Abstract
Drinking behavior and social context are intimately intertwined. Peer relations can promote drinking. Conversely, alcohol promotes social interaction. The present study tested female mice for ethanol-induced conditioned partner preference. Ovariectomized (OVX) C57Bl/6 females with chronic estradiol replacement (OVX+E) received saline or ethanol (1, 2 or 4 g/kg) ip and were paired 4x for 30 min each with 1 of 2 stimulus females. The test female was paired with the CS− stimulus female following saline, and was paired with the CS+ female following ethanol. After pairing, we measured proximity of the test female to the CS+ and CS− females in a 10′ test. In a second study, OVX and OVX+E females were tested for conditioned partner preference (CS+ vs CS−) in response to 2.5 g/kg ethanol. In separate groups of mice, both test and stimulus females (IS+) received ethanol during pairing to determine if test mice develop conditioned partner preference for another intoxicated mouse. OVX+E test females showed conditioned partner preference for the CS+ female in response to ethanol at 2 g/kg (change in preference score for CS+: +86.6±30.0 sec/10 min), but not at 0, 1 or 4 g/kg. At 2.5 g/kg ethanol, OVX+E females developed conditioned partner preference for either IS+ (+63.6±24.0 sec) or CS+ females (+93.8±27.1 sec). OVX test females demonstrated ethanol-induced conditioned partner preference only for the IS+ female (+153.8±32.0 sec). These data demonstrate that ethanol promotes social preference in female mice, and that estradiol enhances this effect.
Keywords: alcoholic intoxication, association learning, conditioning, classical, estradiol, social behavior
INTRODUCTION
Drinking is a social pastime, and this is reflected even in the language we use. “Social drinking” refers to casual intermittent ethanol consumption, as distinguished from problematic ethanol dependence. It is widely recognized that the company you keep while drinking (peers, family) influences ethanol consumption [1], especially in women [2]. Similarly, in animal studies, mice paired with an intoxicated cage-mate will voluntarily consume more ethanol than mice paired with a sober companion [3]. This indicates that social interactions promote ethanol intake. The opposite is also true. That is, ethanol promotes social interactions in humans, at least at moderate doses [4]. The present study determined if ethanol promotes social preference in female mice, using conditioned partner preference as a model
Conditioned partner preference combines elements of conditioned place preference (CPP) and partner preference. CPP is a form of Pavlovian conditioning in which the animal is repeatedly paired with the unconditioned stimulus (US; typically food or drugs) in a unique, but unfamiliar environment (the conditioned stimulus, CS+, reviewed in [5]). With repeated pairings, the animal will prefer the CS+ over another environment (CS−) paired with a control stimulus (e.g. vehicle). The advantage of CPP is that preference for the CS+ is not confounded by the US. A limitation is that CPP tests individual animals in isolation.
Partner preference takes advantage of the mouse’s natural tendency to approach and investigate conspecifics [6]. Partner preference is most often used to test sexual motivation (reviewed in [7]). Typically, the test animal is allowed to interact with tethered stimulus animals of the opposite sex (e.g. gonad-intact vs gonadectomized individuals), and preference is determined. Unlike CPP, partner preference does not depend on prior conditioning. However, the test is conducted in the presence of the US (the stimulus animals).
Conditioned partner preference incorporates a social element into a conditioned response. With this approach, the CS+ and CS− are individual stimulus animals. Previous studies have tested conditioned partner preference in the context of sexual reward. Quail form conditioned preference for sexual partners based on visual stimuli [8]. Male and female rats use olfactory stimuli to form conditioned sexual partner preference [9, 10]. For males, copulation to ejaculation is the US; paced mating is the US for females. In these studies, non-sexual odors (baking extracts) further distinguish the CS+ and CS− stimulus rats. Importantly, male rats demonstrate a generalized preference to ejaculate with an unfamiliar female scented with the CS+.
Conditioned partner preference has not previously been used to test the rewarding properties of drugs or ethanol. The present study tested the hypothesis that ethanol induces conditioned partner preference in female mice. Furthermore, because estradiol promotes social behavior [11], we have explored the interaction of estrogen with ethanol in social preference. Specifically, we determined if estrogen replacement in ovariectomized females enhances ethanol-induced conditioned partner preference.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice (8–10 weeks) were obtained from Charles River Laboratories (Wilmington, MA). This strain shows high voluntary ethanol intake in a 2-bottle preference test [12], and relatively low aversion to ethanol [13]. Mice were group-housed 4 per cage on a 14:10 LD photoperiod with access to food and water ad libitum, except during pairing and testing. Behavior was evaluated under dim illumination during the first 4h of the dark phase. Experimental procedures were approved by USC’s Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, revised 1985, Office of Sciences and Health Reports, DRR/NIH, Bethesda, MD 20205).
Experiment 1: Dose-response for ethanol
Separate groups of females (n=8–10 each) were tested for conditioned partner preference in response to ethanol at 0, 1, 2 or 4 g/kg. Ethanol has been shown previously to facilitate CPP in male mice across this range of doses (1–4 g/kg, [14]), and female C57BL/6 mice show increased consumption of ethanol compared with males [15]. To eliminate endogenous fluctuations in ovarian steroids across the estrous cycle during pairing and testing, all test and stimulus females were ovariectomized (OVX) via bilateral dorsal flank incision under 2, 2, 2-Tribromoethanol (Avertin) anesthesia (250 mg/kg), and received chronic estradiol replacement (OVX+E) via Silastic implant sc (o.d. 2.16 mm, i.d. 1.02 mm, Dow Corning, Midland, MI). The 5 mm implant was filled with a 1:1 mixture of crystalline 17b-estradiol and cholesterol, and the ends were sealed with silicone adhesive. As determined by uterine weights, this regimen provides physiologic levels of estrogen [16, 17]. Females were allowed to recover from surgery up to 2 weeks before testing and pairing.
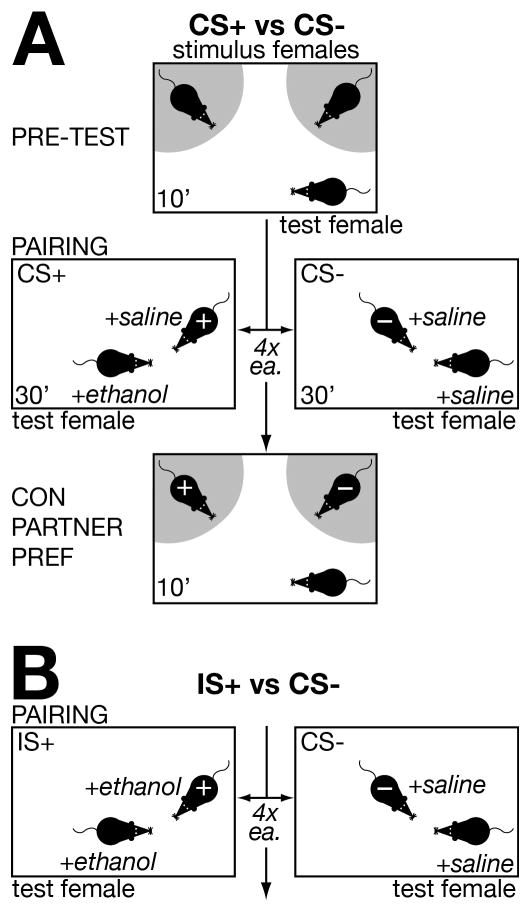
Like CPP, a conditioned partner preference test has three phases: pre-test, pairing, and partner preference (see Figure 1A). All pairing and testing was conducted in identical plastic arenas (40×50×40 cm). During the pre-test, 2 unfamiliar stimulus females were tethered on opposite sides of the area. Stimulus females were lightly restrained by taping the end of the tail to a flexible metal chain. Each stimulus female was further distinguished by a topical odor (coconut or lemon, McCormick baking extracts, Sparks MD). A cotton swab was dipped in extract solution and rubbed on the fur over the scapular region. There was no substantial pre-test preference for the two odors: half of the test females (19/37, 51%) showed a positive preference score for the coconut-scented stimulus female. A test female was introduced, and the amount of time spent in proximity to each stimulus female was recorded for 10 minutes by an observer blind to the treatment groups. This measure is similar to time spent in the choice compartments of a CPP chamber. Proximity was defined when the test female had all 4 paws within reach of the stimulus female (shaded area, Figure 1A). This reflects contact with the stimulus female, as well as exposure to urine and scent gland secretions.
Figure 1.
Experimental design. A: Testing conditioned partner preference for a stimulus female mouse (CS+) paired with ethanol vs another stimulus female (CS−) paired with saline. See Methods for details. B: In separate groups of mice (IS+ vs CS−), the conditioned stimulus female (IS+) also receives ethanol before pairing.
For the next 8 days, test females were paired individually for 30 min with each stimulus female in the testing arena on alternate days (4 pairings each). 30 minutes before pairing, each test female received an injection ip of ethanol or an equivalent volume of saline, and was temporarily housed individually in a clean cage. Ethanol for injection was prepared daily from a commercially-available neutral grain spirit (Everclear, Luxco, St. Louis MO; [18]).
One stimulus female was designated as the CS−. Following a saline injection, the test female was paired with the CS− female. On alternate days, the test female received an ethanol injection, and was paired with the other stimulus female (CS+). The order of pairing (beginning with ethanol or saline) was balanced, and both stimulus females received an injection of saline 30 min before pairing. During pairing, stimulus females were scented with baking extract, but they were unrestrained to facilitate interaction with the test female. The day after the final pairing, conditioned partner preference was measured in test females under conditions identical to the pre-test.
Lastly, we determined if repeated pairing with a scented stimulus female while intoxicated induces a generalized preference in the test female for unfamiliar stimulus females bearing the same scent. Generalization of preference for an unfamiliar scented partner has been demonstrated for mating-induced conditioned partner preference in rats [9, 10]. Whether this applies also to ethanol-induced conditioned partner preference in mice among same-sex conspecifics has not been tested. Accordingly, we retested partner preference, substituting unfamiliar females scented with the same baking extracts for the CS+ and CS− stimulus females.
Experiment 2: Effects of estradiol in test females, effects of ethanol in stimulus females
Experiment 2 extended our initial observations to investigate the interactions of estradiol, ethanol, and social preference. Conditioned partner preference was tested in response to 2.5 g/kg ethanol in ovariectomized females with and without estradiol replacement. Methods were as described above for Experiment 1, except that OVX females received empty Silastic implants, and stimulus females were unfamiliar mice of the same hormonal status (OVX or OVX+E) as test females.
As shown in Fig 1B, we further evaluated the effects of ethanol exposure in a stimulus female (Intoxicated Stimulus: IS+) on ethanol-induced conditioned partner preference in OVX and OVX+E test females. In this comparison, the CS− female received a saline injection as in Experiment 1, but the test female and IS+ female each were injected with 2.5 g/kg ethanol 30 minutes before pairing. This comparison determined preference for IS+ vs CS−.
Data Analysis
Preference score (time with CS+ minus CS−, or time with IS+ minus CS−, in seconds) and total interaction (time with CS+ plus CS−, or time with IS+ plus CS−) were determined for each test female during pre-test, conditioned partner preference, and generalization tests. For Experiment 1, preference scores and total interaction among the different ethanol doses were evaluated by repeated measures ANOVA (RM-ANOVA) with time (pre-test, conditioned partner preference, generalization) as the repeated measure. Subsequently, changes in preference score and total interaction during pre-test, conditioned partner preference, and generalization were evaluated at each ethanol dose by ANOVA, with post-hoc comparison vs pre-test values by Dunnett’s test. For Experiment 2, the effects of estradiol (OVX vs OVX+E) and intoxication of the stimulus female (IS+ vs CS+) on preference scores and total interaction were evaluated by repeated measures ANOVA (RM-ANOVA) with time (pre-test and conditioned partner preference) as the repeated measure. Changes in preference score and total interaction during pre-test and conditioned partner preference were evaluated in each experimental group by paired t-test. For all analyses, p<0.05 was considered significant.
RESULTS
Experiment 1: Dose-response for ethanol
For test females paired with ethanol at 0, 1, 2, or 4 g/kg, there was a significant effect of ethanol dose on preference score by RM-ANOVA [F(3, 31)= 3.681; p<0.05]. However, there was no significant effect of time and no interaction of dose × time. For total interaction, there was a significant of time [F(2, 30)= 3.438; p<0.05] and a dose × time interaction [F(6, 60)= 3.331; p<0.05], but no effect of ethanol dose (p>0.05). Preference scores and total interaction are shown in Figure 2.
Figure 2.
Top: Preference score (mean±SEM) for the conditioned stimulus female mouse (CS+) paired with 0, 1, 2 or 4 g/kg ethanol vs the CS− stimulus female during pre-test (open bars), conditioned partner preference (closed bars), and generalization tests (gray bars) in ovariectomized female mice with estrogen replacement (OVX+E). Bottom: Total interaction (seconds/10 min) with both stimulus female mice during pre-test, ethanol-induced conditioned partner preference, and generalization tests. Asterisk indicates significant difference by Dunnett’s test vs pre-test values at the same ethanol dose.
Repeated pairing with saline (0 g/kg ethanol) had no effect on preference score. Pre-test preference score for the CS+ stimulus female averaged −21.6±37.4 seconds (mean±SEM), while the preference score during conditioned partner preference testing was 9.1±20.1 seconds (p>0.05). When retested with unfamiliar stimulus females, the preference score for the coconut-scented female was −3.0±25.9 seconds. However, the total interaction with both stimulus females varied across the 3 tests [F(2, 20)= 5.303; p<0.05]. After pairing, total interaction with both stimulus females decreased significantly (p<0.05), from 357.9±26.1 seconds during pre-test to 216.6±30.3 seconds for conditioned partner preference testing. When retested with unfamiliar stimulus females, total interaction (253.4±37.9 seconds) was not different from pre-test values (p>0.05). At 1 and 4 g/kg, ethanol also failed to induce a conditioned partner preference for the CS+ stimulus female (p>0.05), and total interaction was unchanged.
There was a significant effect of 2 g/kg ethanol on preference score [F(2, 26)= 3.455, p<0.05]. Preference scores averaged −3.7±14.9 seconds during pre-test, and 82.9±26.8 seconds after pairing, a 86.6±30.0 second increase (p<0.05). When tested with unfamiliar stimulus females, preference score for the coconut-scented female (43.8±26.2 seconds) was not significantly different from pre-test values (p>0.05). However, total interaction did not vary during pre-test (306.3±33.3 seconds), conditioned partner preference (368.4±35.1 seconds) or generalization testing (290.7±28.0 seconds, p>0.05).
Experiment 2: Effects of estradiol in test females, effects of ethanol in stimulus females
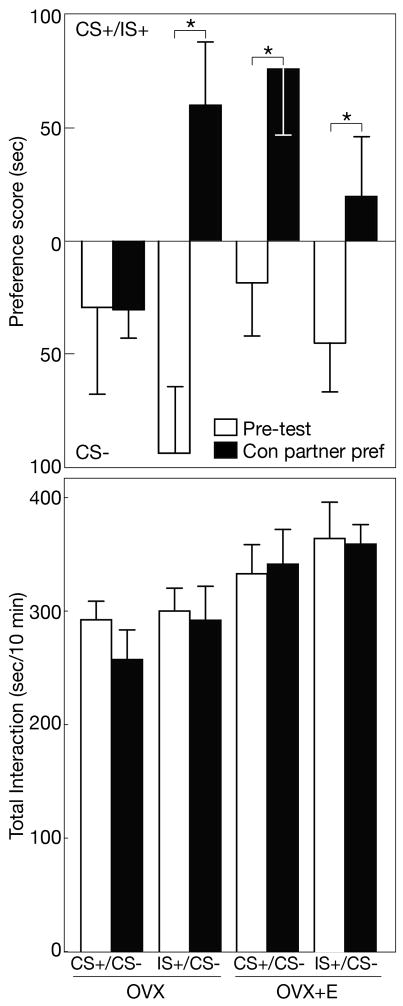
As determined by RM-ANOVA, there was a significant effect of time (pretest vs conditioned partner preference) on preference score in response to ethanol in OVX and OVX+E test females when paired with CS+ or IS+ stimulus females [F(1, 30)=22.735; p<0.05, Figure 3]. As well, there was a significant interaction of time, estradiol (OVX vs OVX+E) and ethanol treatment of the stimulus female (CS+ vs IS+) [F(1, 30)= 8.083; p<0.05)]. For total interaction, there was a significant effect of estradiol [F(1, 30)=8.457; p<0.05], with OVX+E females showing more interaction with the 2 stimulus females.
Figure 3.
Top: Preference score (mean±SEM) for the conditioned stimulus female mouse (CS+ or IS+) paired with 2.5 g/kg ethanol vs the CS− stimulus female during pre-test (open bars) and conditioned partner preference testing (closed bars) in ovariectomized female mice, with (OVX+E) or without (OVX) estrogen replacement. Bottom: Total interaction (seconds/10 min) with both stimulus female mice during pre-test and ethanol-induced conditioned partner preference. Asterisk indicates significant difference by paired t-test vs pre-test values at the same ethanol dose.
Among OVX+E test females, the pre-test preference score for the CS+ stimulus female was −18.8±24.4 seconds. As with 2 g/kg ethanol in Experiment 1, repeated pairing with 2.5 g/kg ethanol in Experiment 2 significantly increased the preference score during conditioned partner preference testing to 75.0±28.8 seconds (p<0.05), a 93.8±27.1 second increase. Likewise, OVX+E test females demonstrated ethanol-induced conditioned partner preference when paired with the IS+ stimulus female. Pre-test preference scores averaged −45.0±21.6 seconds. When tested for conditioned partner preference, preference scores increased by 63.6±24.0 seconds to 18.6±26.0 seconds (p<0.05). For both groups of OVX+E test females, total interaction with the 2 stimulus females after pairing with ethanol was unchanged from pre-test values (p>0.05).
In OVX test females, the mean pre-test preference score for the CS+ stimulus female was −29.4±38.4 seconds. When tested for conditioned partner preference, there was no significant change in preference score (−30.4±12.7 seconds, p>0.05). However, OVX test females did develop a conditioned partner preference when ethanol was paired with the IS+ stimulus female. The mean preference score increased from −93.9±29.1 seconds during pre-test to 59.9±28.0 seconds during conditioned partner preference testing (p<0.05). For both groups of OVX females, total interaction with the two stimulus females was unchanged after pairing.
DISCUSSION
The present study used conditioned partner preference as a model to test the rewarding effects of ethanol in a social context in female mice. After repeated pairing with ethanol at 2 or 2.5 g/kg, OVX+E test females expressed a preference for the stimulus female with whom they had previously been intoxicated. Lower and higher doses of ethanol were ineffective. This study further demonstrates an interaction of ethanol and estradiol to facilitate social preference. OVX+E females developed a conditioned partner preference for the ethanol-paired stimulus female regardless of whether the stimulus female was also intoxicated (IS+) or not (CS+). By contrast, OVX females without estradiol replacement demonstrated conditioned partner preference for IS+ vs CS−, but not for CS+ vs CS−.
Experiment 1 tested ethanol-induced conditioned partner preference at doses sufficient to induce CPP [14]. The development of conditioned partner preference at 2 and 2.5 g/kg mirrors the effects on CPP. The absence of a conditioned partner preference in response to 4 g/kg ethanol was not surprising, owing to severe intoxication in test females. However, it is notable that test females did not display a significant conditioned partner preference at the lowest ethanol dose (1 g/kg). It is possible that conditioning would develop in these animals after additional pairing with ethanol and saline [19].
While CPP has been used extensively to test reward with both drugs and natural reward (food, sex) [5], conditioned partner preference has only a limited history. In the present study, stimulus female mice were scented with baking extract to facilitate comparison with previous studies of conditioned partner preference induced by sexual behavior in rats [9, 10]. As in the present study, neutral odors (baking extracts) applied to the rat sexual partners served as the CS+ and CS−. In these earlier studies, rats of both sexes developed a conditioned partner preference for the CS+ odor cue paired with sexual reward. In particular, male rats also preferred to ejaculate with an unfamiliar female scented with the CS+ [10]. By contrast, OVX+E female mice in the present study did not show a similar generalization for novel stimulus females bearing the same scents. Our findings suggest that conditioned preference for the CS+ stimulus female represents individual recognition rather than a conditioned response to baking extract. This may reflect the relative stimulus intensity and reinforcing efficacy of ejaculation vs ethanol injection.
Experiment 2 explored the role of estradiol, and its potential interaction with ethanol in social preference. OVX+E females represent a model for adult cycling females, while avoiding fluctuation in ovarian steroid levels and the resulting effects on behavior. OVX females approximate the hormonal milieu before puberty and after reproductive senescence [20]. Notably, OVX females paired with ethanol did not form a conditioned partner preference for the CS+ stimulus female over the CS− female. Furthermore, total interaction was greater among OVX+E females than in OVX females. Estrogen is known to promote social interaction, as well as learning and memory, in mice [11, 21]. Estradiol itself is reinforcing in CPP [22]. Ovarian estrogen levels rise at puberty, and ethanol use in teen girls correlates with circulating estradiol levels [23]. Together, socially-induced ethanol consumption and ethanol-induced social preference in the presence of estradiol could promote ethanol dependence in females.
Ethanol and estradiol may work through separate mechanisms. On the other hand, studies of pair-bonding in voles have implicated central vasopressin systems, particularly the vasopressin V1a receptor, in affiliative behavior [24–26]. Both affiliative behavior and vasopressin are sensitive to estradiol and ethanol [24, 26, 27]. Therefore, ethanol and estradiol have potential to promote social preference through similar neurochemical systems. Other candidate mechanisms contributing to conditioned partner preference include oxytocin, dopamine acting via D2-type receptors, and serotonin. In this regard, oxytocin works in concert with vasopressin to facilitate partner preference in prairie voles [28], and oxytocin also contributes to same-sex social behavior in female meadow voles [29]. Oxytocin appears to act on the nucleus accumbens, where dopamine binding to D2-type receptors is essential for partner preference in female prairie voles [30]. Oxytocin may also promote social interaction by modifying serotonin release [31]. The role of these and other potential mechanisms remain to be explored in ethanol-induced social preference. However, it is noteworthy that oxytocin has recently been suggested as a possible modulator of drug addiction [32].
The present study adds to our understanding that sex steroids and sex differences modify non-reproductive behaviors, including affiliative behavior and responses to drugs and alcohol [33, 34]. Ethanol-induced conditioned partner preference in males or mixed-sex groups is harder to predict. Female mice will extensively investigate an unfamiliar conspecific, whereas male mice attack each other [26]. Furthermore, both testosterone and EtOH increase aggression in males [16, 35]. Even so, aggression is reinforcing, at least for the winner [36]. Thus, male mice might demonstrate ethanol-induced conditioned partner preference, despite also displaying aggression. Anecdotal observations of human males suggest that EtOH enhances social bonding among men [37], as it does in female mice. In a related study, when rats are tested for voluntary ethanol consumption in mixed-sex groups, the females consume significantly more than males, and subordinate males consume significantly more than dominant males [38]. This suggests that males may be more sensitive than females to low doses of EtOH. Indeed, a recent study observed that adolescent male mice form CPP in response to 1.25 g/kg ethanol, a dose that was ineffective in females [39].
Conditioned partner preference has potential for use in evaluating and comparing the social dimensions of many drugs of abuse. Existing animal models for drug reward, including self-administration, brain-stimulation reward and CPP, test individual animals in isolation. Thus, they do not take into account the role of social interactions in substance abuse [1]. While social components of ethanol consumption are well-documented, other drugs of abuse are also used in a social context. In particular, N-Methyl-3,4-methylenedioxyamphetamine (ecstasy) is commonly reported by users to promote a sense of social connection [40], while phencyclidine (PCP) reduces social interaction [41]. It would be of interest to contrast the rewarding effects of different drugs using conditioned partner preference and CPP to evaluate reward in social and non-social tests.
Highlights.
We tested effects of ethanol on conditioned partner preference in female mice to explore social dimensions of substance abuse.
Test females showed a conditioned preference for a stimulus female (CS+) paired with ethanol.
Conditioned partner preference was maintained if the CS+ female was also intoxicated.
Estrogen replacement in ovariectomized females facilitated ethanol-induced conditioned partner preference.
Acknowledgments
We thank Dr. Eleni Antzoulatos, Ms. Christina Zeitounsyan, and Mr. Christopher A. Ortega for assistance with experimental design and animal handling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ali MM, Dwyer DS. Social network effects in alcohol consumption among adolescents. Addict Behav. 2010;35(4):337–342. doi: 10.1016/j.addbeh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Vidal JM, Molina JC. Socially mediated alcohol presences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–241. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Källmén H, Gustafson R. Alcohol and disinhibition. Eur Addict Res. 1998;4(4):150–162. doi: 10.1159/000018948. [DOI] [PubMed] [Google Scholar]
- 5.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 6.Winslow JT. Mouse social recognition and preference. Curr Protoc Neurosci. 2003;(Unit 8.16) doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- 7.Paredes RG. Evaluating the neurobiology of sexual reward. Ilar Journal. 2009;50(1):15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Nash S, Domjan M. Learning to discriminate the sex of conspecifics in male Japanese quail (Coturnix coturnix japonica): Tests of “biological constraints”. J Exp Psychol Anim Behav Proc. 1991;17:342–353. doi: 10.1037//0097-7403.17.3.342. [DOI] [PubMed] [Google Scholar]
- 9.Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119(3):716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- 10.Kippin TE, Pfaus JG. The nature of the conditioned response mediating olfactory conditioned ejaculatory preference in the male rat. Behav Brain Res. 2001;122(1):11–24. doi: 10.1016/s0166-4328(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 11.Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30(4):442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama N, Crabbe JC, Ford MM, Murilloa A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broadbent J, Muccino KJ, Cunningham CL. ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- 14.Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology. 2008;201(1):97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17(3):185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 16.Barkley MS, Goldman BD. The effects of castration and Silastic implants of testosterone on intermale aggression in the mouse. Horm Behav. 1997;9:32–48. doi: 10.1016/0018-506x(77)90048-4. [DOI] [PubMed] [Google Scholar]
- 17.Jacob DA, Temple JL, Patisaul HB, Young LJ, Rissman EF. Coumestrol antagonizes neuroendocrine actions of estrogen via the estrogen receptor alpha. Exp Biol Med. 2001;226:301–306. doi: 10.1177/153537020122600406. [DOI] [PubMed] [Google Scholar]
- 18.Crabbe JC, Cameron AJ, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008;42:9.26.1–19. doi: 10.1002/0471142301.ns0926s42. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- 20.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: Current theories, hypotheses, and research models. Exp Biol Med. 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- 21.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27(2):217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Frye CA, Rhodes ME. Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res. 2006;1067(1):209–215. doi: 10.1016/j.brainres.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Martin CA, Mainous AG, 3rd, Curry T, Martin D. Alcohol use in adolescent females: correlates with estradiol and testosterone. Am J Addictions. 1999;8(1):9–14. doi: 10.1080/105504999306036. [DOI] [PubMed] [Google Scholar]
- 24.Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–414. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- 25.Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3(1):20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Takeo S, Tsujimoto G, Tanoue A. Alcohol preference in mice lacking the Avpr1a vasopressin receptor. Am J Physiol. 2008;294(5):R1482–1490. doi: 10.1152/ajpregu.00708.2007. [DOI] [PubMed] [Google Scholar]
- 28.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169(2):665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114(1):173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neuroscience. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61(3):331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J Physiol Sci. 2008;58(4):213–420. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- 34.Rajasingh J, Bord E, Qin G, Ii M, Silver M, Hamada H, Ahluwalia D, Goukassian D, Zhu Y, Losordo DW, Kishore R. Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology. 2007;148(8):3618–3624. doi: 10.1210/en.2006-1357. [DOI] [PubMed] [Google Scholar]
- 35.Chermack ST, Giancola PR. The relation between alcohol and aggression: an integrated biopsychosocial conceptualization. Clin Psychol Rev. 1997;17(6):621–649. doi: 10.1016/s0272-7358(97)00038-x. [DOI] [PubMed] [Google Scholar]
- 36.Fish EW, DeBold JF, Miczek KA. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology. 2005;182:116–127. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- 37.Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29(6):535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28(4):437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- 39.Roger-Sánchez C, Aguilar MA, Rodríguez-Arias M, Aragon CM, Miñarro J. Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotox Teratol. 2012;34:108–115. doi: 10.1016/j.ntt.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207(1):73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sams-Dodd F. Automation of the social interaction test by a video-tracking system: behavioural effects of repeated phencyclidine treatment. J Neurosci Meth. 1995;59(2):157–167. doi: 10.1016/0165-0270(94)00173-e. [DOI] [PubMed] [Google Scholar]