Abstract
Specific contributions of frontal and parietal regions to visuospatial encoding and attention remain controversial. This study used fMRI to examine associative encoding of sequentially-presented spatial cues and object stimuli. The cue preceded the centrally-displayed object by a jittered-delay-interval to better isolate attempt and success in episodically integrating pure location information with separately presented objects. Superior parietal response was modulated by attempted location binding, while superior/middle frontal response was predictive of successful location binding.
Keywords: fMRI, human, frontoparietal, visuospatial
Real-world objects tend to have associated locations, and commonly in daily life, an object needs to be remembered along with its location. Prefrontal and medial temporal regions have often been the focus of studies examining general subsequent memory effects (beginning with [1–2]); however, more recently, the contribution of parietal regions to episodic encoding has gained attention (see [3] for review), particularly when spatial processing is required (see [4] for review). Frontoparietal activity has been widely reported in tasks involving spatial working memory (e.g., [5–9]) and visuospatial associative encoding (e.g., [10–19]). Neuroimaging studies of object-in-place encoding, in which subjects were shown pictures of objects in particular locations and were subsequently tested on these spatial associations, found subsequent memory effects for object-location binding in superior parietal and prefrontal regions [10–12, 18]. Additionally, a recent study examining patients with focal damage due to ischemic or hemorrhagic stroke reported impairment in object-location binding in patients with left posterior parietal cortex lesions [19]. This frontoparietal involvement in spatial encoding has also been salient in reviews of findings from human [16] and rat [15] studies.
Although frontoparietal regions have been strongly implicated in visuospatial encoding, the contribution of particular subregions has been a matter of discussion. Some recent reviews have described a dorsal-ventral distinction in frontoparietal involvement in episodic memory through its role in attention [3, 20]. Corbetta and Shulman [21] performed an extensive review of neurophysiological and psychological findings regarding visual attention. They established and described two separate attention systems: a dorsal frontoparietal network for goal-directed, top-down attention and a ventral frontoparietal network for stimulus-driven, bottom-up attention. Additionally, they discussed superior parietal and frontal involvement in top-down visuospatial attention. Extending this dual-process model for attention to episodic memory, Cabeza et al. [20] described their attention to memory model, AtoM, which complements and applies the dorsal-ventral distinction within the frontoparietal attention network. In a recent review, Uncapher and Wagner [3] acknowledged the additional involvement of parietal regions in successful encoding and addressed this role in light of the dual-attention perspective on parietal activity. They presented converging evidence that dorsal posterior parietal cortex is more active during the encoding of subsequently remembered events of all stimulus types, but particularly when involving spatial attention.
Despite this evidence supporting a dorsal frontoparietal network for controlling goal-directed attention within visuospatial encoding, unanswered questions remain regarding the specific contributions of frontal and parietal regions to the different components of this memory formation process. The current study was designed to examine the regional brain contributions underlying the attempt versus the success of object-location encoding with temporal separation between the location cue and the presentation of the object. By controlling the presence or absence of location information in the cue, the neural bases of attempt and success in episodically pairing location with subsequently presented individual objects could be examined.
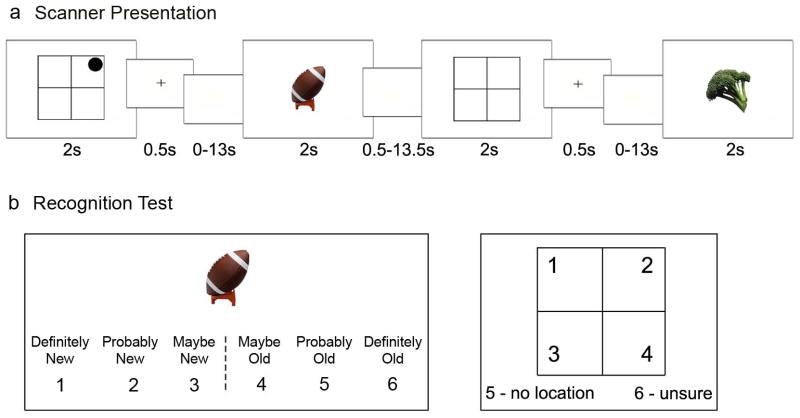
Seventeen healthy volunteers (mean age = 25.3 ± 2.8 years, seven males) were recruited from the University of California, San Diego (UCSD) community and the surrounding area. All subjects gave informed consent approved by the UCSD Institutional Review Board, had normal or corrected vision, and received monetary compensation for their time. Three subjects were excluded from analyses, two for having poor behavioral performance (with three and zero ‘correct location’ trials while included subjects had at least ten ‘correct location’ trials) and one for having magnetic resonance signal artifact that interfered with analysis and led to insufficient quality of functional data. Stimuli consisted of 168 color images of everyday objects [22]. While undergoing functional magnetic resonance imaging (fMRI), subjects were sequentially shown single 2 × 2 grids, with or without an identified quadrant, followed by an object. Fig. 1a illustrates the timing of the 128 location-object pair trials. Subjects were instructed that if the grid had a circle in one of the quadrants (‘location’ trial), to imagine and remember the following object as located in that quadrant, and if the grid was blank (‘no-location’ trial), to remember the object without spatial information. Subjects received an equal number of ‘location’ and ‘no-location’ trials. Following the scan, subjects performed a self-paced recognition test of object and spatial memory for each of the 128 objects. Subjects were asked to make object-memory and spatial-memory judgments for each of the 128 objects presented during the scan, and 40 additional novel objects were included for the object-memory judgment only (see Fig. 1b). Prior to scanning, each subject was shown one grid-object pair (not used during testing) as a practice trial to ensure understanding of the instructions.
Figure 1.
Experimental design. a, Example of the encoding task used in the scanner illustrating the sequential presentation of a spatial cue (with present or absent location information) and an object. A trial consisted of a 2-s spatial cue grid, followed by a 0.5-s associative cue (plus-sign), a blank screen jittered delay of 0 to 13 s, and finally a 2-s object. A blank screen was present during an intertrial interval of 0.5 to 13.5 s. The jittered delay periods and intertrial intervals were calculated to optimize the study design for modeling the hemodynamic response to trials [25]. When a circle was present in one of the grid quadrants, subjects were instructed to imagine and remember the following object (e.g., football) as located in that quadrant. When the grid was blank, subjects were instructed to just remember the following object (e.g., broccoli). b, Example of the post-scan recognition test. Subjects were shown each object previously viewed during the encoding task as well as novel objects, and they were asked to rate their confidence that the picture (e.g., football) was new or that it was shown during the scan (old) on a scale from “definitely new” to “definitely old.” If the object was included in the encoding task, subjects were asked a follow-up question regarding the object’s associated spatial information. Subjects responded 1–4 to identify the object’s associated location, 5 to select that the object had no location, or 6 to say they were unsure about the object’s location.
Subjects were scanned using a 3T GE scanner at the Keck Center for Functional MRI at the University of California, San Diego. Functional images were acquired using gradient-echo, echo-planar, T2*-weighted pulse sequence (repetition time = 2.5 s; one shot per repetition; echo time = 30; flip angle = 90°; bandwidth = 31.25 MHz). Forty slices covering the brain were obtained perpendicular to the long axis of the hippocampus with 3.4 × 3.4 × 4 mm voxels during the four 395-second functional runs. Field maps were acquired to measure and correct for static field inhomogeneities. A T1-weighted structural scan was acquired in the same plane and with the same voxel size as the functional scans. A high resolution structural scan was also acquired sagittally using a T1-weighted (1 × 1 × 1 mm) inversion recovery prepared fast spoiled gradient recalled sequence. Structural data were transformed into Talairach space by AFNI using nearest-neighbor interpolation [23] after standard landmarks, including the anterior and posterior commissures, were manually defined on the anatomical scans.
For two subjects, one functional run (out of four) was discarded due to scanner malfunction or excessive subject motion identified visually during pre-analysis quality checks. After functional data from each run were field-map corrected, slices were temporally aligned and co-registered using a three-dimensional image alignment algorithm, voxels outside the brain were eliminated using a threshold mask of the functional data, and functional runs were corrected for motion and concatenated all using the AFNI suite of programs [23]. An 8.0 mm FWHM Gaussian filter was also applied to smooth the functional data from each run. A general linear model was constructed using multiple regression analysis; six motion regressors obtained from the registration process were included along with 10–12 behavioral regressors based on subsequent memory performance. Subjects’ behavioral trials were sorted based on object-memory accuracy and confidence and spatial-memory accuracy. The objects remembered with high confidence (“definitely old”) were divided into four trial types based on the accuracy of the spatial judgment: (1) the correct location quadrant was identified, ‘correct location;’ (2) the correct location quadrant was not identified, ‘incorrect location;’ (3) the lack of a location quadrant was correctly identified, ‘correct no-location;’ (4) the lack of a location quadrant was not identified, ‘incorrect no-location.’ All trial types were modeled, but only ‘correct location,’ ‘incorrect location,’ and ‘correct no-location’ trials were further analyzed in group analyses, since ‘incorrect no-location’ trials represent false alarm errors for location memory which was not a focus of this study. Additional trials, modeled but not included in further analyses, consisted of all objects rated as “new,” which were grouped as ‘forgotten’ trials, and objects rated as “maybe old” or “probably old,” which were grouped as ‘weak-memory’ trials. Signal deconvolution with tent basis functions and a defined time window of 15 seconds following the onset of each cue and each object was used to derive hemodynamic response functions from the fMRI data [23]. Multiple linear regression analyses were used to separately examine the activity for all three conditions.
Whole brain voxel-wise t-tests (p < 0.05, two-tailed, corrected) carried out across all 14 subjects were conducted to examine regional responses to ‘location’ versus ‘no-location’ trials and to ‘correct location’ versus ‘incorrect location’ trials. These analyses were conducted both during the cue period and during the object period. AlphaSim (using AFNI) was used to correct for multiple comparisons inside the brain and to obtain cluster significance values; functional clusters of least 62 contiguous voxels were identified in each analysis to yield a whole brain significance value of p < 0.05 corrected for all comparisons. Percent signal change was extracted for each condition for the 15-second time period following the onset of the location cue.
Object memory did not differ between ‘location’ and ‘no-location’ trials, as subjects confidently remembered 72% (± 4% SEM) of the objects that had a location and 70% (± 4% SEM) of the objects that did not have a location (t(13) = 0.63, p > 0.5). For ‘location’ and ‘no-location’ trials combined, most of the objects were confidently remembered, with 13% (± 2% SEM) of the objects remembered weakly (rated as “probably old” or “maybe old”) and 21% (± 3% SEM) of the objects forgotten (rated as “definitely new,” “probably new,” or “maybe new”). False alarm rate was 6% (± 1% SEM). Of the confidently remembered objects, 55% (± 5% SEM) had a correctly identified associated quadrant location and 60% (± 4% SEM) had a correct ‘no-location’ judgment. Location responses were selected from six choices, one of which was an ‘unsure’ judgment (Fig. 1b).
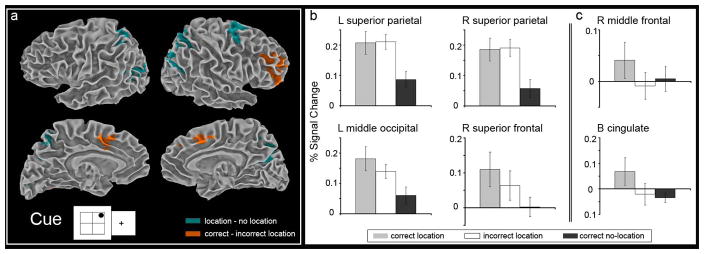
During the scan, the presentation of the visual object was temporally separated from the spatial cue thereby allowing improved behavioral isolation of the cue and object portions of the task. Despite this advantage, the lack of temporal overlap does not assure independence of the respective functional activity associated with cue and object, since cue is maintained in working memory up until the time of the object stimulus. The purpose of temporal separation was not to provide a direct comparison between cue and object activity, but rather to maximize location-related activity in the brain that occurs even before the object is presented. The analysis targets the question of how encoding that includes a directed attempt to episodically encode location differs from encoding that does not include such an attempt, as well as examining the brain bases of success and failure in these attempts. During the cue period, brain regions were identified where the size of the BOLD response was greater when a location cue was present than when a location cue was absent (‘location’ minus ‘no-location’ trials, p < 0.05, corrected for multiple comparisons). Additionally, for trials in which a location was presented, regional activity predictive of subsequent successful object-location binding was identified (‘correct location’ minus ‘incorrect location’ trials, p < 0.05, corrected for multiple comparisons). Regions identified by these contrasts are listed in Table 1. Greater response for ‘location’ trials was seen in right superior frontal, bilateral superior parietal, and left middle occipital regions (Fig. 2a). Greater response predictive of subsequent object-location memory was seen in right middle frontal and bilateral cingulate regions (Fig. 2a). Percent signal change for the three conditions, ‘correct location,’ ‘incorrect location,’ and ‘correct no-location,’ show that the greatest response in bilateral superior parietal cortex was for maintaining location information in order to associate spatial information with object encoding. In this region, increased activity was seen for successful object encoding when location information was to be associated, regardless of whether the location was remembered or forgotten (Fig. 2b). Therefore, these findings suggest that simple intention to link spatial components to a subsequent, successfully encoded, object strongly drives superior parietal lobe regardless of binding success. In contrast, the predominant response in right middle frontal cortex was for successful object-location binding. In this region, increased activity was seen for successful object-location binding relative to successful encoding of objects for which associated location information was absent or forgotten (Fig. 2c). In other words, greater involvement of middle frontal was predictive of object-location binding success.
Table 1.
Regions activated by the object-location associative encoding task during the cue period.
| Region | BA | Volumea | x | y | z | t-values |
|---|---|---|---|---|---|---|
| Location > No-Locationb | ||||||
| R superior frontal gyrus | 6 | 7744 | 14 | −7 | 52 | 4.68 |
| L middle occipital | 31 | 14656 | −26 | −73 | 16 | 4.56 |
| R superior parietal | 31 | 19648 | 26 | −65 | 24 | 4.45 |
| L superior parietal | 7 | 7616 | −14 | −57 | 48 | 4.41 |
| Correct Location > Incorrect Locationb | ||||||
| R middle frontal gyrus | 10 | 7488 | 34 | 35 | 16 | 5.54 |
| L cerebellum | -- | 4224 | −38 | −57 | −32 | 4.14 |
| B cingulate | 32 | 5568 | 2 | 15 | 40 | 3.97 |
cluster volumes (mm3); coordinates correspond to the voxel of maximum intensity for each cluster
p<0.05 corrected for multiple comparisons
Figure 2.
Superior parietal and middle frontal regions are differently engaged by object-location binding attempt versus success. a, Statistical activation maps for regions showing increased activity during the cue period for ‘location’ versus ‘no-location’ trials (teal) and ‘correct location’ versus ‘incorrect location’ trials (orange). All contrasts (p < 0.05, corrected for multiple comparisons) are overlaid on the smooth white matter surface of the Talairach and Tournoux N27 average brain. b, Graphs depict the percent signal change for ‘correct location,’ ‘incorrect location,’ and ‘correct no-location’ trials during the cue period within the brain regions identified by the ‘location’ versus ‘no-location’ trials contrast (clusters of activation shown in teal): left superior parietal, right superior parietal, left middle occipital, and right superior frontal regions. Bilateral superior parietal regions show similar levels of activity for both location trials regardless of subsequent object-location memory. c, Graphs depict the percent signal change for ‘correct location,’ ‘incorrect location,’ and ‘correct no-location’ trials during the cue period within the brain regions identified by the ‘correct location’ versus ‘incorrect location’ trials contrast (clusters of activation shown in orange): right middle frontal and bilateral cingulate. Left middle frontal gyrus shows differential activity based on memory for object-location associations. The error bars represent the standard error of the mean.
In daily life, people encounter objects that need to be associated with a different location from where that event is occurring. This study is believed to be the first to explore long-term memory for the association between objects and locations separated in time. The involvement of frontal and parietal regions to location encoding were disentangled, as parietal regions were modulated by presence of additional location information to be bound to an object regardless of success, while frontal region responses were predictive of successful binding of place to an object.
Although some prior studies have reported subsequent object-location memory effects in both frontal and parietal regions (e.g., [10, 18]), these studies have used object stimuli that were presented to subjects in the cued location. The current study, however, found differences in how frontal and parietal regions were modulated during object-location encoding when the location cue was not visually concurrent with the presented object. Therefore, when a cued location was provided, subject needed to maintain that location across the delay in order to associate and encode the following object with that location. Given the established role of parietal regions in attention, and especially spatial attention, it is possible that the responses seen in parietal regions in the present study are attentional; however, this would suggest that increased location attention did not influence subsequent object-location binding success, which would be in contrast to previous studies which found that selective attention influenced binding success (e.g., [3, 24]). Additionally, even low level processes encompassed by attempted location encoding, such as saccades, may contribute to the pattern of activation attributed to “binding attempt,” whereas activity attributed to “binding success,” or long-term memory for the location, would, at least intuitively, be less susceptible to such low level or motoric influences. Nevertheless, these findings suggest frontal and parietal contribution to object-location encoding, in which attempted binding of location with subsequent object information engages superior parietal regions, while binding success is predicted by additional middle frontal response. The unique design of the current study allowed examination of the specific contributions of parietal and frontal regions to object-location associative encoding and demonstrated that these regions are differentially engaged by location binding attempt versus success.
HIGHLIGHTS.
Frontoparietal involvement in sequential object-location associative encoding
Superior parietal modulated by attempted visuospatial binding regardless of success
Medial / middle frontal response predictive of successful visuospatial binding
Dissociated parietal and frontal engagement in spatial binding attempt and success
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke K23 NS050305 and the University of California, San Diego Departments of Neurosciences and Radiology. JBH is supported by the National Science Foundation through the Graduate Research Fellowship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–7. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 3.Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–54. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–6. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 6.Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: contributions from functional imaging studies. Behav Brain Res. 2010;214:172–9. doi: 10.1016/j.bbr.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. Neuroimage. 2000;12:85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- 8.Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–91. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- 9.Schon K, Tinaz S, Somers DC, Stern CE. Delayed match to object or place: an event-related fMRI study of short-term stimulus maintenance and the role of stimulus pre-exposure. Neuroimage. 2008;39:857–72. doi: 10.1016/j.neuroimage.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–56. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- 11.Gould RL, Brown RG, Owen AM, Bullmore ET, Williams SC, Howard RJ. Functional neuroanatomy of successful paired associate learning in Alzheimer’s disease. Am J Psychiatry. 2005;162:2049–60. doi: 10.1176/appi.ajp.162.11.2049. [DOI] [PubMed] [Google Scholar]
- 12.Gould RL, Brown RG, Owen AM, ffytche DH, Howard RJ. fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage. 2003;20:1006–19. doi: 10.1016/S1053-8119(03)00365-3. [DOI] [PubMed] [Google Scholar]
- 13.Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–24. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991;88:1621–5. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesner RP. The posterior parietal cortex and long-term memory representation of spatial information. Neurobiol Learn Mem. 2009;91:197–206. doi: 10.1016/j.nlm.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postma A, Kessels RP, van Asselen M. How the brain remembers and forgets where things are: the neurocognition of object-location memory. Neurosci Biobehav Rev. 2008;32:1339–45. doi: 10.1016/j.neubiorev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Sommer T, Rose M, Glascher J, Wolbers T, Buchel C. Dissociable contributions within the medial temporal lobe to encoding of object-location associations. Learn Mem. 2005;12:343–51. doi: 10.1101/lm.90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommer T, Rose M, Weiller C, Buchel C. Contributions of occipital, parietal and parahippocampal cortex to encoding of object-location associations. Neuropsychologia. 2005;43:732–43. doi: 10.1016/j.neuropsychologia.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.van Asselen M, Kessels RP, Frijns CJ, Kappelle LJ, Neggers SF, Postma A. Object-location memory: a lesion-behavior mapping study in stroke patients. Brain Cogn. 2009;71:287–94. doi: 10.1016/j.bandc.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 22.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–2. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 24.Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci. 2009;29:8270–9. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]