Abstract
The ultimate goal of hair cell regeneration is to restore functional hearing. Because birds begin perceiving and producing song early in life, they provide a propitious model for studying not only whether regeneration of lost hair cells can return auditory sensitivity but also whether this regenerated periphery can restore complex auditory perception and production. They are the only animal where hair cell regeneration occurs naturally after hair cell loss and where the ability to correctly perceive and produce complex acoustic signals is critical to procreation and survival. The purpose of this review article is to survey the most recent literature on behavioral measures of auditory functional return in adult birds after hair cell regeneration. The first portion of the review summarizes the effect of ototoxic drug induced hair cell loss and regeneration on hearing loss and recovery for pure tones. The second portion reviews studies of complex, species-specific vocalization discrimination and recognition after hair cell regeneration. Finally, we discuss the relevance of temporary hearing loss and recovery through hair cell regeneration on complex call and song production. Hearing sensitivity is restored, except for the highest frequencies, after hair cell regeneration in birds, but there are enduring changes to complex auditory perception. These changes do not appear to provide any obstacle to future auditory or vocal learning.
1. Introduction
Understanding how molecular and genetic cues regulate and control hair cell regeneration is critical if we are to discover a way to regenerate hair cells in humans. Ultimately, though, the goal is to restore functional hearing. Birds offer a unique model for achieving this goal. They are the only animal model where it is possible to restore hearing through hair cell regeneration and then examine the effect of these newly created hair cells on the recovery of complex auditory perception that supports vocal learning and production. Several studies have shown that both young and adult birds experience hair cell loss in response to acoustic trauma or ototoxic insult. This loss is subsequently followed by restoration of hair cell numbers through a mitotic or conversion response and culminates in physiological and even behavioral recovery of auditory sensitivity within a matter of weeks (Corwin and Cotanche 1988; Ryals and Rubel 1988; Tucci et al. 1990; Girod et al. 1991; Hashino et al. 1991; Lippe et al. 1991; Saunders et al. 1992; Saunders et al. 1996; Ryals et al. 1999). Recent reviews of the recovery of auditory function following hair cell regeneration in birds have focused primarily on electrophysiological measures of the auditory system (compound action potential (CAP), auditory brainstem response (ABR), etc.) or changes in hair cell responses using otoacoustic emissions (e.g. Smolders et al. 1999; Saunders and Salvi 2008). While these physiological measures are highly correlated with the return of hearing, behavioral measures of hearing most directly address the actual recovery of auditory perception. Dooling et al (2008) reviewed both physiological and behavioral studies of the return of hearing after hair cell regeneration. The current review summarizes some of these same behavioral studies (Dooling et al 2008) with a clear focus on the relation between auditory function and vocal production as the “gold standard” for understanding the true behavioral consequences of recovery following hair cell regeneration.
The two primary methods by which hair cells have been damaged in order to induce the regenerative response in birds are acoustic overstimulation and otototoxic injury. Both of these conditions result in loss of hair cells; in the case of acoustic overstimulation other inner ear structures such as supporting cells, tegmentum vasculosum and neural synapses are also damaged or destroyed. Because acoustic overstimulation does not selectively damage hair cells, and because it has been difficult to damage a continuous sheath of hair cells along the epithelium without causing damage to other inner ear structures using noise exposure, studies of functional hearing return after acoustic trauma have not been particularly informative about the ability of regenerated hair cells to restore functional hearing. In fact, Saunders and Salvi in a recent review (2008) conclude that hair cell regeneration likely plays a relatively minor role in the recovery of physiological function following acoustic trauma in birds. On the other hand, studies using ototoxic injury are much more structurally specific to hair cells and can damage an extensive and continuous sheath of hair cells along the basilar papilla. Thus the current review will focus on behavioral studies of the recovery of auditory sensitivity and complex perception after ototoxic injury.
Finally, several studies have taken advantage of the avian model to study the relationship between the return of auditory function and changes in vocal learning or production. The interplay between auditory learning and vocal production has great relevance to the ultimate goal of restoration of complex communication through a regenerative therapy and so these studies will also be a focus of the review. In addition, we will explore the potential role of genetic hearing loss on the ability for regenerated hair cells to provide recovery of functional hearing by reviewing auditory sensitivity and perception in the Belgian Waterslager canary (BWS). The Belgian Waterslager canary has a genetic hearing loss but also retains the ability to regenerate hair cells. This unique model may provide insight into the potential influences or limitations that underlying genetic pathology has on the ability to restore auditory function through regeneration.
2. Changes in Auditory Sensitivity
In order for hair cells to be functional they must form and express the appropriate ionic channels and currents. Levic et al (2007) showed that individual regenerated hair cells harvested after ototoxic injury in adult chickens recapitulated the ionic currents shown in developing hair cells. Other less direct measures of regenerated hair cell viability, such as the cochlear microphonic and distortion product otoacoustic emissions, have also shown some return of function following regeneration (see Saunders and Salvi, 2008 for review). The cochlear microphonic shows substantial but incomplete recovery 11–14 weeks after ototoxic injury and hair cell regeneration (Chen et al 2001; Sun et al 2002), confirming recovery of transduction currents in regenerated hair cells. Distortion product otoacoustic emission (DPOAE) thresholds, input-output responses and amplitudes show partial to full recovery with the most consistent lingering decline at the highest frequencies (Chen et al 2001; Norton and Rubel, 1990; Durham et al 2000). Because avian hair cells apparently lack the somatic motility of mammalian outer hair cells (He et al 2003), it has been suggested that these measures of cochlear non-linearity are a function of stereocilia bundle resonance (Koppl 2011). Hair cell bundle irregularities have been reported in regenerated hair cells (Duckert and Rubel 1993) and Saunders and Salvi (2008) suggest that these continuing abnormalities may be responsible for the lack of full DPOAE recovery, especially at the highest frequencies. ABR and CAP measures of auditory function suggest that threshold sensitivity returns to normal levels within 30–70 days after cessation of ototoxic drug administration for all but the highest frequencies (see for Smolders 1999 and Saunders and Salvi 2008 for review). Thus physiological evidence predicts that behavioral threshold sensitivity should return to normal levels for all but the highest frequencies after ototoxic injury.
We know that hair cells are restored in birds through supporting cell mitotic division and differentiation as well as a through direct conversion or transdifferentiation (Shang et al 2010). Electrophysiological studies have yet to work out a way to differentiate the functional contribution of hair cells restored through either of these mechanisms separately. However, ABR thresholds showed recovery of sensitivity in some but not all birds in a recent study where cell division was blocked in vivo (Lin et al 2008). Further, mammalian hair cell restoration initiated through transdifferentiation resulted in some recovery of auditory brainstem evoked potential sensitivity (Izumikawa et al 2005).
While physiological measurements might be able to help tease out the contribution of particular anatomical elements to changes in sensitivity, behavioral responses reveal the perceptual consequences of those changes. Figure 1 shows behavioral audiograms measured after ototoxic drug administration in budgerigars (A), European starlings (B) and canaries (C). The findings are similar in all three species; hearing loss is greatest at the highest frequencies initially after antibiotic injection and recovery is most complete for the lowest frequencies. Recovery appears to plateau by 8 weeks post-injection. This functional loss and recovery follows a frequency pattern predicted by the hair cell loss which initially occurs at the base (highest frequencies on the place frequency map) and proceeds to more apical (lower frequency) regions of the basilar papilla (BP). Interestingly, hair cell numbers recover to within 1 standard deviation of normal within 2–3 months after antibiotic injections but behavioral thresholds continue to show some degree of threshold shift. This permanent threshold shift is greatest at the highest frequencies, however all frequencies tested show some residual threshold elevation.
Figure 1.
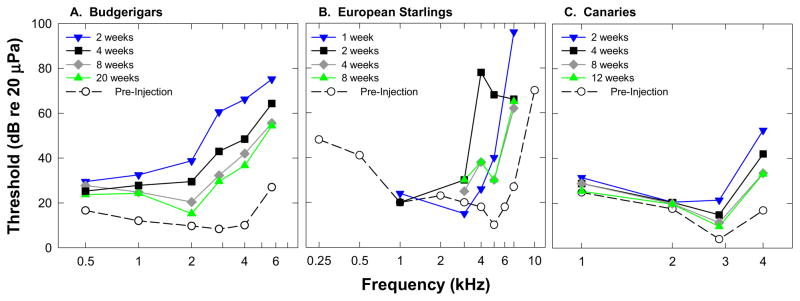
Behavioral pure tone threshold data are shown for A) budgerigars (Dooling et al. 2006), B) European Starlings (Marean et al. 1993), and C) canaries (unpublished data).
One early study of behavioral changes in pure tone sensitivity reported an anomalous pattern of maximal threshold shift for low frequencies in budgerigars (Hashino and Sokabe 1989), however this pattern has not been seen in more recent behavioral studies in this species (Dooling et al 1997; Dooling et al 2006; Dooling et al 2008), in European starlings (Marean et al. 1993), or in canaries (Dooling et al 1998). While hearing recovery after ototoxicity and hair cell regeneration generally seems to follow the pattern predicted by hair cell loss, there were some slight differences in the amount of threshold shift as a function of frequency and species. Audiometric threshold results reported for budgerigars (Figure 1) show that frequencies were differentially affected by injection of the ototoxic antibiotic kanamycin such that initial threshold shifts were greater at high frequencies (50–60dB above 2kHz) than at low frequencies (10–30dB below 2kHz). All frequencies tested showed some threshold shift two weeks following initial injections but thresholds gradually recovered to within 5–10dB of normal below 2,000Hz and within 20–30dB of normal above 2,000Hz (Dooling et al 2006). The pattern of threshold shift and recovery was similar in canaries, but interestingly thresholds at 1000Hz and 2,000Hz were only slightly elevated (<10dB) two weeks after injections and these lower frequency thresholds showed relatively little recovery over the next 12 weeks. European starlings (Marean et al 1993) showed a similarly negligible change in threshold for frequencies below 3000Hz two weeks after injections. In all three species, however, thresholds above 2–3kHz were initially elevated by 40dB–70dB but recovered to an asymptotic permanent threshold shift of less than 30dB over the next 8–10 weeks. While the mechanism underlying this permanent threshold shift at the highest frequencies is unclear, we know that even at 2–3 months after ototoxic dose, some hair cell abnormalities remain in the basal portion of the papilla. These lingering abnormalities include multiple and/or abnormal stereocilia bundles and abnormal stereocilia bundle orientation, some immature hair cells, and an irregular pattern of hair cells (e.g. Cotanche, 1999). Finally, factors beyond the hair cell could affect neural function and/or basilar membrane mechanics that may contribute to the permanent high frequency threshold shift.
In summary, results from physiological and behavioral measures of auditory sensitivity in several species of birds suggest that when a broad sheath of hair cells are lost through ototoxic damage and then replaced through either mitotic or transdifferentiation mechanisms, hearing thresholds are initially elevated and then recover to smaller asymptotic level of threshold shift within 8 to 10 weeks. Hair cell loss proceeds from base to apex following ototoxic drug administration. Threshold shifts follow a similar patter with highest frequencies, corresponding to the basal region of the basilar papilla, showing small permanent threshold shifts, while thresholds return to normal or nearly normal for lower frequencies corresponding to more apical regions of the BP. While some species differences in overall sensitivity to ototoxicity exists, this general pattern of frequency distribution of hearing loss and recovery is similar across species.
3. Belgian Waterslager Canary – changes in threshold sensitivity in an avian model of hereditary deafness
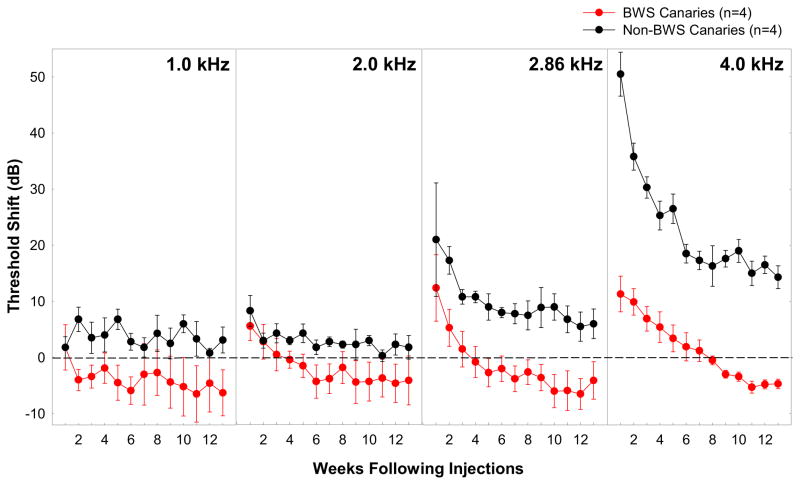
Canaries (Serinus canaria) have been selectively bred for specific song traits and plumage/body shape for well over a hundred years, and as a result there are many different strains of these domesticated songbirds (Stresemann, 1923; Guttinger, 1985). One strain, the Belgian Waterslager (BWS) canary, has become particularly noted for its loud, low-pitched song (Marler and Waser, 1977; Waser and Marler, 1977; Guttinger, 1985) and very poor hearing at high frequencies (Okanoya and Dooling, 1985; 1987; see also Gleich et al., 1994b, 1995). Thresholds at frequencies above 2 kHz are generally 20–40 dB higher than thresholds from other canary strains and birds (see review in Dooling et al., 2001). These elevated thresholds have been found behaviorally even in young (4–8 months old) BWS canaries (Okanoya and Dooling, 1987; Okanoya et al., 1990) and in auditory brainstem (ABR) recordings in 14 day old BWS canaries (Brittan-Powell et al., 2010). Although mammalian species with a hearing deficiency include numerous strains of mice with a variety of inner ear mutations (Battey, 2001), this is the first known hearing deficiency in a strain of bird where recovery from hearing loss through hair cell regeneration is at least possible. BWS canaries, like other birds (Cruz et al., 1987; Corwin and Cotanche, 1988; Ryals and Rubel, 1988), have the capacity to regenerate hair cells. Unlike other birds, they regenerate hair cells in the absence of external insult or trauma. Dooling and colleagues (1998) tested whether stimulating the hair cell regenerative response above the level of on-going cell death and regeneration in BWS might result in better hearing sensitivity than normal for these birds. They predicted that the synchronization of the loss of many hair cells in one area after ototoxic insult in adulthood might overwhelm ongoing cell death throughout the papilla in adult BW canaries and result in a sheath of synchronously regenerated hair cells and functional recovery to better than normal levels. Figure 2 shows the results of behavioral threshold sensitivity changes as a function of frequency and time after kanamycin injection (100mg/kg first day followed by 200mg/kg nine additional days) in BWs and non-BWS canaries.
Figure 2.
Mean threshold shifts at 1, 2, 2.86, and 4 kHz for four BWS (red circles) and four non-BWS (black circles) canaries for thirteen weeks following injections. Threshold shifts were calculated for each individual relative to their pre-injection threshold at that frequency. The horizontal dotted lines represent those pre-injection thresholds for both groups at 0 dB. Error bars represent between-subject standard errors. (data from three BWS published previously Dooling et al. 1997)
Before injections, behavioral thresholds at 4.0 kHz were significantly higher for BWS canaries (18 dB SPL) than non-BWS canaries (58 dB SPL). Following treatment with kanamycin, thresholds began to increase in both canary strains by injection day 4 until they peaked at week 1 following the cessation of injections. The amount of threshold shift observed at 4.0 kHz was not the same for the two canary strains however; BWS canary thresholds, already elevated, increased by 11.4 dB while non-BWS canary thresholds increased by 50.5 dB, a shift similar to that seen in other birds (Figure 1). For both strains, threshold recovery at 4 kHz began immediately following cessation of injections, until it reached asymptote at about week 6 for the non-BWS canaries and week 11 for the BWS canaries. The amount of recovery was much greater for the non-BWS canaries than the BWS canaries (40 dB improvement versus 15 dB improvement), but the non-BWS canaries had a permanent threshold shift (PTS) of about 15 dB while the BWS canaries showed threshold recovery that was almost 10 dB better than thresholds prior to injections with kanamycin. Although threshold shifts for the BWS canaries were smaller at 1, 2, and 2.86 kHz than at 4 kHz, and recovery occurred at a similar rate, the improvement following recovery was not significant at the lower frequencies. That is, the enhanced detection thresholds only occurred at 4 kHz. Dooling and colleagues (1998) had hypothesized that changes in hair cell number or morphology might correlate with these improved thresholds, however there were no morphological correlates in BWS to suggest that hair cells had returned in greater numbers than normal or that their surface morphology was more normal. The absence of superficial morphological changes of course does not address changes that might be present within the cells themselves or at the neural level. We do not know the number of functional cells within the BWS BP prior to kanamycin injection and cannot determine that number after kanamycin simply on the basis of absolute number and surface morphology. It may well be that kanamycin injections induced new hair cell production and those new hair cells remained more functional, despite the continuing presence of cells with abnormalities. Because BWS have fairly normal hair cell numbers and morphology at hatch it seems possible that the genetic basis of the hair cell abnormality is one of maintenance rather than production (Brittan-Powell et al 2010). Our results suggest that hair cell loss and recovery in adulthood results in improved auditory sensitivity despite the presence of an inherited inner ear abnormality. This improvement may be mediated by a renewed proliferation of hair cells which are capable of maintaining better functionality even in the face of the original genetic disorder. These results suggest that at least in the case of the BWS canary, genetic hearing loss does not preclude the possibility of improved hearing through stimulation of regenerative or transformational mechanisms.
4. Changes in Spectral, Intensity, and Temporal Resolution
Behavioral studies of auditory resolution have been made in both budgerigars and European starlings during and after hair cell regeneration (Hashino and Sokabe, 1989, Marean et al 1998, Dooling et al 2006). Critical ratios have been shown to increase immediately following KM treatment in budgerigars (Hashino and Sokabe 1989), and auditory filter widths were significantly wider at 5kHz in starlings following kanamycin ototoxicity induced hair cell loss and regeneration (Marean et al. 1998). In spite of mild permanently elevated absolute thresholds following kanamycin ototoxicity and hair cell regeneration, frequency difference limens tested at suprathreshold levels were only slightly elevated at 1000 and 2860kHz in budgerigars.
Marean et al. (1998) used temporal modulation transfer functions (TMTFs) to estimate temporal resolution following kanamycin ototoxicity and hair cell regeneration. In general they found little effect of kanamicin on temporal modulation. While some decrement in temporal resolution was noted for the highest frequencies, that may have been a function of poor signal audibility during temporary threshold shifts. Dooling et al (2006) tested difference limens for intensity in budgerigars at suprathreshold levels and found no significant difference 4–6 weeks post-injection. In summary, behavioral studies of auditory resolution for simple auditory stimuli suggest that frequency and temporal resolution may not fully recover after hair cell regeneration, especially at the highest frequencies, but that intensity resolution returns to normal within 1–2 months of hair cell regeneration.
5. Changes in Perception of Complex Auditory Stimuli
5.1 Closed Set Identification: Discrimination of complex vocal calls
While measures of absolute auditory sensitivity and frequency and intensity resolution give an initial indication of the influence of regenerated hair cells on the return of functional hearing, investigations of the perception of more complex sounds such as vocalizations are critical for our understanding of the ecological influence of hair cell regeneration. Birds provide an especially useful model for understanding these more salient auditory properties as they have extremely complex, learned vocal repertoires for acoustic communication, critical to their procreation and survival.
Dooling and colleagues (2006) studied changes in auditory discrimination and recognition of complex vocal calls in budgerigars after kanamycin induced hair cell regeneration. Budgerigars have an advantage over some other songbirds in that they learn new vocalizations throughout life, (Dooling 1986; Dooling et al. 1987; Farabaugh et al. 1994; Hile et al. 2000) and require auditory feedback in order to maintain the full range of adult vocal repertoire (Dooling et al 1987; Heaton et al. 1999). Moreover, budgerigars increase their vocal intensity when auditory feedback is reduced because of background noise, suggesting that they monitor vocal output in real time (Manabe et al. 1998).
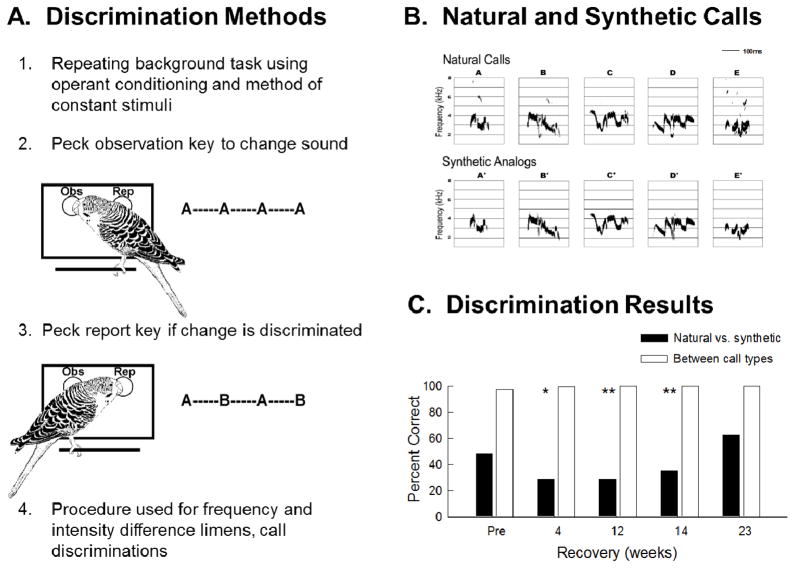
Adult budgerigars were behaviorally tested on their ability to discriminate between pairs of species-specific contact calls and their synthetic analogs before and after kanamycin treatment (see Figure 3). Discriminations between different contact calls were relatively easy while discrimination between a natural call and its synthetic analogue was difficult. Further analysis of the bird’s response latency to calls showed continued distortion in perceptual space one month after kanamycin injections had ceased (Dooling et al 2006). Discrimination of these synthetic complex call analogues took 4–5 months to return to normal levels, long after pure tone thresholds had recovered and hair cell regeneration was complete. This suggests that processes beyond peripheral sensitivity may be important for functional return of complex perception. In order to address this issue further, experiments are described in the next section on using an “open set” or recognition task of previously learned contact calls.
Figure 3.
(A) Cartoon describing operant conditioning paradigm used for behavioral training during discrimination task. (B) Sonograms of contact calls from five different birds and their synthetic analogs plotted as frequency by time (taken from Dooling and Okanoya 1995). (C) The average percent correct for 3 budgerigars discriminating among the five natural contact calls and their synthetic analogs before treatment with kanamycin and at 4, 12, 14, and 23 weeks after injections. (revised from Dooling et al 2008)
5.2 Open Set Identification: Recognition of Contact Calls
The previous experiments focused on the birds’ ability to discriminate between two presented complex vocalizations. This is a much simpler task than recognizing and identifying a particular complex signal in isolation. In this second set of experiments, Dooling et al (2006) tested whether budgerigars recognized contact calls with which they had previously been familiarized. One might consider this as analogous to recognizing a previously learned word or element of speech. This task is inherently more difficult than the discrimination task because the birds had to remember from trial to trial which call was a previously familiar call (“go” call) and which call was a new, unfamiliar, call (“no go” call).
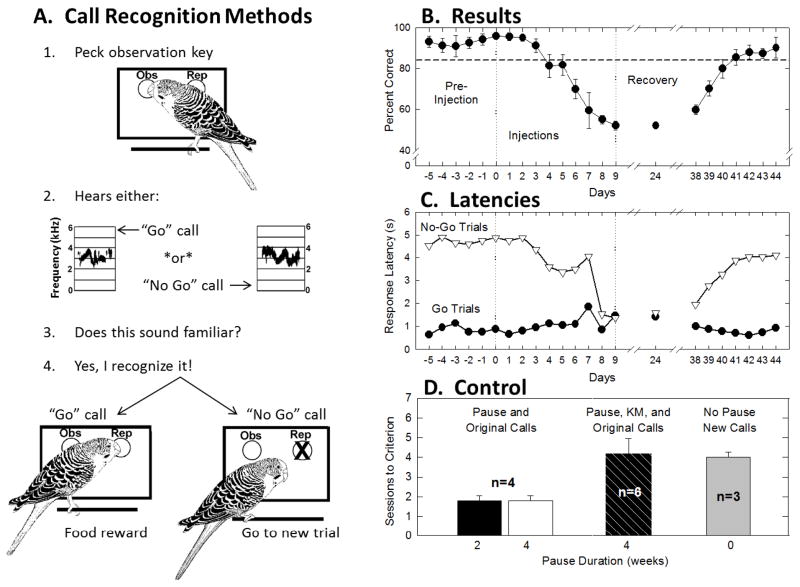
Six birds were trained to recognize two natural contact calls on a “go/no go” task (see Dooling et al 2006 for details) prior to kanamycin injections. Figure 4A shows a cartoon of the experimental protocol. Figure 4B shows the average percent correct before, during and after ten days of kanamycin injections. Prior to injections, birds were able to correctly recognize the “go” call well above an eighty-five percent criterion level. However, by nine days post-injection performance had fallen to chance (≤50%) and remained at chance when tested in single 100-trial test sessions 24 and 38 days later. At approximately one month post-injection, birds were retrained and tested on the same task in 100-trial daily sessions. Birds returned to pre-injection performance four days after retraining began.
Figure 4.
(A) Cartoon describing operant conditioning paradigm used for behavioral training during call recognition task. (B) Average percent correct responses of six budgerigars on a Go/No-Go recognition task involving two contact calls before, during and after treatment with kanamycin. Four weeks following the end of treatment, recognition performance returns to pre-injection levels after 4 days of testing. Error bars represent standard errors. (C) The average response latencies to both “go” and “no-go” stimuli for the six budgerigars before, during and after kanamycin treatment. (D) Summary of number of trials to reach criterion in the kanamycin experiment and various control experiments indicating that kanamycin treatment renders previously familiar calls unrecognizable. Error bars represent standard errors. (revised from Dooling et al 2008)
In order to determine whether birds’ response reduction to chance might be due to changes in overall health after kanamycin injections response latencies for the “go/no go” stimuli were examined (Figure 4C). Response latencies for the “go” stimuli remained below 1 second and were relatively unchanged throughout the experiment, suggesting that birds’ overall health did not change during testing. Response latencies for the “no go” calls were much longer (5seconds) indicating a clear difference between responsivity for the two stimuli prior to kanamycin injections. One week after kanamycin injections, response latencies for both the “go” and “no go” stimuli were similar (about 1 second) indicating that the stimuli were heard but that birds did not recognize one call as distinct from the other (responding at chance levels).
Four weeks post-injection, birds required an average of over four 100-trial sessions to reach criterion again. These results suggest that one month after kanamycin injections, when mature regenerated hair cells are present and have made synaptic contact (Duckert and Rubel 1990), birds are unable to recognize previously learned calls and instead treat them as new calls they are hearing for the first time. Control experiments to confirm these results are summarized in panel D of Figure 4. Four birds not injected with kanamycin were trained to recognize the “go” calls and then given either a 2-week or a 4-week pause in testing. Following the pause, these birds quickly reached criterion within one to two sessions. The kanamycin treated birds, on the other hand, given the same 4-week pause, took over four sessions to reach criterion. Interestingly, birds given no pause but completely new calls required the same number of sessions as the kanamycin treated birds. This suggests that these birds heard the original calls as completely new calls following hair cell regeneration. Four weeks into recovery, these birds were able to relearn the classification of previously familiar contact calls but they did so with a time course that suggests that these previously familiar calls now sound unfamiliar. In other words, although the ability to detect, discriminate, and classify complex acoustic sounds approaches pretreatment levels 4 weeks into recovery, the perceptual world of vocalizations is not the same as before hair cells were lost. These findings have relevance for hearing restoration efforts in humans since they suggest an enduring change in an organism’s auditory world of vocalizations despite a newly regenerated auditory periphery and relatively normal hearing sensitivity (Dooling et al 2006).
6. Changes in vocal production
While the recovery of auditory sensitivity and perception is a prime objective after hair cell regeneration, the concomitant effects of hearing loss on speech production cannot be overlooked. Prelingual deafness in humans has a profound effect on the development of speech production and even when auditory input is provided early the consequences on vocal precision remain significant. Similar consequences on vocal production are seen in birds, again making them a unique model for studies on hearing loss and recovery on both the perception and production of complex vocal signals.
In a series of experiments, Dooling et al (1997) and Manabe et al (1998) developed a procedure using operant conditioning with food reward to train budgerigars to reliably and consistently produce species-specific contact calls with a high degree of precision. They then tested birds before, during and after kanamycin injections. In these experiments contact call precision was determined through digital comparison with a stored template. Contact calls that matched the stored template resulted in food reinforcement, while calls that did not match the template were not rewarded.
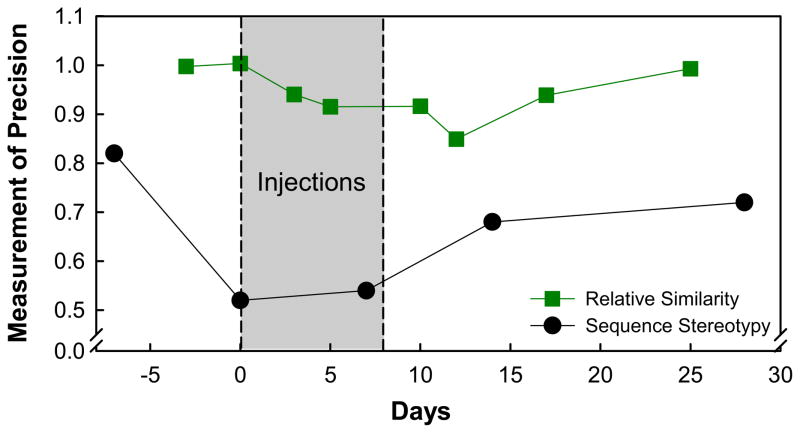
Figure 5 shows the precision with which contact calls were reproduced before, during and after an 8-day course of kanamycin. While there was a good deal of variability across these three birds, all showed some loss in vocal precision during kanamycin treatment. All three birds recovered to pre-treatment levels within 10–15 days following the injections and long before pure tone sensitivity had fully returned.
Figure 5.
The relative similarity of contact calls produced by three different budgerigars to their respective templates before, during, and up to 25 days after 8 days of kanamycin injections (green squares) from Manabe et al (1998) and song note sequence stereotypy for eight Bengalese finches that sang degraded song before, during, and after Amikacin injections (black circles) from Woolley and Rubel (2002).
Woolley and Rubel (2002) also examined vocal production, memory and vocal learning in adult Bengalese finches following hair cell loss and regeneration. They used a combination of noise and ototoxicity (amikacin) to produce substantial hair cell loss and song degradation (see Woolley et al., 2001). While their results were somewhat less straightforward than the results from budgerigars, they generally supported the finding that syllabic structural precision can be degraded by hearing loss and that song patterns can be relearned after hair cell regeneration. Figure 5 shows degradation and then recovery in syllable order of songs in Bengalese finches from Woolley and Rubel (2002). Finches, unlike budgerigars, have been shown to have a stable, unchanging song pattern throughout life. Woolley and Rubel (2002) used this same hair cell loss and regeneration model to also look at whether adult finches could learn new song patterns. In other words they asked whether the presence of a newly regenerated auditory periphery would provide an opportunity for plasticity in song learning. They found that, at least for some birds, new song patterns could be learned after regeneration and auditory experience with vocalizing cage mates (tutors). Taken together, results from budgerigars and finches suggest that the neural circuitry necessary for complex vocal pattern production is retained after a period of hearing loss and regeneration, and there is sufficient plasticity in the system to learn new song through auditory experience.
7. Conclusions
Results from both physiological and behavioral studies show that auditory sensitivity recovers after hair cell regeneration. The degree to which thresholds recover is frequency dependent; lower frequencies recover to pre-regeneration levels while higher frequency hearing recovers less completely. The ultimate goal for hair cell regeneration as a therapeutic approach to the treatment of hearing loss is to not only recover sensitivity, but also to provide clear resolution of the signal so that complex acoustic signals, like speech, can be understood and subsequent precise vocal production can be learned or retained. Behavioral studies of auditory resolution for simple stimuli suggest that intensity discrimination remains unchanged after hair cell regeneration, while frequency and temporal resolution remain compromised. Perhaps more importantly, behavioral studies of the perception of acoustically complex natural vocalizations suggests that the world indeed sounds different after hair cell regeneration. Budgerigars trained to recognize species specific contact calls respond to these same contact calls after regeneration as though they were “new” calls being heard for the first time. Significantly, birds were able to be re-trained to recognize contact calls after hair cell regeneration suggesting that neural pathways necessary for auditory learning remain intact after hair cell regeneration. So even though auditory memory for the previous signals is impaired, re-learning through the regenerated auditory channel is not.
Vocal precision and vocal learning in both budgerigars and finches are impacted by hair cell and hearing loss. Budgerigars trained to produce specific contact calls under operant control show a transient degradation of vocalization during hair cell loss and regeneration but recover vocal precision well before hearing thresholds have fully recovered. Similarly, Bengalese finches are able to reproduce complex syllabic structure in song after hair cell loss and regeneration. Both of these studies suggest that a number of neurological processes such as long-term memory, etc. play an important role in guiding vocal production in birds, as it does in humans. Finally, studies from Bengalese finches, a bird noted for new song learning only during early development (a critical period for vocal learning) show that hair cell regeneration may provide a window of opportunity for new vocal learning. Mechanisms for such plasticity include the possibility that regeneration of sensory cells in the periphery might promote plasticity in other neural circuits, making them more labile and available once more for learning through an auditory/vocal feedback channel.
We now know that while hair cell regeneration may restore hearing sensitivity, there are subtle, enduring changes to complex auditory perception. These changes do not appear to provide any obstacle to future auditory or vocal learning. Further, there is evidence that at least some types of hereditary deafness may not preclude benefit from hair cell regeneration. Of course there continue to be many unanswered questions about functional recovery after hair cell regeneration. For example, all of the behavioral studies described in this review have involved hair cell regeneration following transient hearing loss after an acute ototoxic injury in young or adult birds. Research thus far has done little to increase our understanding of the effects of hair cell regeneration on the restoration of hearing in the face of long-term hair cell damage and hearing loss. Experiments addressing the influence of the onset (young birds prior to song learning) or duration of hearing loss could provide important information leading to advances in treating hearing loss in humans, determining when it is most beneficial to install cochlear implants, and ultimately restoring hearing in humans through regenerated hair cells.
Highlights.
We review behavioral studies of hearing recovery after ototoxic induced hair cell regeneration
Pure tone sensitivity and resolution returns to normal levels for all but the highest frequencies
Hereditary hearing loss does not prevent hearing improvement in Belgian Waterslager Canaries
We found enduring changes in the perception of complex, species specific vocalizations
These perceptual difficulties did not preclude future auditory or vocal learning
Acknowledgments
The authors would like to thank Elizabeth Brittan-Powell for comments on earlier drafts of this article and Amanda Lauer for other assistance. This work was supported in part by grant DC-001372 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health to RJD and BMR and P30DC004664.
Abbreviations
- CAP
compound action potential
- ABR
auditory brainstem response
- BWS
Belgian Waterslager canary
- DPOAE
distortion product otoacoustic emission
- BP
basilar papilla
- SPL
sound pressure level
- PTS
permanent threshold shift
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brenda M. Ryals, Email: ryalsbm@jmu.edu.
Micheal L. Dent, Email: mdent@buffalo.edu.
Robert J. Dooling, Email: dooling@psyc.umd.edu.
References
- Battey JF. Human hereditary hearing impairment: research progress fueled by the human genome project and mouse models. In: Willott JF, editor. Handbook of Mouse Auditory Research. CRC Press; New York: 2001. pp. 391–400. [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Ryals BM, Gleich O. Electrophysiological and morphological development of the inner ear in Belgian Waterslager canaries. Hear Res. 2010;269:56–69. doi: 10.1016/j.heares.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Sun W, Salvi RJ. Electrically evoked otoacoustic emissions from the chicken ear. Hear Res. 2001;161(1–2):54–64. doi: 10.1016/s0378-5955(01)00353-7. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Structural recovery from sound and aminoglycoside damage in the avian cochlea, Audiol. Neuro-Otol. 1999;4:271–285. doi: 10.1159/000013852. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PM, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Hearing in birds and reptiles. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative Hearing: Birds and Reptiles. Springer-Verlag; New York: 2001. pp. 308–359. [Google Scholar]
- Dooling RJ. Perception of vocal signals by budgerigars. Exp Biol. 1986;45:195–218. [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Manabe K. Recovery of hearing and vocal behavior after hair cell regeneration. Proc Natl Acad Sci. 1997;94:14206–14210. doi: 10.1073/pnas.94.25.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Dent ML, Reid TL. Perception of complex sounds in budgerigars (Melopsittacus undulatus) with temporary hearing loss. J Acoust Soc Am. 2006;119:2524–2532. doi: 10.1121/1.2171839. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Gephart BF, Price PH, McHale C, Brauth SE. Effects of deafening on the contact call of the budgerigar, Melopsittacus undulatus. Anim Behav. 1987;35:1264–1266. [Google Scholar]
- Dooling RJ, Dent ML, Lauer AM, Ryals BM. Functional Recovery Following Hair Cell Regeneration in Birds. In: Salvi RJ, Popper AN, Fay RR, editors. Hair Cell Regeneration, Repair, and Protection. Springer Science+Business Media, LLC; New York: 2008. pp. 117–140. [Google Scholar]
- Dooling RJ, Ryals BM, Presson J. The paradox of the Belgian Waterslager canary: congenital hair cell abnormalities and hearing loss despite post mitotic hair cell replacement. In: Palmer AR, Rees E, Summerfield AQ, Meddis R, editors. Psychophysical and Physiological Advances in Hearing. Whurr Publishers; London: 1998. pp. 145–152. [Google Scholar]
- Duckert LG, Rubel EW. Morphological correlates of functional recovery in the chicken inner ear after gentamycin treatment. J Comp Neurol. 1993;331:75–96. doi: 10.1002/cne.903310105. [DOI] [PubMed] [Google Scholar]
- Duckert LG, Rubel EW. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear Res. 1990;42(1–2):175–194. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- Durham D, Park DL, Girod DA. Central nervous system plasticity during hair cell loss and regeneration. Hear Res. 2000;147:145–59. doi: 10.1016/s0378-5955(00)00128-3. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsittacus undulatus): Evidence for social factors in the learning of contact calls. J Comp Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- Girod DA, Tucci DL, Rubel EW. Anatomical correlates of functional recovery in the avian inner ear following aminoglycoside ototoxicity. Laryngoscope. 1991;101:1139–1149. doi: 10.1288/00005537-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Gleich O, Klump GM, Dooling RJ. Hereditary sensorineural hearing loss in a bird. Naturwissenschaften. 1994;81:320–323. doi: 10.1007/BF01131950. [DOI] [PubMed] [Google Scholar]
- Gleich O, Klump GM, Dooling RJ. Peripheral basis for the auditory deficit in Belgian Waterslager canaries (Serinus canarius) Hear Res. 1995;82:100–8. doi: 10.1016/0378-5955(94)00166-n. [DOI] [PubMed] [Google Scholar]
- Guttinger HR. Consequences of domestication on the song structures in the canary. Behaviour. 1985;94:254–278. [Google Scholar]
- Hashino E, Sokabe M. Kanamycin induced low-frequency hearing loss in the budgerigar (Melopsittacus undulatus) J Acoust Soc Am. 1989;85:289–294. doi: 10.1121/1.397736. [DOI] [PubMed] [Google Scholar]
- Hashino E, Sokabe M, Tanaka R. Function-structure correlation during recovery from aminoglycoside ototoxicity in the avian auditory system. In: Dancer AL, Henderson D, Salvi RJ, Hammernik RP, editors. Noise-Induced Hearing Loss. St. Louis: Mosby Year Book; 1991. pp. 228–236. [Google Scholar]
- He DZ, Beisel KW, Chen L, Ding DL, Jia S, Fritzsch B, Salvi R. Chick hair cells do not exhibit voltage-dependent somatic motility. J Physiol. 2003;546(Pt 2):511–520. doi: 10.1113/jphysiol.2002.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JT, Dooling RJ, Farabaugh SM. Effects of deafening on the calls and warble song of adult budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 1999;105:2010–2019. doi: 10.1121/1.426734. [DOI] [PubMed] [Google Scholar]
- Hile AG, Plummer TK, Streidter GF. Male vocal imitation produces call convergence during pair bonding in budgerigars (Melopsittacus undulatus) Anim Behav. 2000;59:1209–1218. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Koppl C. Birds—same thing, but different? Convergent evolution in the avian and mammalian auditory systems provides informative comparative models. Hear Res. 2011;273(1–2):65–71. doi: 10.1016/j.heares.2010.03.095. [DOI] [PubMed] [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci USA. 2007;104(48):19108–13. doi: 10.1073/pnas.0705927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V, Lee GS, Rubel EW, Stone J. Hearing recovery via direct transdifferntiation in an avian model. Assoc Res Otol. 2008:abstract 922. [Google Scholar]
- Lippe WR, Westbrook EW, Ryals BM. Hair cell regeneration in the chick cochlea following aminoglycoside ototoxicity. Hear Res. 1991;56:203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- Manabe K, Sadr EI, Dooling RJ. Control of vocal intensity in budgerigars (Melopsittacus undulatus): differential reinforcement of vocal intensity and the Lombard effect. J Acoust Soc Am. 1998;103:1190–1198. doi: 10.1121/1.421227. [DOI] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW. Hair cell regeneration in the European starling (Sturnus vulgaris): Recovery of pure-tone detection thresholds. Hear Res. 1993;71:125–136. doi: 10.1016/0378-5955(93)90028-y. [DOI] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW. Auditory perception following hair cell regeneration in European starling (Sturnus vulgaris): Frequency and temporal resolution. J Acoust Soc Am. 1998;103:3567–3580. doi: 10.1121/1.423085. [DOI] [PubMed] [Google Scholar]
- Marler P, Waser MS. Role of auditory feedback in canary song development. J Compar Physiological Psychol. 1977;91:8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Norton SJ, Rubel EW. Active and passive ADP components in mammaliean and avian ears. In: Dallos P, Geisler CD, Matthews JA, Ruggero MA, Steele CR, editors. Mechanics and Biophysics of Hearing. New York: Springer-Verlag; 1990. pp. 219–226. [Google Scholar]
- Okanoya K, Dooling RJ. Colony differences in auditory thresholds in the canary (Serinus canarius) J Acoust Soc Am. 1985;78:1170–6. doi: 10.1121/1.392885. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Hearing in Passerine and Psittacine birds: A comparative study of masked and absolute auditory thresholds. J Comp Psych. 1987;101:7–15. [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ, Downing JD. Hearing and vocalizations in hybrid Waterslager-Roller canaries (Serinus canarius) Hear Res. 1990;46:271–5. doi: 10.1016/0378-5955(90)90008-d. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Dooling RJ, Westbrook E, Dent ML, MacKenzie A, Larsen ON. Avian species differences in susceptibility to noise exposure. Hear Res. 1999;131:71–88. doi: 10.1016/s0378-5955(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Saunders SS, Adler H, Pugliano F. The structural and functional aspects of hair cell regeneration in the chick as a result of exposure to intense sound. Exp Neurol. 1992;115:13–17. doi: 10.1016/0014-4886(92)90213-a. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Doan DE, Cohen YE, Adler HJ, Poje CP. Recent observations on the recovery of structure and function in the sound damaged chick ear. In: Salvi RJ, Henderson D, Fiorino F, Colletti V, editors. Auditory System Plasticity and Regeneration. New York: Thieme Medical Publishers; 1996. pp. 62–84. [Google Scholar]
- Saunders SS, Salvi RJ. Recovery of Function in the Avian Auditory System after Ototrauma. In: Salvi RJ, Popper AN, Fay RR, editors. Hair Cell Regeneration, Repair, and Protection. Springer Science+Business Media, LLC; New York: 2008. pp. 77–116. [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J. Supporting cell division is not required for regeneration of auditory hair cels after ototoxic injury in vitro. J Assoc Res Otol. 2010;11:2-3-222. doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders JW. Functional recovery in the avian ear after hair cell regeneration. Audiol Neurootol. 1999;4:286–302. doi: 10.1159/000013853. [DOI] [PubMed] [Google Scholar]
- Stresemann E. Zur Geschichte eineger Kanarienvogel-Rasen. Ornithol Mon ber. 1923;31:103–106. [Google Scholar]
- Sun W, Chen L, Salvi RJ. Acoustic modulation of electrically evoked otoacoustic emission in chickens, Audiol. Neurootol. 2002;7(4):206–13. doi: 10.1159/000063737. [DOI] [PubMed] [Google Scholar]
- Tucci DL, Rubel EW. Physiological status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990;103:443–445. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- Waser MS, Marler P. Song learning in canaries. J Compar Physiological Psychol. 1977;91:1–7. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Rubel EW. Vocal memory and learning in adult Bengalese Finches with regenerated hair cells. J Neurosci. 2002;22:7774–87. doi: 10.1523/JNEUROSCI.22-17-07774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Wissman AM, Rubel EW. Hair cell regeneration and recovery of auditory thresholds following aminoglycoside ototoxicity in Bengalese finches. Hear Res. 2001;153:181–195. doi: 10.1016/s0378-5955(00)00217-3. [DOI] [PubMed] [Google Scholar]