Abstract
The current study examined the long-term effects of neonatal amygdala lesions on emotional and hypothalamic-pituitary-adrenal (HPA) axis reactivity to an acute stressor in rhesus monkeys. Rhesus monkeys received either bilateral MRI-guided ibotenic acid amygdala (Neo-Aibo; n = 6) or sham (Neo-C; n = 7) lesions between 7–14 days of age. Emotional reactivity was assessed using the Human Intruder paradigm at 2 months, 4.5 months, and 6–8 years of age, whereas stress neuroendocrine response was only assessed in adulthood (6–8 years). The modulation of defensive and emotional behaviors based on the gaze direction of the intruder emerged between 2–4 months of age in surrogate-peer reared sham-operated infant monkeys, as already shown for mother-reared infants. Although neonatal amygdala lesions did not impair the ability to exhibit defensive and emotional behaviors, it altered the modulation of these responses based on the intruder’s gaze direction. The changes in emotional reactivity after neonatal amygdala lesions emerged in infancy and persisted throughout adulthood when they were associated with a reduction of basal cortisol levels and a blunted cortisol response to the stressor. These changes are reminiscent of those found after adult-onset amygdala lesions, demonstrating little functional compensation following early amygdala damage.
Keywords: Amygdala, Emotion, Development, HPA-axis
When a potential danger is encountered, the ability to assess its magnitude, select an appropriate response, and modulate internal physiology accordingly is essential to survival. Studies in rodents, monkeys, and humans have shown that the amygdala is critical for the modulation of behavioral and physiological responses according to the magnitude of threat signals (Aggleton, 2000). Thus, when faced with a potential threat, adult monkeys with amygdala lesions express less freezing, as well as less fearful and aggressive behaviors toward a novel conspecific (Meunier, et al., 1999; Kalin, et al., 2001), and approach an unfamiliar human more willingly (Meunier, et al., 1999; Kalin, et al., 2004; Machado & Bachevalier, 2008), although no changes were found in two studies (Kalin, et al., 2001; Izquierdo, et al., 2005). The amygdala also has an excitatory influence on the neuroendocrine HPA-axis response to potential threats through both direct projections to the lateral hypothalamus and indirect projections to the paraventricular nucleus (Herman, et al., 2003; Freese & Amaral, 2009). Electrical stimulation of the amygdala increases cortisol secretion (Mason, 1959), whereas complete lesions of the amygdala or lesions restricted to the central nucleus decrease HPA-axis response to a stressor in both rodents (review Herman, et al., 2003) and primates (Machado & Bachevalier, 2008; Kalin, et al., 2004). To date, the putative role of the amygdala in the regulation of defensive behaviors and neuroendocrine responses is derived from studies of adult animals in which both the amygdala and hypothalamus are fully developed. Given the upsurge of interest on how the amygdala contributes to emotional and neuroendocrine regulation in developmental neuropsychiatric disorders, such as autism, schizophrenia, anxiety, and depression (Britton, et al., 2011; Schumann, et al., 2011), further investigation of its role in the development of behavioral and neuroendocrine stress is warranted.
Few nonhuman primate studies have shown that neonatal amygdala damage yields abnormal emotional reactivity to objects and social partners (Thompson, 1981; Prather, et al., 2001; Bauman, et al., 2004a, 2004b; Bliss-Moreau, et al., 2011) but normal HPA-axis response to pharmacological challenges (Goursaud, et al., 2006), and none have investigated the modulation of threat signals together with HPA-axis reactivity. In the present study, we assessed the effects of neonatal amygdala lesions on the development of behavioral and neuroendocrine stress reactivity to different magnitudes of threat using the Human Intruder paradigm. This paradigm has proven to be a robust, rapid, and precise experimental tool for measuring specific defensive responses in monkeys based on the salience of the threat stimulus used (Kalin et al., 1991). In the Alone condition, when monkeys are separated from their cagemates and placed into a novel environment, they respond by emiting coo vocalizations and exploratory behavior in an attempt to reunite with cagemates (Kalin, et al., 2005). In the Profile condition [No Eye Contact (NEC) in other publications] the unfamiliar human presents his/her profile and avoids eye contact with the animals denoting a mild threat that triggers fearful defensive or anti-predator detection behaviors, such as freezing (Kalin, et al., 2005). Finally, in the Stare condition, the most salient threat, the unfamiliar human makes direct eye contact with the animals which elicits hostile defensive and anxious behaviors (Kalin et al., 1991). Threatening stimuli, such as the Human Intruder paradigm, also initiate significant activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in rapid increases in neuroendocrine measures, such as ACTH and cortisol in blood (Herman, et al., 2003, Jahn, et al., 2010). Thus, given that, around 3–4 months of age, infant monkeys begin to detect and respond appropriately to social cues from faces (Mendelson, et al., 1982) and modulate their defensive behaviors based on the salience of threat signals from a Human Intruder (Kalin, et al., 1991), we predicted that the modulation of defensive and emotional behaviors in infant rhesus monkeys will emerge between 2–4 months of age and that neonatal amygdala lesions will alter or delay the emergence and pattern of these behaviors as well as reduce activation of the HPA-axis in response to the Human Intruder stressor.
Methods
Subjects
Twelve rhesus monkeys (Macaca mulatta) surrogate-peer reared in a socially enriched environment that promoted species-specific socio-emotional skills (Sackett, et al., 2002; Goursaud & Bachevalier, 2007; Rommeck et al., 2011;) were used in this study. Animals received neonatal neurotoxic lesions of the amygdala (Neo-Aibo; males = 3, females = 3) or sham operations (Neo-C; males = 3, females = 3) between one to two weeks of age. The surgical procedures as well as the behavioral testing during infancy and adolescence were performed at the University of Texas Health Science Center (UTHSC, Houston, TX). Behavioral testing and neuroendocrine measures during adulthood were performed at the Yerkes National Primate Research Center (YNPRC, Atlanta, GA). At both institutions, animals were housed under a 12 hour light/dark cycle and all procedures were approved by the respective Institutional Animal Care and Use Committees of the UTHSC and of Emory University.
Following surgeries, emotional reactivity to a Human Intruder was assessed during infancy (at 2 months and 4.5 months of age) to assess short-term effects, and during early adulthood (6–8 years) to examine long-term effects. Other testing not reported here included examination of social behavior (at 3 months, 6 months, and 3 years), goal-directed behaviors (at 3 months, 3 years and 4–5 years), and memory abilities (at 6, 8, 9, and 18 months, and 2, 4, 6–8 years). Procedures for rearing condition, neuroimaging, neurosurgery, and estimation of lesion extent have been previously described in detail elsewhere (Goursaud & Bachevalier, 2007; Kazama & Bachevalier, 2012) and will be briefly summarized below.
Surrogate Peer-rearing Conditions
Animals were selected from six cohorts of four age-matched animals each that included one Neo-C animal and one Neo-Aibo animal along with two other animals that participated in other ongoing studies.
Housing
Infants were housed individually in wire cages (40 cm X 30 cm X 40 cm) under open radiant incubators with contact comfort provided by a synthetic plush surrogate (approximately 30 cm in length) until 1 months of age after. The wire cages allowed for somato-sensory contact between infants in adjacent cages as well as visual, auditory, and olfactory contacts with all other infants in the nursery. At 3 months of age, animals were transferred to larger quad cages and housed individually, but visual and physical contact was possible between pairs of infants through the large central mesh separating two adjacent cages. Animals were housed in quads in large enclosures at approximately 7 months of age and then in pairs when they reached 12 months of age.
Socalization
Daily care of animals was provided by a principal human caregiver who fed and handled them several times a day from the day they arrived in the primate nursery. The principal human caregiver spent approximately 6 h daily, 5 days a week in the primate nursery with the infants. On weekends, familiar human caregivers fed, handled and played with the infants 2–3 times a day for a total of 2–4 h both days. In addition to social contact with human caregivers, at 1 month of age until approximately 12 months of age, all infants received daily socializations (3–4 h, 5 days/week) with three other age- and sex-matched peers of the same or different cohorts. Socialization took place in a large play cage, containing toys and towels, located in the primate nursery.
Imaging and Surgical Procedures
Magnetic Resonance Imaging (MRI)
On the day of surgery, the infant was removed from its home cage and anesthetized (isoflurane, 1–2% to effect). Its head was shaved and secured in a nonferromagnetic stereotaxic apparatus. Three MRI sequences were obtained with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 3-inch surface coil. First, a high resolution T1-weighted spin-echo sequence (echo time [TE] = 11 ms, repetition time [TR] = 450 ms, contiguous 4 mm thick images, 12 cm field of view [FOV], 256 x 256 matrix) was taken in the sagittal plane and was used to align the two subsequent series. A 3D T1-weighted fast spoiled gradient (FSPGR)-echo sequence (TE = 2.6ms, TR = 10.2ms, 25° flip angle, contiguous 1 mm thick images, 256 x 256 matrix) was used to determine the three-dimensional coordinates for each neurotoxin injection site in the amygdala for group Neo-Aibo. Animals in group Neo-Aibo also received a Fluid Attenuated Inversion Recovery (FLAIR) sequence (TE = 140 ms, TR = 10000 ms, inversion time [TI] = 2200, contiguous 3 mm thick images, 12 cm FOV, 256 x 256 matrix). The FLAIR images reveal tissue T2 prolongation with cerebrospinal fluid suppression and were compared to the post-surgical FLAIR images to accurately indicate localized areas of edema and estimate the extent of lesion (Nemanic et al., 2002). Animals in group Neo-C received a Fast Spin-Echo – Inversion Recovery (FSE-IR) series (TE = 20ms, TR = 4500/250ms, ETL =6, BW = 32 kHz, contiguous 1.5 mm thick images, 12 cm FOV, 256 x 256 matrix, 2 NEX). The latter series was used as part of a separate study to track the developmental trajectory of several brain structures (Payne, et al., 2010).
Neurosurgery
After all structural neuroimaging was completed, the infant was kept anesthetized in the stereotaxic apparatus and immediately brought to the surgical suite. An intravenous drip of 5% dextrose and 0.45% sodium chloride was started to maintain normal hydration during surgery. Vital signs (heart rate, respiration, blood pressure, expired CO2) were monitored throughout the surgical procedures. Body temperature was maintained via a warm air blanket placed around the animal and attached to a Bair Hugger® Therapy warming unit. The scalp was disinfected with Nolvasan solution and a local anesthetic (Marcain 25%, 1.5 ml) was injected subcutaneously along the incision line to reduce pain during skin incision. Under aseptic conditions, the skin was opened and connective tissue was gently displaced to expose the skull. For all animals, two small bilateral craniotomies were made above the amygdala. The dura was cut and retracted to expose the brain.
For Neo-C animals, the surgical procedures stopped here and no injections were made. For Neo-Aibo animals, injections of ibotenic acid (Biosearch Technologies, Novato, CA; 10 mg/ml in phosphate buffer saline, pH 7.0–7.4) were made at 4–6 sites (2 mm apart) within the center of the amygdala using 10 μl Hamilton syringes. The needles were lowered simultaneously in both hemispheres and a total of 0.8–1.6 μl of ibotenic acid was manually injected at a rate of 0.2 μl/30 seconds. After each injection, the needles were left in place for an additional 3-minute period to allow complete diffusion of the neurotoxin at the tip of the needle and minimize its spread in the needle track during retraction of the needles.
At completion of the surgical procedures, the dura was closed with silk sutures and the bone opening was covered with Surgicel NU-KNIT (absorbable hemostat). The connective tissues and skin were closed in anatomical layers. The animal was removed from anesthesia, and placed in a temperature controlled incubator ventilated with oxygen until full recovery from anesthesia. All animals received acetaminophen (10 mg/kg), cephazolin (25 mg/kg), and dexamethasone sodium phosphate (0.4 mg/kg) to reduce pain, prevent infection and edema, respectively. A topical antibiotic ointment (bacitracin-neomycin-polymyxin) was applied to the wound daily.
Lesion Verification
Estimation of the extent of intended and unintended damage for Neo-Aibo animals was accomplished using MRI scans obtained 7–10 days post-surgery. The animal was again anesthetized, repositioned into the stereotaxic frame, and underwent high resolution T1 FSPGR and FLAIR scans described above. Pre- and post-surgical MRI scans were compared to estimate the extent of lesion using previously established procedures (Nemanic, et al., 2002). The high-resolution T1 images were used to help identify the borders of each structure. Lower resolution FLAIR images were used to identify extent of the hypersignals showing edema and to estimate lesion extent. Hypersignals were plotted onto corresponding coronal drawings from a normalized infant rhesus monkey brain (J. Bachevalier, unpublished atlas) using Adobe Photoshop software. Drawings were imported into an image analysis program (Image-J) to measure the surface area (in pixels2) of intended amygdala damage. Unintended damage for all surrounding structures (entorhinal and perirhinal cortices, and hippocampus) was also measured. The volume of amygdala damage was then divided by the normal volume of the amygdala (obtained from the template brain in a similar manner) and multiplied by 100 to estimate a percentage of the total volume damaged. The same procedure was applied to estimate potential damage to structures adjacent to the amygdala.
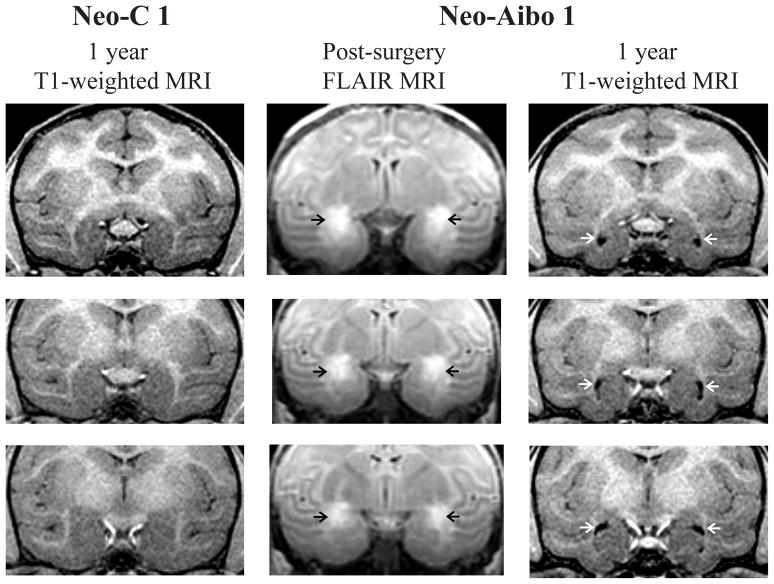
Extent of bilateral damage to the amygdala, estimated from the FLAIR images, averaged 62.5% across both hemispheres (see Table 1), but varied from case to case. Three cases (Neo-Aibo-1, -4, and -6) received substantial symmetrical damage to the amygdala in both hemispheres (from 63.8% to 76%), whereas the remaining three cases (Neo-Aibo-2, -3, and -5), received more extensive damage to the right amygdala (61.1% to 77.6%) than to the left (33.0% to 42%). In all cases, however, the damage included the central, medial, accessory basal, and dorsal areas of the basal and lateral nuclei, but spared the ventral portion of the lateral and basal nuclei. Extent of unintended damage to the perirhinal and entorhinal cortical areas, anterior portion of the hippocampus, and tail of the putamen were negligible for all cases. Figure 1 illustrates the location and extent of hypersignals resulting from the neurotoxin injections seen on FLAIR images (middle column), and the bilateral amygdala volume reduction seen on the 1-year post-surgical T1-weighted images from one representative case (Neo-Aibo-1; see right column) as compared to a control animal (Neo-C-1; left column). Note the location of the hypersignals, which were more intense in the dorsal than in the ventral amygdala and the enlarged ventricles resulting from reduction of amygdala volume. Extent of amygdala lesions for two other cases was illustrated in Goursaud & Bachevalier (2007, see Figure 2) and Kazama & Bachevalier (2012, see Figure 2), respectively.
Table 1.
Intended and unintended damage after neurotoxic lesions of the amygdala
| Intended Damage
|
Unintended Damage
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala
|
Hippocampus
|
Perirhinal
|
||||||||||
| Subjects | Lf% | Rt% | X% | W% | Lf% | Rt% | X% | W% | Lf% | Rt% | X% | W°/o |
| Neo-Aibo-1 | 89.0 | 59.8 | 74.4 | 53.2 | 5.1 | 3.1 | 4.1 | 0.2 | 2.0 | 10.1 | 6.0 | 0.2 |
| Neo-Aibo-2 | 42.0 | 77.6 | 59.8 | 32.6 | 0.0 | 0.8 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-3 | 33.0 | 61.1 | 47.1 | 20.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-4 | 62.1 | 90.0 | 76.0 | 55.9 | 1.9 | 3.0 | 2.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-5 | 41.2 | 66.6 | 53.9 | 27.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-6 | 52.1 | 71.6 | 63.8 | 39.3 | 5.6 | 10.3 | 8.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 71.8 | 53.2 | 62.5 | 38.1 | 2.9 | 2.1 | 2.5 | 0.1 | 1.7 | 0.3 | 1.0 | 0.03 |
Rt%: percent damage in the right hemisphere; Lf%: percent damage in the left hemisphere; X%: average damage to both hemispheres; W%: weighted average damage to both hemispheres (W%=(Lf%×Rt%)/100). Neo-A-F: female amygdala lesion subject, Neo-A-M: male amygdala lesion subject.
Figure 1.
Coronal sections through the anterior (top) to posterior (bottom) extent of the amygdala. Left column: T1-weighted MR images through the amygdala at 1-year post-surgery in one sham-operated control (Neo-C-1). Middle column: FLAIR images illustrating the location and extent of hypersignals (black arrows) within the amygdala in a representative case with neonatal amygdala lesion (Neo-Aibo-1). Rigtht column: T1-weighted MR images through the amygdala at 1-year post-surgery in case Neo-Aibo-1 illustrating the enlargement of ventricles (white arrows) resulting from amygdala volume reduction.
Figure 2.
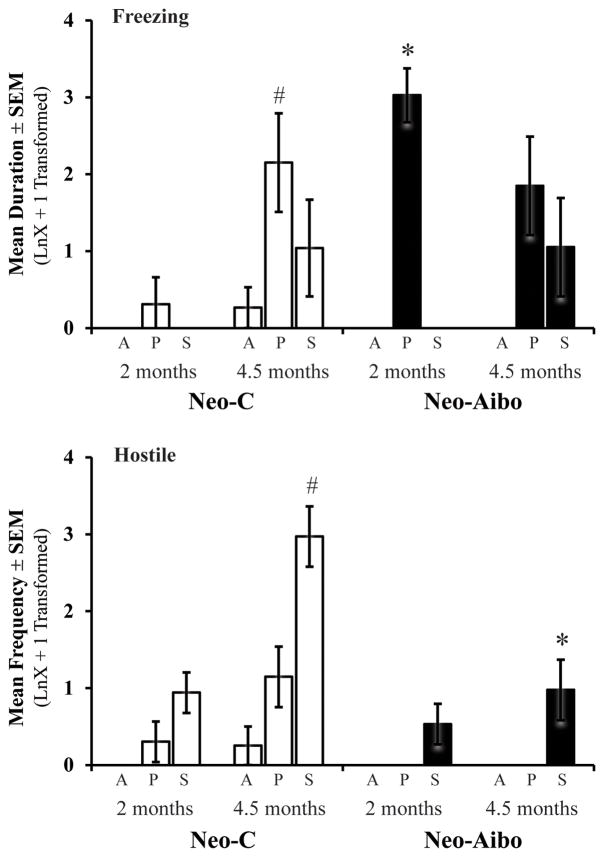
Bars represent the mean ± SEM for 4 animals in each group tested at both 2 and 4.5 months of age on the Human Intruder paradigm. Freezing and hostile defensive behaviors during the Alone (A), Profile (P), and Stare (S) conditions for animals with sham operations (Neo-C, open bars) and animals with neonatal amygdala lesions (Neo-Aibo, black bars). Note that scores were log-transformed (LnX + 1). # indicates significant age effect and * indicates significant group differences (p < 0.05).
Experiment 1
The Human Intruder paradigm was used to measure the development of defensive behaviors in the sham-operated controls and to assess the effects of neonatal amygdala lesions on this development. This paradigm is sensitive to changes in defensive behaviors following amygdala lesions in adult rhesus macaques (Kalin, et al., 2001, 2004; Izquierdo, et al. 2004, 2005; Machado & Bachevalier, 2008). Given that the modulation of emotional reactivity emerges between 2 and 4 months of age in mother-reared rhesus macaques (Kalin, et al. 1991), we predicted that a similar developmental time course will be seen in surrogate-peer reared infant monkeys, but would be disrupted or delayed in infant monkeys with neonatal amygdala lesions.
Human Intruder Paradigm
Twelve animals (Neo-C, n=6; Neo-Aibo, n=6) were tested at 2 months of age, whereas only four of the six subjects in each group were tested at 4.5 months. At both ages, animals were transported to a testing room adjacent to their housing room, and then transferred to a stainless steel cage (53 cm x 53 cm x 55 cm) with one side made of clear lexan plastic for video recording. The cage was positioned on an elevated platform so that the animal was at the same level as the intruders’ face. The Human Intruder paradigm lasted 43 minutes and consisted of four 10-minute conditions (Alone 1, Profile [No Eye Contact in other publications], Stare, Alone 2). Animals were tested on two consecutive days during which the order of the Profile and Stare conditions were counterbalanced across the two days. For each day, the animal first remained alone in the cage for 10 minutes (Alone 1) to acclimate to the environment and obtain a baseline level of behavior. Then, the intruder (experimenter wearing a human rubber mask) entered the room, stood two meters from the test cage while presenting his/her profile to the animal for 10 minutes (Profile condition). The intruder then left the room while the animal remained in the cage alone for a 3-min period, after which the intruder reentered the room and stared directly at the animal for 10 minutes (Stare condition). Finally, the intruder left the room leaving the animal alone for another 10 minutes (Alone 2). To avoid habituation to the Human Intruder task during the second testing phase at 4.5 months, the intruder wore a different rubber mask depicting a different person. The animal’s behavioral reactivity during all conditions was videotaped and later coded using a detailed ethogram (Table 2) and the Observer 5 software (Noldus Inc.). Two trained experimenters, both unaware of the animals’ lesion group, coded the videotapes. The two experimenters had an inter-rater reliability of Cohen’s Kappa = .81 and an average intra-rater reliability of Cohen’s Kappa = 0.97.
Table 2.
Behavioral Ethogram
| Category and specific behavior | Measurement | Brief Definition |
|---|---|---|
| Fearful Defensive Behaviors | Cumulative Frequency | |
| Freeze | frequencya | Rigid, motionless posture except slight head movement |
| Crouch | frequencya | Whole body or just front bent with head near floor |
| Withdrawal | frequency | Quick, jerky motion away from intruder (jump back) |
| Fear Grimace | frequency | Refracted lips, exposed clenched teeth |
| Hostile Defensive Behaviors | Cumulative Frequency | |
| Threat Bark Vocalization | frequency | Low pitch, high intensity, rasping, guttural |
| Scream Vocalization | frequency | High pitch, high intensity screech or loud chirp |
| Threat (facial expression) | frequency | Any of the following: open mouth (no teeth exposed), head-bobbing, or ear flapping |
| Cage Aggression | frequency | Vigorously slaps, shakes or slams body against cage |
| Lunge | frequency | A quick, jerky movement toward the intruder |
| Anxious Behaviors | Cumulative Frequency | |
| Scratch | frequency | Rapid scratching of body with hands or feet |
| Body Shake | frequency | Whole body or just head and shoulder region shakes |
| Tooth Grind | frequencya | Repetitive, audible rubbing of upper & lower teeth |
| Yawn | frequency | Open mouth widely, exposing teeth |
| Stereotypies | Cumulative Duration | |
| Pacing | duration | Repetitive motor pattern around the test cage |
| Motor stereotypy | duration | Repetitive, abnormal voluntary or involuntary motor patterns (swinging, twirling, floating limb) |
| Self-directed | duration | Sucking thumb, eye poke, self-bite |
| Affiliative Behaviors | Cumulative Frequency | |
| Coo Vocalization | frequency | Clear soft pitch and intensity, sounds like “oooooh” |
| Grunt Vocalization | frequency | Deep, muffled, low intensity, almost gurgling sound |
| Lipsmack | frequency | Rapid movement of pursed lips and smacking sound |
| Present | frequency | Rigid posture (knees locked) with tail elevated and rump oriented toward intruder |
| Self-Soothing Behaviors | Cumulative Duration | |
| Self-groom | duration | Use of hands or mouth to pick through its hair |
| Self-clasp | duration | Non-manipulatory enclosing or holding of a body part |
List of all behaviors scored, how they are measured and a brief definitions.
Behavior for which total duration was also measure
Data Analysis
At each age, preliminary analyses were first performed to compare emotional reactivity during the two Alone conditions (Alone 1 & Alone 2). Repeated measures ANOVA (Group X Testing Day) revealed no significant main effects or interactions. Therefore data from the two Alone conditions over the two days of testing were averaged to create a single Alone condition at each age. Similarly, data on the Profile and Stare conditions from the first testing day were compared to data obtained on the second testing day using repeated measures ANOVA (Group X Testing Day). Again, no significant differences were detected between the two days of testing and, for each animal, a single measure combining the scores from the two Profile and the two Stare conditions were calculated. Thus, for the final analyses (see below), the combined scores of each animal for each of the 3 conditions (Alone, Profile, Stare) were used and transformed using an LnX + 1 constant to achieve normality.
First, to assess the normal development of defensive behaviors between 2 and 4.5 months of age in group Neo-C, repeated measures ANOVA with Condition (Alone, Profile, Stare) as the main factors and Age (2 and 4.5 months) as the repeated factor were performed for each behavior listed in Table 2. To examine differences between Neo-C and Neo-Aibo animals, MANOVAs with Group (2) and Condition (3) as main factors, and Age (2) as within subjects factors with repeated measures were used. Planned comparisons were performed with one-way ANOVA’s to compare group differences at each age separately.
Results
Development of Normal Defensive Behaviors
Previous research has shown that mother-reared rhesus monkeys are able to modulate their defensive behaviors based on the gaze direction of the intruder by 3 – 4 months of age (Kalin, et al., 1989, 1991a). Sham-operated surrogate-peer reared rhesus monkeys also developed species typical defensive behaviors by 4.5 months of age (see Figure 2; Supplementary Table 1), as revealed by an increase in the duration of freezing behavior during the Profile condition (Age: F[1,9] = 7.68, p = 0.022 ,η2 = 0.46; Figure 2a), as well as an increase in the frequency of hostile defensive behaviors during the Stare condition (Age X Condition: F[1,9] = 4.19, p = 0.05 ,η2 = 0.48; Figure 2b). Although the Age X Condition interaction did not reach significance for freezing, there was a trend for the main effect for Condition with a large effect size (F[1,9] = 3.19, p = 0.08 ,η2 = 0.42), suggesting that the time Neo-C animals spent displaying freezing behavior depended on the presence and gaze direction of the Human Intruder.
Effect of Neonatal Amygdala Lesions on Development of Defensive Behaviors
As shown on Figure 2 (see also Supplementary Table 1), comparison of freezing behavior in Groups Neo-C and Neo-A revealed only a significant effect of the intruder’s gaze direction (Condition: F[2,18] = 15.05, p < 0.001, η2 = 0.63) but no significant interactions. Planned comparisons for the Profile condition at the two ages separately demonstrated that Neo-Aibo animals spent significantly more time freezing than Neo-C animals at 2 months of age (Group: F[1,11] = 19.624, p = 0.001, η2 = 0.66), but not at 4.5 months of age (Group: F[1,7] = 0.061, p = 0.81, η2 = 0.01; Figure 2a). For hostile defensive behaviors, there was a significant Group X Age interaction (F[1,18] = 8.90, p = 0.008, η2 = 0.33; Figure 2b). Planned comparisons indicated that both groups exhibited an increase in hostile defensive behaviors with age during the Stare condition, although the magnitude of this increase was significantly greater in the Neo-C animals than Neo-Aibo animals at 4.5 months of age (Group: F[1,7] = 9.42, p = 0.022, η2 = 0.61). No significant main effects and no significant interactions were noted in any other behaviors (Group effect for fearful defensive F[1,18] = 1.39, p = 0.25, η2 = 0.07; affiliative F[1,18] = 0.003, p = 0.96, η2 = 0.001; coo vocalizations F[1,18] = 0.29, p = 0.60, η2 = 0.02; anxious F[1,18] = 0.13, p = 0.73, η2 = 0.007; stereotypy F[1,18] = 1.38, p = 0.25, η2 = 0.07; self-soothing F[1,18] = 0.45, p = 0.51, η2 = 0.02).
Experiment 2
Results of Experiment 1 demonstrate that defensive behaviors have emerged by 4.5 months of age in both sham-operated and amygdalectomized surrogate-peer reared monkeys. Yet, animals with neonatal amygdala lesions exhibited more freezing at 2 months of age and less hostile behaviors at 4.5 months of age compared to sham-operated controls. These group differences suggest that the amygdala lesions did not abolish the development of these species typical defensive behaviors, but altered their normal expression. To investigate whether the effects of early amygdala lesions on emotional reactivity dissipate or persist and become more profound with further maturation, all animals were retested on the Human Intruder paradigm as adults (between 6–8 years of age). Furthermore, to assess whether changes in emotional reactivity after neonatal amygdala lesions were associated with alterations in HPA-axis stress reactivity, we also used the Human Intruder paradigm as an acute stressor and measured ACTH and cortisol levels in plasma just prior to and immediately after the task. To ensure that group differences in hormone secretion were due to the effects of the stressor and not merely the effect of handling or blood sampling, ACTH and cortisol levels were examined on a baseline day (non-stressor). Furthermore, the diurnal HPA-axis rhythm was investigated to replicate an unexpected finding we observed in the baseline day samples.
Human Intruder Paradigm
To collect blood quickly in an unanestethized monkey, the testing cage (65 cm X 65 cm x 150 cm) was modified to train the animal to voluntarily present its leg through an opening for awake saphenous venipuncture. In addition, the presentation of the Human Intruder paradigm was slightly modified by reducing the length of the task to 30 minutes in order to capture the peak of ACTH and cortisol stress responses. During these 30 minutes, the three conditions (Alone, Profile, Stare) were presented in that order for 9 minutes each and were separated by a brief 3-min break. The paradigm was given only for one day with the 3 conditions presented in the same order for all animals. Emotional reactivity to the Human Intruder was assessed via videotape recording for later coding using the Observer XT 10 software (Noldus Inc.) and the ethogram described in Experiment 1. One experimenter coded all of the videotapes, but had a high degree of inter-rater reliability (Cohen’s Kappa = .84) with the previous experimenters that coded behavior at 2 and 4.5 months of age, for consistency across ages. This experimenter also had an average intra-rater reliability of Cohen’s Kappa = 0.98.
Procedures for Assessment of Neuroendocrine Function
Training for blood collection procedures
Elevations in plasma cortisol concentration can be readily detected within 10 minutes of initial disturbance. Therefore, animals received a training procedure to ensure that baseline blood samples could be collected within 10 minutes after initial disturbance (experimenter entering the housing room). Positive reinforcement was used to progressively shape the animal to voluntarily present a leg to the experimenter for awake blood collection from the saphenous vein using established protocols at the YNPRC demonstrating that under these conditions hormonal levels reliably reflect basal levels (Blank, et al. 1983).
Blood Sampling
All animals were tested at the same time of day (Lights-ON: 0700) and blood samples were collected immediately before (0700) and after Human Intruder paradigm (0730), serving as an acute stressor. To control for individual variability and determine that changes in hormone levels were not due to handling or sampling technique, two days prior to the Human Intruder paradigm, two blood samples were collected at the same time of day (0700 & 0730) without the presence of the acute stressor. Approximately one year thereafter, the diurnal rhythm of cortisol secretion was characterized by collecting blood samples for each animal at Lights-ON (0700), Mid-day (1300), and Lights-OFF (1900). All blood samples were collected in pre-chilled 2-ml vacutainer tubes containing EDTA (3.6 mg) and immediately placed on ice. Samples were centrifuged at 3,000 rpm for 15 minutes in a refrigerated centrifuge (4°C) and plasma samples were stored at −80°C until assayed.
Plasma Hormonal Assays
All assays were performed by the YNPRC Biomarker Core Laboratory. Plasma samples during the Human Intruder task and on baseline days were assayed for both ACTH and cortisol. Plasma concentrations of ACTH were assayed in duplicate by radioimmunoassay (RIA) using commercially available kits (DiaSorin, Inc., Stillwater, MN). The sensitivity of the DiaSorin assay was 7.10 pg/ml and intra- and inter-assay coefficients of variation in each assay were < 9.2%. Plasma concentrations of cortisol were assayed in duplicate by RIA using commercially available kits (DSL kit: Diagnostic Systems Laboratories, Webster, TX). The sensitivity of the DSL assay was 1.25μg/dl and intra- and inter-assay coefficients of variation in each assay were < 10%.
Given that Diagnostic Systems Laboratories discontinued their cortisol RIA kits in 2010, plasma samples for the diurnal cortisol rhythm were assayed using liquid chromatography – mass spectroscopy (LC-MS). LC-MS analyses were performed via reverse phase chromatography on an LTQ-Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA). Quantitation was achieved using a deuterated cortisol internal standard (CDN Isotopes, Cortisol-9,11,-12,12-d4). The assay range was 2.5–60 ug/dl with intra- and inter-assay coefficients of variation < 15%.
Data Analysis
One Neo-C male was unavailable for testing as an adult and was replaced with a female, which was added to group Neo-C when she was 1 year of age. Preliminary analyses showed that this female did not differ from the other surrogate-peer reared Neo-C animals in either behavior responses or hormone levels. Thus, 12 animals (Neo-C, n = 6; Neo-Aibo, n = 6) were tested at this age. Prior to analysis, the behavioral data were transformed to achieve normality using an LnX + 1 constant. Normal expression of defensive and emotional behaviors in adulthood was examined in group Neo-C using one-way repeated measures ANOVA with Condition (Alone, Profile, Stare) as the repeated factor. Repeated measures ANOVA with Group (2) and Condition (3) were used to assess differences between Neo-C and Neo-Aibo animals. Planned comparisons were performed with one-way ANOVA’s to compare group differences in freezing during the Profile and hostile behaviors during the Stare condition. Additionally, the ability of Neo-Aibo animals to modulate behaviors across the three conditions was examined with a one-way Repeated Measures ANOVA. Finally, we performed a test of equivalence (Rogers et al., 1993; Wellek, 2003) to further examine whether behavioral scores between two conditions (Alone vs. Profile and Profile vs Stare) within each group were small enough to be considered equivalent. Statistical equivalency/similarity between conditions was tested with a confidence interval of 95% and an equivalence interval of Δ = ± 1. Any condition difference falling within the equivalence interval indicates that the difference is not meaningful and the conditions can be treated as equivalent/similar.
Changes in stress hormone levels were assessed using repeated measures ANOVA with Group (2) as between subjects factor and Time as the within subjects repeated measures (e.g. pre- and post-stressor OR Lights-ON and + 30 min for baseline day). Percent change in cortisol concentrations from pre-stress to post-stress was also calculated ([post-stress – pre-stress]/pre-stress * 100) for each animal and analyzed by GLM ANOVA with Group (2) as the between subjects factor and percent change in cortisol level as the dependent variable. Diurnal cortisol rhythm was analyzed using repeated measures ANOVA with Group (2) as between subjects factor and Time of Day (Lights-ON, Mid-day, Lights-OFF) as the within subjects repeating measures. Significance level was set at p < 0.05 for all analyses.
Results
Emotional reactivity in the sham-operated controls when reaching adulthood
Sham-operated animals modulate their freezing and fearful defensive behaviors depending on the presence and gaze direction of the intruder, showing greater number of these behaviors in the Profile conditions than in the other two conditions (Condition: F[2,10] = 489.08, p < 0.001, η2 = 0.99; F[2,10] = 20.38, p < 0.001, η2 = 0.80, respectively; Figure 3). Neo-C animals also exhibited a modulation in the expression of hostile and anxious behaviors, with an increase in both behaviors during the Stare condition as compared to the other two conditions (Condition: F[2,10] = 88.68, p < 0.001, η2 = 0.95; F[2,10] = 16.47, p = 0.001, η2 = 0.77, respectively; Figure 3, Supplementary Table 2).
Figure 3.
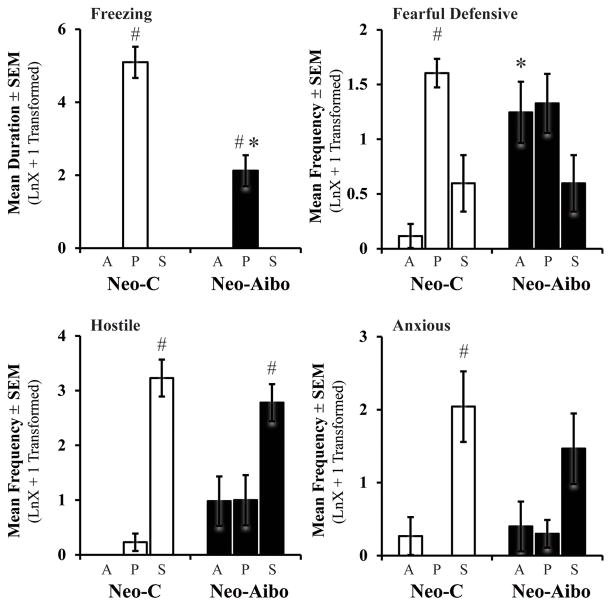
Bars represent the mean ± SEM for Freezing, Fearful defensive, Hostile, and Anxious behaviors obtained in adulthood for each group during the three conditions of the Human Intruder paradigm. # indicates significant differences between conditions (p < 0.05). All other abbreviations as in Figure 2.
Long-term effects of neonatal amygdala lesions on emotional reactivity
Neonatal amygdala lesions had long-term effects on emotional reactivity to the presence and gaze direction of the Human Intruder (see Figure 3, Supplementary Table 2). The Group X Condition interactions were significant for both freezing and fearful defensive behaviors (F[2,20] = 24.24, p < 0.001, η2 = 0.71; F[2,20] = 5.38, p = 0.014, η2 = 0.35, respectively). For freezing specifically, Neo-Aibo animals displayed more freezing in the Profile condition than in the other conditions, yet, levels were still significantly lower than for controls (F[1,10] = 24.24, p = .001, η2 = 0.70; Figure 3). As for fearful defensive behaviors, unlike Neo-C animals, Neo-Aibo animals displayed more fearful defensive behaviors during the Alone condition, whereas during the Profile and Stare they were not significantly different from Neo-C animals (repeated contrast: Alone vs Profile, F[1,10] = 17.97, p = 0.002, η2 = 0.64; Profile vs Stare, F[1,10] = 0.43, p = 0.52, η2 = 0.04; Figure 3). For fearful behaviors in Neo-C animals, the difference between Alone-Profile and Profile-Stare both reached significance (t[5] = 8.68, p < 0.001, d = 4.9; t[5] = 4.17, p = 0.009, d = 1.7, respectively), and for both contrasts the test of equivalence did not reveal similarity (see Supplementary Figure 1). By contrast, Fearful behaviors in Neo-Aibo animals did not differ between Alone-Profile (t[5] = 0.29, p = 0.78, d = 0.1) and between Profile-Stare conditions (t[5] = 2.09, p = 0.09, d = 1.2) and the test of equivalence revealed similarity for both contrasts (Supplementary Figure 1). Thus, both statistical tests concurred and suggest that Neo-Aibo animals were unable to express and/or modulate anti-predator detection behaviors, such as high freezing and fearful defensive behaviors during the Profile as compared to the other two conditions.
For hostile behaviors (Figure 3), both groups expressed high levels of hostility during the Stare condition (Condition: F[2,20] = 53.09, p < 0.001, η2 = 0.84) and the Group X Condition interaction was just short of significance with a large effect size (F[2,20] = 3.95, p = 0.057, η2 = 0.28). Thus, as compared to Neo-C animals that exhibited increased hostility almost exclusively during the Stare condition (repeated contrast: Profile vs Stare, F[1,10] = 7.44, p = 0.02, η2 = 0.43), Neo-Aibo animals expressed equal amount of hostility in the first two conditions (repeated contrast: Alone vs Profile, F[1,10] = 0.01, p = 0.93, η2 = 0.001). Test of equivalence revealed that the difference in hostility between Alone-Profile did not reach significance for both groups (Neo-C: t[5] = 1.58, p = 0.18, d = 0.9; Neo-Aibo: t[5] = 0.81, p = 0.94, d =0.01) and the test of equivalence indicated similarity for both groups. However, the Profile-Stare contrast for Neo-C animals reached significance (t[5] = 8.41, p < 0.001, d = 5.7) and did not meet the criteria for similarity (Suppl. Fig. 1). The Profile-Stare contrast for the Neo-Aibo animals reached significance (t[5] = 2.98, p = 0.03, d = 1.4) and met criteria for similarity. Thus, both statistical analyses suggest that Neo-C animals modulated their behavior to primarily express hostility during the Stare condition, whereas Neo-Aibo animals displayed a weaker modulation of these behaviors.
Finally, both groups expressed more anxious behaviors during the Stare condition (Condition: F[2,20] = 12.97, p < 0.001, η2 = 0.57), but neither the group effect nor interaction were significant (F[1,10] = 0.03, p = 0.87, η2 = 0.003; F[2,20] = 0.91, p = 0.42, η2 = 0.08, respectively). However, as shown in Figure 3, anxious behaviors occurred more frequently in the Stare condition for controls (Condition: F[2,10] = 16.47, p = 0.001, η2 = 0.77; repeated contrast: Alone vs Profile F[1,5] = 1.00, p = 0.36, η2 = 0.17; Profile vs Stare F[1,5] = 17.23, p = 0.009, η2 = 0.78), whereas the amount of anxious behaviors was roughly equal in all three conditions for the Neo-Aibo animals (Condition: F[2,10] = 2.56, p = 0.13, η2 = 0.34). For anxious behaviors, the Alone-Profile contrasts did not reach significance for both groups (Neo-C: t[5] = 1.0, p = 0.36, d = 0.6; Neo-Aibo: t[5] = 0.20, p = 0.84, d =0.1) and the test of equivalence indicated similarity between conditions for both groups (Supplementary Fig. 1). The Profile-Stare contrast for Neo-C animals was statistically significant (t[5] = 4.15, p = 0.009, d = 2.4) and the test of equivalence did not reveal similarity. By contrast, the Profile-Stare contrast for Neo-Aibo animals reached significance (t[5] = 3.51, p = 0.017, d = 1.3) but met the criteria for similarity. Overall, these results suggest that, although Neo-C animals modulated their anxiety across the conditions, this modulation was weaker in Neo-Aibo animals.
Both groups exhibited an increase in affiliative behavior during the Stare condition (Condition: F[2,20] = 8.61, p = 0.004, η2 = 0.46; repeated contrasts Alone vs Profile F[1,10] = 1.44, p = 0.26, η2 = 0.13; Profile vs Stare F[1,10] = 19.57, p = 0.001, η2 = 0.66) and Neo-Aibo animals did not differ from controls (Group: F[1,10] = 0.26, p = 0.62, η2 = 0.03). No significant interactions or group differences were found for any other behavior (Group effect: coo vocalizations F[1,10] = 1.36, p = 0.27, η2 = 0.12; stereotypy F[1,10] = 3.37, p = 0.09, η2 = 0.25; self-soothing F[1,10] = 2.36, p = 0.16, η2 = 0.19).
Long-term effects of neonatal amygdala lesions HPA-axis function
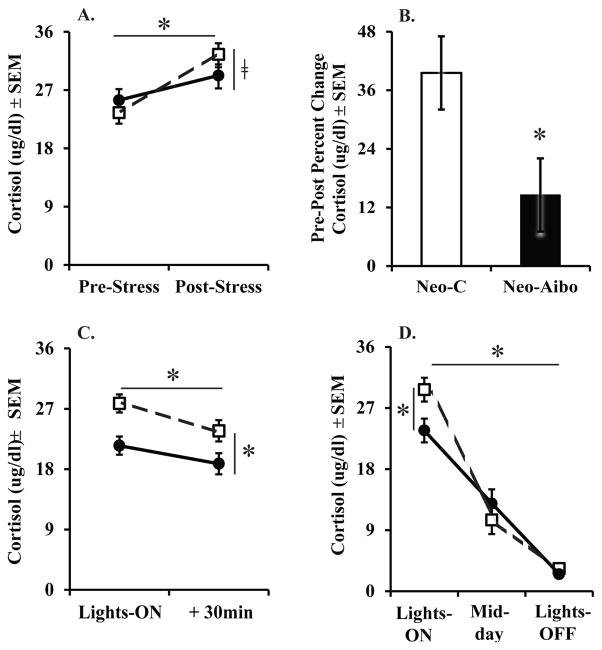
Together with alterations of emotional reactivity, the neonatal amygdala lesions had also a significant impact on HPA functioning in adulthood (Figure 4). Both groups exhibited a significant increase in both ACTH and cortisol secretion from pre- to post-stress blood samples (Time: F[1,10] = 6.27, p = 0.031, η2 = 0.39; F[1,10] = 25.50, p < 0.001, η2 = 0.72, for ACTH and cortisol, respectively), and the Group X Time interaction for cortisol fell just short of significance with a large effect size (F[1,10] = 4.19, p = 0.06, η2 = 0.30; see Figure 4A). Therefore, this trend for a Group X Time interaction was investigated by examining the percent change in cortisol secretion from pre- to post-stress blood samples. The results revealed a greater percent change in cortisol secretion for the Neo-C animals than the Neo-Aibo animals (F[1,12] = 5.57, p = 0.04, η2 = 0.36; see Figure 4B), demonstrating that the neonatal amygdala-operated animals were hypo-responders to the stressor.
Figure 4.
Scores are mean ± SEM of cortisol in adulthood during Human Intruder stressor (A), Percent Change from pre- to post-stress (B), Non-stress day (C), as well as Diurnal rhythm (D). Neo-C is represented with open squares with dashed lines or open bars, Neo-Aibo is represented with black circles with solid lines or black bars. * indicates significance of p < 0.05 and □ indicates a trend of p = 0.057.
To ensure that group differences in hormone secretion were due to the effects of the stressor and not merely the effect of handling or sampling technique, ACTH and cortisol levels were examined on a baseline day, two days prior to the Human Intruder stressor. There were no significant effects of Group or Time on ACTH levels on the baseline day. For cortisol levels, both groups exhibited a small but significant decrease in cortisol levels from Lights-ON to 30 minutes later (Time: F[1,10] = 30.14, p < 0.001, η2 = 0.75; Figure 4C), although at both times cortisol levels in Neo-Aibo animals were slightly but significantly lower than those in the Neo-C animals (Group: F[1,10] = 7.95, p = 0.018, η2 = 0.44). This lower cortisol levels among Neo-Aibo animals on the baseline (non-stress) day was unexpected, thus the diurnal rhythm of cortisol secretion was investigated to replicate these changes in basal cortisol secretion. A similar group difference also occurred for the diurnal rhythm of cortisol secretion. Again, Neo-Aibo animals had slightly, but significantly, lower cortisol levels at Lights-ON than Neo-C animals, although they did not differ from Neo-C at Mid-day or Lights-OFF (Group X Time: F[2,20] = 4.74, p = 0.021, η2 = 0.32; see Figure 4D).
Discussion
The present study provides important new findings. First, similar to mother-reared infant monkeys (Kalin, et al., 1991), the modulation of defensive behaviors in surrogate-peer reared rhesus infant monkeys emerged by 4.5 months of age. Second, neonatal amygdala lesions did not impair or delay the emergence of defensive behaviors in early infancy, but instead altered their modulation depending on the gaze direction of the intruder. Third, the changes in emotional reactivity after neonatal amygdala lesions persisted in adulthood, and were associated with both a reduction in basal cortisol levels and a blunted cortisol response to the acute stressor. Finally, the behavioral and neuroendocrine changes after neonatal amygdala lesions were reminiscent to those found after adult-onset lesions, suggesting little functional compensation after early amygdala damage.
Emotional and neuroendocrine responses to a stressor in surrogate-peer reared monkeys
Extensive evidence exists on the role of the mother and other members of the social environment in the development of normal behavioral and physiological responses to stress in primates (Boyce, et al., 1995; Shannon, et al. 1998; Capitanio, et al., 2005; Karere et al., 2009; Nelson, et al., 2009; Rommeck et al., 2011; Corcoran, et al., 2012). Therefore, it was important to first investigate the emotional and physiological responses to stress by sham-operated surrogate-peer reared infant monkeys. As mother-reared infant monkeys (Kalin et al. 1991), the surrogate-peer reared rhesus macaques exhibited an age-dependent increase in organization of contextual patterns of defensive behaviors from 2 to 4.5 months, reflected by an increase in freezing responses during the Profile condition and in hostile behaviors during the Stare condition. Although there is evidence for changes in stress-reactivity in infant monkeys reared in a nursery, our surrogate-rearing conditions did not significantly impact defensive behaviors at least when measured by the human intruder task. In fact, a recent study (Rommeck et al., 2011) demonstrated that nursery-reared infant monkeys that received continuous rotation peer rearing (a rearing condition similar to that given to the monkeys of the present study) displayed behavioral and temperament measures similar to those of mother-reared controls. Thus, the surrogate-peer reared infant monkeys were able to modulate the expression of defensive behaviors based on the presence and gaze direction of the intruder. This modulation of defensive behaviors in sham-operated monkeys persisted throughout adulthood, when they also exhibited a significant modulation of anxious behaviors according to the level of threat presented. The changes in emotional reactivity to an acute stressor in the surrogate-peer reared animals were associated with normal physiological alterations of the HPA-axis, i.e. increased ACTH and cortisol secretion after the stressor.
Neonatal Amygdala Lesions and Defensive Behaviors
Although Neo-Aibo animals exhibited defensive behaviors in response to the Human Intruder, the expression of these behaviors differed at the three ages tested (2 and 4.5 months, and 6–8 years) as compared to controls. First, at 2 months, Neo-Aibo animals showed more freezing during the Profile condition as compared to Neo-C controls. Although this group difference disappeared by 4.5 months, Neo-Aibo animals did not modulate freezing behaviors according to the intruder’s gaze directions (i.e. more freezing in the Profile than in the Stare conditions). Instead, they displayed more freezing in both conditions. This increased freezing at 2 months in Neo-Aibo animals might appear to be contradictory to the traditional role played by the amygdala in fear learning and hypo-reactivity to stressors. Yet, this pattern is exhibited early in infancy, when the amygdala and its connections are still undergoing striking and fast developmental changes (Freese & Amaral, 2009; Payne, et al., 2010; Chareyron, et al., 2012). The current findings are reminiscent to those reported in rodents with early amygdala lesions. Rodents have a sensitive period in development (postnatal day 1–9) when pups are less able to learn aversions or express fear, such that pups do not express freezing to unfamiliar male/predator odor (Takahashi, 1992) or to conditioned odor-shock pairings (Moriceau, et al., 2006) until after the sensitive period has ended. Lesions of the amygdala during this sensitive period result in pup’s fear learning and avoidance of aversive stimuli (Moriceau, et al., 2006). A similar mechanism may take place in infant primates and may provide a reason for why neonatal amygdala lesions resulted in greater defensive avoidance behaviors at 2 months (although the same damage at a later age may result in a different behavioral profile).
Upon reaching adulthood, group differences were noted in the pattern of behavioral expression. First, unlike Neo-C controls that exhibited greater freezing and fearful responses in the Profile condition as compared to the other two conditions, Neo-Aibo animals had a blunted freezing response but heightened fearful behaviors in the Alone condition. These results parallel the increased fearful responses found in earlier studies when animals with neonatal amygdala lesions interacted with social partners (Thompson, 1981; Prather et al., 201; Bauman et al., 2004a). Second, similar to Neo-C controls, Neo-Aibo animals exhibited an increase in hostile behaviors during the Stare condition; however, they also expressed hostility during both the Alone and Profile conditions. This increased hostility (or lack of its contextual modulation) is reminiscent to the higher life history of aggression reported among healthy human subjects with smaller amygdala volumes (Matthies, et al., 2012). Additionally, some patients with Urbach-Weithe disease (resulting in calcification of the medial temporal lobe, including the amygdala) exhibit incidents of rage (Emsley & Paster, 1985; Kleinert, et al., 1987) and perceive anger more intensely in images of angry faces as compared to healthy controls (Siebert, et al., 2003), whereas some others, such as the patient SM, show alterations in threat detection (Feinstein, et al., 2011). Finally, neonatal amygdalectomy altered the animals’ ability to modulate anxious behaviors based on the level of threat presented. This lack of modulation also parallels findings from people with Urbach-Weithe disease that show increased rates of mood and anxiety disorders compared to healthy, age-matched, controls (Thornton, et al., 2008). Taken together, these behavioral changes after neonatal amygdala lesions (decreased freezing, increased fearful defensive behaviors, and inability to modulate hostile and anxious behaviors) suggest long lasting disruption of the normal expression of defensive and emotional behaviors, leading to emotional dysregulation.
The reduction in freezing behaviors after amygdala damage has mostly been linked to damage to the central nucleus, which has strong anatomical connections with the periaqueductal gray matter, a brain structure crucial for freezing behavior in rodents (Kalin, et al., 2001; Izquierdo, et al., 2005; Walker & Davis, 2008). Given that, in the present study, all cases received damage located to the dorsal portion of the amygdala, which includes the central nucleus (see Figure 1), it is likely that the alterations in freezing could have resulted from damage to this specific amygdaloid nucleus. Alternatively, the duration of freezing has also been linked to metabolic activity in the basal forebrain in primates (Kalin, et al., 2005) and basal forebrain lesions (Knox & Bernston, 2006) or inactivation (Fendt, et al., 2003) reduce conditioned and unconditioned freezing in rodents. Thus, unintended damage to the basal forebrain may likewise have resulted in the reduction in freezing seen in the current study (see Figure 1). Post-mortem histological examination of the brains of the neonatal amygdala-operated animals will help validate the specific neural structures responsible for the changes in freezing behaviors.
The absence of a functional amygdala early in life did not totally abolish emotional reactivity to danger, but resulted in an inability to modulate behaviors based on the magnitude of threat presented by the Human Intruder. The same animals in this study have also shown impairments on other emotional regulation tasks, such that they were unable to flexibly modulate their behavior in an appetitive task (Kazama, O’Malley, & Bachevalier, 2007); inhibit their retrieval of a food reward located in front of an aversive stimulus (e.g. doll with large eyes, fake snake; Raper, Kazama, & Bachevalier, 2009); and exhibit a retarded acquisition of fear conditioned cues (Kazama, et al., 2012). Taken together these data indicate that the amygdala may not be the structure responsible for the production of emotional responses. Rather the amygdala regulates the activity of other brain structures (Freese & Amaral, et al., 2009) to modulate emotional reactivity depending on contextual information. Evidence from fear conditioning literature has shown that animals with amygdala damage can acquire conditioning to cues if the training is extended beyond that of normal intact animals (Ponnusamy, et al., 2007; Kazama, et al., 2012), suggesting alternative neural pathways to learning fear conditioned cues (Ponnusamy, et al., 2007). As reported by others, the bed nucleus of the stria terminalis (BnST) and prefrontal cortices are important for emotional processing (Izquierdo, et al., 2005; Laviolette, et al., 2005; Kalin, et al., 2007) and provide alternative neural routes enabling animals to express species typical emotional behavior in the absence of a functional amygdala (Ponnusamy, et al., 2007). The BnST has been shown to modulate anxiety behaviors in rodents (see review Walker & Davis, 2008), as well as freezing during the Human Intruder paradigm in primates (Kalin, et al., 2005). In turn, prefrontal cortices (e.g. anterior cingulate, orbital frontal cortex) are critical for guiding behavior based on contextual information (Bechara, et al., 1994; Izquierdo, et al., 2005; Kalin, et al., 2007). Therefore, in the absence of a functional amygdala, activation of the BnST and prefrontal cortices could explain the production of emotional reactivity to threatening stimuli, although the modulation of emotional behaviors to contextual information may be reduced or even absent.
Neonatal Amygdala Lesions and HPA-axis functioning
As compared to controls, Neo-Aibo animals exhibited blunted stress-induced cortisol reactivity to an acute stressor, strengthening the view that the amygdala has an excitatory influence on the HPA-axis. Neonatal amygdalectomy also led to a flattened basal cortisol rhythm as reflected by lower cortisol levels at Lights-ON in both the baseline (non-stress) day and the diurnal rhythm. Few studies have examined the effects of amygdala lesions on basal HPA functions, particularly in primates, and among those none have reported changes in basal cortisol secretion (Kalin, et al., 2004; Goursaud, et al., 2006; Machado & Bachevalier, 2008). Differences in timing of blood collection may explain the different findings. In the previous studies, blood samples were collected at a time (around mid-morning or mid-day) when we showed no changes in cortisol levels after amygdala lesions as compared to the lower levels found in early morning samples.
Brain plasticity and functional compensation
A final, important point that the present study unveiled is that, despite the greater potential for brain plasticity early in life, neonatal amygdala lesions resulted in pervasive changes in emotional reactivity as well as altered HPA axis functioning that were reminiscent to those reported in adult-onset amygdala lesions. Reduced freezing and lack of contextual modulation of anxious behaviors observed when the animals with neonatal amygdala lesions reached adulthood paralleled some studies of monkeys with adult-onset amygdala lesions (Kalin, et al., 2004; Machado & Bachevalier, 2008), but not others (Kalin, et al., 2001; Izquierdo, et al., 2005). Additionally, there were no differences between neonatal and adult-onset amygdala lesions (Kalin, et al., 2001, 2004; Izquierdo, et al., 2005; Machado & Bachevalier, 2008) in the amount of hostile behaviors expressed during the Stare condition. Finally, the neonatal amygdala lesions resulted in blunted cortisol response to stressors as previously reported after adult-onset amygdala lesions (review Herman, et al., 2003; Kalin, et al., 2004; Machado & Bachevalier, 2008). Thus, despite greater potential for compensatory functional and structural reorganization after early brain lesions, the loss of amygdala function early in life has long-lasting, negative effects on emotional reactivity and HPA-axis functioning. The present results have important clinical significance given that dysregulation of emotional reactivity, anxiety, and HPA-axis functioning are defining characteristics of developmental neuropsychiatric disorders, such as depression, anxiety disorders, autism, and schizophrenia. Thus, developmental studies examining the neural circuitry subserving the expression and regulation of emotions in nonhuman animals provide a foundation for understanding the neuroanatomical and neuropathological basis of human mood and anxiety disorders.
Supplementary Material
Supplementary Figure 1. Equivalencies between the Alone (A), Profile (P), and Stare (S) conditions of the Human Intruder for Fearful Defensive, Hostile, and Anxious behaviors in adulthood. The horizontal T-shaped bars depict 95% confidence intervals for both Group Neo-C (dashed bars) and Group Neo-Aibo (solid bars). The grey vertical lines indicate the equivalence interval of Δ = ± 1. Confidence intervals that fall within the grey vertical lines are considered equivalent/similar. Confidence intervals that do not include zero and extend beyond the 1 point difference are considered to represent a meaningful difference and are indicated by an asterisk (*).
Acknowledgments
Authors are grateful to Jairus O’Malley, Tammy Humbird, Keith Kline, M.S., and Alyson Zeamer, PhD for their assistance with data collection and coding. Additional thanks goes to Sarah Pruett, PhD in the Yerkes BioMarker Core Laboratory for the use of equipment and assistance with the hormone assays.
Funding Sources
This research was supported by the National Institute for Mental Health (MH58846 & MH050268) and the National Institute for Child Health and Human Development (NICHD35471). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIMH, or the National Institutes of Health. The studies were also supported by the Center for Behavioral Neuroscience (NSF IBN 9876754), and Integrated Training in Psychobiology and Psychopathology Fellowship (NIMH T32 MH732505), as well as by the National Center for Research Resources to the Yerkes National Research Center (P51 RR00165; YNRC Base grant) which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Footnotes
Conflicts of Interest
None of the authors have any conflicts of interest in the conduct or reporting of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jessica Raper, Email: jraper@emory.edu.
Mark Wilson, Email: mwils02@emory.edu.
Mar Sanchez, Email: mmsanch@emory.edu.
Christopher J. Machado, Email: cjmachado@ucdavis.edu.
Jocelyne Bachevalier, Email: jbachev@emory.edu.
References
- 1.Aggleton JP, editor. The amygdala. A functional analysis. 2. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 2.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;40:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 3.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004a;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 4.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci. 2004b;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank MS, Gordon TP, Wilson ME. Effects of venipuncture on serum levels of prolactin, growth hormones, and cortisol in outdoor compound-housed female rhesus monkeys. Acta Endocrinol. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- 6.Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev Psychobio. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- 9.Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobi. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- 10.Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P. Postnatal development of the amygdala: a stereological study in macaque monkeys. J Comp Neurol. 2012;520:1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, Friedman DP, Bennett AJ. Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Devel Psychobio. 2012;54:546–555. doi: 10.1002/dev.20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emsley RA, Paster L. Lipoid proteinosis presenting with neuropsychiatric manifestations. J Neurol Neurosur Psychi. 1985;48:1290–1292. doi: 10.1136/jnnp.48.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein JS, Adolphs R, Damasio AR, Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21:34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Gilford Press, Inc; 2009. pp. 1–42. [Google Scholar]
- 16.Goursaud APS, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala, and orbital frontal cortex. Behav Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Goursaud APS, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuoendocrin. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- 20.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychi. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Bio Psychi. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychi. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci. 1998;112:251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- 27.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- 28.Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiat. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazama AM, Heuer E, Davis M, Bachevalier J. Effects of neonatal amygdala lesions on fear learning, conditioned inhibition, and extinction in adult macaques. Behav Neurosci. 2012 doi: 10.1037/a0028241. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazama AM, O’Malley J, Bachevalier J. Neonatal amygdala and orbital frontal cortex lesions disrupt flexible decision-making in adult macaques. Poster presentation at Society for Neuroscience Meeting; San Diego, CA. 2007. [Google Scholar]
- 31.Kleinert R, Cervòs-Navarro J, Kleinert G, Walter GF, Steiner H. Predominantly cerebral manifestation in Urbach-Wiethe’s syndrome (lipoid proteinosis cutis et mucosae): a clinical and pathomorphological study. Clin Neuropathol. 1987;6:43–45. [PubMed] [Google Scholar]
- 32.Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesions on fear-like and anxiety-like behavior. Behav Neurosci. 2006;120:307–312. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- 33.Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal, or orbital frontal lesions in monkeys. Psychoneuroendo. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason JW. Plasma 17-hydroxycorticosteroid levels during electrical stimulation of the amygdaloid complex in conscious monkeys. Am J Physiol. 1959;196:44–48. doi: 10.1152/ajplegacy.1958.196.1.44. [DOI] [PubMed] [Google Scholar]
- 36.Matthies S, Rüsch N, Weber M, Lieb K, Philipsen A, Tuescher O, Ebert D, Hennig J, Tebartz van Elst L. Small amygdala – high aggression? The role of the amgydala in modulating aggression in healthy subjects. World J Biol Psychia. 2012;13:75–81. doi: 10.3109/15622975.2010.541282. [DOI] [PubMed] [Google Scholar]
- 37.Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 38.Mendelson MJ, Haith MM, Goldman-Rakic PS. Face scanning and responsiveness to social cues in infant rhesus monkeys. Dev Psychol. 1982;18:222–228. [Google Scholar]
- 39.Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learing and stress. Dev Psychobio. 2010;52:651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, Delaney D, Ernst M, Fox NA, Suomi SJ, Winslow JT, Pine DS. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Bio Psychi. 2009;66:702–704. doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Meth. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 43.Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponnusamy R, Poulos AM, Fanselow MS. Amygdaladependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 46.Raper J, Kazama AM, Bachevalier J. Blunted fear reactivity after neonatal amygdala and orbital frontal lesions in rhesus monkeys. Poster presented at the Annual Society of Neuroscience Meeting; Chicago, IL. 2009. [Google Scholar]
- 47.Rommeck I, Capitanio JP, Strand SC, McCowan B. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta) Am J Primatol. 2011;73:692–701. doi: 10.1002/ajp.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers JL, Howard KI, Vessey JT. Using significance tests to evaluate equivalence between two experimental groups. Psyc Bull. 1993;113:553–565. doi: 10.1037/0033-2909.113.3.553. [DOI] [PubMed] [Google Scholar]
- 49.Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtail monkeys (Macaca nemestrina) Am J Primatol. 2002;56:165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- 50.Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav. 1992;52:493–498. doi: 10.1016/0031-9384(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi LK, Rubin WW. Corticosteroid induction of threat-induced behavioral inhibition in preweanling rats. Behav Neurosci. 1993;107:860–866. doi: 10.1037//0735-7044.107.5.860. [DOI] [PubMed] [Google Scholar]
- 55.Thompson CI. Learning in rhesus monkeys after amygdalectomy in infancy or adulthood. Behav Brain Res. 1981;2:81–101. doi: 10.1016/0166-4328(81)90039-5. [DOI] [PubMed] [Google Scholar]
- 56.Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsych Clin Neurosci. 2008;20:86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- 57.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 58.Wellek S. Testing statistical hypotheses of equivalence. Chapman and Hall/CRC Press LLC; Boca Raton, FL: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Equivalencies between the Alone (A), Profile (P), and Stare (S) conditions of the Human Intruder for Fearful Defensive, Hostile, and Anxious behaviors in adulthood. The horizontal T-shaped bars depict 95% confidence intervals for both Group Neo-C (dashed bars) and Group Neo-Aibo (solid bars). The grey vertical lines indicate the equivalence interval of Δ = ± 1. Confidence intervals that fall within the grey vertical lines are considered equivalent/similar. Confidence intervals that do not include zero and extend beyond the 1 point difference are considered to represent a meaningful difference and are indicated by an asterisk (*).