Abstract
Background
Mood stabilizers used for treating bipolar disorder (BD) selectively downregulate arachidonic acid (AA) turnover (deacylation-reacylation) in brain phospholipids, when given chronically to rats. In vitro studies suggest that one of these, valproic acid (VPA), which is teratogenic, reduces AA turnover by inhibiting the brain acyl-CoA synthetase (Acsl)-4 mediated acylation of AA to AA-CoA. We tested whether non-teratogenic VPA analogues might also inhibit Acsl-4 catalyzed acylation, and thus have potential anti-BD action.
Methods
Rat Acsl4-flag protein was expressed in E. coli, and the ability of three VPA analogues, propylisopropylacetic acid (PIA), propylisopropylacetamide (PID) and N-methyl-2,2,3,3-tetramethylcyclopropanecarboxamide (MTMCD), and of sodium butyrate, to inhibit conversion of AA to AA-CoA by Acsl4 was quantified using Michaelis-Menten kinetics.
Results
Acsl4-mediated conversion of AA to AA-CoA in vitro was inhibited uncompetitively by PIA, with a Ki of 11.4 mM compared to a published Ki of 25 mM for VPA, while PID, MTMCD and sodium butyrate had no inhibitory effect.
Conclusions
PIA's ability to inhibit conversion of AA to AA-CoA by Acsl4 in vitro suggests that, like VPA, PIA may reduce AA turnover in brain phospholipids in unanesthetized rats, and if so, may be effective as a non-teratogenic mood stabilizer in BD patients.
Keywords: bipolar disorder; valproate; arachidonic acid; acyl-CoA synthetase 4; mood stabilizer; Acsl4; brain; MTMCD; N-methyl-2,2,3,3-tetramethylcyclopropanecarboxamide; PIA; propylisopropylacetic; PID; propylisopropylacetamide; rat; butyrate; inhibition; uncompetitive; enzyme; anticonvulsant
Introduction
Valproic acid (VPA; 2-propylpentanoic acid; di-n-propylacetic acid, Figure 1), an eight-carbon, branched side-chain dicarboxylic acid, is an anticonvulsant that also is FDA-approved to treat bipolar disorder (BD) [1, 2]. However, VPA can produce unwanted clinical side effects, including hepatotoxicity, weight gain and metabolic disturbances [3–7]. It also is teratogenic, because it inhibits the chromatin-modifying enzyme, histone deacetylase [8, 9]. As such, it poses a significant fetal risk in pregnant women taking the drug [10], thus justifying the need for a non-teratogenic yet equipotent mood-stabilizer that may act by the same mechanism as VPA. Identifying a pharmacological brain target of VPA with regard to BD could lead to the rational development of effective VPA-like compounds with fewer side effects, including teratogenicity.
Figure 1.
Structures of VPA, PIA, PID and MTMCD.
One suggested target of VPA, as well as of the other FDA-approved mood stabilizers, lithium, carbamazepine and lamotrigine, is the brain arachidonic acid (AA, 20:4n–6) cascade [11–13]. This suggestion is based on evidence that VPA as well as the other mood stabilizers, when given chronically to rats to produce therapeutically relevant plasma concentrations, downregulate markers of the brain AA cascade [11–13]. Since markers of the cascade are upregulated in the postmortem BD brain, in association with excitotoxicity, neuroinflammation, apoptosis and synaptic loss [14–16], dampening by the drugs of the brain AA cascade may contribute to their efficacy in BD [12, 13].
AA can be released from membrane phospholipid by an AA-selective calcium-dependent cytosolic phospholipase A2 (cPLA2) in response to excitotoxicity or inflammation associated with microglial activation and increased cytokine production [17–21], and these neuropathological processes are found in BD [14–16]. AA also is liberated as a second messenger at post-synaptic neuronal membranes during neurotransmission via dopaminergic D2 receptors, muscarinic M1,3,5, serotonergic 5-HT2A/2C and glutamatergic N-methyl-D-aspartate receptors, all of which are coupled to cPLA2. Neurotransmission involving these receptors is disturbed in BD [13, 22–24]. After being hydrolyzed from the stereospecifically number-2 position of membrane phospholipid by a PLA2, a portion of the released AA is converted into pro-inflammatory lipid mediators including prostaglandin (PG)E2 and multiple other bioactive metabolites [11, 25], whereas the majority (~97%) is reincorporated into phospholipid via the serial actions of Acsl and acyltransferase.
When given chronically to rats to produce therapeutically relevant plasma levels, lithium and carbamazepine, in addition to VPA, downregulated turnover (deacylation-reacylation [26]) of AA but not of docosahexaenoic acid (DHA, 22:6n-6) or palmitic acid (16:0) in brain phospholipid [27–30]. Downregulation of AA turnover by lithium and carbamazepine was associated with decreased brain expression of cPLA2 IVA via reduced activity of one of its transcription factor, activator protein-2. Chronic VPA did not affect this enzyme or transcription factor, but its effect has been ascribed to uncompetitive inhibition of brain acyl-CoA synthetase (Acsl, long-chain-fatty-acid--CoA ligase, E.C.6.2.1.3) 4, which preferentially converts unesterified AA to acyl-CoA compared to other long chain fatty acids, palmitic acid or DHA [31–33]. This was demonstrated by kinetic studies on a rat brain microsomal fraction, and by using recombinant Acsl4. Rat tissue contains at least 5 ACSL genes (ACSL1, ACSL3, ACSL4, ACSL5 and ACSL6v1 and ACSL6v2 splice variants) [34], and the protein product of ACSL4, Acsl4, preferentially acylates AA [32, 33] and is found in cell mitochondria, peroxisomes, microsomes and endoplasmic reticulum (http://www.genecards.org/cgi-bin/carddisp.pl?gene=ACSL4&search=ACSL4). Acsl4 is the rate-limiting enzyme that regulates AA reincorporation into brain phospholipid within the AA deacylation-reacylation cycle [35, 36].
ACSL4 is highly expressed in newborn and adult mouse brain, especially in granule cells of the dentate gyrus and the pyramidal cell layer of CA1 in the hippocampus, and the granular cell layer and Purkinje cells of the cerebellum [37]. Additionally, a deficiency of the ACSL4 gene has been associated with X-linked mental retardation, microcephaly and other congenital malformations in humans [38, 39]. The Alport syndrome with intellectual disability is a contiguous gene deletion syndrome involving several genes on Xq22.3 including ACSL4 [40].
Using recombinant plasmids for the main ACSL's found in rat brain (ACSL3, ACSL4, ACSL6v1 and ACSL6v2), we reported that VPA selectively and uncompetitively inhibited incorporation of AA into AA-CoA by Acsl4 [32]. VPA did not equally reduce activation of palmitate or DHA to their acyl-CoAs, consistent with observations on rat brain microsomal extracts [31, 41]. There also was no inhibitory effect of lithium on AA conversion to AA-CoA [32].
In view of VPA's clinical teratogenic and hepatotoxic side-effects (see above), and of evidence that it reduces AA turnover in rat brain in vivo and uncompetitively inhibits recombinant Acsl4 in vitro, we thought it of interest to test whether non-teratogenic VPA structural analogues also would inhibit conversion of AA to AA-CoA by Acsl4 in vitro, as potential new agents with fewer side effects than VPA for treating BD. To do this, we used in vitro Michaelis-Menten kinetics to test inhibition of Acsl4 by the VPA analogues, propylisopropylacetic acid (PIA, 2-isopropylpentanoic acid), propylisopropylacetamide (PID), and N-methyl-2,2,3,3-tetramethylcyclopropanecarboxamide (MTMCD) (Figure 1). They were chosen because they do not inhibit histone deacetylase at relevant clinical doses tested in mice [42] and should not be teratogenic [43, 44], and because their published pharmacokinetic and anticonvulsant profiles suggest in vivo bioactivity and brain penetration [43, 45]. Each has eight carbon atoms in its chemical structure, like VPA. PID is an amide derivative, MTMCD is an amide cyclopropyl derivative, and PIA is a constitutional isomer of VPA (Figure 1). We also used sodium butyrate as a negative control. Butyrate is a 4-carbon analog of VPA that does inhibit histone deacetylase [42].
Briefly, we found that Acsl4-mediated conversion of AA to AA-CoA was inhibited uncompetitively by PIA, with a inhibitory constant Ki less than reported for VPA [32]. PID, MTMCD or butyrate had no inhibitory action. An abstract of part of this work has been published [46].
Materials and Methods
Reagents
[1-14C]AA (50 mCi/mmol) was purchased from Moravek Biochemicals (Brea, CA). Unlabeled AA, sodium butyrate, coenzyme A, and ATP were purchased from Sigma (St. Louis, MO). Racemic PIA was obtained from the National Institute of Mental Health's Chemical Synthesis and Drug Supply Program (Research Triangle Park, NC). PID and MTMCD were synthesized according to published procedures [47].
Preparation of bacterial lysate
Recombinant plasmids for rat liver ACSL4-Flag were expressed in E. coli strain BL21-codonPlus (DE3)-RIL [48]. As a negative control, the same strain, transformed with the empty vector, was used under identical conditions. Recombinant Acsl-Flag proteins were induced with 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) at A600=1.0. E. coli were grown in Terrific Broth medium supplemented with carbenicillin (final concentration 50 μg/ml) at 37°C and shaken at 206 rpm for 6 h. Cells were harvested by centrifugation at 4000 g for 20 min in a Sorval (Newton, CT) SA-600 rotor at 4°C after the 6-h induction period. The cell pellet was resuspended in a buffer containing 10 mM HEPES (pH 7.8) and 0.5 mM EDTA, and sonicated on ice with six 10-s bursts each followed by a 10-s rest, using a cell disruptor sonicator (Heat Systems Ultrasonics, Farmingdale, NY) at setting 4. Lysate aliquots were stored at −80°C for enzyme assay. Protein concentrations were determined by the Bradford method [49].
As reported earlier [32], we demonstrated using Western blotting and a specific anti-Flag M2 monoclonal antibody, that the enzyme preparation that we are studying was a single Acsl 4 isoenzyme, whereas the empty control showed no immunostaining.
Acsl4 activity assay
Acsl4 activity was measured using 1–3 μg protein as previously described [32]. The assay medium contained 175 mM Tris-HCl pH 7.4, 8 mM MgCl2, 5 mM dithiothreitol, 10 mM ATP, 0.25 mM CoA, 0.01 mM EDTA, and 5 μM [14C]AA in 0.5 mM Triton X-100, and increasing concentrations of unlabeled AA in a total volume of 200 μl. PIA (0, 5, 10 or15 mM in ethanol), PID (10 mM in water) or MTMCD (10 mM in water), was added directly to the reaction mixture during inhibition assays. The drug controls consisted of the respective vehicle without the drug. As an additional negative control, sodium butyrate (a short-chain VPA analog) was added to the reaction mixture at 60 mM [32]. Assays were performed at 37°C for 5 min with shaking. The reaction was started by adding 15 μl bacterial lysate to the reaction mixture, and was terminated by adding 1 ml Dole's Reagent (isopropanol:heptane:1M H2SO4, 80:20:2, by vol). In a preliminary experiment, the pH of reaction mixtures spiked with VPA and sodium butyrate at concentrations of 60 mM was measured using a pH meter. The pH (7.4) remained constant at these drug concentrations.
Unesterified fatty acids were extracted using two 2-ml heptane washes, and acyl-CoA radioactivity was measured by liquid scintillation counting. As a negative control, Acsl enzyme activity of the E. coli cell lysate lacking a gene coding for ACSL-Flag was measured with AA as substrate as described above. The results were corrected for blanks (samples without cell lysates added and samples analyzed in the absence of fatty acids). The negative control (empty vector) activity were compared with Acsl4 to make sure that the signal to noise ratio was adequate between the test and negative control at each concentration of AA.
Analysis and Statistics
Initial reaction velocity V was plotted against AA concentration for each PIA analogue concentration Io, and the plots were fitted by least squares to a hyperbolic Michaelis–Menten model using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Km (μM) and Vmax (nmol/min/mg protein) were calculated by the following equation, in which V is reaction velocity (nmol acyl-CoA formed/min/mg enzyme protein, e.g. nmol/min/mg protein) at a given AA substrate concentration, S (μM)
| (1) |
The model in which the substrate (i.e. AA) inhibits the reaction velocity can be described as [50],
| (2) |
A model that involves both substrate inhibition and uncompetitive inhibition by the inhibitor Io can be represented as,
| (3) |
where Ki is the enzyme inhibition constant.
Data were plotted as a function of inhibitor concentration Io, for PIA, PID and MTMCD or sodium butyrate, and the enzyme inhibition constant (Ki) was derived from the ascending part of the plot. Lineweaver–Burke plots of 1/V vs. 1/S in the presence of different inhibitor concentrations were plotted [50].
Selection of model
To determine which inhibition model best described the data, we utilized the Akaike Information Criterion (AIC) [51],
| (4) |
where k = number of parameters and L = maximized value of the likelihood function of the model. For small sample sizes, the AIC is corrected and is given as AICc [52],
| (5) |
where ss is the sum of squares from the fit, N is the number of experimental observations and K is the number of parameters in the model. As the goodness of fit of a model to the measured data improves, the value of AIC declines. Therefore, AICc is a formal method to evaluate model quality and simplicity.
The probability that the model is correct can be determined by the following equation, where Δ is the difference between AIC scores [52]
| (6) |
For this study with AA as a substrate, the lowest AICc was found for the “uncompetitive inhibition” model, as reported for VPA [32, 50].
Data are presented as mean ± S.D. Linear regression analyses for obtaining Km, Vmax, Ki and other parameters were made using GraphPad Prism Version 5.0 (GraphPad Software,).
Results
As previously described, Acsl4-mediated conversion of AA to AA-CoA showed substrate inhibition [32]. The kinetics of the Acsl4-mediated reactions using AA as a substrate without an inhibitor followed a simple Michaelis-Menten model, with pooled mean Km and Vmax of 4.12 ± 0.56 μM, and 132.6 ± 8.81 nmol/min/mg (n = 3) respectively, among the different experiments. These values are comparable to previously reported means of 4.98 ± 1.41 μM and 143 ± 11.1 nmol/min/mg, respectively, for Acsl4 [32].
PIA inhibited AA to AA-CoA conversion by Acsl4 with a Ki of 11.44 ± 1.28 mM (n = 3) (Figure 2A). When calculating the Lineweaver-Burke plots in Figure 2B, we considered substrate AA concentrations only in the rising phase of the V vs. [AA] curves, from 0 to 35 μM AA, since at higher AA concentrations the enzyme showed substrate inhibition (Figure 2A). Inhibition by PIA, determined by graphical analysis of the Lineweaver-Burke plots showing parallel slopes, was consistent with an uncompetitive inhibition mechanism (Figure 2B) [50]. The difference between AICc values for the uncompetitive and noncompetitive enzyme inhibition models was 3.491 (Eq. 5), which means that the probability that the uncompetitive model was correct, was 85%, compared to 15% for the noncompetitive model (Eq. 6).
Figure 2.
(2A) Initial reaction velocity (V, nmol/min/mg protein) of Acsl-4 plotted against increasing AA concentration [S] in the presence of 0, 5, 10, or 15 mM PIA [I]. Empty vector contains no Acsl enzyme, and shows no activity.
(2B) Typical Lineweaver-Burke plot of the reciprocal of enzyme activity (1/V) against the inverse of substrate concentration, 1/[S] (1/[AA]), with AA concentration range limited to from 0 to 35 μM (see Results). The plot is typical of 3 experiments as indicated in text. Parallel plots are characteristic of uncompetitive inhibition [50] put in correct reference
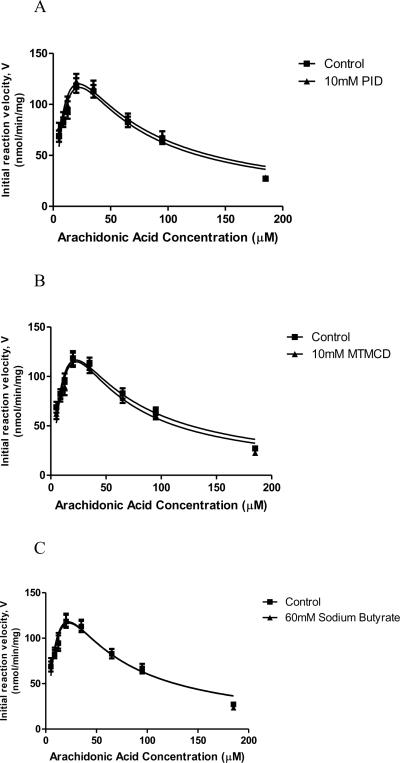
PID and MTMCD did not inhibit Acsl4-mediated conversion of AA to AA-CoA, with inhibitor concentrations as high as 10 mM (Figures 3A and 3B). As an additional control, we measured Acsl4 activity in the presence of sodium butyrate, the 4-carbon analog of VPA that also inhibits histone deacetylase [42]. As reported [32], sodium butyrate did not inhibit Acsl4 activity at a concentration of 60 mM (Figure 3C).
Figure 3.
Initial reaction velocity V of Acsl-4 plotted against increasing AA concentration [S] in the presence of (3A) 0 mM and 10 mM PID, (3B) 0 mM and 10 mM MTMCD, and (3C) 0 mM and 60 mM sodium butyrate.
Discussion
We examined inhibition of the conversion of AA to AA-CoA by rat recombinant Acsl4 in vitro by each of three non-teratogenic VPA analogues, PIA, PID and MTMCD, and of sodium butyrate, a 4-carbon teratogenic analogue, using our previously published method [53]. Similar to VPA, PIA inhibited Acsl4 conversion by an uncompetitive acylation mechanism, whereas PID, MTMCD or butyrate had no measurable inhibitory effect. PIA inhibited Acls4 activity with a Ki of 11.4 mM, half the reported Ki of 25 mM for VPA [32]. An uncompetitive pattern of inhibition using Michaelis-Menten kinetics was consistent with the parallel Lineweaver-Burke plots of Figure 2B, and was demonstrated to have a high probability compared with other mechanisms using the Akaike Information Criterion (AIC). Uncompetitive inhibition implies that PIA binds to the Acsl4-AA substrate complex at a different binding site than does substrate AA, and causes a conformational change that reduces enzyme activity and conversion rate [50]. A similar model was derived for inhibition by VPA of Acsl4 activity [32].
Acsl4 mediated conversion of AA to AA-CoA also showed substrate inhibition (Figure 2A), with best-fit values for Km and Vmax of 4.12 ± 0.56 μM, and 132.6 ± 8.81 nmol/min/mg, respectively, comparable to values of 4.98 ± 1.41 μM and 143.3 ± 11.1 nmol/min/mg, respectively, that were reported previously [32].
PIA inhibited recombinant Acls4 activity with a Ki of 11.4 mM. In comparison, VPA inhibited AA acylation by recombinant Acsl4 in vitro at a Ki of 25 mM, about twice that of PIA, suggesting that PIA would be more effective in vivo on an equi-concentration basis. Rat brain PIA concentrations have not been reported, although penetration occurs, based on its anticonvulsant effects in rats [43], whereas the mean brain VPA concentration is estimated as 1.0 – 1.5 mM after VPA administration at a therapeutically relevant dose (200 mg/kg, i.p.) that selectively reduces AA turnover in rat brain phospholipid [27, 32, 54–56]. The discrepancy between the in vitro concentration required for Acsl4 inhibition and the estimated mean therapeutic brain level for VPA was reconciled by evidence that VPA can accumulate, via a short-chain fatty acid transporter, within cellular mitochondria, microsomes and other organelles in which Acsl4 also is found [37, 39, 57–59]. Similar considerations may apply to PIA, which also is a short chain fatty acid. For both PIA and VPA, their in vitro kinetic inhibition constant for Acsl4 may differ from the actual in vivo value, since it may depend on bath conditions such as pH, temperature, salt and ATP concentrations, and on the absence of fatty acid transport proteins that are present in vivo [60]. At clinical therapeutic levels, VPA can be hepatotoxic, and it can be teratogenic in pregnant women because it inhibits histone deacetylase [8, 9, 43]. PIA is less teratogenic than VPA. It is not teratogenic at 3.6 mmol/kg in mice compared to marked teratogenicity of VPA at this dose, but its teratogenicity at higher doses remains to be further evaluated [43, 61, 62], and it does not inhibit histone deacetylase in vitro [42]. Although PID and MTMCD have equal or better anticonvulsant activity in the rat than does VPA [43, 45], neither compound inhibited Acsl4 in this study. These differences distinguish between anticonvulsant activity and anti-BD activity of these drugs, and suggest that they have different mechanisms of action in each of the two disorders. Similarly, the clinically useful anticonvulsants, topiramate and gabapentin, do not measurably affect rat brain AA metabolism [63–66].
In comparing the structures of VPA and the three analogues used in this study (Figure 1), a free carboxylic group (Figure 1) would appear necessary for Acsl4 inhibition. Thus, PIA's effect was absent when the hydroxyl group of its carboxylic acid moiety was replaced by an amino group (PID and MTMCD). Furthermore, since butyrate did not inhibit Acsl4, a chain of longer than four carbons appears necessary for inhibition.
X-ray crystallography might help to establish structure-activity relations for inhibition of Acsl4 by identifying a common site for PIA and VPA binding. At present, X-ray crystallography-derived structures for mammalian Acsl enzymes are unavailable, although one has been published for the distantly related Acsl from Thermus thermophilus HB8. The fatty acid binding pocket of this latter enzyme is at its N-terminus [67].
Brain AA metabolism and turnover are upregulated in animal models of neuroinflammation and excitotoxicity [68–70], and AA metabolic markers are elevated in association with these neuropathological processes in the postmortem BD brain [14–16]. Because lithium, carbamazepine and VPA downregulate brain AA turnover and other AA metabolic markers in rat brain [21, 28, 30, 71–75], their therapeutic efficacy in BD may depend on suppressing the upregulated brain AA cascade of that disease. It remains to be determined whether the observed inhibition by PIA of AA to AA-CoA conversion by recombinant Acsl4 in vitro corresponds to its ability to also reduce metabolic markers of the AA cascade in vivo [27, 76], which would lend more justification to initiating a clinical trial with PIA in BD.
In conclusion, we have identified PIA as a new uncompetitive Acsl4 inhibitor, similar to VPA. PIA has a lower Ki than does VPA, it does not inhibit histone deacetylase, and it is not teratogenic up to a dose of 3.6 mmol/kg in mice [42, 43, 61, 62]. Thus, PIA may be of interest for treating BD. Showing this also would argue that Acsl4 is a reasonable target for developing new mood stabilizers to treat BD. However, further in vivo experiments are required to claim that PIA would decrease AA turnover in rat brain phospholipids like VPA, lithium and carbamazepine, which would justify the need for a clinical trial.
Highlights
Valproic acid's constitutional isomer, PIA, uncompetitively inhibit Acsl4
Ki of PIA is 11.4 mM, compared to a published Ki of 25 mM for valproic acid.
Like VPA, PIA may reduce AA turnover in brain phospholipids in unanesthetized rats
If so may be effective as a non-teratogenic mood stabilizer in BD patients.
Justification for designing new Acsl4 inhibitors as potential less toxic drugs for treating bipolar disorder.
Acknowledgements
This work was supported by the Intramural Program of the National Institute on Aging, NIH, by NIH Grant DK 59935 (RAC), and by a postdoctoral fellowship from the American Heart Association-Mid-Atlantic Region (L. O. L). We also thank the Chemical Synthesis and Drug Supply Program of the National Institute of Mental Health (Research Triangle Park, NC, USA) for supplying us with propylisopropylacetamide (PID).
Abbreviations
- AA
arachidonic acid
- Acsl
acyl-CoA synthetase
- BD
bipolar disorder
- MTMCD
N-methyl-2,2,3,3-tetramethylcyclopropanecarboxamide
- PIA
propylisopropylacetic acid
- PID
propylisopropylacetamide
- VPA
valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of interest. No author has a financial or other conflict of interest related to this work.
References
- [1].Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG, Jr., Chou JC, Keck PE, Jr., Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group, Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- [2].Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, Ostacher MJ, Morriss R, Alder N, Juszczak E. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375:385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- [3].Chang HH, Yang YK, Gean PW, Huang HC, Chen PS, Lu RB. The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord. 2010;124:319–323. doi: 10.1016/j.jad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [4].Sobaniec-Lotowska ME. Effects of long-term administration of the antiepileptic drug--sodium valproate upon the ultrastructure of hepatocytes in rats. Exp Toxicol Pathol. 1997;49:225–232. doi: 10.1016/S0940-2993(97)80015-2. [DOI] [PubMed] [Google Scholar]
- [5].Loscher W, Nau H, Wahnschaffe U, Honack D, Rundfeldt C, Wittfoht W, Bojic U. Effects of valproate and E-2-en-valproate on functional and morphological parameters of rat liver. II. Influence of phenobarbital comedication. Epilepsy Res. 1993;15:113–131. doi: 10.1016/0920-1211(93)90092-l. [DOI] [PubMed] [Google Scholar]
- [6].Lewis JH, Zimmerman HJ, Garrett CT, Rosenberg E. Valproate-induced hepatic steatogenesis in rats. Hepatology. 1982;2:870–873. doi: 10.1002/hep.1840020622. [DOI] [PubMed] [Google Scholar]
- [7].Zimmerman HJ, Ishak KG. Valproate-induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2:591–597. doi: 10.1002/hep.1840020513. [DOI] [PubMed] [Google Scholar]
- [8].Brown NA, Farmer PB, Coakley M. Valproic acid teratogenicity: demonstration that the biochemical mechanism differs from that of valproate hepatotoxicity. Biochem Soc Trans. 1985;13:75–77. doi: 10.1042/bst0130075. [DOI] [PubMed] [Google Scholar]
- [9].Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- [10].Samren EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, Mannagetta GB, Deichl AW, Gaily E, Granstrom ML, Meinardi H, Grobbee DE, Hofman A, Janz D, Lindhout D. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- [11].Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- [12].Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch. Gen. Psychiatry. 2002;59:592–506. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- [13].Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2011;16:419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- [18].Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007;102:1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- [20].Ramadan E, Rosa AO, Chang L, Chen M, Rapoport SI, Basselin M. Extracellular-derived calcium does not initiate in vivo neurotransmission involving docosahexaenoic acid. J Lipid Res. 2010;51:2334–2340. doi: 10.1194/jlr.M006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- [22].Ramadan E, Basselin M, Taha AY, Cheon Y, Chang L, Chen M, Rapoport SI. Chronic valproate treatment blocks D2-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology. 2011;61:1256–1264. doi: 10.1016/j.neuropharm.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Molecular psychiatry. 2002;7(Suppl 1):S57–63. doi: 10.1038/sj.mp.4001019. [DOI] [PubMed] [Google Scholar]
- [24].Post RM, Jimerson DC, Bunney WE, Jr., Goodwin FK. Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology. 1980;67:297–305. doi: 10.1007/BF00431272. [DOI] [PubMed] [Google Scholar]
- [25].Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- [27].Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- [28].Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- [29].Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2005;182:180–185. doi: 10.1007/s00213-005-0059-7. [DOI] [PubMed] [Google Scholar]
- [30].Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- [31].Bazinet RP, Weis MT, Rapoport SI, Rosenberger TA. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berl) 2006;184:122–129. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- [32].Shimshoni JA, Basselin M, Li LO, Coleman RA, Rapoport SI, Modi HR. Valproate uncompetitively inhibits arachidonic acid acylation by rat acyl-CoA synthetase 4: Relevance to valproate's efficacy against bipolar disorder. Biochim Biophys Acta. 2011;1811:163–169. doi: 10.1016/j.bbalip.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Experimental biology and medicine. 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635–1642. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- [35].Gijon MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem. 2008;283:30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Perez-Chacon G, Astudillo AM, Ruiperez V, Balboa MA, Balsinde J. Signaling role for lysophosphatidylcholine acyltransferase 3 in receptor-regulated arachidonic acid reacylation reactions in human monocytes. J Immunol. 2010;184:1071–1078. doi: 10.4049/jimmunol.0902257. [DOI] [PubMed] [Google Scholar]
- [37].Cao Y, Murphy KJ, McIntyre TM, Zimmerman GA, Prescott SM. Expression of fatty acid-CoA ligase 4 during development and in brain. FEBS Lett. 2000;467:263–267. doi: 10.1016/s0014-5793(00)01159-5. [DOI] [PubMed] [Google Scholar]
- [38].Bhat SS, Schmidt KR, Ladd S, Kim KC, Schwartz CE, Simensen RJ, DuPont BR, Stevenson RE, Srivastava AK. Disruption of DMD and deletion of ACSL4 causing developmental delay, hypotonia, and multiple congenital anomalies. Cytogenet Genome Res. 2006;112:170–175. doi: 10.1159/000087531. [DOI] [PubMed] [Google Scholar]
- [39].Meloni I, Parri V, De Filippis R, Ariani F, Artuso R, Bruttini M, Katzaki E, Longo I, Mari F, Bellan C, Dotti CG, Renieri A. The XLMR gene ACSL4 plays a role in dendritic spine architecture. Neuroscience. 2009;159:657–669. doi: 10.1016/j.neuroscience.2008.11.056. [DOI] [PubMed] [Google Scholar]
- [40].Rodriguez JD, Bhat SS, Meloni I, Ladd S, Leslie ND, Doyne EO, Renieri A, Dupont BR, Stevenson RE, Schwartz CE, Srivastava AK. Intellectual disability, midface hypoplasia, facial hypotonia, and Alport syndrome are associated with a deletion in Xq22.3. American journal of medical genetics. Part A. 2010;152A:713–717. doi: 10.1002/ajmg.a.33208. [DOI] [PubMed] [Google Scholar]
- [41].Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem Res. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- [42].Eyal S, Yagen B, Shimshoni J, Bialer M. Histone deacetylases inhibition and tumor cells cytotoxicity by CNS-active VPA constitutional isomers and derivatives. Biochem Pharmacol. 2005;69:1501–1508. doi: 10.1016/j.bcp.2005.02.012. [DOI] [PubMed] [Google Scholar]
- [43].Shimshoni JA, Bialer M, Wlodarczyk B, Finnell RH, Yagen B. Potent anticonvulsant urea derivatives of constitutional isomers of valproic acid. J Med Chem. 2007;50:6419–6427. doi: 10.1021/jm7009233. [DOI] [PubMed] [Google Scholar]
- [44].Spiegelstein O, Bialer M, Radatz M, Nau H, Yagen B. Enantioselective synthesis and teratogenicity of propylisopropyl acetamide, a CNS-active chiral amide analogue of valproic acid. Chirality. 1999;11:645–650. doi: 10.1002/(SICI)1520-636X(1999)11:8<645::AID-CHIR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [45].Bialer M, Hadad S, Kadry B, Abdul-Hai A, Haj-Yehia A, Sterling J, Herzig Y, Yagen B. Pharmacokinetic analysis and antiepileptic activity of tetra-methylcyclopropane analogues of valpromide. Pharm Res. 1996;13:284–289. doi: 10.1023/a:1016055517724. [DOI] [PubMed] [Google Scholar]
- [46].Modi HR, Shimshoni JA, Mireille B, Li LO, Coleman RA, Rapoport SI. Trans. Soc. Neurochem. Baltimore, MD: Mar 3–7, 2012. PIA (propylisopropylacetic acid) selectively inhibits acyl-CoA synthetase-4; pp. PSM11–12.pp. 118 [Google Scholar]
- [47].Haj-Yehia A, Bialer M. Structure-pharmacokinetic relationships in a series of valpromide derivatives with antiepileptic activity. Pharm Res. 1989;6:683–689. doi: 10.1023/a:1015934321764. [DOI] [PubMed] [Google Scholar]
- [48].Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- [49].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [50].Palmer T. Understanding Enzymes. John Wiley; New York: 1985. [Google Scholar]
- [51].Akaike H. A new look at statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723. [Google Scholar]
- [52].Motulsky CA. Fitting Models to Biological Data using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press; NY: 2004. pp. 143–148. [Google Scholar]
- [53].Shimshoni JA, Dalton EC, Jenkins A, Eyal S, Ewan K, Williams RS, Pessah N, Yagen B, Harwood AJ, Bialer M. The effects of central nervous system-active valproic acid constitutional isomers, cyclopropyl analogs, and amide derivatives on neuronal growth cone behavior. Mol Pharmacol. 2007;71:884–892. doi: 10.1124/mol.106.030601. [DOI] [PubMed] [Google Scholar]
- [54].Deutsch J, Rapoport SI, Rosenberger TA. Valproyl-CoA and esterified valproic acid are not found in brains of rats treated with valproic acid, but the brain concentrations of CoA and acetyl-CoA are altered. Neurochem Res. 2003;28:861–866. doi: 10.1023/a:1023267224819. [DOI] [PubMed] [Google Scholar]
- [55].Loscher W, Fisher JE, Nau H, Honack D. Valproic acid in amygdala-kindled rats: alterations in anticonvulsant efficacy, adverse effects and drug and metabolite levels in various brain regions during chronic treatment. J Pharmacol Exp Ther. 1989;250:1067–1078. [PubMed] [Google Scholar]
- [56].Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem Res. 1999;24:399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- [57].Scism JL, Powers KM, Artru AA, Lewis L, Shen DD. Probenecid-inhibitable efflux transport of valproic acid in the brain parenchymal cells of rabbits: a microdialysis study. Brain Res. 2000;884:77–86. doi: 10.1016/s0006-8993(00)02893-6. [DOI] [PubMed] [Google Scholar]
- [58].Rajaonarison JF, Lacarelle B, Catalin J, Placidi M, Rahmani R. 3'-azido-3'-deoxythymidine drug interactions. Screening for inhibitors in human liver microsomes. Drug Metab Dispos. 1992;20:578–584. [PubMed] [Google Scholar]
- [59].Terlouw SA, Tanriseven O, Russel FG, Masereeuw R. Metabolite anion carriers mediate the uptake of the anionic drug fluorescein in renal cortical mitochondria. J Pharmacol Exp Ther. 2000;292:968–973. [PubMed] [Google Scholar]
- [60].Knudsen J, Neergaard TB, Gaigg B, Jensen MV, Hansen JK. Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J Nutr. 2000;130:294S–298S. doi: 10.1093/jn/130.2.294S. [DOI] [PubMed] [Google Scholar]
- [61].Bojic U, Elmazar MM, Hauck RS, Nau H. Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem Res Toxicol. 1996;9:866–870. doi: 10.1021/tx950216s. [DOI] [PubMed] [Google Scholar]
- [62].Skladchikova G, Berezin V, Bock E. Valproic acid, but not its non-teratogenic analogue 2-isopropylpentanoic acid, affects proliferation, viability and neuronal differentiation of the human teratocarcinoma cell line NTera-2. Neurotoxicology. 1998;19:357–370. [PubMed] [Google Scholar]
- [63].Lee HJ, Ghelardoni S, Chang L, Bosetti F, Rapoport SI, Bazinet RP. Topiramate does not alter the kinetics of arachidonic or docosahexaenoic acid in brain phospholipids of the unanesthetized rat. Neurochem Res. 2005;30:677–683. doi: 10.1007/s11064-005-2756-3. [DOI] [PubMed] [Google Scholar]
- [64].Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double-blind placebo-controlled trials. Bipolar Disord. 2006;8:15–27. doi: 10.1111/j.1399-5618.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- [65].Ben-Menachem E, Sander JW, Stefan H, Schwalen S, Schauble B. Topiramate monotherapy in the treatment of newly or recently diagnosed epilepsy. Clinical therapeutics. 2008;30:1180–1195. doi: 10.1016/s0149-2918(08)80045-8. [DOI] [PubMed] [Google Scholar]
- [66].Reese EA, Cheon Y, Ramadan E, Kim HW, Chang L, Rao JS, Rapoport SI, Taha AY. Gabapentin's minimal action on markers of rat brain arachidonic acid metabolism agrees with its inefficacy against bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2012;87:71–77. doi: 10.1016/j.plefa.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, Yokoyama S, Miyano M. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J Biol Chem. 2004;279:31717–31726. doi: 10.1074/jbc.M400100200. [DOI] [PubMed] [Google Scholar]
- [68].Basselin M, Kim HW, Chen M, Ma K, Rapoport SI, Murphy RC, Farias SE. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. J Lipid Res. 2010;51:1049–1056. doi: 10.1194/jlr.M002469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [69].Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008;49:162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- [70].Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. Erratum in: J Neurochem (2004) 1190, 1255. [DOI] [PubMed] [Google Scholar]
- [71].Chang MCJ, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- [72].Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry. 2002;7:845–850. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- [73].Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–696. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- [74].Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry. 2004;56:248–254. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- [75].Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007;102:761–772. doi: 10.1111/j.1471-4159.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- [76].Shimshoni JA, Dalton EC, Watson P, Boris Y, Bialer M, Harwood AJ. Evaluation of the effects of propylisopropylacetic acid (PIA) on neuronal growth cone morphology. Neuropharmacology. 2009;56:831–837. doi: 10.1016/j.neuropharm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]