Abstract
A hexanucleotide repeat expansion in the chromosome 9 open reading frame 72 (C9ORF72) gene was recently discovered as the cause underlying frontotemporal degeneration (FTD) and/or amyotrophic lateral sclerosis (ALS) linked to chromosome 9 (c9FTD/ALS). In this atypical case of c9FTD/ALS, the proband presented with amnestic mild cognitive impairment which evolved into Alzheimer’s disease (AD)-type dementia and later developed ALS. Fluorodeoxyglucose-positron emission tomography of the brain demonstrated mild hypometabolism involving the medial frontal and lateral temporal lobes, left more so than right, which progressed over time. He was subsequently confirmed to have the C9ORF72 expansion. This report highlights the need to consider mutations in the FTD-associated genes when a familial disorder is suggested and neuroimaging studies reveal findings atypical of an AD pathophysiological process despite the typical anterograde amnestic syndrome.
Keywords: Frontotemporal degeneration, amyotrophic lateral sclerosis, motor neuron disease, Alzheimer’s disease, TDP-43, C9ORF72, c9FTD/ALS
INTRODUCTION
The frontotemporal lobar degenerations (FTLD) comprise a clinically and pathologically heterogenous group of disorders associated with degeneration of the frontal and/or anterior temporal lobes (McKhann et al., 2001). Behavioral variant frontotemporal dementia (FTD), the most common clinical syndrome of the FTLDs, is typically characterized by progressive changes in personality, behavior, insight, judgment, reasoning abilities, or language, with relative perseveration of episodic memory (Rascovsky et al., 2011). There is growing awareness of the overlapping syndromes of FTD, motor neuron disease, parkinsonism, corticobasal syndrome, and progressive supranuclear palsy syndrome (Josephs, 2008). The clinical and pathological overlap of FTD with amyotrophic lateral sclerosis (ALS) is exemplified in the multiple kindreds with FTD and/or ALS associated with deposition of 43 kDa transactive response DNA-binding protein (TDP-43) in the ubiquitinated inclusions (Arai et al., 2006; Hosler et al., 2000; Morita et al., 2006; Neumann et al., 2006). The most common genetic cause known to date in kindreds whose members have developed FTD and/or ALS was recently identified to be a hexanucleotide repeat expansion (GGGGCC) in the chromosome 9 open reading frame 72 (C9ORF72) gene (DeJesus-Hernandez et al., 2011; Renton et al., 2011). These cases are now collectively referred to as c9FTD/ALS (Boeve et al., 2012; DeJesus-Hernandez et al., 2011; Murray et al., 2011).
As genetic testing has expanded to include cases outside of the typical FTD and/or ALS phenotype, atypical cases are being increasingly identified (Khan et al., 2012), although many are lacking with detailed longitudinal characterization. We report detailed longitudinal clinical, neuropsychological, and neuroimaging findings of a proband of a small kindred with the C9ORF72 mutation who initially presented with amnestic mild cognitive impairment which evolved to Alzheimer’s disease (AD)-type dementia and was later followed by the development ALS. This report extends the clinical phenotype associated with the C9ORF72 expansion.
METHODS
Subjects
The subject underwent a clinical evaluation at our institution and was then enrolled in the Mayo Alzheimer Disease Research Center – a Mayo Foundation Institutional Review Board-approved program. The subject and his wife provided written consent for participation. All additional data from affected relatives were collected and analyzed.
Clinical and Neuropsychological Data
His neurological and neuropsychological data were reviewed. Neuropsychological testing was performed using standard measures (Fields, Ferman, Boeve, & Smith, 2011). Index Scores were converted to Mayo Older American Normative Studies (MOANS) age-adjusted scaled scores (mean of 10 and standard deviation of 3) (Ivnik et al., 1992; Ivnik, Malec, Smith, Tangalos, & Petersen, 1996; Lucas et al., 1998; Machulda et al., 2007; Pedraza et al., 2010). Clinical diagnoses were based on published criteria for mild cognitive impairment (MCI), FTD, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS) (Brooks, Miller, Swash, & Munsat, 2000; McKhann et al., 1984; Neary et al., 1998; Rascovsky et al., 2011; Petersen et al., 2001).
Neuroimaging
MRIs were performed either at 1.5 Tesla or 3 Tesla (GE Healthcare). At 1.5 Tesla, a 3D high resolution spoiled gradient recalled acquisition in steady state (SPGR) and at 3 Tesla magnetization prepared rapid gradient echo acquisition were used for the high resolution T1 weighted images. A fluid attenuated inversion recovery (FLAIR) sequence was performed at both 1.5 and 3 Tesla.
All positron emission tomography (PET) scans were acquired using a PET/computed tomography scanner (DRX; GE Healthcare) operating in 3-dimensional mode. A computerized tomography image was obtained for attenuation correction. The subject was injected with both 11C-Pittsburgh compound B (PiB) (average, 715 MBq; range, 713–718 MBq) and 18F-fluorodeoxyglucose (FDG) (average, 564 MBq; range, 449–691 MBq). The images were acquired on the same day, one hour apart. Following a 40-minute PiB uptake period, a 20-minute PiB scan was obtained. PiB PET acquisition consisted of four 5-minute dynamic frames. Following a 40-minute FDG uptake period, an 8-minute FDG scan was obtained. Image acquisition consisted of four 2-minute dynamic frames. Standard corrections were applied, and standard acquisition and vendor reconstruction parameters were used. FDG-PET scans were processed using CortexID software (GE Healthcare). The activity in each FDG-PET data set was normalized to the pons and compared with an age-segmented normative database, yielding 3-dimensional z-score metabolic maps. The uptake on PiB-PET was calculated using an averaged multiregional cortical to cerebellar ratio described previously (Lowe et al., 2009), with a ratio below 1.5 being considered “PiB-PET negative.”
Genetic Analyses
Genomic DNA (gDNA) was extracted from peripheral blood samples using standard procedures. The gDNA was screened for the presence of the expanded hexanucleotide repeat in C9ORF72 using the repeat primed PCR method as previously described. (DeJesus-Hernandez et al., 2011) Genetic analysis and DNA sequencing for C9ORF72, MAPT, and PGRN were performed as previously described (DeJesus-Hernandez et al., 2011). Apolipoprotein E (ApoE) genotyping was performed as previously described (Tsai et al., 1994).
RESULTS
Clinical, Neuropsychological, and Neuroimaging Data
The proband began experiencing mild forgetfulness of recent events and difficulty recalling names at age 67 without any additional cognitive deficit or change in behavior. He had been started on donepezil and memantine without any significant benefit. His parent had been diagnosed with Parkinson’s disease at age 70 years. There was no additional family history of neurologic or psychiatric disease.
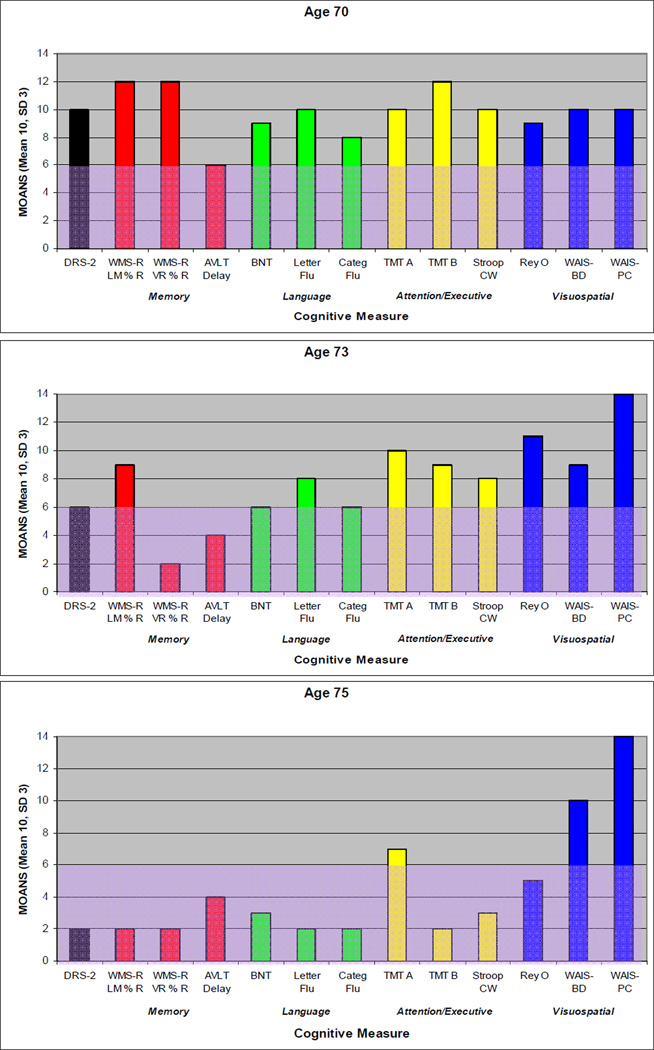
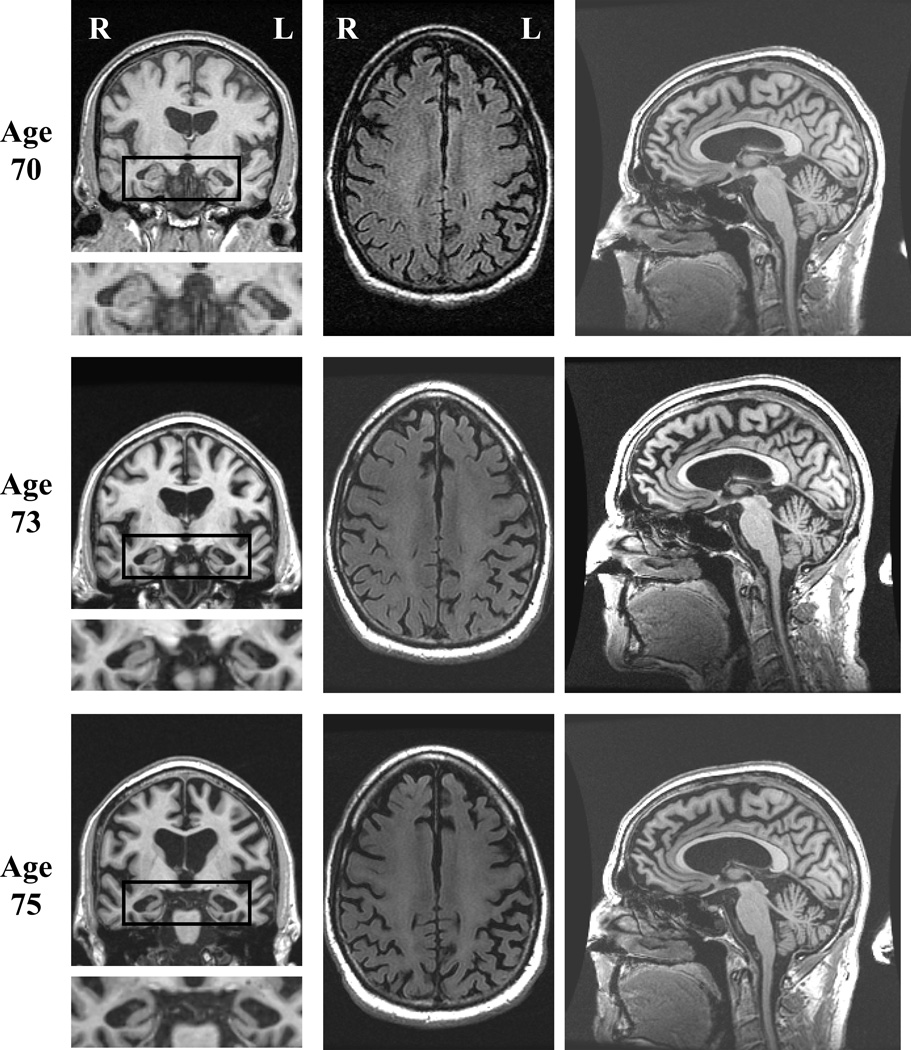
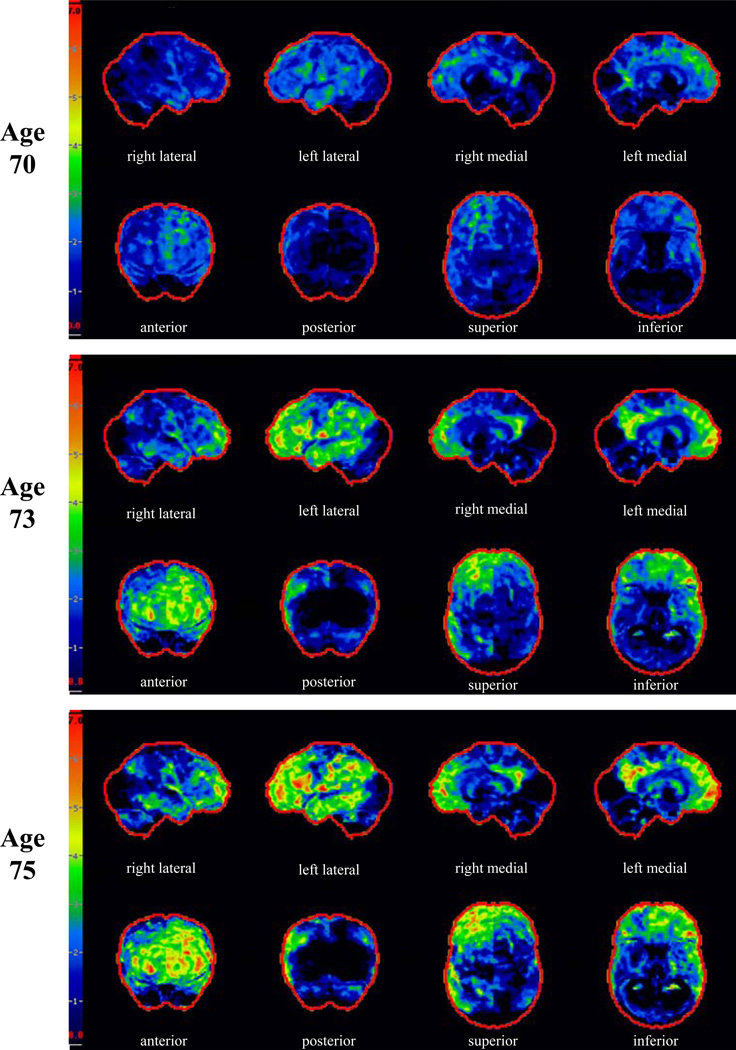
On our first encounter with the proband at age 70, he expressed frustrations over short term memory, and his wife corroborated problematic forgetfulness for details of recent events and upcoming appointments. He had a total score of 32 out of 38 on the Kokmen Short Test of Mental Status (STMS) (Kokmen, Naessens, & Offord, 1987) with one out of four objects recalled after a five minute delay. He had dysnomia on confrontation naming, but otherwise language evaluation was within normal limits. He demonstrated preserved recognition of famous faces, but had difficulty stating their names. He had mild distal sensory loss to vibration in stocking distribution and hyporeflexic Achilles tendon reflex bilaterally. Motor and cerebellar examinations were normal. Neuropsychological assessment showed weakness in verbal retention on serial list learning with an adequate learning curve (Figure 1). Executive functioning was above average. Based on his clinical presentation and his performance on neuropsychological testing, he was diagnosed with amnestic MCI, realizing that his delayed recall for paragraphs and figures was relatively preserved. MRI of the brain showed mild generalized cortical atrophy which was slightly more apparent in the medial frontal regions, as well as more obvious hippocampal atrophy, more pronounced on the left (Figure 2). FDG-PET showed mild asymmetric hypometabolism involving the mesial and dorsolateral frontal and temporal lobes, left much more so than right (Figure 3).
Figure 1. Neuropsychological Profile of Impairment.
The subject’s performance on serial neuropsychological tests are grouped according to cognitive domain and displayed graphically. Mayo Older American Normative Studies (MOANS) age-adjusted scaled scores are shown in which 10 represents the mean and the standard deviation is 3. Shaded areas represent scores in the abnormal range. Note the isolated weakness in word list memory task early in the course. Performance on all memory tests declined over time, followed by decline in language and executive functioning. Although percent retention of paragraph length information (WMS-R LM % R) at age 73 years appears to be preserved this is somewhat misleading as his immediate retention was significantly impaired. See text for details.
Abbreviations: DRS-2 = Dementia Rating Scale-2; WMS-R LM % R = Wechsler Memory Scale-Revised Logical Memory Percent Retention; WMS-R VR % R = Wechsler Memory Scale-Revised Visual Reproductions Percent Retention; AVLT LT % R = Auditory Verbal Learning Test Long Term Percent Retention; BNT = Boston Naming Test; Letter Flu = Letter Fluency; Categ Flu = Category Fluency; TMT A = Trail Making Test Part A; TMT B = Trail Making Test Part B; Stroop CW = Stroop Color-Word; ReyO = Rey-Osterrieth Complex Figure Test; WAIS-BD = Wechsler Adult Intelligence Scale Block Design; WAIS-PC = Wechsler Adult Intelligence Scale Picture Completion.
Figure 2. Brain MRI.
MRI scans at ages 70, 73 and 75 demonstrating the topography of atrophy. Left column of images involves T1-weighted coronal slices (with mesial temporal lobe structures enlarged and placed below each coronal image), middle column of images involves FLAIR axial slices, and right column of images involves T1-weighted midsagittal slices. Note the left more so than right hippocampal atrophy and medial frontal atrophy that progressed over time. Atrophy in the posterior cerebrum and cerebellum is minimal to absent.
Figure 3. FDG-PET Scan.
FDG-PET scans at ages 70, 73 and 75. The images from left to right represent Z-score projection maps. Top rows: right lateral, left lateral, right medial, and left medial. Bottom rows: Anterior, posterior, superior, and inferior where the right side of the brain corresponds to the left side of each image. Note that the initial FDG-PET scan shows mild hypometabolism primarily involving the medial and dorsolateral frontal and lateral temporal lobes, left more so than right. Over time, the scans show progression of fronto-temporal hypometabolism, worse on the left, with involvement of the posterior cingulate cortex. The cerebellum is slightly affected at ages 73 and 75 as well.
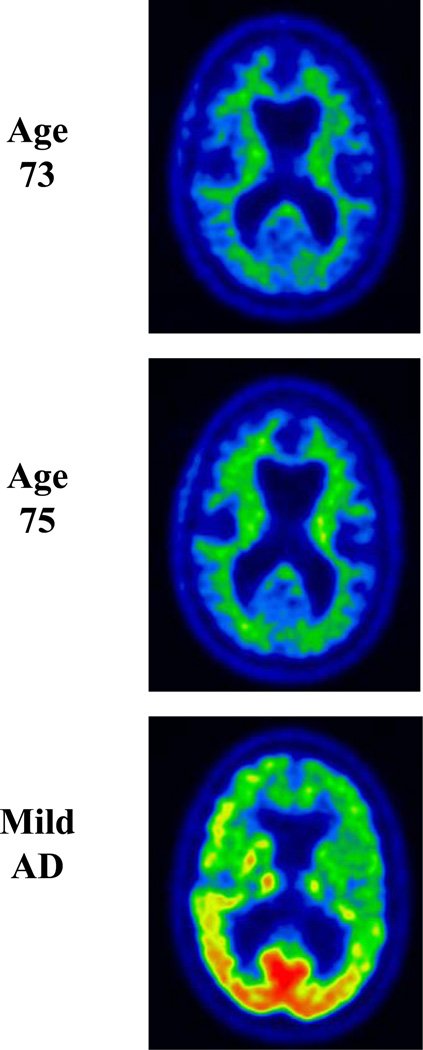
Over the subsequent years his short-term memory difficulties dominated his symptomatology. At the age of 73 years, he developed mild apathy, but other changes in behavior and personality were absent. He started to have mild navigational problems when driving. He noted mild clumsiness involving his left leg without associated weakness, numbness, or cramps. His examination revealed a score of 26 out of 38 on STMS with most prominent difficulties on delayed recall and mild difficulties on tests of attention and construction. He had mild spasticity, mild hyperreflexia, and decreased alternating motion rates of the left lower limb. There was no evidence of parkinsonism, weakness, or fasciculations. Neuropsychological assessment demonstrated a marked decline in learning of paragraph length information as well as visually presented information (Figure 1). There was relative preservation of verbally learned material on delayed recall, as compared with visually presented information for which there was zero percent retention. There was a marked decline in learning and recall of serial list information. There were also mild but significant declines in object naming and mental flexibility. Performance on other tests of language and executive function declined minimally, while visuospatial functioning remained strong. MRI of the brain showed continued progression of cortical and hippocampal atrophy (Figure 2). FDG-PET showed interval progression of the hypometabolism involving the frontal and temporal lobes, far greater on the left, and also hypometabolism involving the left medial parietal lobe and cingulate cortex (Figure 3). PiB-PET showed no significant cortical uptake of the radiotracer, and the ratio was 1.1 (Figure 4). He was diagnosed with mild dementia. Based on his symptomatology, neuropsychological test results, and MRI demonstrating hippocampal atrophy, a clinical diagnosis of probable AD was made as the clinician was blinded to PiB-PET scan results.
Figure 4. PiB-PET Scan.
PiB-PET scan at age 73. Note the absence of significant PiB uptake in the cortex (ratio 1.1) in the proband compared to the AD case example.
At the age of 75 years, he was experiencing progressive difficulties with his short-term memory with marked difficulties recalling conversations or events minutes after they had occurred. He complained of more prominent word-finding difficulties in conversation and dysnomia for names of people had progressed. There were no problems with reading, writing, or comprehension. He had difficulty with complex decision-making and became dependent for most instrumental activities of daily living, such as managing the finances, driving, and house-work. His family reported mild apathy and an increased craving for sweets. There were otherwise no reported changes in behavior or personality and no disinhibition. In the preceding year, he developed a left foot drop for which he required an ankle-foot orthosis. He was also noted to be moving slower. He demonstrated limited insight into his cognitive and motor difficulties. On his examination, his STMS score was 15 out of 38. His examination revealed difficulties with verbal learning, delayed recall, calculation, and abstract reasoning. He had mild hypomimia, but no dysarthria. Strength in the cranial and cervical segments was normal. He had weakness at the iliopsoas bilaterally, worse on the left than on the right. He also demonstrated significant weakness of the following muscles of his left lower limb: anterior tibialis, toe extensors, extensor hallucis longus, peronei, posterior tibialis, toe flexors, and gastrocnemius. There was mild gegenhalten of his upper limbs and mild spasticity of his lower limbs bilaterally. He had a mild tremor with action of his left upper limb. Fasciculations were present diffusely in his upper and lower limbs, including bilateral triceps, biceps, brachioradiales, quadriceps, and gastrocnemii. Fasciculations of the left latissimus dorsi were noted as well. Aside from the left foot drop, gait evaluation was normal. He had difficulty standing from a seated position without the use of his arms. There was no postural instability. He was diffusely hyperreflexic, except hyporeflexic at the Achilles tendon bilaterally, with flexor plantar responses. Neuropsychological assessment showed a considerable decline in tests of memory, language, and executive functioning (Figure 1). MRI of the brain demonstrated progression of atrophy (Figure 2). FDG-PET showed mild progression of hypometabolism involving the frontal and temporal lobes, again with the left side affected far more so than the right (Figure 3). PiB-PET was performed again, and the ratio was unchanged (1.1). Electromyogram was performed due to the suspicion of motor neuron disease and showed fibrillation potentials, fasciculations and large polyphasic motor unit potentials with reduced recruitment in the thoracic paraspinals, left deltoid, left triceps brachii, left first dorsal interosseous, left tibialis anterior, left medial gastrocnemius, left vastus medialis and left gluteus medius muscles. The clinical diagnosis was changed to atypical frontotemporal lobar degeneration plus amyotrophic lateral sclerosis.
Genetic Findings
DNA was available for analysis in our subject and the hexanucleotide expansion in C9ORF72 was detected. No mutation was present in MAPT or PGRN. ApoE genotype was 3/4.
DISCUSSION
The phenotype associated with the C9ORF72 mutation is typically behavioral variant FTD with or without parkinsonism and/or ALS (Boeve et al., 2012). Our subject’s clinical features were atypical for this mutation, with the onset of memory decline and mild dysnomia without associated change in behavior, personality, or comportment early in his course. He initially presented with a picture consistent with amnestic MCI that evolved into an AD-type dementia which was followed by the insidious development of motor neuron dysfunction about six to seven years after the onset of cognitive difficulties. Mild parkinsonism evolved several years after disease onset. The changes in behavior were seen later in his clinical course and consisted of apathy and increased craving of sweets. The progression of his cognitive decline was gradual until the onset of motor neuron dysfunction, at which point he had a dramatic decline in both his performance on neuropsychological tests and in function. Even granting that our neuropsychological battery may not have used the most sensitive tests for executive functions, our patient scored well above expectation on executive tasks, suggesting that deficits in this domain were very mild at worst initially.
When both ALS and FTD are present in an individual with c9FTD/ALS, the features tend to emerge within the first two years (Boeve et al., 2012). Parkinsonism, as well, tends to become evident within the first two years of the onset of clinical symptoms (Boeve et al., 2012). Our subject had a much longer duration between the onset of ALS and parkinsonism from his initial symptom onset. ALS developed over the course of one to two years, during which time his cognitive symptoms also dramatically declined. Although it has been shown that survival in c9FTD/ALS is shorter in those with features of FTD and ALS versus FTD alone (Boeve et al., 2012), rates of cognitive decline in these two groups have not been previously studied.
The neuroimaging findings in our case are somewhat atypical for what has been previously described in c9FTD/ALS. The majority of studies have reported symmetric atrophy and hypometabolism involving the bilateral frontal and/or temporal cortex in c9FTD/ALS cases (Boeve et al., 2012; Hsiung et al., 2012; Mahoney et al., 2012; Whitwell et al., 2012). One study noted more variability in left–right sided asymmetries and reported cases with both symmetric and asymmetric atrophy and/or hypoperfusion in cases with progressive aphasia or FTD (Snowden et al., 2012). Similar to our case, the asymmetric cases were most often associated with left predominant atrophy or hypoperfusion. Based on the FDG-PET findings, one would have presumed that clinical features consistent with corticobasal syndrome and/or a more obvious progressive aphasia syndrome would have developed, but this did not occur. This case is consistent with other rare cases with atypical FDG-PET findings (Boeve et al., 2012), and exemplifies that asymmetric neuroimaging findings should not exclude consideration of the C9ORF72 expansion as a cause of an atypical dementing illness.
Clinicopathologic studies have demonstrated that the underlying substrate of amnestic MCI that progresses to dementia is most often AD (Jicha et al., 2006). The proband’s initial MRI showed a moderate degree of mesial temporal atrophy which was slightly asymmetric, left more than right, and progressed over time. This was consistent with the prominent anterograde amnestic presentation and course initially. On the other hand, FDG-PET scans performed early in the course of his disease showed asymmetric hypometabolism, left more than right, involving the medial frontal regions and the lateral temporal lobes to a lesser degree, which also progressed. This pattern of hypometabolism was suggestive of an alternative diagnosis in the FTLD spectrum of disorders rather than AD (Foster et al., 2007). It was not until he developed ALS and he was screened for the C9ORF72 expansion that the diagnosis of an FTLD-spectrum disorder could be made confidently as he did not meet clinical criteria for behavioral variant frontotemporal dementia or any of the progressive aphasia variants (Neary et al., 1998; Rascovsky et al., 2011; Gorno-Tempini et al, 2012). His ApoE genotype was 3/4, but his PiB PET did not show increased amyloid uptake, making coexisting AD pathology an unlikely explanation for his clinical features. Early amnestic features have been present with FTLD associated with a mutation in PGRN in those who carry at least one ApoE4 allele (Rademakers et al., 2007). In a study that compared sporadic FTD with cognitively normal control subjects, ApoE 3/4 genotype appeared to be overrepresented in cases of behavioral variant FTD and PPA (Seripa et al., 2011). ApoE4 carrier status has been associated with disease-specific patterns of grey matter atrophy, and in FTD more pronounced grey matter atrophy in the bilateral medial, dorsolateral, orbital frontal, cingulate cortices, and anterior insula with right predominance has been seen (Agosta et al., 2009). A similar pattern of atrophy was seen in our patient, but with left hemisphere predominance. Based on these studies, the ApoE genotype may play an important role in modifying the phenotype of FTLD including those associated with the C9ORF72 expansion, and this may be mediated by loss of grey matter volume. Understanding the role of ApoE in FTLD requires further investigation.
Clinicopathologic studies of C9ORF72 have found an AD phenotype in a minority of cases (Boeve et al., 2012; Hsiung et al., 2012; Mahoney et al., 2012). As these studies screened for the C9ORF72 mutation in patients with a clinical diagnosis of one of the FTD syndromes, the true frequency of an AD-phenotype is likely underestimated. A recent report found the C9ORF72 repeat expansion to account for a very small percentage of cases diagnosed with probable AD who also had a family history of late onset-AD (Majounie et al., 2012). Another study that included all patients with a diagnosis of AD, but did not limit their selection of cases to those with a positive family history, did not identify the C9ORF72 expansion in their large cohort of patients (Rollinson et al., 2012). In a pathologic study of twenty cases of c9FTD/ALS, there was pathological heterogeneity in the distribution and type of TDP-43 pathology (Murray et al., 2011). In addition, some cases of c9FTD/ALS had evidence of moderate to severe hippocampal sclerosis, all of whom had a clinical diagnosis of AD (Murray et al., 2011). Larger studies are needed to study this possible relationship.
Although the accuracy of the diagnosis of AD made in these studies may be questioned as clinical details are minimally provided, our case report provides the detailed clinical course of a patient who met criteria for amnestic MCI and then probable AD who later developed ALS at which point screening for a mutation in C9ORF72 demonstrated the pathologic expansion. The clinicians caring for this subject were troubled by the discrepancy between the FDG-PET findings and the clinical phenotype that evolved early in his course. Yet this case also emphasizes the importance of regular follow-up of patients with cognitive impairment evaluated early in their course, particularly when imaging findings “don’t fit,” as clinical syndromes may evolve in an atypical manner which may prompt consideration of alternate diagnoses.
A broad spectrum of intrafamilial and interfamilial phenotypic variability has been associated with the mutation in C9ORF72 (Boeve et al., 2012; Hsiung et al., 2012; Simon-Sanchez et al., 2012; Snowden et al., 2012). This case demonstrates that an amnestic dementia indistinguishable from AD dementia should be included in the spectrum of phenotypic expression of the C9ORF72 expansion. An AD-like anterograde amnestic syndrome has been associated with mutations in other FTD associated genes, such as the genes encoding progranulin and microtubule associated protein tau (Kelley et al., 2010; Lindquist et al., 2008; Rademakers et al., 2003). C9ORF72 expansion should be added to the list of mutations that may present with an AD-like phenotype. This case demonstrates the importance of considering mutations in the FTD-associated genes in cases when a familial disorder is suggested and neuroimaging studies reveal findings which are atypical of AD despite the classic AD phenotype. Future studies are needed to learn what factors determine the clinical presentation in c9FTD/ALS and to what degree the C9ORF72 expansion length, ApoE genotype, TDP-43 subtype, and hippocampal neuronal loss and gliosis contribute to the clinical heterogeneity.
ACKNOWLEDGEMENTS
This work was supported by the “Mayo Alzheimer’s Disease Research Center” (P50 AG016574), the "Identifying Mechanisms of Dementia: Role for MRI in the Era of Molecular Imaging" (RO1 AG011378), the ALS Association (R.R), and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program of the Mayo Foundation. R.R. is also funded by NIH grants R01 NS065782 and R01 AG026251.
We thank the patient and his family for participating in aging and neurodegenerative disease research.
ABBREVIATIONS
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ApoE
Apolipoprotein E
- c9FTD/ALS
frontotemporal dementia and/or amyotrophic lateral sclerosis linked to chromosome 9
- C9ORF72
gene encoding the mutation in chromosome 9 open reading frame 72
- FDG
18F-fluorodeoxyglucose
- FLAIR
fluid attenuated inversion recovery
- FTD
frontotemporal degeneration
- FTD/ALS
frontotemporal dementia and/or amyotrophic lateral sclerosis
- gDNA
genomic DNA
- GGGGCC
the hexanucleotide expansion of guanine-guanine-guanine-guanine-cytosine-cytosine
- MOANS
Mayo's Older Americans Normative Studies
- MCI
mild cognitive impairment
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PiB
11C-Pittsburgh compound B
- STMS
Short Test of Mental Status
- TDP-43
TAR DNA binding protein of molecular weight 43 kDa
Footnotes
DISCLOSURES
Dr. Adeli - nothing to disclose
Dr. Savica - nothing to disclose
Dr. Knopman serves as Deputy Editor for Neurology®; has served on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH (R01 AG011378 [Coinvestigator], P50 AG016574 [Coinvestigator], U01 AG006786 [Coinvestigator], AG029550 [Coinvestigator], AG032306 [Coinvestigator], and U01 096917 [Coinvestigator]).
Dr. Vemuri - nothing to disclose
Ms. DeJesus-Hernandez - nothing to disclose
Dr. Rademakers - nothing to disclose
Dr. Fields - nothing to disclose
Dr. Crum - nothing to disclose
Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Eli Lilly & Company, GE Healthcare, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., Pfizer Inc, the NIH/NIA (R01 AG011378 [Principal investigator], P50 AG016574 [Coinvestigator]), and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock/stock options in Johnson & Johnson.
Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society.
Dr. Petersen serves on Safety Monitoring Committees for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and as a consultant for Elan Pharmaceuticals and GE Healthcare; receives royalties from the publication of a book entitled Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH (P50 AG016574 [Principal Investigator], U01 AG006786 [Principal Investigator], R01 AG011378 [Coinvestigator], and U01 AG024904 [Coinvestigator]).
Dr. Boeve serves as an investigator for clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals, and GE Healthcare; receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009); and receives research support from the NIH (P50 AG016574 [Coinvestigator], U01 AG006786 [Coinvestigator], and R01 AG032306 [Coinvestigator]), and the Mangurian Foundation.
REFERENCES
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, et al. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Nat Acad Sci USA. 2009;106:2018–2022. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Comm. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Ferman TJ, Boeve BF, Smith GE. Neuropsychological assessment of patients with dementing illness. Nature Reviews Neurology. 2011;7:677–687. doi: 10.1038/nrneurol.2011.173. [DOI] [PubMed] [Google Scholar]
- Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain. 2007;130:2616–2635. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler BA, Siddique T, Sapp PC, Sailor W, Huang MC, Hossain A, et al. Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21-q22. JAMA. 2000;284:1664–1669. doi: 10.1001/jama.284.13.1664. [DOI] [PubMed] [Google Scholar]
- Hsiung GY, Dejesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik R, Malec J, Smith G, Tangalos E, Petersen R, Kokmen E. Mayo’s Older American Normative Studies: WAIS-R, WMS-R, and AVLT norms for ages 56–97. The Clin Neuropsychol. 1992 [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, JLO. The Clin Neuropsychol. 1996;10:262–278. [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, Knopman DS, et al. Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol. 2010;67:171–177. doi: 10.1001/archneurol.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psych. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clinic Proceedings. 1987;62:281–288. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- Lindquist SG, Holm IE, Schwartz M, Law I, Stokholm J, Batbayli M, et al. Alzheimer disease-like clinical phenotype in a family with FTDP-17 caused by a MAPT R406W mutation. European Journal of Neurology. 2008;15:377–385. doi: 10.1111/j.1468-1331.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. Journal of Nuclear Medicine. 2009;50:878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo's older Americans normative studies: category fluency norms. J Clin Exper Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ivnik RJ, Smith GE, Ferman TJ, Boeve BF, Knopman D, et al. Mayo's older Americans normative studies: Visual form discrimination and copy trial of the Rey-Osterrieth complex figure. J Clin Exper Neuropsychol. 2007;29:377–384. doi: 10.1080/13803390600726803. [DOI] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, et al. Repeat expansion in C9ORF72 in Alzheimer's disease. New England Journal of Medicine. 2012;366:283–284. doi: 10.1056/NEJMc1113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathologica. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Lucas JA, Smith GE, Petersen RC, Graff-Radford NR, Ivnik RJ. Robust and Expanded Norms for the Dementia Rating Scale. Arch Clin Neuropsychol. 2010;25:347–358. doi: 10.1093/arclin/acq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Dermaut B, Peeters K, Cruts M, Heutink P, Goate A, et al. Tau (MAPT) mutation Arg406Trp presenting clinically with Alzheimer disease does not share a common founder in Western Europe. Human Mutation. 2003;22:409–411. doi: 10.1002/humu.10269. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Baker M, Gass J, Adamson J, Huey ED, Momeni P, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: an international initiative. Lancet Neurol. 2007;6:857–868. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinson S, Halliwell N, Young K, Callister JB, Toulson G, Gibbons L, et al. Analysis of the hexanucleotide repeat in C9ORF72 in Alzheimer's disease. Neurobiol Aging. 2012;33:1846, e1845–e1846. doi: 10.1016/j.neurobiolaging.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Seripa D, Bizzarro A, Panza F, Acciarri A, Pellegrini F, Pilotto A, et al. The APOE gene locus in frontotemporal dementia and primary progressive aphasia. Arch Neurol. 2011;68:622–628. doi: 10.1001/archneurol.2011.90. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain. 2012;135:723–735. doi: 10.1093/brain/awr353. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Tangalos EG, Petersen RC, Smith GE, Schaid DJ, Kokmen E, et al. Apolipoprotein E: risk factor for Alzheimer disease. American Journal of Human Genetics. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]