Abstract
Mucosa-associated lymphoid tissue (MALT) lymphomas are known to occur in Sjögren syndrome (SS) patients, but reported cases in labial salivary glands (LSG) are rare. We report a case of 60-year-old female patient with SS who developed MALT lymphoma in the labial salivary glands during a 2-year time interval when she was participating in the Sjögren’s International Clinical Collaborative Alliance (SICCA). SICCA is an ongoing longitudinal multisite observational study funded by the National Institutes of Health (NIH) of the United States. At follow-up exam, LSG biopsy showed atypical diffuse infiltration by mononuclear cells of variable size and atypical nuclei affecting the whole specimen with destruction of glandular architecture, leading to a diagnosis of B-cell MALT lymphoma. Computed tomography and bone marrow biopsy failed to show additional evidence of disease. Clinical, serological, ocular, histologic and immunohistochemical findings are presented. A “watch and wait” policy was adopted with regular examinations.
Keywords: MALT lymphoma, Sjögren’s syndrome, Labial salivary gland biopsy, Salivary glands
Primary Sjögren’s syndrome (SS) is a chronic autoimmune disease affecting the exocrine glands, other organs, and producing circulating antibodies. Lymphocytic infiltration of the salivary and lacrimal glands leads to the progressive loss of the glandular parenchyma with decrease of function resulting in chronic salivary hypofunction and keratoconjunctivitis sicca, two of the major components of the syndrome.1 The oral dryness increases the risk of caries and the mucosa is more prone to candidiasis and/or bacterial infections. Hernández et al.2 reported a 37% prevalence of atrophic chronic candidiasis in 246 patients with SS. Frequently the gastrointestinal tract is compromised, with immunophenotype of the lymphoid cells that infiltrate this mucosa being very similar to that found in the minor salivary glands.3 Also gastric infection by Helicobacter pylori (Hp) has been reported in patients with SS. Aragona et al.4 communicated a significantly higher prevalence of antibodies against Hp and its heat-shock protein 60 (HSP60) in the serum of patients with SS than that found in a group of patients with other autoimmune diseases and healthy controls. These authors concluded that the hypothetical role of HSP60 in the development of the immune response both in primary and secondary SS seems linked to the prevalence rate of Hp infection. The most likely mode of transmission of this bacterium is from person to person, mainly orally, since Hp DNA is present both in saliva and dental plaque.5
The evolution of SS is usually indolent, although affected patients have a higher risk of developing non-Hodgkin lymphoma (NHL) than the general population, estimated to be 44 times greater.6 This is the most serious complications of SS. The incidence of lymphomas described by Voulgarelis et al.7 in patients with SS, is 4.3 % while according to Sutcliffe et al.,8 it varies between 5 and 10%. In most cases, they are extranodal B-cell lymphomas of low-grade and mucosa-associated lymphoid tissue (MALT) lymphoma, also known as extranodal marginal zone B-cell lymphoma. This kind of lymphoma has unique clinicopathologic characteristics.9
The World Health Organization divides marginal zone B-cell lymphomas (MZL) into three subtypes: a) extranodal MZL (MALT type lymphoma); b) splenic MZL; and c) nodal MZL (with/without monocitoid cells).10 The majority of the extranodal lymphomas affects mainly the digestive system and are originated from the lymphoid tissue of the mucosa and acquired secondary to a chronic inflammatory process. This inflammatory process produces the recruitment of type T and B lymphocytes to the affected site, and acquires the structure of organized lymphoid tissue, similar to normal MALT found in Peyer’s patches. 9, 10 In the gastric mucosa MALT lymphoma is frequently associated with Hp infection.11 In the same way, cutaneous MZL has been associated with infection by Borrelia burgdorferi, ocular adnexa MZL to Chlamydia psittaci, small bowel MZL to Campylobacter jejuni and MZL of the salivary glands to the hepatitis C virus. 12 In addition, autoimmune diseases such as SS are a predisposing factor for its development in specific sites of head and neck, including salivary glands.13, 15
In SS, MALT lymphoma originates mainly in the major salivary glands, particularly the parotid.12–25 Reported cases in the minor salivary glands are very rare. Speight et al.26 in their study of lower lip minor labial salivary glands reported the early detection of four cases of MALT lymphoma by in situ hybridization for Kappa and Lambda light chain mRNA, in 14 cases of SS; Van Mello et al.27 have reported three cases in the same location, and Sakuma et al.28 reported one case in minor salivary glands of the hard palate. Table 1 summarizes MALT lymphoma cases of salivary glands of patients with SS, found in the English literature in the last 15 years.
TABLE 1.
MALT lymphomas in salivary glands of patients with Sjogren’s Syndrome
| AUTHORS | YEAR | N° | LOCALIZATION | DIAGNOSTIC CRITERIA (#) |
|---|---|---|---|---|
| Speight PM et al (26) | 1994 | 4 | MINOR LABIAL SALIVARY GLANDS | κ and λ light chain. In situ hybridization for mRNA |
| Royer B et al (14) | 1997 | 3 | PAROTID | REAL classification CD20, CD3, κ and λ light chain, Bcl2 |
| Nishimura M et al (15) | 2000 | 1 | PAROTID | Immunohistochemical. Southern blotting immunoglobulin gene rearrangement |
| Biasi D et al (16) | 2001 | 6 | PAROTID & SUBMAXILLAR | REAL. classification Histomorphology and immunohistochemial study (**) |
| Queneau PE et al (17) | 2002 | 1 | PAROTID | CD20 Southern blot |
| Klussmann JP et al (18) | 2003 | 1 | PAROTID | (**) |
| Dunn P et al (20) | 2004 | 4 | PAROTID | WHO classification CD3, CD20, CD-45RO, κ and λ light chain |
| Ambrosetti A et al (12) | 2004 | 15 | PAROTID | REAL/WHO classification CD20, CD5, CD21, CD10, CD3, κ and λ light chain |
| Streubel B et al (19) | 2004 | 15 | PAROTID & SUBMAXILLAR | WHO classification Reverse transcriptase-PCR Fluorescence in situ hybridation |
| Van Mello NM et al (27) | 2005 | 3 | MINOR LABIAL SALIVARY GLANDS | Monoclonal κ and λ light chain CD 43, CD 20, CD3 |
| Sakuma H et al (28) | 2006 | 1 | MINOR SALIVARY GLAND HARD PALATE | CD20, CD3, CD5, CD10, CD23, cyclin D1, immunoglobulin light chain |
| Arcaini L et al (21) | 2006 | 4(*) | PAROTID | WHO classification Immunophenotypic profile was used to exclude other type of indolent low-grade B cell lymphomas (**) |
| Lewis K et al (22) | 2007 | 2 | PAROTID & SUBMAXILLAR | CD20, CD5, CD10, CD23, cyclin D1, κ light chain |
| Roh JL et al (24) | 2008 | 1(*) | PAROTID | REAL classification CD3, CD5, CD10, CD20, CD23, CD43, CD-45RO, CD79a, κ and λ light chain, Bcl2, Bcl6, Ki67 |
| Suh C et al (13) | 2008 | 2 | PAROTID & SUBMAXILLAR (both) | WHO classification CD3, CD5, CD10, CD20, CD21, CD43, CD79a, cyclin D1, κ and λ light chain, bcl2, bcl6, KI 67 |
| Hu S et al (23) | 2009 | 6 | PAROTID | WHO classification CD3, CD20, CD10, Bcl6, κ and λ, IgM, IgG, IgA |
Classification and Immunohistochemial markers used
only patients with SS was considered
Non specified
In this paper, we present a case of MALT lymphoma in lower labial minor salivary glands found during the follow-up of a patient who had previously been diagnosed with SS according to SICCA protocol. SICCA (Sjögren’s International Clinical Collaborative Alliance) is a multicenter observational interventionist study originated at the University of California, funded by the National Institutes of Health (NIH) of the United States. Its purpose is to establish diagnostic criteria for Sjögren’s Syndrome and to create a data and specimen bank for future research on this disease.29 In each participating center a multidisciplinary team evaluates patients with symptoms compatible with SS for diagnostic purposes including oral, rheumatologic, and eye exams; sialometry and minor salivary gland biopsy of the lower lip; as well as blood test determination of levels of rheumatoid factor (RF), IgG, IgA, IgM, C3, C4, antinuclear antibodies (ANA), anti-SS-A and anti-SS-B antibodies, and anti-hepatitis C. A follow-up assessment is carried out two years later with repeat of all studies.
CLINICAL CASE
A 60-year-old female patient presented at the Oral Medicine clinic of the School of Dentistry of the University of Buenos Aires in December 2004, with dry mouth symptoms and burning sensation. She reported xerophthalmia for 15 years and xerostomia for four years and had not used anticholinergic drugs. Clinical examination revealed marked dryness and atrophy of the buccal mucosa, with the presence of traumatic erosions associated with lack of moisture. Given these clinical characteristics, she was a candidate for and consented to be enrolled in the SICCA study. The initial oral examination was performed and revealed clinical manifestations of chronic candidiasis, confirmed by culture, and marked xerostomia. Saliva expressed from both parotid ducts was thick and there was no expression from the submandibular ducts. The patient wore maxillary and mandibular complete dentures. Sialometry showed total unstimulated salivary flow rate of 0 ml/5 min. (RR: 2gr/5min) and a stimulated parotid salivary flow rate of 0 ml/5min (RR: 0.1 gr/5min).
At the rheumatologic examination, the patient stated not having pain and presented with mild asthenia. At the time of the visit the patient was taking the following drugs: paracetamol 500 mg/day, alprazolam 0.25 mg (3 times a week); and used artificial tears. Ocular examination revealed keratoconjunctivitis sicca demonstrated by Schirmer test: 0 mm; Tear Break-Up Time (BUT): 1 second; corneal staining with fluorescein at 0.5 %: 6 (five patches of confluent staining and staining of pupillary area); and conjunctival staining with lissamine green at 1%: 6 in both eyes.
Blood test showed levels of RF, IgA, IgG, IgM, C3 and C4 within normal limits. The ANA was high at 1: 1280 (range 1–40), with a speckled pattern, while the anti-SSA and anti-SSB were negative. Anti-hepatitis C was non-reactive. In addition, serum anti-HP IgG antibodies, and secretory IgA antibodies were requested for evaluation of the presence of HP. IgG was negative with a value of 2.57 (range >15) and secretory IgA was also negative with a value of 18 (range 7–18).
Histopathology of the biopsied labial salivary glands showed multiple dense foci (n=18) of typical lymphocytes with periductal and perivascular orientation and no evidence of germinal centers or lymphoepithelial lesions. The diagnosis was focal lymphocytic sialadenitis. The evaluated glandular area was 7.5 mm2 and the calculated focus score was 8.5. Based on the working standard used at the time within the SICCA registry for patient management, the patient was informed that she had both the ocular and oral components of SS.
The patient periodically returned to the Oral Medicine clinic for follow-up and management of her oral dryness and associated complications. In March 2007, the second evaluation was completed according to the project protocol. The oral examination revealed fissures on the dorsal tongue. In addition to the lack of expression of saliva from the submandibular ducts, as observed in the initial visit, no saliva was expressed from the parotid ducts. This was consistent with the sialometric values, of 0 ml/5min, as on the initial evaluation.
Upon rheumatologic clinical examination asthenia and no pain were reported, there was absence of purpura or other systemic manifestations. The serology results were the same as in the first visit except for the RF. Even though the value of 8 IU/ml was within normal limits (< 14 IU/ml), in this second occasion it doubled the initial value.
On ocular examination, keratoconjunctivitis sicca was observed like in the initial examination, and similar results were obtained for the Schirmer test, BUT and corneal staining with fluorescein at 0.5 %, with the only difference that filaments could be observed. The results of the conjunctival staining with lissamine green at 1% were unchanged in both eyes.
The follow-up minor labial salivary gland biopsy showed atypical diffuse infiltration by mononuclear cells of variable size with little cytoplasm, heteromorphic and hyperchromatic nuclei, affecting the whole specimen with destruction of glandular architecture. Histomorphologic studies included the use of an immunohistochemical panel for the determination of the phenotype. The result of the immunohistochemistry was that the lymphocytes were CD20+, CD3-, CD5- and CD23-. Evidence of Kappa light chain restriction and positive staining for Bcl-2 was observed. Ki-67, measurement of cellular proliferation, was positive in <5% of neoplastic cells (Fig. 1). The diagnosis of B-cell MALT lymphoma of low grade, was made. Since the patient did not present clinical signs or symptoms of alteration in the parotid glands no image diagnostic tests were requested.
Fig. 1.
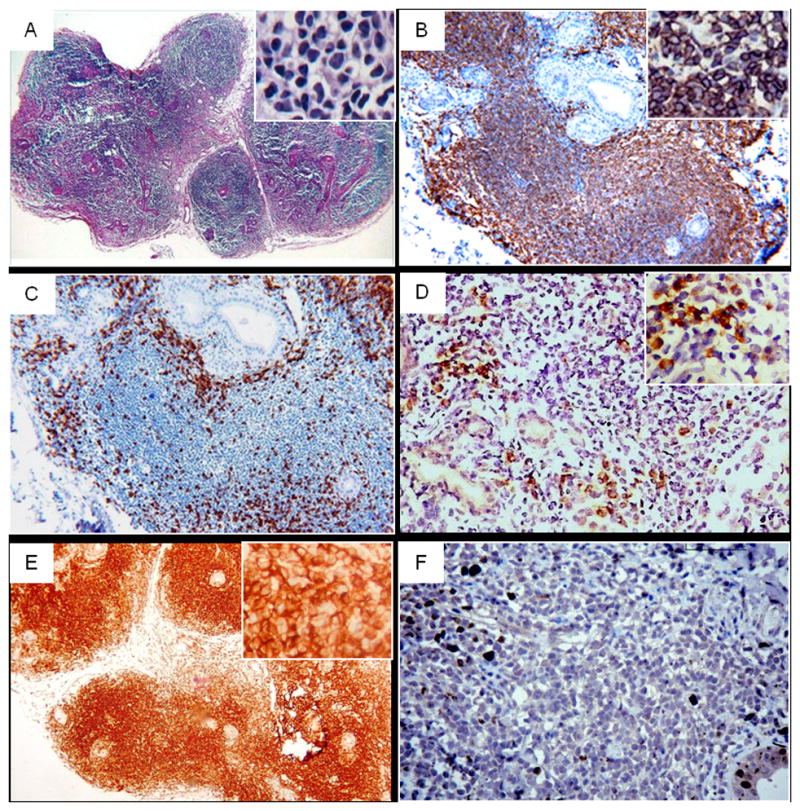
Histomorphology and immunohistochemistry of MALT lymphoma in minor salivary gland follow up biopsy; A: Hematoxylin-eosin (Original magnification ×40), B: CD20 (positive) in most lymphocytes exhibiting a diffuse pattern, C: CD3 (negative) identifies scattered T-cells of the lymphocytic infiltrate, D: Kappa light chain restriction, E: Bcl-2 positive staining, F: Ki-67 positive expression <5% of neoplastic cells. (Original magnification ×100).
Due to the presence of a gastric acid sensitive syndrome she was referred to Gastroenterology for study. An endoscopy was performed and the gastric biopsy showed chronic gastritis with presence of Hp. Antibacterial treatment with: amoxicillin-clavulanic acid (1g twice daily), plus clarithromycin (500 mg twice daily) for 8 days, and pantoprazol (40 mg daily) for 8 weeks, was prescribed. The general evaluation of the patient including computed tomography and bone marrow biopsy showed no additional evidence of disease. The endoscopy and gastric biopsy performed post treatment showed mild chronic gastritis and absence of Hp. A “watch and wait” policy was adopted. Local and systemic evaluation was performed every 6 months during the first year and then, once a year with no evidence of disease dissemination to date.
DISCUSSION
Development of NHL is the most serious complication of SS. Several histological subtypes of NHL have been described in patients affected with this syndrome, most commonly MALT lymphoma. This type of lymphoma has characteristic clinicopathologic features and is rare in the oral cavity. Generally has a relatively indolent course and remains localized to the original site for prolonged periods of time.30 This sometimes leads to mistaking it for an inflammatory process. However, in extra-gastrointestinal locations, the clinical development and biologic behavior is heterogeneous, and may compromise multiple sites of the mucosa. This occurrence is most probable in head and neck MALT lymphomas.13, 31 Histologically it is composed of morphologically heterogeneous small B cells including marginal zone (centrocyte-like) cells, others resembling monocytoid cells, small lymphocytes, and scattered immunoblasts and centroblast-like cells. The neoplastic cells infiltrate around reactive B cell follicles, in a marginal zone distribution and spread into the interfollicular areas. 9, 10 The determination of the immunophenotype, as well as the presence of immunoglobulin or Bcl-2 gene rearrangements, aid in the diagnosis, especially when it is difficult to distinguish from reactive lymphocytic infiltration.13 It has been suggested that prolonged autoimmune inflammation, as in SS, or persistent antigenic stimulation caused by Hp and/or hepatitis C virus could play a role in the development of this type of lymphoma.12, 15 The labial salivary gland biopsy is one of the diagnostic tests for SS and aids in detection of lymphoma. The evaluation must be very thorough, especially when the infiltration of mononuclear cells is very marked and uniform. In this event the use of immunohistochemical techniques is necessary to determine the diagnosis.
In this case, scheduled recall allowed early detection of the presence of a MALT lymphoma of the labial salivary glands. General evaluation of the patient performed after this diagnosis, showed no evidence of disease dissemination. The biopsy performed during follow-up gastroscopy after anti-Hp treatment revealed mild chronic gastritis, and absence of Hp. There is strong evidence of the association between gastric MALT lymphoma and infection by Hp and remission can be obtained with appropriate antibacterial treatment.32 Such association has not been reported in salivary glands although some authors have reported the remission of a case of parotid MALT lymphoma in a patient with SS after having received anti-Hp treatment because of chronic gastritis associated with Hp.33, 34 The treatment suggested for disease localized in the head and neck is local therapy of either surgery or radiotherapy.35 Systemic chemotherapy is not required to prevent recurrence but, periodic local and systemic evaluation for a prolonged period of time is necessary.13 However, some centers prefer the “watch and wait” approach in patients with localized disease because it may be many years before evidence of disease progression occurs.22, 36 In our case the latter option was preferred because after general evaluation there was no additional evidence of disease.
CONCLUSION
Repeat examinations, including labial salivary gland biopsies as part of the SICCA protocol, enable standardized and thorough evaluation of disease progress, in patients with SS. In this particular case, this protocol allowed early detection of a MALT lymphoma in a patient that did not present with clinical signs related to a neoplastic lesion.
Acknowledgments
Supported by NIH contract NOI-DE-32636 (Principal Investigators: 2004–2010, T Daniels and J Greenspan; 2010-Present, C Shiboski and L Criswell).
We would like to thank Dr. Troy Daniels, from the University of California, San Francisco, for his collaboration in some of the immunohistochemical techniques and for providing some of the micrographs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daniels T, Fox P. Salivary and oral components of Sjögren’s syndrome. Rheum Dis Clin North Am. 1992;18:571–89. [PubMed] [Google Scholar]
- 2.Hernández YL, Daniels TE. Oral candidiasis in Sjögren’s syndrome: prevalence, clinical correlations, and treatment. Oral Surg Oral Med Oral Pathol. 1989;68:324–9. doi: 10.1016/0030-4220(89)90218-1. [DOI] [PubMed] [Google Scholar]
- 3.Ferraccioli GF, Sorrentino D, De Vita S, Casatta L, Labombarda A, Avellini C, Dolcetti R, Di Luca D, Beltrami CA, Boiocchi M, Bartoli E. B cell clonality in gastric lymphoid tissues of patients with Sjögren’s syndrome. Ann Rheum Dis. 1996;55:311–6. doi: 10.1136/ard.55.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragona P. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjögren’s syndrome. J Rheumatol. 1999;26:1306–11. [PubMed] [Google Scholar]
- 5.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 6.Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, Costa J, Decker JL, Chused TM. Increased risk of lymphoma in sicca syndrome. ANN Intern Med. 1978;89:888–92. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- 7.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 1999;42:1765–72. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe N, Inanc M, Speight PM, Isenberg DA. Predictors of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 1998;28:80–7. doi: 10.1016/s0049-0172(98)80040-1. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson PG, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Isaacson PG, Chott A, Nakamura S, Müller-Hermelink HK, Harris NL, Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon: IARC Press; 2008. pp. 214–217. [Google Scholar]
- 11.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosetti A, Zanotti R, Pattaro C, Lenzi L, Chilosi M, Caramaschi P, Arcaini L, Pasini F, Biasi D, Orlandi E, D’Adda M, Lucioni M, Pizzolo G. Most cases of primary salivary mucosa-associated lymphoid tissue lymphoma are associated either with Sjögren syndrome or hepatitis C virus infection. Br J Haematol. 2004;126:43–9. doi: 10.1111/j.1365-2141.2004.04993.x. [DOI] [PubMed] [Google Scholar]
- 13.Suh C, Huh J, Rohm JL. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the extracranial head and neck region: A high rate of dissemination and disease recurrence. Oral Oncol. 2008;44:949–955. doi: 10.1016/j.oraloncology.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X. Lymphomas in patients with Sjögren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–75. [PubMed] [Google Scholar]
- 15.Nishimura M, Miyajima S, Okada N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjögren’s Syndrome. J Dermatol. 2000;27:450–2. doi: 10.1111/j.1346-8138.2000.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 16.Biasi D, Caramaschi P, Ambrosetti A, Carletto A, Mocella S, Randon M, Bambara LM. Mucosa-associated lymphoid tissue lymphoma of the salivary glands occurring in patients affected by Sjögren’s syndrome: report of 6 cases. Acta Haematol. 2001;105:83–8. doi: 10.1159/000046539. [DOI] [PubMed] [Google Scholar]
- 17.Queneau PE, Helg C, Brundler MA, Frossard JL, Spahr L, Girardet C, Armenian B, Hadengue A. Diagnosis of a gastric mucosa-associated lymphoid tissue lymphoma by endoscopic ultrasonography-guided biopsies in a patient with a parotid gland localization. Scand J Gastroenterol. 2002;37:493–6. doi: 10.1080/003655202317316150. [DOI] [PubMed] [Google Scholar]
- 18.Klussmann JP, Wagner M, Guntinas-Lichius O, Müller A. Detection of HHV-8 sequences and antigens in a MALT lymphoma associated with Sjögren’s syndrome. J Oral Pathol Med. 2003;32:243–5. doi: 10.1034/j.1600-0714.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 19.Streubel B Huber D, Wöhrer S, Chott A, Raderer M. Frequency of Chromosomal Aberrations Involving MALT1 in Mucosa-Associated Lymphoid Tissue Lymphoma in Patients with Sjögren’s Syndrome. Clinical Cancer Research. 2004;10:476–80. doi: 10.1158/1078-0432.ccr-0873-03. [DOI] [PubMed] [Google Scholar]
- 20.Dunn P, Kuo TT, Shih LY, Lin TL, Wang PN, Kuo MC, Tang CC. Primary salivary gland lymphoma: a clinicopathologic study of 23 cases in Taiwan. Acta Haematol. 2004;112:203–8. doi: 10.1159/000081273. [DOI] [PubMed] [Google Scholar]
- 21.Arcaini L, Burcheri S, Rossi A, Passamonti F, Paulli M, Boveri E, Brusamolino E, Orlandi E, Molteni A, Pulsoni A, Cox MC, Orsucci L, Fabbri A, Frezzato M, Voso MT, Zaja F, Montanari F, Pascutto C, Morra E, Cortelazzo S, Lazzarino M. Nongastric marginal-zone B-cell MALT lymphoma: prognostic value of disease dissemination. Oncologist. 2006;11:285–91. doi: 10.1634/theoncologist.11-3-285. [DOI] [PubMed] [Google Scholar]
- 22.Lewis K, Vandervelde C, Grace R, Ramesar K, Williams M, Howlett DC. Salivary gland mucosa-associated lymphoid tissue lymphoma in 2 patients with Sjögren’s syndrome: clinical and sonographic features with pathological correlation. J Clin Ultrasound. 2007;35:97–101. doi: 10.1002/jcu.20280. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Zhou M, Jiang J, Wang J, Elashoff D, Gorr S, Michie SA, Spijkervet FK, Bootsma H, Kallenberg CG, Vissink A, Horvath S, Wong DT. Systems biology analysis of Sjögren’s syndrome and mucosa-associated lymphoid tissue lymphoma in parotid glands. Arthritis Rheum. 2009;60(1):81–92. doi: 10.1002/art.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh Jl, Huh J, Suh C. Primary non-Hodgkin’s lymphoma of the mayor salivary glands. J Surg Oncol. 2008;97:35–9. doi: 10.1002/jso.20901. [DOI] [PubMed] [Google Scholar]
- 25.Voulgarelis M, Moutsopoulos HM. Mucosa-associated lymphoid tissue lymphoma in Sjögren’s syndrome: risks, management, and prognosis. Rheum Dis Clin North Am. 2008;34:921–33. doi: 10.1016/j.rdc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Speight PM, Jordan R, Colloby P, Nandha H, Pringle JH. Early detection of lymphomas in Sjögren’s syndrome by in situ hybridization for kappa and lambda light chain mRNA in labial salivary glands. Eur J Cancer B Oral Oncol. 1994;30B:244–7. doi: 10.1016/0964-1955(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 27.Van Mello NM, Pillemer SR, Tak PP, Sankar V. B cell MALT lymphoma diagnosed by labial minor salivary gland biopsy in patients screened for Sjögren’s syndrome. Ann Rheum Dis. 2005;64:471–3. doi: 10.1136/ard.2004.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakuma H, Okabe M, Yokoi M, Eimoto T, Inagaki H. Spontaneous regression of intraoral mucosa-associated lymphoid tissue lymphoma: molecular study of a case. Pathol Int. 2006;56:331–5. doi: 10.1111/j.1440-1827.2006.01967.x. [DOI] [PubMed] [Google Scholar]
- 29.Daniels TE, Criswell LA, Shiboski C, Shiboski S, Lanfranchi H, Dong Y, Schiødt M, Umehara H, Sugai S, Challacombe S, Greenspan JS Sjögren’s International Collaborative Clinical Alliance Research Groups. An Early View of the International Sjögren’s Syndrome Registry. Arthritis Rheum. 2009;61:711–4. doi: 10.1002/art.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, Felman P, Coiffier B. Mucosa- associated lymphoid tissue gastrointestinal and non gastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624–30. doi: 10.1200/JCO.1997.15.4.1624. [DOI] [PubMed] [Google Scholar]
- 31.Raderer M, Vorbeck F, Formanek M, Osterreicher C, Valencak J, Penz M, Kornek G, Hamilton G, Dragosics B, Chott A. Importance of extensive staging in patients with mucosa-associated lymphoid tissue (MALT)-type lymphoma. Br J Cancer. 2000;83:454–7. doi: 10.1054/bjoc.2000.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wündisch T, Ehninger G, Stolte M, Bayerdörffer E. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350–55. doi: 10.1093/jnci/89.18.1350. [DOI] [PubMed] [Google Scholar]
- 33.Alkan S, Karcher DS, Newman MA, Cohen P. Regression of salivary gland MALT lymphoma after treatment for Helicobacter pylori. The Lancet. 1996;348 (9022):268–9. doi: 10.1016/s0140-6736(05)65578-x. [DOI] [PubMed] [Google Scholar]
- 34.Iwai H, Nakamichi N, Nakae K, Konishi M, Inaba M, Hoshino S, Baba S, Amakawa R. Parotid mucosa-associated lymphoid tissue lymphoma regression after Helicobacter pylori eradication. Laryngoscope. 2009;119:1491–4. doi: 10.1002/lary.20258. [DOI] [PubMed] [Google Scholar]
- 35.Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Patterson BJ. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–64. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 36.Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Medicine (Baltimore) 2012;91:1–9. doi: 10.1097/MD.0b013e31824125e4. [DOI] [PubMed] [Google Scholar]


