Abstract
Background
HIV-infected children may require the use of combination antiretroviral treatment (cART) into adulthood. However, regimens are limited to first- and second-line in many African settings. Therefore, understanding the long-term rate of virologic failure and drug resistance during prolonged antiretroviral treatment is important for establishing treatment strategies in African pediatric cohorts.
Methods
Children ages 18 months to 12 years initiated first-line cART and were followed every 1–3 months, for up to 5.5 years. Treatment was switched to second-line based on clinical and immunologic criteria according to national guidelines. Virologic failure was determined retrospectively as defined by ≥2 viral loads >5000 copies/mL. Drug resistance was assessed during viral failure by population-based sequencing.
Results
Among 100 children on first-line cART followed for a median 49 months, 34% experienced virologic failure. Twenty-three (68%) of the 34 children with viral failure had detectable resistance mutations, of whom 14 (61%) had multi-class resistance. Fourteen (14%) children were switched to second-line regimens and followed for a median of 28 months. Retrospective analysis revealed that virologic failure had occurred a median of 12 months prior to the switch to second-line. During prolonged first-line treatment in the presence of viral failure, additional resistance mutations accumulated, however, only 1 (7%) of 14 children had persistent viremia during second-line treatment.
Discussion
Virologic suppression was maintained on first-line cART in two-thirds of HIV-infected children for up to 5 years. Switch to second-line based on clinical/immunologic criteria occurred ~1 year after viral failure, but the delay did not consistently compromise second-line treatment.
BACKGROUND
Combination antiretroviral therapy (cART) has transformed the natural course of pediatric HIV-1 from a rapidly fatal illness into a chronic disease.1,2 In Africa where the majority of the world’s 2 million HIV-1 infected children reside, improved access to early infant HIV-1 diagnosis, rapid scale-up of antiretroviral drug programs, and current guidelines that recommend initiating treatment in all infants irrespective of CD4 count or clinical disease stage, have all contributed to better survival.3 As children with HIV-1 survive longer on cART, greater emphasis is being placed on the importance of long-term viral suppression. Two recent pooled analyses on the effectiveness of ART in resource-constrained settings found that between 40% and 81% of children have complete virologic suppression by 12 months of treatment.4,5 While there is substantial literature describing outcomes during the first year of therapy, there is a scarcity of data on longer-term outcomes among cART–treated African children.6,7 Similarly, data on the frequency and pattern of genotypic resistance mutations that arise in response to first-line therapy in this population is largely limited to the first year of treatment.8,9
The standard of care in resource-limited settings does not include virologic monitoring and instead relies on clinical and immunologic criteria to indicate failing regimens.3 However, increasing evidence suggests that clinical and immunologic failure may not adequately detect failing regimens in HIV-1 infected children10,11 and that prolonged treatment on failing regimens may accelerate the emergence of multi-class resistance.12,13 It is anticipated that a large number of the children currently on first-line cART will require second-line therapy in the next few years, and therefore it is important to define the pattern of resistance mutations that arise in African cohorts where HIV-1 non-subtype B is predominant. We describe the pattern of virologic failure and genotypic resistance in a cohort of Kenyan children followed for 3–5 years after treatment initiation.
METHODS
The Pediatric Adherence Study is a prospective cohort established in 2004 to study long-term outcomes of HIV-1 infected Kenyan children initiating cART as previously described.14,15 Children were recruited from the Kenyatta National Hospital (KNH) pediatric wards and HIV Care Clinic and were enrolled after receiving written informed consent from their legal guardians. Antiretroviral-naïve HIV-1 infected children aged 18 months to 12 years who met clinical (WHO stage 3–4) or immunologic (CD4 <15%) criteria, which were the WHO recommended criteria for starting cART at the time the study was conducted, were started on NNRTI-based cART. Thus, initiation of cART and the follow-up in this cohort was similar to what other Kenyan children received at the time, except that entry into the study depended on being hospitalized at KNH, and therefore this cohort represents children that were sick at the time of enrollment. The specific drugs used in first-line regimens were selected as previously described.14 The decision to switch to a second-line regimen was based on clinical or immunologic criteria according to the current Kenyan National Guidelines.16 Children were followed prospectively at the KNH research clinic at monthly intervals in the first year, and 3-monthly visits subsequently. At every visit, clinical assessment was performed and self-reported adherence was obtained from the caregiver by 3-day and 2-week recall of missed doses. Caregivers were asked to bring the medication, including empty bottles, to each clinic visit. In all cases, the caregiver was either a parent or close family member including grandparent, uncle or aunt. Overall adherence was the average percent adherence for all clinic visits. CD4 counts were determined using FACSCOUNT® BD Biosciences (Franklin Lakes, NJ) and CD4% determined using a dual platform for absolute lymphocyte count from the Humalyser® hematology analyzer using blood collected at enrollment, months 3, 6, 15, and every 6 months thereafter.
Viral Load Testing and Virologic Failure
Plasma samples that were collected every 3-months during the first year and 6-monthly thereafter were frozen and shipped to Seattle, Washington in liquid nitrogen and stored at −80°C until use. HIV-1 RNA levels were measured by the Gen-Probe HIV-1 viral load assay (Gen-Probe, San Diego, CA), which has been validated on the subtypes prevalent in Kenya.17 We considered a child to have virologic suppression if their viral load dropped and remained below 5000 copies/mL after treatment initiation based on the current WHO definition of viral failure in children.3 Virologic failure was classified into two categories: Incomplete viral suppression in which a child’s viral load failed to drop below 5000 copies/ml after ≥3 months of therapy, and viral rebound in which a child’s viral load rose above 5000 copies/ml for ≥2 viral load measurements after a period of initial suppression, or if the last sample available was >5000 copies/ml.3
Genotypic Resistance Testing
For all children who experienced virologic failure, we performed genotypic resistance testing at baseline (pre-ART) as well as on either the first or second sample that had a viral load >5000 copies/mL. In children with detectable resistance at the initial point of viral failure, resistance testing was also performed on the last sample available during first-line cART (prior to initiating second-line cART or at the end of follow-up in children who were not switched). To detect mutations known to confer drug resistance, population-based sequencing was performed on HIV-1 RNA extracted from 140ul of plasma as previously described18. Briefly, a 645bp region of HIV-1 pol was amplified in duplicate using nested RT-PCR on RNA normalized to 500 viral copies per reaction. Three sequencing reactions were performed on each duplicate PCR product. The sequences were analyzed using Sequencher, Version 4.5 (Gene Codes Co., Ann Arbor, Michigan). To differentiate mixed peaks from background noise, a line was drawn such that 95% of secondary peaks were below the line. A site was defined as a “mixed peak” if the secondary peak was above background in at least 3 of 4 sequences. A consensus sequence was submitted to the Stanford University HIV Drug Resistance Database (http://hivdb.stanford.edu/) for interpretation of drug resistance. In replicate reactions of known mixtures of wild-type and mutant sequences, we reliably detected mutant sequences present at ≥20% of total sequence with this method (data not shown).
Statistical Methods
We compared baseline characteristics in children who failed cART to those who did not fail using Pearson Chi Square and Mann-Whitney tests for categorical and continuous variables, respectively. A linear mixed effects model was performed to model the association of immunologic response with virologic response. We performed univariate Cox proportional hazards to model factors associated with virologic failure. The Cox proportional hazard assumptions were confirmed by comparing slopes of the log-log survival plots for each variable and by the global test for proportional hazards based on the schoenfeld residuals. In children who experienced viral failure, univariate logistic regression was performed to model the association with resistance mutations. All analyses were performed with Stata version 9.2 (College Station, Texas, USA).
RESULTS
One hundred forty-nine children were enrolled and initiated cART between August 2004 and December 2006. Of those enrolled, 14 children did not have a baseline viral load sample available, and 35 children either died or were lost to follow-up before their next scheduled appointment. The remaining 100 children had viral load results available at baseline as well as a median of 9 (range: 1–14) viral load results after cART initiation and were included in the analysis. During follow-up on first-line treatment, 3 of the 100 children died and 16 were lost to-follow-up, and 1 of 14 children died during second-line treatment. The median follow-up of the 100 children on cART was 49 months (IQR 35–60 months).
Baseline characteristics of these 100 children are shown in Table 1. At enrollment, the median age was 4.5 years, and 33 (33%) of the children were <3 years of age. Fifty-three percent were female and 89% were classified as WHO clinical stage 3–4. The median baseline CD4% was 6.8 and median viral load was 6.0 log10 copies/mL. None of the children’s mothers had received antiretroviral drugs to prevent mother-to-child transmission (PMTCT). In addition, all but one of the children were antiretroviral naïve at cART initiation, as they were infected before prophylaxis for PMTCT was widely available. The first-line cART regimen in this cohort consisted of zidovudine (ZDV) plus lamivudine (3TC) with an NNRTI in 75 (75%) children while stavudine (D4T) plus 3TC in combination with an NNRTI was used in 25 (25%) children. The NNRTI backbone consisted of nevirapine (NVP) and efavirenz (EFV) in 57 (57%) and 34 (34%) children, respectively.
Table 1.
Baseline characteristics comparing children with virologic success versus virologic failure
| Characteristics | Number of children* |
Overall cohort n=100 Median (IQR) or N (%) |
Virologic success n = 66 Median (IQR) or N (%) |
Virologic failure n = 66 Median (IQR) or N (%) |
p-value# |
|---|---|---|---|---|---|
| Age (years) | 100 | 4.5 (2.6, 6.2) | 4.7 (2.9, 6.7) | 3.3 (2.2, 5.7) | 0.07 |
| Female | 100 | 53 (53%) | 36 (55%) | 17 (50%) | 0.67 |
| Lost one or both parents | 100 | 28 (28%) | 17 (26%) | 11 (32%) | 0.49 |
| Hospitalized pre-ART | 99 | 69 (69%) | 46 (70%) | 23 (68%) | 1.00 |
| WHO clinical stage 3–4 | 97 | 86 (89%) | 59 (89%) | 27 (79%) | 0.35 |
| Weight-for-age z-score | 98 | −2.3 (−3.0, −1.4) | −2.3 (−3.2, −1.4) | −2.3 (−2.8, −1.7) | 0.75 |
| Weight-for-height z-score | 92 | −1.1 (−1.9, −0.2) | −1.2 (−1.9, −0.2) | −1.0 (−1.9, −0.3) | 0.98 |
| Height-for-age z-score | 97 | −2.2 (−3.1, −1.2) | −2.1 (−3.5, −1.2) | −2.4 (−3.0, −1.0) | 0.86 |
| CD4 count cells/µl | 99 | 344 (102, 666) | 300 (82, 650) | 387 (156, 666) | 0.27 |
| CD4 cell percent | 97 | 6.8 (3.7, 11.4) | 6.6 (3.3, 10.9) | 7.0 (3.8, 13.0) | 0.53 |
| HIV-1 RNA log10 copies/ml | 100 | 6.0 (5.5, 6.4) | 5.9 (5.3, 6.5) | 6.1 (5.7, 6.4) | 0.10 |
| Haemoglobin g/dl | 99 | 10.3 (9.2, 11.7) | 10.2 (9.0, 11.8) | 10.4 (9.7, 11) | 0.58 |
| Started on Efavirenz | 100 | 34 (34%) | 26 (39%) | 8 (24%) | 0.11 |
| Started on ZDV | 100 | 75 (75%) | 51 (77%) | 24 (71%) | 0.47 |
| Adherence <100% | 100 | 60 (60%) |
number of children with data for each baseline characteristic included in the analysis
p-value compares children with viral success to those with viral failure
Virologic Response
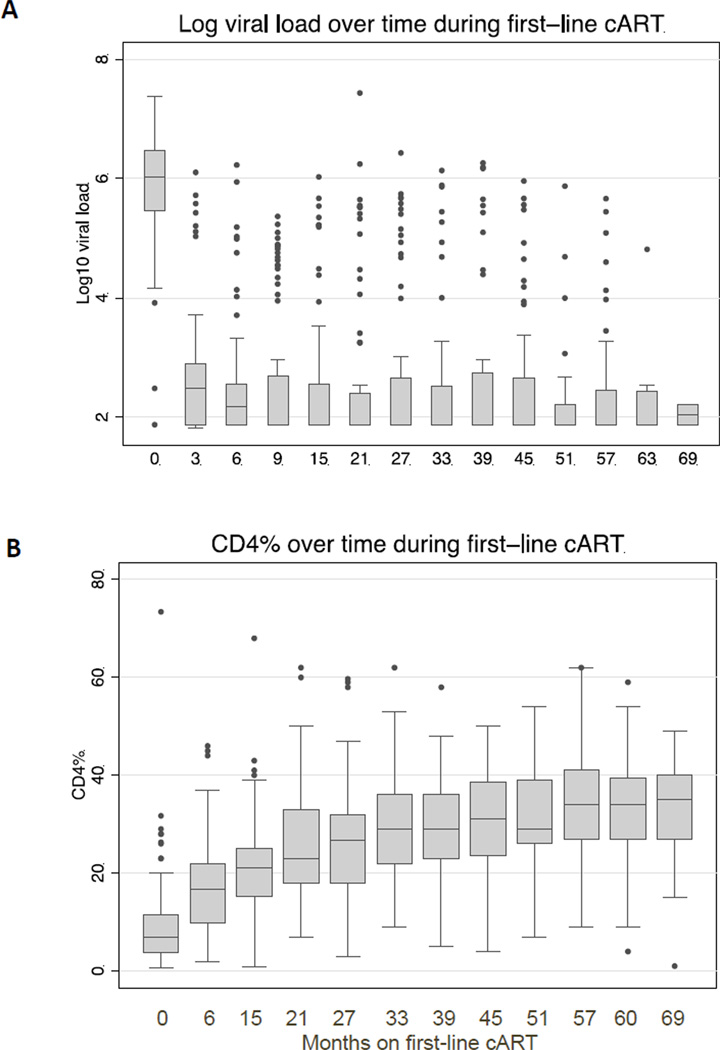
In the majority of the 100 children who initiated treatment, virus levels were successfully suppressed during first-line cART (Figure 1A). Thirty-four children (34%) had virologic failure at a median of 9 months from cART initiation (IQR 6–20 months). Twenty (59%) of the 34 children who experienced virologic failure did so during the first year of cART while an additional 8 (24%) failed during year 2. Twenty-five (74%) children with virologic failure initially suppressed their virus and later experienced viral rebound, while the remaining 9 (26%) never had complete viral suppression.
Figure 1. Viral load and CD4 kinetics during first-line cART.
Changes in (A) log10 viral load and (B) CD4 percent during first-line cART in the 100 children included in the study. The horizontal bar at the center of each box plot represents the median value, the top and bottom of each box are the 75th and 25th percentiles, respectively. The upper bound and lower bounds of the whiskers are the largest data point ≤ 75th percentile + 1.5*IQR and the smallest data point ≥ 25th percentile − 1.5*IQR, respectively. Observed data points beyond these bounds are plotted as filled circles.
Immunologic Response Associated with Virologic Outcome
Out of the 100children, CD4 results were available both at baseline and after 6 months of cART for 81 children, after 15 months of cART for 77 children, and in 59 children by 57 months of first-line cART. In all children with available CD4 results during first-line cART, the median CD4% rose from 6.9% at baseline to 17% at 6 months, to 21% by 15 months, and 34% by 57 months (Figure 1B). Based on a linear mixed effects model, children who experienced viral failure had a trend towards a lower CD4% at baseline (12.7% vs 15.9%, p=0.08). Over time during first-line, the rate of increase in CD4% was lower in those with viral failure compared to children who continued to suppress their virus while on first-line (increase of 2.5% versus 3.3% per year, p=0.016). This suggests that children with virologic failure experienced a significantly poorer CD4 response.
Predictors of Virologic Failure
Children who experienced virologic failure were younger at enrollment than those with viral suppression (median age 3.3 versus 4.7 years), but the difference was not statistically significant (Mann-Whitney: p=0.07, Table 1). When age was dichotomized to above or below 3 years, children <3 years at cART initiation, had increased likelihood of experiencing virologic failure (HR=2.25 (95%CI 1.14, 4.42), p=0.02) using either a univariate cox proportional hazard model (Table 2) or a chi-squared analysis (data not shown). None of the other baseline characteristics including sex, WHO clinical stage, weight-for-height Z-score, log viral load, CD4%, type of NRTI or NNRTI in first-line regimen, or adherence were predictive of virologic failure (Tables 1 and 2). When multivariate analysis was done using Cox proportional hazard models, the results were similar (data not shown).
Table 2.
Predictors of virologic failure using univariate Cox hazard models#
| Baseline Characteristic | N* | HR | (95% CI) | p-value |
|---|---|---|---|---|
| Age < 3 years | 100 | 2.25 | 1.14, 4.42 | 0.02 |
| Sex (female) | 100 | 0.84 | 0.43, 1.64 | 0.60 |
| WHO Clinical Stage 3–4 | 97 | 0.76 | 0.29, 1.97 | 0.57 |
| Weight-for-height z score <−2 | 92 | 0.97 | 0.40, 2.38 | 0.95 |
| Log10 HIV-1 RNA >6 | 100 | 1.76 | 0.88, 3.51 | 0.11 |
| CD4 percent <15 % | 97 | 1.01 | 0.44, 2.33 | 0.98 |
| Adherence by caregiver report <100 % | 100 | 0.64 | 0.33, 1.26 | 0.19 |
Time zero for the Cox hazard models was the time of first-line treatment initiation. Censoring included: viral failure, switch to second-line cART, loss to follow-up, or last follow-up visit available at the time of analysis.
The number of children included in each univariate analysis
Genotypic Resistance at Virologic Failure During First-line Treatment
Twenty-three (68%) of the 34 children with virologic failure had mutations associated with drug resistance at the initial point of virologic failure. This constitutes 23% of the overall cohort. Of children with initial viral suppression followed by rebound, 72% had detectable resistance mutations, while only 56% of those whose virus was never suppressed had resistance (p=0.37). We performed univariate logistic regression to assess potential predictors of resistance and found no significant associations. However only 34 children with viral failure were tested for resistance, and therefore, power was quite limited. Overall adherence was not associated with development of resistance as 11/23 (48%) children with resistance mutations were less than 100% adherent, while 7/11 (64%) children without resistance mutations were <100% adherent (p=0.39).
Table 3 provides a list of all 23 children with specific resistance mutations. Overall, 2 (9%) of the 23 children with resistance had mutations to NRTIs only, 7 (30%) children had resistance to NNRTIs only, and 14 (61%) had multi-class resistance (Table 3). Multi-class resistance was prevalent (n=13, 52%) in children that experienced viral rebound, but was rare (n=1, 11%) in children with incomplete viral suppression (p=0.03, ^ in Table 3). The most common resistance mutation was M184V present in 15, followed by K103N and G190A/S in 11 and 7 children, respectively. Four children had thymidine analogue resistance mutations (TAMS)19, including M41L, D67N, K70R, T215Y, and K219EQ. Only one child had resistance to the first-line regimen detectable at baseline, with a single mutation, V179D (data not shown) that confers low-level resistance to NNRTIs.
Table 3.
Resistance mutations in children with virologic failure
| ID | subtype | first-line regimen |
log VL at failure |
months post cART initiation |
NRTI resistance mutations |
NNRTI resistance mutations |
multi-class resistance |
|---|---|---|---|---|---|---|---|
| PADX1* | A2/D | D4T+3TC+NVP | 4.14 | 7 | K65R | Y181C | + |
| PADX2* | A | D4T+3TC+NVP | 6.14 | 33 | M184MV | K103N | + |
| PADX8* | D | D4T+3TC+NVP | 4.90 | 9 | none | K103N | − |
| PADX9* | A | D4T+3TC+NVP | 4.76 | 7 | M184V | K101E, G190A | + |
| PADC1* | A | AZT+3TC+EFV | 4.47 | 20 | D67G, M184V | K101H, G190S | + |
| PADD9* | D | AZT+3TC+NVP | 4.02 | 6 | M184V | Y181C | + |
| PADF2* | A | AZT+3TC+NVP | 5.07 | 20 | D67N, M184V | K103N | + |
| PADG5* | A | AZT+3TC+NVP | 5.41 | 9 | D67N, K70R, M184V, K219EQ | K103S | + |
| PADH3* | A | AZT+3TC+NVP | 5.19 | 15 | M184V | Y181C, G190A | + |
| PADK4* | A | D4T+3TC+NVP | 5.54 | 9 | L74V, Y115F, M184V | Y181C, H221Y | + |
| PADM8* | A | AZT+3TC+NVP | 5.00 | 9 | M184IV | K103N | + |
| PADA1# | A | D4T+3TC+NVP | 4.86 | 8 | none | K103N | − |
| PADA5# | A | AZT+3TC+NVP | 5.10 | 11 | M184V | K103KN | + |
| PADB1# | C | AZT+3TC+NVP | 4.82 | 9 | M184V | Y181C, G190AG | + |
| PADI3# | A | AZT+3TC+EFV | 4.60 | 55 | M184V | K103N | + |
| PADJ6# | A | AZT+3TC+EFV | 5.33 | 21 | none | G190S | − |
| PADO0# | C | D4T+3TC+NVP | 3.71 | 6 | none | K103KN | − |
| PADP6# | A | AZT+3TC+ABC | 4.55 | 11 | M184IMV | none | − |
| PADB2#,^ | A | AZT+3TC+EFV | 5.19 | 6 | none | K103N | − |
| PADC9#,^ | D | AZT+3TC+NVP | 5.58 | 3 | none | K103KN | − |
| PADG6#,^ | A2/D | D4T+3TC+ABC | 5.03 | 3 | M184V | none | − |
| PADH8#,^ | A2/D | AZT+3TC+NVP | 6.03 | 14 | M41L, M184V, T215Y | K101EK, V179E, G190A | + |
| PADL8#,^ | A | AZT+3TC+NVP | 5.21 | 4 | none | K103KN, G190AG | − |
switched to PI-based cART based on clinical or immunological criteria
remained on first-line cART during follow-up
never suppressed their virus during treatment
Accumulation of resistance during extended first-line treatment in the presence of unrecognized viral failure
The decision to switch to second-line therapy, which included three new drugs according to Kenyan Ministry of health guidelines, were based on clinical and/or immunologic criteria because viral load testing was not routinely available in 2004 when this cohort began (and is still not widely available in many parts of Kenya). Fourteen children (14%) were switched to second-line regimens (ritonavir-boosted lopinavir) due to clinical and/or immunologic failure at a median of 30 months (IQR 18–36), 12 of whom experienced viral failure prior to switch (Supplementary Figure 1A). Using archived samples, we observed that 12 (86%) of the children that had been switched based on clinical criteria had experienced virologic failure at a median of 9 months (IQR 8–17), indicating that virologic failure occurred well before clinical and immunologic deterioration. The delay between viral failure and switch to 2nd-line treatment can be seen in Supplementary Figure 1B. Eleven (92%) of these 12 children (* in Table 3) had resistance detectable at the initial point of viral failure, 10 (91%) of which had multi-class resistance. The median delay on first-line cART in the presence of unrecognized virologic failure was 12.5 months (IQR 10–19). In addition, of 34 children with viral failure, 22 were not switched to second-line but had evidence of viral failure using retrospective samples. However, only 12 (55%) of these 22 (denoted by # in Table 3) had detectable resistance mutations at the initial point of viral failure, 4 (33%) of which had multi-class resistance. After extended first-line treatment in the presence of unrecognized viral failure, we performed resistance testing on the last sample during first-line treatment in 23 children with samples available. Eighteen of these 23 children accumulated additional mutations during the extended time on first-line cART (Table 4). While the majority of children already had multi-class resistance at the initial point of viral failure (Table 3), 6 of 10 children tested that initially had either no mutations or only single-class resistance, accumulated multi-class resistance after extended first-line cART.
Table 4.
Accumulation of resistance mutations during the lag between viral and immunologic failure on first-line cART
| ID§ | months on 1st line after viral failure |
Resistance following extended 1st line treatment during viral failure□ |
Accumulation of new resistance mutations |
|---|---|---|---|
| PADX1* | 9 | T69N, Y181C, M184V | + Ψ |
| PADX2* | 12 | K103N, V108IV, M184V | + |
| PADX9* | 8 | K101E, M184V, G190A | − |
| PADC1* | 13 | D67del, T69G, K70R, K101H, M184V, G190S, T215F, K219E | + |
| PADD9* | 8 | M41L, D67N, K101EK, Y181C,M184V, T215F | + |
| PADF2* | 6 | D67N, K70R, K103N,M184V, K219E | + |
| PADG5* | 17 | D67N, K70R, K103S, M184V, T215F, K219E | + |
| PADH3* | 40 | D67N, T69N, K70R, A98G, M184V, G190A, T215F, K219Q | + Ψ |
| PADK4* | 6 | L74V, Y115F, Y181C, M184V | − Ψ |
| PADM8* | 17 | K103N, M184V, T215TF | + |
| PADA1# | 27 | K103N, M184V | + |
| PADJ6# | 23 | K101EQ, M184V, G190S | + |
| PADP6# | 27 | K103KN | + Ψ |
| PADB2# | 3 | K101EK, K103N, M184V | + |
| PADC9# | 7 | K101E, M184V, G190A | + Ψ |
| PADG6# | 50 | L74V, Y115F, M184V | + |
| PADH8# | 35 | M41L, D67N, V179E, M184V, G190A, L210W, T215Y | + |
| PADL8# | 1 | K103KN, G190AG | − |
| PADL4#, ^ | 23 | K103N, M184V | + |
| PADP4#, ^ | 26 | A98AG, K101EQ, M184V, G190A | + |
| PADA7#, ^ | 19 | K103N, V108VI, M184V | + |
| PADC6#, ^ | 34 | none | − |
| PADD1#, ^ | 3 | none | − |
Some ID numbers present in Table 3 are not included here because some children did not have additional follow-up samples on first-line cART
Switched to PI-based cART based on clinical or immunological criteria
Remained on first-line cART during follow-up
No resistance detected at the initial point of viral failure
Mutations in bold were not present at the initial point of viral failure
Had a mutation at the initial point of failure that was no longer detectable after extended 1st-line cART (compare to Table 3)
Virologic Suppression and Resistance After Switch to Second-line treatment
In children who were switched to ritonavir-boosted lopinavir, the median duration of virologic follow-up on this regimen was 28 months, during which time 5 (38%) children had at least one viral level above 5000 copies/mL. However during the entire follow-up period on second-line treatment, only one child had sustained viral levels >5000 copies/mL, while the remaining 4 had only intermittent viremia. Only one of these 5 children had evidence of protease resistance during intermittent viremia and at baseline, with a minor mutation (L10I) which can occur in untreated individuals and is only associated with resistance to protease inhibitors (PIs) when present with other mutations.
DISCUSSION
In this cohort of HIV-1 infected Kenyan children, we observed a virologic failure rate of 34% during a median of 49 months on first-line NNRTI-based cART. This is comparable to the viral failure rates seen in other pediatric cohorts in similar settings.7,8,20 The median time to virologic failure on first-line treatment in our study was 9 months, and it is notable that 82% of those that experienced virologic failure did so during the first 2 years on cART. Thus, the rate of failure was low in children who maintained viral suppression at 2 years. The major strength of our study is the long follow-up, which demonstrates that durable virologic response is an achievable goal in at least two-thirds of HIV-1 infected children treated with first-line cART in similar settings. Nevertheless, the proportion of children who experienced early virologic failure is cause for concern and indicates the need to further optimize adherence, especially in the initial months of treatment.
In this cohort, younger children had a higher likelihood of virologic failure even after controlling for baseline viral load, similar to previous findings.21 This could result from sub-therapeutic drug levels in younger children due to lower adherence or differences in pharmacokinetics. We did not monitor drug levels and therefore cannot confirm the possibility of sub-therapeutic treatment. However, younger children are fully dependent on a caregiver for drug administration and only 36% of our cohort reported disclosure to other family members, implying that the pool of potential caregivers able to administer medication in the event of the primary caregiver’s absence was limited. Although we inquired about missed doses and spitting out of medications, this information was based on self-report and may not be accurate. A study in the same facility found that self-report overestimates true adherence when compared to pharmacy records.22 In addition, pharmacokinetic data for most antiretroviral drugs is poorly defined for young children and under-dosing may occur. These findings suggest that younger children should be prioritized for virologic testing in settings where access to viral monitoring is available on a limited basis.
Aside from age, no other baseline characteristics were associated with viral failure, however our sample size was limited to 100 children total, and only 34 experienced viral failure, thus power was limited. In contrast to findings from a study in Uganda, we did not find that low baseline CD4 or type of NRTI backbone predicted virologic failure.8 In the Ugandan cohort (n=222), with shorter follow-up (12 months), lower baseline CD4 counts, male sex, and use of D4T-based treatment was associated with virologic failure. The smaller size of our study and homogeneity of baseline CD4 may explain the lack of detecting a similar association.
Two-thirds of the children with viral failure had resistance detectable at the point of failure, the majority of whom had 2 or more clinically relevant mutations resulting in multi-class resistance. At the point of virologic failure, the two most common mutations found were M184V, which confers high-level resistance to lamivudine, and K103N, which confers resistance to all first-generation NNRTIs. This is similar to findings from studies in Uganda, Central America, and Cote d’Ivoire and is in part due to the low genetic barrier to resistance for lamivudine and NNRTIs.23–25 The virus that bears the M184V mutation has been found to be relatively unfit, incapable of rapid replication, and has increased susceptibility to zidovudine, which may explain why a number of children in our cohort who remained on ZDV in the presence of virologic failure were clinically stable.26 In fact, in children in our cohort on ZDV-based cART, viral load was significantly lower at rebound compared to baseline in children with detectable M184V compared to children whose mutations did not include M184V (data not shown, P=0.01). The WHO guidelines were recently revised to retain lamivudine in second-line pediatric regimens due to the high prevalence and poor replicative capacity of M184V, and our findings confirm the relevance of these guidelines for Kenya.
TAMs and K65R were found at viral failure in 4 and 1 child, respectively, which was less frequent than the prevalence of NNRTI-associated mutations in our cohort, but higher than the prevalence observed in a large cohort in South Africa.27 These mutations limit the choice of second-line regimens and therefore present a challenge to children failing thymidine-based first-line.27 The Kenyan national guidelines were revised to give preference to abacavir over zidovudine in first-line ART to lower the potential for development of TAMs.16
One-third of children in our cohort who experienced virologic failure had no detectable resistance at the initial point of viral failure. The most plausible explanation for lack of viral suppression in these children is poor adherence. In the absence of resistance, it is possible for children to achieve virologic suppression if adherence is improved. Previous studies provide evidence that targeted counseling can lead to viral suppression, averting the need for second-line regimens.28,29 Therefore, as virologic testing becomes increasingly available in these settings, optimizing adherence should be the first approach to addressing viral failure when resistance testing is not available.
In our study, 22 children who did not meet the clinical criteria to switch to second-line cART had evidence of viral failure upon retrospective testing. However, only 12 (55%) of these children had evidence of antiretroviral resistance at viral failure. Thus, for 10 (45%) children viral suppression could possibly have been achieved with better adherence. These findings underscore the importance of resistance assays, which when available, add critical information to viral load assays to guide treatment.
Switch to 2nd-line treatment was based on clinical or immunologic failure, which lagged viral failure by an average of 12 months. This extended period on first-line treatment in the presence of unrecognized viral failure resulted in the accumulation of additional resistance mutations in 18 of 23 children, and multi-class resistance often developed in children who had only single-class or no resistance at the onset of viral failure. There is evidence from other studies that this lag in switching to second-line treatment is associated with increased mortality rates, particularly when the first-line is NNRTI-based.30 A recent study found viral loads of >5000 copies/ml was associated with a nearly doubled risk of developing a WHO stage 3–4 event, independent of CD4 count, hemoglobin level and body mass index,31. Thus, our study suggests that increased access to virologic testing may be useful for early detection of treatment failure and could improve treatment outcomes.
The number of children in our cohort who were switched to PI-ART was relatively small (n=14). However, a long follow-up (median 28 months after switch) showed that persistent virologic failure on second-line was rare. This was true although 10 of the 14 children that switched to PI-ART had detectable resistance to both NRTIs and NNRTIs prior to the switch, suggesting that PI-monotherapy may be effective in some children as shown in recent studies.32,33 Despite the lag following virologic failure on first-line, most sustained viral suppression on PI-therapy well beyond 2 years, and the emergence of detectable protease resistance was rare. This is reassuring in settings where third-line regimens, including second-generation boosted PIs or integrase inhibitors, are not feasible due to high cost.
Limitations of this study include the fact that the cohort was established primarily for research, which may somewhat limit generalizability. Resistance was assessed by population-based sequencing, which only detects resistant virus that comprises >20% of the viral population, and therefore it is possible that we missed resistance mutations present at lower frequencies in these children. In addition, the cohort was established in the pre-PEPFAR period, when access to ART was critically limited and therefore may represent very sick children and self-selected survivors. Baseline CD4% at cART initiation in this treatment program has progressively risen from 5%, when this cohort was established, to about 13% currently. Finally, this cohort did not have children with perinatal antiretroviral exposure and hence the findings may be less relevant to children with prior PMTCT exposure. Strengths of the study include the long follow-up with serially detailed viral and resistance data.
In summary, approximately a third of long-term cART-treated children experienced virologic failure during ~4 year follow-up, the majority of whom had antiretroviral drug resistance. Viral load assays may decrease the lag to treatment switch, and thus lessen the accumulation of additional mutations. However, without resistance assays it is not possible to distinguish failure due to non-adherence from viral rebound due to resistance. Children had excellent suppression on second-line therapy despite the lag in detection of viral failure.
Supplementary Material
(A) Kaplan Meier curves showing the proportion of children with immunologic or clinical failure between children who had virologic success (black line) and virologic failure (grey line) on first-line cART. Time zero is time of cART initiation. (B) Delay between time of viral failure and switch to 2nd-line cART in the 11 children who experienced viral failure prior to immunologic/clinical failure. Three other children were switched to 2nd-line but not included here because they either did not experience viral failure on 1st-line cART (n=2) or they were switched at the time of viral failure (n=1). Time zero is time of viral failure.
Acknowledgements
The authors thank the research personnel, laboratory staff, and data management teams in Nairobi, Kenya and Seattle, Washington; the research clinic at the Kenyatta National Hospital, Nairobi or their participation and cooperation: the Divisions of Obstetrics and Gynaecology and Paediatrics at Kenyatta National Hospital for providing facilities for laboratory and data analysis: Katie Odem-Davis and Ken Tapia for advice on statistical analysis. Most of all we thank the children and their caregivers who participated in the study.
Sources of Funding
This work was supported by grants from the National Institutes of Health (R01-TW007632, K24-HD054314, and AI076105) as well as Fogarty (D43-TW000007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest.
Author Contributions
DW and DAL were joint lead authors in the development and writing of the manuscript. All co-authors contributed to the writing and editing of the manuscript. DW led onsite implementation of the study including recruitment and follow-up. DAL and DW conducted the statistical analysis of the data. DAL, SBN and MG conducted resistance assays for genotypic testing and interpreted the resistance data. DW, GJS and CF designed the study including all epidemiologic aspects. RG and EMO provided clinical service to children in the cohort with EMO guiding management of complicated cases. JO provided overall leadership for the virologic aspects of the study including viral load and resistance testing.
REFERENCES
- 1.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010 Jan 1;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K, Hernán MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008 Jun 1;46(11):1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access. [Accessed, August 2011]; Recommendations for a public health approach, 2010 revision. 1–206. from http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. [PubMed]
- 4.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009 Dec 15;49(12):1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. The Lancet Infectious Diseases. 2008 Aug 1;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 6.Barth R, Tempelman H, Smelt E, Wensing A, Hoepelman A, Geelen S. Long-term Outcome of Children Receiving Antiretroviral Treatment in Rural South Africa. Pediatr Infect Dis J. 2011 Jan 1;30(1):52–56. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- 7.Rouet F, Fassinou P, Inwoley A, et al. Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006 Nov 28;20(18):2315–2319. doi: 10.1097/QAD.0b013e328010943b. [DOI] [PubMed] [Google Scholar]
- 8.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 9.Lwembe R, Ochieng W, Panikulam A, et al. Anti-retroviral drug resistance-associated mutations among non-subtype B HIV-1-infected Kenyan children with treatment failure. J Med Virol. 2007 Jul 1;79(7):865–872. doi: 10.1002/jmv.20912. [DOI] [PubMed] [Google Scholar]
- 10.Kantor R, Diero L, Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis. 2009 Aug 1;49(3):454–462. doi: 10.1086/600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health. 2009 Aug;14(8):856–861. doi: 10.1111/j.1365-3156.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 12.ART-LINC of IeDEA Study Group. Keiser O, Tweya H, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009 Sep 10;23(14):1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The PENPACT-1 (PENTA 9/PACTG 390) Study Team. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. The Lancet Infectious Diseases. 2011 Mar 18;11(4):273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatrics. 2010 Jan 1;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenya Ministry of Health. National AIDS and STI Contro Program, Guidelines for Antiretroviral Therapy in Kenya. [Accessed April 2011];2011 :1–203. from: http://www.nascop.or.ke.
- 17.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. Journal of clinical microbiology. 2000 Jul;38(7):2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman DA, Chung MH, Mabuka JM, et al. Lower risk of resistance after short-course HAART compared with zidovudine/single-dose nevirapine used for prevention of HIV-1 mother-to-child transmission. J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):522–529. doi: 10.1097/QAI.0b013e3181aa8a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanford University HIV drug resistance database. [Accessed August 2011]; from: http://hivdbstanfordedu/. [Google Scholar]
- 20.van Dijk JH, Sutcliffe CG, Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS ONE. 2011 Jan 1;6(4):e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frange P, Briand N, Avettand-fenoel V, et al. Lopinavir/ritonavir-based antiretroviral therapy in human immunodeficiency virus type 1-infected naive children: rare protease inhibitor resistance mutations but high lamivudine/emtricitabine resistance at the time of virologic failure. Pediatr Infect Dis J. 2011 Aug 1;30(8):684–688. doi: 10.1097/INF.0b013e31821752d6. [DOI] [PubMed] [Google Scholar]
- 22.Ngeno B, Wamalwa DC, Nduati R. Comparison of self-report and pharmacy records as measures of adherence to antiretroviral therapy in HIV-1 infected children in Kneyatta National Hospital, Nairobi. Presented at: 5th IAS Conference on HIV Pathogenesis, treatmend and prevention; Cape Town, South Africa. 2001. [Google Scholar]
- 23.Adjé-Touré C, Hanson DL, Talla-Nzussouo N, et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Côte d'Ivoire. AIDS Research and Human Retroviruses. 2008 Jul 1;24(7):911–917. doi: 10.1089/aid.2007.0264. [DOI] [PubMed] [Google Scholar]
- 24.Holguín A, Erazo K, Escobar G, et al. Drug resistance prevalence in human immunodeficiency virus type 1 infected pediatric populations in Honduras and El Salvador during 1989–2009. Pediatr Infect Dis J. 2011 May 1;30(5):e82–e87. doi: 10.1097/INF.0b013e3182117289. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds SJ, Kityo C, Mbamanya F, et al. Evolution of drug resistance after virological failure of a first-line highly active antiretroviral therapy regimen in Uganda. Antivir Ther (Lond) 2009 Jan 1;14(2):293–297. doi: 10.1177/135965350901400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeger LK, Margot NA, Miller MD. Increased drug susceptibility of HIV-1 reverse transcriptase mutants containing M184V and zidovudine-associated mutations: analysis of enzyme processivity, chain-terminator removal and viral replication. Antivir Ther (Lond) 2001 Jun 1;6(2):115–126. [PubMed] [Google Scholar]
- 27.Barth RE, Wensing AM, Tempelman H, Moraba R, Schuurman R, Hoepelman A. Rapid accumulation of nonnucleoside reverse transcriptase inhibitor-associated resistance: evidence of transmitted resistance in rural South Africa. AIDS. 2008 Oct 18;22(16):2210–2212. doi: 10.1097/QAD.0b013e328313bf87. [DOI] [PubMed] [Google Scholar]
- 28.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther (Lond) 2007 Jan 1;12(1):83–88. [PubMed] [Google Scholar]
- 29.Wilson D, Keiluhu AK, Kogrum S, et al. HIV-1 viral load monitoring: an opportunity to reinforce treatment adherence in a resource-limited setting in Thailand. Trans R Soc Trop Med Hyg. 2009 Jun 1;103(6):601–606. doi: 10.1016/j.trstmh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks S. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008 Oct 18;22(16):2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira R, Krauss M, Essama-Bibi S, et al. Viral load predicts new world health organization stage 3 and 4 events in HIV-infected children receiving highly active antiretroviral therapy, independent of CD4 T lymphocyte value. Clin Infect Dis. 2010 Dec 1;51(11):1325–1333. doi: 10.1086/657119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosalaraksa P, Ananworanich J, Puthanakit T, et al. Lopinavir/ritonavir Monotherapy in HIV-infected Children; week 144 results. Presented at: 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. Abstract #962. [Google Scholar]
- 33.Neth O, Falcon-Neyra L, Ruiz-Valderas R, et al. Simplified human immunodeficiency virus maintenance therapy in virologically suppressed children with Ritonavir-boosted protease inhibitor monotherapy. Pediatr Infect Dis J. 2011 Oct 1;30(10):917. doi: 10.1097/INF.0b013e318223bc56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Kaplan Meier curves showing the proportion of children with immunologic or clinical failure between children who had virologic success (black line) and virologic failure (grey line) on first-line cART. Time zero is time of cART initiation. (B) Delay between time of viral failure and switch to 2nd-line cART in the 11 children who experienced viral failure prior to immunologic/clinical failure. Three other children were switched to 2nd-line but not included here because they either did not experience viral failure on 1st-line cART (n=2) or they were switched at the time of viral failure (n=1). Time zero is time of viral failure.