. [PubMed] [Google Scholar]
- 33.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Iabearing epidermal Langerhans cells. J Immunol. 1978;121:2005–2013. [PubMed] [Google Scholar]
- 34.Haley K, Igyarto BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012;188:4334–4339. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakajima S, Igyarto BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, Watanabe N, Ziegler SF, Tomura M, Inaba K, et al . Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol. 2012;129:1048–1055. e1046. doi: 10.1016/j.jaci.2012.01.063.. Authors report that epicutaneous application of antigen on mouse skin drives a TSLP-dependent Th2 response that required the presence of TSLPresponsive Langerhans
- 36.Shklovskaya E, O'Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, Fazekas de St Groth B. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci U S A. 2011;108:18049–18054. doi: 10.1073/pnas.1110076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018.. Using LC isolated from human skin and donor-matched skin-resident memory T cells, the authors demonstrated that in the absence of inflammation, LC promoted proliferation of Treg. The addition of Candida albicans to the cultures induced a switch to Th17 proliferation.


