Abstract
The ventral medial prefrontal cortex (vmPFC) controls vulnerability to the negative effects of chronic or uncontrollable stress. Dominance status alters responses to social defeat in the conditioned defeat model, which is a model characterized by loss of territorial aggression and increased submissive and defensive behavior following an acute social defeat. We have previously shown that dominant individuals show a reduced conditioned defeat response and increased defeat-induced neural activation in the vmPFC compared to subordinates. Here, we tested the hypothesis that defeat-induced activation of the vmPFC is necessary to confer resistance to conditioned defeat in dominants. We paired weight-matched male Syrian hamsters (Mesocricetus auratus) in daily 5-min aggressive encounters for two weeks and identified dominants and subordinates. Twenty-four hours after the final pairing, animals were bilaterally injected with 200 nl of the GABAA receptor agonist muscimol (1.1nmol) or 200 nl of saline vehicle 5 minutes prior to social defeat. Defeat consisted of 3, 5-min encounters with resident aggressor hamsters at 10-min intervals. Twenty-four hours following social defeat, animals received conditioned defeat testing which involved a 5-min social interaction test with a non-aggressive intruder. Muscimol injection prior to social defeat prevented the reduced conditioned defeat response observed in vehicle-treated dominants. Further, there was no effect of muscimol injection on the conditioned defeat response in subordinates or controls. These data support the conclusion that activation of the vmPFC during social defeat is necessary for the protective effects of dominant social status on the acquisition of conditioned defeat.
Keywords: social defeat, resilience, social stress, dominance, anxiety, infralimbic cortex
1. Introduction
Animal models of stress have traditionally aimed to minimize genetic, physiological, and behavioral variability between individuals. While this approach has produced a wealth of data on the biological basis of the stress response, it has also created a gap in our understanding of the biological basis of individual differences. Variability in the effects of stress has been shown in humans as not all individuals who experience a traumatic event experience long-term negative consequences [1]. Thus, a growing number of researchers are focused on the mechanisms controlling individual coping styles and vulnerability to the negative consequences of stressful events.
One brain region implicated in resilience to stress is the medial prefrontal cortex (mPFC). The mPFC is a brain region known to be important for executive control, integrating information from multiple sensory modalities and guiding appropriate behavioral responses to stimuli. Human imaging studies show that the suppression of negative emotion is associated with increased mPFC activation [2, 3]. In humans, deficits in mPFC activation are associated with symptoms of depression [2] and anxiety disorders such as post-traumatic stress disorder [4, 5]. In animals, the mPFC acts as an inhibitory modulator of the emotional responses produced by aversive stimuli such as restraint [6], forced swim [7], and punished stimuli in tests such as the Vogel punished-licking test [8]. Additionally, the mPFC can inhibit the neuroendocrine stress response via a GABAergic relay that projects to the paraventricular nucleus through an indirect connection in the bed nucleus of the stria terminalis [9, 10]. Altogether, these findings indicate that the mPFC is an important source of inhibitory control over behavioral and neuroendocrine responses to stressors.
The ventral portion of the mPFC (vmPFC) has been studied in a variety of models that examine the mechanisms by which past experience alters susceptibility to future stressors. For example, the vmPFC plays a critical role in the stress-buffering effects of environmental enrichment. Mice housed in enriched environments prior to chronic social defeat stress show a reduction in the behavioral consequences of defeat, which include increased anxiety-like behavior on the light/dark test, tail suspension test, forced swim test, and social avoidance test compared to non-enriched mice [11]. Mice living in enriched housing conditions also show increased defeat-induced neural activation compared to non-enriched controls in both the prelimbic (PL) and infralimbic (IL) subregions of the vmPFC. Further, lesions of the IL prior to enriched housing abolished housing-associated resistance to social defeat stress [11]. A similar role for the vmPFC has been found in resistance associated with stressor controllability. Rats that are able to “escape” tail-shock by turning a wheel to terminate shock are protected against the negative effects of later inescapable tail-shock. Inescapable tail-shock produces escape deficits in the shuttle box test, an effect known as learned helplessness. Pharmacological inactivation of the vmPFC with the GABAA receptor agonist muscimol during the escapable tail-shocks blocks the protective effect of control against subsequent inescapable tail-shock [12]. Further, pharmacological activation of the vmPFC with the GABAA antagonist picrotoxin during inescapable tail-shock blocks the escape deficit produced by later inescapable tail-shock [13]. These pharmacological studies suggest that the resistance to learned helplessness provided by prior experience with controllable tail-shocks requires neural signaling in the vmPFC. Altogether, these models suggest that the vmPFC controls various forms of experience-dependent stress resistance.
Conditioned defeat is a model of social defeat stress in Syrian hamsters in which normal territorial aggression is replaced by submissive and defensive behavior in future social encounters following an acute social defeat [14–17]. We have recently extended the conditioned defeat model to study the effects of prior dominance status on the conditioned defeat response. Our previous work indicates that dominant individuals show less submissive and defensive behavior during conditioned defeat testing than do subordinates [18, 19]. Further, we found that dominant individuals have increased c-Fos immunoreactivity in the IL following social defeat stress compared to subordinates [19]. In the current study, we examined whether the increased defeat-induced neural activity in the vmPFC of dominant individuals is necessary for their resistance to conditioned defeat. We hypothesized that inactivation of the vmPFC prior to social defeat would increase submissive and defensive behavior of dominant individuals at testing but would not alter conditioned defeat in subordinates or controls.
2. Materials and Methods
2.1. Subjects
Subjects were male Syrian hamsters (Mesocricetus auratus) obtained from our breeding colony that was derived from Charles River Laboratories (Wilmington, MA) stock. Subjects were 8–9 weeks old (120–180 g) at the start of the study and were individually housed. Older hamsters (> 6 months old, >190 g) were individually housed and used as resident aggressors for social defeat training. Younger hamsters (approx. 2 months old, <120 g) were housed in groups of three or four and used as non-aggressive intruders for conditioned defeat testing. All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Food and water were available ad libitum. Cages were not changed for one week prior to dominant-subordinate encounters to allow individuals to scent mark their territory. Subjects were handled daily for a week prior to dominant-subordinate encounters to habituate them to the stress of human handling. Animals were housed in a temperature controlled colony room (21 ± 2 °C) and kept on a 14:10 hr light:dark cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Stereotaxic Surgery
Hamsters were anesthetized with isoflurane and stereotaxically implanted with a 26-gauge double-barrel cannula with 1 mm between each barrel and aimed at the IL subregion of the vmPFC. The stereotaxic coordinates were +3.6 mm anterior and −0.5 mm lateral to bregma, and −1.7 mm below dura. During microinjection, a 33-gauge double-barrel injection needle was inserted that projected 2 mm below the guide cannula for a final projection of 3.7 mm below dura. After surgery, dummy stylets that projected 0.5 mm below the guide cannulae were inserted into the cannulae to maintain patency. Animals were given 7 days to recover from surgery before dominant-subordinate encounters began and were handled daily.
2.3. Behavioral Protocols
2.3.1. Dominant-Subordinate Encounters
One week following surgery, animals were weight-matched in resident-intruder dyads and paired in daily aggressive encounters for 14 days (Fig. 1). Subjects were randomly assigned as a resident or intruder, and all encounters occurred in the resident’s home cage. The encounter on day 1 was 10 min in duration, while all subsequent encounters were 5 min. We have previously determined that a 10 min encounter on day 1 facilitates the formation of a dominance relationship, and that 5 min encounters on subsequent days maintain the dominance relationship and reduce the chance of wounding [18, 19]. Dominant and subordinate animals were identified by the direction of agonistic behavior within each dyad. In male Syrian hamsters, dominant animals reliably display aggression only, while subordinate animals reliably display submissive behavior only. Daily encounters were digitally recorded for later behavioral analysis. We quantified agonistic behavior during daily encounters using the ethogram described below for conditioned defeat testing. We also included empty cage controls, which were exposed to a clean, empty cage each day for seven days. We chose seven days because it is more analogous to our standard conditioned defeat protocol and 14 days of empty cage exposure may increase territorial aggression in controls [14, 16, 20].
Figure 1.
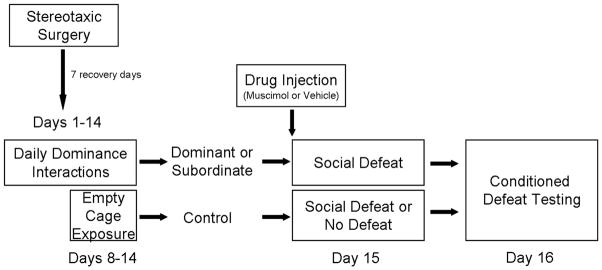
A schematic representation of the experimental design. All subjects received an injection of muscimol or saline vehicle into the vmPFC 5 min prior to social defeat or no defeat.
2.3.2. Social Defeat Training
Following two weeks of daily aggressive encounters or one week of empty cage exposure, all dominants and subordinates and half of the empty cage controls were socially defeated. They were placed in the home cages of three different resident aggressors for three separate 5-min social defeats, which occurred at 10-min intervals. Resident aggressors are older, heavier male hamsters that have been singly housed for a prolonged period of time and display reliable territorial aggression when faced with intruders. Dominants often fought back against the resident aggressor during the first defeat but eventually lost and did not fight back during subsequent defeats. To correct for potential variation in the amount of aggression subjects received, we defined social defeat as starting at the resident aggressor’s first attack that was accompanied by submissive behavior in the subject. We digitally recorded all social defeat sessions and quantified behavior using Noldus Observer software (Noldus Information Technology, Wageningen, Netherlands). Half of the empty cage controls were not socially defeated, but instead were placed in the empty home cages of three different resident aggressors for three separate 5-min exposures. We placed no defeat controls in dirty resident aggressor cages to control for the effect of exposure to olfactory cues that may impact behavior at testing. One subject from the dominant muscimol group was excluded from analysis due to wounds sustained during social defeat training, and one subject from the dominant vehicle group was excluded because he defeated two resident aggressors.
2.3.3. Conditioned Defeat Testing
Conditioned defeat testing occurred 24 hours after social defeat stress and consisted of a single 5-minute social interaction test during which a non-aggressive intruder was placed in the subject’s home cage. Non-aggressive intruders are younger, group-housed animals that display social and nonsocial behavior during conditioned defeat testing. We digitally recorded all testing sessions and quantified the behavior of subjects using Noldus Observer software. We quantified the total duration of the following categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend, head flag); aggressive (chase, attack including bite, upright and side offensive postures); nonagonistic social (sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding). We also recorded the frequency of attacks, flees, and stretch-attend postures. A researcher blind to the experimental conditions performed all behavioral scoring. On a subset of videos, inter-rater reliability on the duration of submissive/defensive behavior was greater than 90%, while inter-rater reliability using the more strict Cohen’s kappa measure was r = 0.62, which is good agreement. One subject from the dominant vehicle group was excluded from analysis because it was attacked during conditioned defeat testing.
2.4. Drugs and Drug Injection
The GABAA receptor agonist muscimol (Sigma Aldrich) was dissolved in sterile saline for a final concentration of 1.1 nmol in 200 nl. This dose was chosen because it has successfully been used to alter the acquisition and/or expression of conditioned defeat behavior in prior studies [21, 22]. All subjects were injected with either muscimol or vehicle 5 min prior to social defeat stress. Dummy stylets were removed and subjects were gently restrained while a bilateral injection needle was inserted into the cannula. A 1 μl syringe (Harvard Instruments) was used to connect the bilateral injection needle to a Harvard Syringe Pump (Harvard Instruments). Injection of 200 nl of muscimol or vehicle per side took place over a 1 min period, and needles were left in place for 1 min after the injection to allow for drug diffusion. One subject from the subordinate vehicle group and one subject from the dominant vehicle did not receive successful bilateral injections due to clogged cannulae and were excluded from analysis.
2.5. Verification of Injection Site
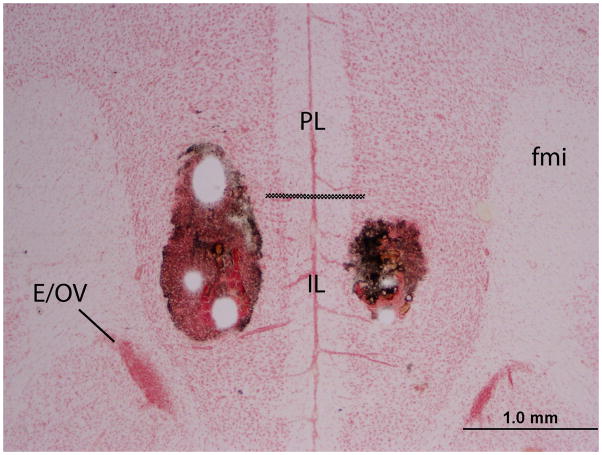
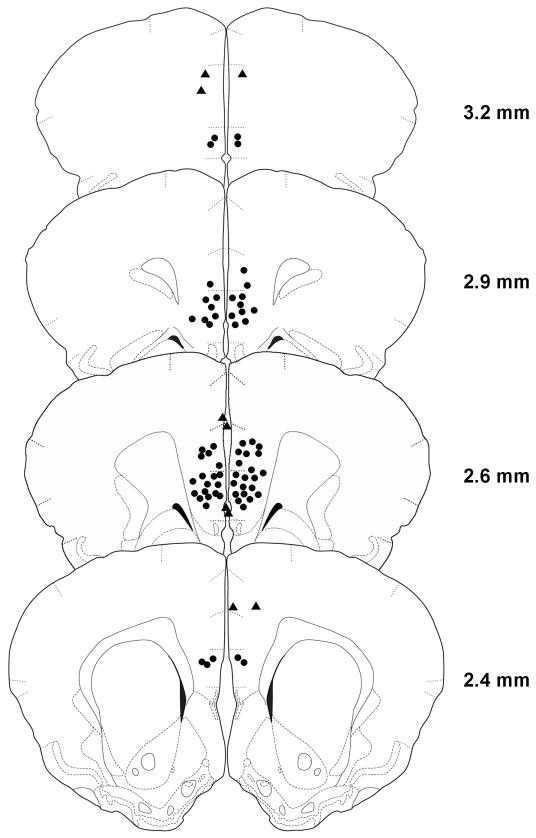
Following conditioned defeat testing, animals were given a lethal cocktail of 93% sodium pentobarbital and 7% isopropyl alcohol (Sleep Away, Webster Veterinary) and infused with 200 nl of India ink into the IL. Brains were then removed, frozen on dry ice, and stored at −80°C. Brains were sliced at 30 μm on a cryostat, and sections were stored on microscope slides. Sections were stained with neutral red and coverslipped prior to being examined under a light microscope for evidence of ink in the IL (Fig. 2). Subjects with bilateral injections sites > 300 μm from the IL or with injection sites between the two hemispheres were excluded from analysis (Fig. 3).
Figure 2.
A representative photomicrograph shows a coronal brain section of the vmPFC injected with India ink and stained with neutral red. The bilateral injection sites are clearly visible within the vmPFC. PL – prelimbic cortex, IL – infralimbic cortex, fmi – forceps minor corpus callosum, E/OV – ependyma & subependymal layer/olfactory ventricle. The dashed line represents the approximate division between the PL and IL.
Figure 3.
The location of vmPFC injection sites is shown using illustrations adapted from a hamster stereotaxic atlas [55]. The distances shown for each illustration are relative to bregma. Black circles indicate the approximate placement of injection sites within the vmPFC. Black triangles represent misplaced injection sites. Circles and triangles may represent more than one individual.
2.6. Data Analysis
Our experimental design included three independent variables (dominance status, drug dose, social defeat). Because we did not have a complete factorial design, we used one set of 2-way analysis of variance (ANOVA) tests to investigate dominance status (dominant, subordinate, empty cage control) and drug dose (vehicle vs. muscimol) and another set of 2-way ANOVAs to investigate social defeat (defeat vs. no defeat) and drug dose (vehicle vs. muscimol). We also used independent samples t-tests as planned comparisons to separately assess the effect of drug treatment on dominants, subordinates, and empty cage controls. The occurrence of counter attacking during social defeat training was analyzed using a Chi-square test. All statistical tests were two-tailed, and the α level was set at p ≤ 0.05.
3. Results
3.1. Dominant-Subordinate Encounters
Dominance relationships were formed quickly and were stable throughout the daily agonistic encounters. On average, a dominance relationship formed between a pair on day 1.9 (± 0.3). Subordinates displayed a greater duration of submissive/defensive behavior during agonistic encounters compared to dominants, which never showed submissive/defensive behavior (subordinates: 118.6 ± 18.7 sec per day, N = 24; dominants: 0.0 ± 0.0 sec per day, N = 22). Further, subordinates showed overt submissive/defensive behavior, such as fleeing, throughout the agonistic encounters (1.3 ± 0.3 flees per day). Dominants displayed a greater duration of aggressive behavior during daily agonistic encounters compared to subordinates (subordinates: 0.0 ± 0.0 sec per day, N = 24; dominants: 70.9 ± 14.2 sec per day, N = 22). Similarly, dominants attacked subordinates 1.8 (± 0.3) times per day, while subordinates never attacked dominants.
3.2. Social Defeat Training
There was no significant difference between the groups in the total amount of aggression received during social defeat (Table 1). However, there were effects of dominance status and of muscimol treatment on the behavior of subjects during social defeat. During the first defeat session, subjects responded differently to the initial attack of the resident aggressor. We found that 16 out of 22 dominant individuals counter attacked the resident aggressor, which is significantly more than the proportion of subordinates that counter attacked (0 out of 24 subordinates; χ2 (1, N = 46) = 26.8, p < 0.001) and the proportion of empty cage controls that counter attacked (3 out of 19 controls; χ2 (1, N = 41) = 13.3, p < 0.001). Additionally, the proportion of empty cage controls that counter attacked was significantly higher than the proportion of subordinates that counter attacked (χ2 (1, N = 41) = 4.1, p < 0.05). Muscimol treatment did not alter the proportion of dominants, subordinates, or empty cage controls that counter attacked resident aggressors (dominant vehicle: 9 out of 11; dominant muscimol: 7 out of 11; subordinate vehicle: 0 out of 11; subordinate muscimol: 0 out of 13; empty cage control vehicle: 1 out of 10; empty cage control muscimol: 2 out of 9). Also, there was an effect of both dominance status and muscimol treatment on the total amount of submissive and defensive behavior displayed during all three defeat sessions (Table 2). Specifically, muscimol treatment resulted in an increase in the display of submissive and defensive behavior compared to vehicle treatment (F(1,59) = 5.65, p = 0.021). Also, dominant individuals showed significantly less submissive and defensive behavior throughout social defeat training than did subordinates (F(2,59) = 5.17, p = 0.008; Tukey, p = 0.005; Table 2).
Table 1.
Total duration (mean sec ± SEM) of aggression received during social defeat training
| 0 nmol Muscimol | 1.1 nmol Muscimol | |
|---|---|---|
| Dominant | 435 ± 43 (N = 11) | 416 ± 47 (N =11) |
| Subordinate | 413 ± 28 (N = 11) | 457 ± 31 (N =13) |
| Empty Cage | 426 ± 24 (N = 10) | 464 ± 25 (N = 9) |
Table 2.
Total duration (mean sec ± SEM) of submissive behavior displayed during social defeat training
| 0 nmol Muscimol | 1.1 nmol Muscimol* | |
|---|---|---|
| Dominanta | 717 ± 37 (N = 11) | 747 ± 25 (N = 11) |
| Subordinateb | 797 ± 32 (N = 11) | 870 ± 43 (N = 13) |
| Empty Cagea,b | 721 ± 22 (N = 10) | 812 ± 19 (N = 9) |
Note: An asterisk (*) indicates a main effect of drug dose (p < 0.05). There was also a main effect of dominance status, as indicated by unshared superscript letters (p < 0.05).
3.3. Conditioned Defeat Testing
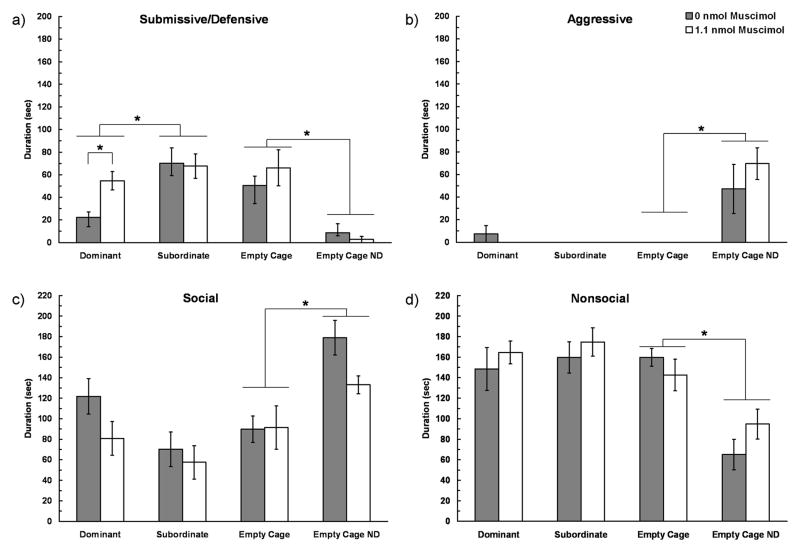
We found that injection of muscimol into the vmPFC prior to social defeat training increased the acquisition of conditioned defeat in dominants but not in subordinates or controls (Fig. 4). Among defeated individuals, there was a main effect of dominance status on the duration of submissive/defensive behavior (F(2,59) = 4.35, p = 0.017). Specifically, dominant individuals showed significantly less submissive/defensive behavior than did subordinates (Tukey, p = 0.014). Further, analysis with independent samples t-tests showed that vehicle-treated dominants displayed significantly less submissive/defensive behavior at testing than did muscimol-treated dominants (t(1,20) = 3.42, p = 0.003). However, muscimol treatment did not alter the duration of submissive/defensive behavior in either subordinates or empty cage controls compared to vehicle treatment (p > 0.05). Among defeated individuals, we found that there was no effect of status or muscimol treatment on the duration of aggressive, social, or nonsocial behavior displayed at testing (p > 0.05, Fig. 4b–d).
Figure 4.
Mean durations (sec ± SEM) of a) submissive/defensive, b) aggressive, c) nonagonistic social and d) nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Social defeat animals received an injection of muscimol (dominant, N = 11; subordinate, N = 13; empty cage control, N = 9) or vehicle (dominant, N = 11; subordinate, N = 11; empty cage control, N = 10) into the vmPFC 5 min prior to social defeat training. Empty cage no defeat (ND) controls received an injection of muscimol (N = 10) or vehicle (N = 9) into the vmPFC 5 min prior to the no defeat procedure. An asterisk (*) indicates a difference of p < 0.05 between the bracketed bars.
To analyze the effect of drug treatment on agonistic behavior, we included empty cage control subjects that were not socially defeated. Among empty cage control individuals, there was a main effect of defeat experience on submissive/defensive behavior (F(1,34) = 29.90, p < 0.0001), such that defeated individuals displayed significantly more submissive/defensive behavior at testing than did no defeat controls (Fig. 4a). There was not a main effect of muscimol treatment or an interaction of muscimol treatment x defeat experience on the duration of submissive/defensive behavior at testing among empty cage control subjects (p > 0.05). Additionally, we found a main effect of social defeat on the duration of aggressive behavior (F(1,34) = 21.02, p < 0.0001), the duration of social behavior (F(1,34) = 18.59, p < 0.0001), and the duration of nonsocial behavior (F(1,34) = 27.84, p < 0.0001). Thus, defeated empty cage control individuals displayed less aggressive, less social, more nonsocial and more submissive/defensive behavior than did non-defeated empty cage control individuals.
4. Discussion
We have shown that inactivation of the vmPFC with the GABAA receptor agonist muscimol during social defeat results in an increase in submissive and defensive behavior at testing in dominants only. Importantly, the effect of muscimol in dominants was specific to the duration of submissive and defensive behavior. This suggests that inactivation of the vmPFC does not increase submissive and defensive behavior by non-selectively altering other types of social or nonsocial behavior. Additionally, muscimol treatment at social defeat training does not alter submissive and defensive behavior in no-defeat controls at testing, indicating that inactivation of the vmPFC does not interfere with the production of agonistic behavior in the absence of social defeat. Further, the effect of muscimol treatment in dominants was not a result of a differential social defeat experience, as muscimol treatment prior to social defeat did not alter the amount of aggression received by subjects. While muscimol treatment did not affect the behavior of resident aggressors, muscimol treatment increased the submissive and defensive behavior displayed by subjects during social defeat. However, this muscimol-dependent increase in submissiveness during social defeat was not associated with a universal increase in conditioned defeat. Only dominant individuals showed an increased conditioned defeat response. Altogether, these results suggest that neural activity in the vmPFC during social defeat is necessary for dominants to show a resistant-like behavioral phenotype at testing.
While inactivation of the vmPFC during social defeat altered the conditioned defeat response in dominants, it did not affect the initial coping style that they employed during social defeat, as indicated by whether or not they counter attacked the resident aggressor. Specifically, a similar rate of counter attacking was observed in both vehicle-treated and muscimol-treated dominants as well as in subordinates and empty cage controls. In this and other studies, we have found that dominant individuals reliably counter attack the resident aggressor during social defeat [14, 18]. The occurrence of counter aggression may indicate that dominants are displaying a proactive coping style, as offensive aggression is associated with proactive coping on a variety of measures in rats and mice [23]. In previous studies, coping style has been found to predict behavioral responses to future stressors. For example, in rats, the use of a proactive coping style during social defeat leads to reduced negative effects of social defeat, including decreased conditioned fear when exposed to the social defeat arena 24 hours later [24]. Our current findings suggest that counter aggression in the absence of vmPFC activation is not sufficient to produce resistance to social defeat in hamsters. It is possible that both counter attacking and level of submissive and defensive behavior at testing are independent characteristics of the resistance phenotype seen in dominant individuals, and are not causally related.
In our previous study, dominants showed increased neural activation in the IL subregion of the vmPFC following social defeat compared to subordinates [19]. In the same study, we found a similar, albeit nonsignificant, trend in the PL, making it unclear whether resistance to conditioned defeat is mediated by the IL or PL. While our stereotaxic coordinates were aimed at the IL in the current study, we cannot say with certainty that the drug stayed within the boundaries of the IL. When we excluded animals with drug injections into the PL, our main findings remained unchanged such that muscimol increased conditioned defeat in dominant animals only (data not shown). However, we did not have enough injections directly into the PL to analyze these animals separately. Altogether, these findings suggest that the IL mediates resistance to conditioned defeat in dominant hamsters.
Anatomical studies point to distinct functional connections for the vmPFC subregions. Studies examining afferent and efferent projections suggest that the IL projects to and receives input from brain regions that are important in visceral and motor control, while the PL projects to and receives input from regions that are more important in directly controlling limbic and cortical functions [25–27]. Importantly, both the IL and PL send efferent projections to the basolateral complex of the amygdala (BLA) where they differentially modulate conditioned fear. Electrical stimulation of the PL has been shown to increase the expression of conditioned fear [28], whereas electrical stimulation of the IL reduces conditioned fear [29]. Also, brain-derived neurotrophic factor (BDNF) signaling in the PL is necessary for the acquisition and consolidation of fear memories [30]. On the other hand, pharmacological inactivation of the IL, but not the PL, impairs extinction memory [31]. The vmPFC is also a critical neural substrate for stress resistance in models using environmental enrichment [11] and stressor controllability [12]. Voluntary wheel running also protects individuals against the anxiety-like consequences of uncontrollable stress [32–34], and the vmPFC is a likely candidate for mediating the exercise-induced resistance to stress [35]. Environmental enrichment in mice appears to confer resistance to future social defeat through activity in the IL. Lesions of the IL, but not the PL, prior to housing in an enriched environment block the stress resiliency conferred by enrichment [11]. In contrast, neural activity in the PL appears critical for stress resistance conferred by controllable shock exposure. For example, controllable shock selectively activates a PL-dorsal raphe nucleus (DRN) circuit [36] and increases the excitability of PL pyramidal neurons [37].
While there are similarities in the brain regions involved in the neural circuitry of conditioned defeat and learned helplessness, neural activation patterns and hypothesized locations of neural plasticity differ. A key node in the acquisition of learned helplessness is the DRN. While the vmPFC mediates resistance to learned helplessness, it is thought to do so by altering neural plasticity within the DRN [38], although recent work suggests that neural plasticity in the vmPFC may also contribute to resistance [37]. In conditioned defeat, activation of serotonin neurons in the DRN is necessary for both the acquisition and expression of the conditioned defeat response [39]. Further, we have shown that social defeat decreases 5-HT1A mRNA in the DRN, indicating a role for defeat-induced plasticity in the DRN in the conditioned defeat response [20]. The vmPFC sends glutamatergic projections to GABAergic interneurons in the DRN [40, 41], and it is possible that dominant individuals have enhanced inhibition of the DRN and thus decreased 5-HT release into forebrain regions. Additionally, defeat-induced neural activation in brain regions important for the analysis of social information, such as the medial amygdala (MeA) [22], and regulation of aggressive behavior, such as the lateral ventromedial hypothalamus (VMHL) [42, 43], has been associated with conditioned defeat resistance [19]. Resistance to learned helplessness does not involve social information or social behavior, and no role has been found for brain regions such as the MeA and VMHL. In contrast, brain regions important for producing escape behavior, such as the dorsal straitum [44], are critical parts of the neural circuitry controlling resistance to learned helplessness, although their role in conditioned defeat is unknown.
The BLA is a key region for the neural plasticity underlying conditioned defeat [15, 45–47]. Both subregions of the vmPFC project to the amygdala and the vmPFC is capable of inhibiting amygdala output [48]. This inhibition is thought to be mediated through activation of BLA GABAergic interneurons, which would cause a decrease in spontaneous firing and a decrease in responsiveness to other inputs [49]. There is also evidence for other possible mechanisms controlling vmPFC-BLA signaling. For example, the vmPFC might inhibit the output of BLA neurons by activating the GABAergic neurons within the intercalated cell mass of the amygdala [48, 50]. One possible mechanism of resistance to conditioned defeat is that increased activity in the vmPFC of dominants during social defeat decreases BLA activity and perhaps disrupts the defeat-induced neural plasticity that supports the acquisition of conditioned defeat. Alternatively, increased vmPFC activation may lead to the facilitation of the extinction of conditioned defeat in dominant hamsters. As mentioned earlier, neural activation within the IL is known to be critical for the extinction of fear memories [31, 51–53], and this process may be facilitated in dominant individuals.
In the current study, inactivation of the vmPFC during social defeat had no effect on the acquisition of conditioned defeat in either subordinates or control subjects. This indicates that activation of the vmPFC during social defeat is not necessary for species-typical conditioned defeat. Our results are in contrast with a recent study showing that inactivation of the vmPFC with muscimol during social defeat significantly enhanced the acquisition of conditioned defeat [54]. It is not initially clear why these two studies are not in agreement about the role of the vmPFC in conditioned defeat, as there were only minor differences in the drug dose and intensity of social defeat. In both studies, subjects were exposed to social defeat for a total of 15 min. However, Markham and colleagues used a single defeat experience with one resident aggressor and we used three, 5 min exposures to three different resident aggressors. It is possible that our subjects experience a more intense social defeat, and this could have resulted in a ceiling effect on the duration of submissive and defensive behavior at testing. However, in previous studies, acute social defeat has produced durations of submissive and defensive behavior at testing greater than 100 sec, indicating that a ceiling effect is unlikely [15, 16, 18, 39]. The most apparent difference is that Markham et al. (2012) delivered 2.2 nmol of muscimol into one side of the vmPFC, while we delivered 1.1 nmol of muscimol into each side. It is possible that the dose used in our study was not high enough to alter conditioned defeat in our subordinates and controls, and that dominants are more sensitive to lower doses of muscimol treatment. Another important finding is that delivery of the protein synthesis inhibitor anisomycin into the vmPFC prior to social defeat does not alter the acquisition of conditioned defeat [54]. This finding indicates that the vmPFC does not directly alter the acquisition of conditioned defeat, but that activation of the vmPFC may alter defeat-induced neural plasticity elsewhere in the brain.
Conclusion
We found that pharmacological blockade of the vmPFC prior to social defeat increases the acquisition of conditioned defeat in dominant hamsters only. These data indicate that defeat-induced activation of the vmPFC is necessary for the protective effects of dominant social status on the development of conditioned defeat. Because our data indicate a critical role for the IL, dominance status might lead to resiliency by facilitating the extinction of stress-induced changes in social behavior.
Highlights.
Dominant individuals show reduced conditioned defeat compared to subordinates.
Blockade of vmPFC activation during social defeat blocks resistance to defeat.
Blockade of vmPFC activation during social defeat does not affect coping style.
Acknowledgments
We thank our team of undergraduate students, most notably Travis Goode and Jenna Hagermaker. This work was supported by NIH grant R21 MH098190 and a University of Tennessee Professional Development Award.
Abbreviations
- 5-HT
serotonin
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- DRN
dorsal raphe nucleus
- IL
infralimbic cortex
- mPFC
medial prefrontal cortex
- PL
prelimbic cortex
- vmPFC
ventral medial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agaibi CE, Wilson JP. Trauma, PTSD, and resilience: a review of the literature. Trauma Violence and Abuse. 2005;6:195–216. doi: 10.1177/1524838005277438. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research. 2008;167:171–86. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 6.Blanco E, Castilla-Ortega E, Miranda R, Begega A, Aguirre JA, Arias JL, et al. Effects of medial prefrontal cortex lesions on anxiety-like behaviour in restrained and non-restrained rats. Behavioural Brain Research. 2009;201:338–42. doi: 10.1016/j.bbr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Scopinho AA, Scopinho M, Lisboa SF, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behavioural Brain Research. 2010;214:437–42. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Resstel LB, Souza RF, Guimaraes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav. 2008;93:200–5. doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. Journal of Neuroscience. 2009;29:7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. Journal of Comparative Neurology. 2005;481:363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. The Journal of Neuroscience. 2011;31:6159–73. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. The Journal of Neuroscience. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–86. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison KE, Cooper MA. A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacol Biochem Behav. 2012;100:592–600. doi: 10.1016/j.pbb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behavioural Brain Research. 2011;217:55–9. doi: 10.1016/j.bbr.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper MA, Huhman KL. Blocking corticotropin-release factor-2 receptors, but not corticotropin-releasing factor-1 receptors of glucocorticoid feedback, disrupts the development of conditioned defeat. Physiol Behav. 2010;101:527–32. doi: 10.1016/j.physbeh.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, et al. Conditioned defeat in male and female syrian hamsters. Hormones and Behavior. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Morrison KE, Swallows CL, Cooper MA. Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiol Behav. 2011;104:283–90. doi: 10.1016/j.physbeh.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience. 2012;210:168–78. doi: 10.1016/j.neuroscience.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–90. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behavioural Brain Research. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learning and Memory. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Frontiers in Neuroendocrinology. 2010;31:307–21. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Walker FR, Hinwood M, Masters L, Deilenberg RA, Day TA. Individual differences predict susceptibility to conditioned fear arising from psychosocial trauma. Journal of Psychiatric Research. 2008;42:371–83. doi: 10.1016/j.jpsychires.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 26.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 27.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 28.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning and Memory. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral Neuroscience. 2004;118:389–94. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 30.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–80. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 2011;39:140–9. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European Journal of Neuroscience. 2009;20:1111–6. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. Journal of Neuroscience. 2012;32:12848–53. doi: 10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Research. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–47. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. Journal of Comparative Neurology. 2004;468:518–29. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- 41.Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 42.Delville Y, Mansour K, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–6. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 43.Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav. 1996;60:25–9. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- 44.Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, et al. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–44. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–34. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behavioral Neuroscience. 2005;119:1125–30. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- 47.Markham CA, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learning and Memory. 2010;17:109–16. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. The Journal of Neuroscience. 2005;25:7429–37. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiology & Behavior. 2002;77:489–93. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- 50.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–53. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learning and Memory. 2010;17:591–9. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 54.Markham CM, Luckett CA, Huhman KL. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology. 2012;62:933–9. doi: 10.1016/j.neuropharm.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego: Academic Press; 2001. [Google Scholar]