Abstract
Background
Magnetic resonance imaging (MRI) is a noninvasive technology that can quantitatively access ACL graft size and signal intensity. However, how those properties relate to reconstructed or repaired ligament strength during the healing process is yet unknown.
Purpose
We hypothesized that MR derived measures of graft volume and signal intensity are significant predictors of the structural properties of a healing ACL or ACL graft after 15 weeks and 52 weeks of healing.
Study Design
Controlled Laboratory Experiment
Methods
The current data were gathered from two experiments evaluating ACL reconstruction and repair techniques. In the first experiment, pigs underwent unilateral ACL transection and received: 1) ACL reconstruction, 2) ACL reconstruction with collagen platelet composite (CPC), or 3) no treatment. The surgical legs were harvested following 15 weeks of healing. In the second experiment, pigs underwent ACL transection and received: 1) ACL reconstruction, 2) ACL reconstruction with CPC, 3) bio-enhanced ACL primary repair with CPC, or 4) no treatment. The surgical legs were harvested after 52 weeks. The harvested knees were imaged using a T2* weighted 3D-CISS sequence. Each ligament was segmented from the scans, and the intra-articular volume and the median grayscale values were determined. Mechanical testing was performed to establish the ligament structural properties.
Results
Volume significantly predicted the structural properties (maximum load, yield load, linear stiffness) of the ligaments and grafts (R2 = 0.56, 0.56, 0.49; p≤0.001). Likewise, the median grayscale values significantly predicted the structural properties of the ligaments and grafts (R2 = 0.42, 0.37, 0.40; p<0.001). The combination of these two parameters in a multiple regression model improved the predictions (R2 = 0.73, 0.72, 0.68; p≤0.001).
Conclusion
Volume and grayscale values from high resolution T2* weighted MRI images are predictive of structural properties of the healing ligament or graft in a porcine model.
Clinical Relevance
This study provides a critical step in the development of a non-invasive method to predict the structural properties of the healing ACL graft or repair. This technique may prove beneficial as a surrogate outcome measure in pre-clinical animal and clinical studies.
Key Terms: MRI, ACL, Reconstruction, Structural Properties, Linear Regression
Introduction
Magnetic resonance (MR) imaging is widely used as a clinical tool for qualitatively monitoring anterior cruciate ligament (ACL) graft health following surgical reconstruction.12 With the advent of stronger magnets and new imaging protocols, MR can now accurately measure the geometry of complex structures, such as the ACL. In addition, MR imaging can also provide information about the tissue quality by using different sequences to determine water content, fiber alignment and tissue density11. Therefore, in this study, we hypothesized that MR imaging also has the potential to provide a quantitative method for assessing the structural (failure) properties of an ACL graft or repaired ligament during healing, in vivo. A non-invasive MR method for predicting the structural properties of the graft or ligament would allow researchers to document functional healing within a subject in pre-clinical animal studies and clinical trials.
Determining the structural properties of the native ACL or reconstructed graft, ex vivo, is a common method for evaluating graft strength and documenting healing.7,15,20 However, these methods require destructive testing and are not suitable for in vivo longitudinal assessment. Alternatively, signal intensity (also termed grayscale), a MR parameter that is a function of tissue type and water content, has been used to evaluate ACL graft integrity and maturation following ACL reconstruction surgery in humans.3,8,13,16,18 The use of grayscale as an outcome measure is founded on research showing that the graft grayscale values decrease with time post-operatively,18,20 and negatively correlates with its structural properties in an ovine model.20 It has recently been established that graft volume when measured in situ via MR imaging, also correlates with the graft structural properties, and that the correlation could be improved by normalizing the volume to the graft T2 relaxation time three months post-operatively in the caprine model.5 While these findings are promising, the relationship between graft volume, grayscale value, and the graft structural properties throughout the healing process remains undocumented. Because bio-enhanced primary repair of the ACL is now proving to be efficacious in animal models,19 it is also important to validate the relationships between graft volume, grayscale and the structural properties of repaired ligaments.
To date there have been no studies evaluating volume (a measure depicting the amount of tissue) and grayscale (a surrogate measure of tissue quality) as separate quantifiable MR variables to predict the structural properties of an ACL graft or ACL repair over the course of the healing process. The objective of this study was to assess a novel, non-invasive method for predicting the structural properties of a porcine ACL reconstructed ligament (graft) and a bio-enhanced ACL suture repair using 3-D models derived from T2*-weighted MR imaging at two time points in the healing process. We hypothesize that intra-articular graft or ligament volumes and grayscale values will be significant predictors of the structural properties of the reconstructed ligament and ACL repairs after 15 weeks and 52 weeks of healing. Additionally, we hypothesize that the combination of volume and grayscale values, as two separate defining characteristics of the ligament, will improve that prediction in a multiple linear regression model.
Methods
Animal Model
Approval was obtained from the Institutional Animal Care and Use Committee (IACUC) prior to performing these studies.
15 Week Animals
Fifty adolescent Yucatan minipigs (approximately 15 weeks of age) underwent either ACL transection (10 animals), ACL transection immediately followed by ACL reconstruction with a patellar tendon allograft (10 animals), or ACL transection immediately followed by bio-enhanced ACL reconstruction using patellar tendon allograft and a collagen-platelet composite (30 animals) as previously described.4 All surgical procedures were performed by the same orthopaedic surgeon. After 15 weeks of healing, the animals were euthanized and the surgical legs were harvested distal to the hip. MR imaging was performed before the joints were frozen and stored for mechanical testing.
52 Week Animals
As part of a different study utilizing the same animal model, a separate group of 32 Yucatan adolescent minipigs (approximately 15 weeks of age) underwent either ACL transection (8 animals), ACL transection immediately followed by ACL reconstruction with a patellar tendon allograft (8 animals), ACL transection immediately followed by bio-enhanced ACL reconstruction using patellar tendon allograft and a collagen-platelet composite (8 animals), or ACL transection immediately followed by bio-enhanced ACL repair with collagen-platelet composite (8 animals), as previously described.4,10 All procedures were performed by the same orthopaedic surgeon. After 52 week of healing, the knees were harvested, imaged, and stored for mechanical testing.
MR Imaging
A surface knee coil on a 3T MR scanner (Siemens TIM Trio, Erlangen, Germany) was used to image the joints. A T2* weighted 3D-CISS sequence (Constructive Interference in the Steady State; TR/TE/FA, 12.9/6.5/ 35°; FOV, 160 mm; matrix 512×512, slice length/gap, 0.8mm/0; avg 1) was selected. This sequence produces high contrast between the soft tissues and joint fluid,11,12 which optimizes the boundaries of the ligament or graft for manual segmentation from the image stack (Fig. 1). For the 15 week animals, two scans from the ACL reconstruction group and six scans from the ACL reconstruction with collagen-platelet composite were omitted due to magnetic susceptibility artifact. For the 52 week animals, three scans from the ACL reconstruction group and two scans from the ACL reconstruction with collagen-platelet composite group were also omitted due to magnetic susceptibility artifact. It should also be noted that one transected animal in the 52 week time group was euthanized following surgery due to respiratory complications and was excluded.
Fig. 1.
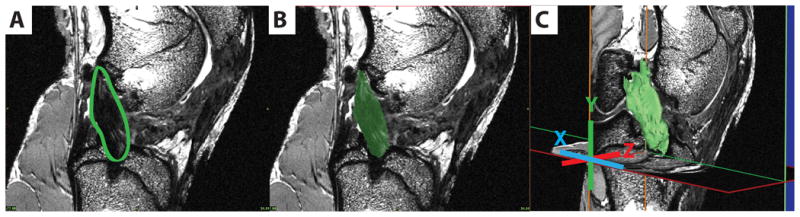
3-D segmentation process illustrated on one sagittal slice of the image stack. A) 2D Graft location; B) Graft segmented; C) 3D model of graft.
3-D Model and Volume Generation
Using commercially available software (Mimics 13.1, Materialise, Ann Arbor, Michigan), the reconstructed, repaired, and untreated ACL transected ligaments were segmented from the MR image stacks in both the coronal and sagittal planes (Fig. 1). Three-dimensional surface models and grayscale volumes were created from the segmented images on a voxel by voxel basis. Intra-articular volumes and median grayscale values were determined for the reconstructed ligaments, repaired ligaments, and untreated transected ligaments (note, that residual healing does occur in the transected ligaments in this animal model so segmentation is possible). The median grayscale values from the ligaments or grafts were normalized to the grayscale value of femoral cortical bone to account for any inter-scan variability.2,17
Structural Properties of the ACL/graft
An established tensile testing protocol was used to determine the structural properties of the reconstructed, repaired and transected ACLs.4,14 The specimens were thawed to room temperature. The femur was transected just distal to the hip while the tibia was transected just proximal to the ankle to preserve the length of the long bones. The soft tissues were dissected from the tibia and femur while leaving the joint capsule intact. The proximal end of the femur and the distal end of the tibia were potted in 6 inch and 4 inch lengths of 38.1 mm PVC pipe, respectively, using a urethane resin (Smooth-On, Easton, Pennsylvania). All residual soft tissue and joint structures were then removed from the joint leaving only the femur-ligament-tibia complex intact. Using a servohydraulic material testing system (MTS 810; Prairie Eden, MN), the tensile loads were applied at 20 mm/min to failure as previously reported.14 Initially, the joint was placed at 30 degrees of flexion so that the mechanical axis of the ligament was collinear with the direction of pull of the tensile testing actuator. Starting with a tibiofemoral compressive force of 5 N, the entire load-displacement curve was recorded until a precipitous drop in load occurred. Yield load, maximum load, and linear stiffness values of the ligaments were calculated as previously described.14
Data Analysis
Relationship between MRI-derived parameters and structural properties
Because the grayscale values were not normally distributed, the log base 2 transforms of the grayscale values were used for all subsequent analyses. The reconstructed, repaired, and untreated transected ligaments at both 15 and 52 week time points were grouped together and analyzed as a single data set. First, linear regression models were used to separately test the relationships between: a) MR volume and structural properties and, b) median grayscale value and structural properties. Subsequently, both volume and median grayscale were included in a multiple linear regression model to predict the structural properties (a fit plane). The R-square values for models were reported as indicators of the strength of the relationships since p-values alone may indicate a highly consistent or non-random relationship even in the presence of relatively poor prediction. The individual p-values of the covariates of the volume and median grayscale value in the regression were used to test the contribution of these variables to the regression and as a check against concerns over multi-collinearity. As an additional check of the model fit, the predicted maximum loads across specimens were plotted against the actual experimental maximum loads to visualize the standard error of the regressions.
Volume and Grayscale differences between 15 and 52 week treatment groups
The ability of the volume and grayscale parameters to detect differences between the 15 week and 52 week treatment groups was tested using a one-way analysis of variance (ANOVA). Two ANOVAs were used, one using volume as the dependant variable and the other using the log base two of median grayscale value, comparing the 15 week and 52 week treatment groups.
Results
Relationship between MRI-derived parameters and structural properties
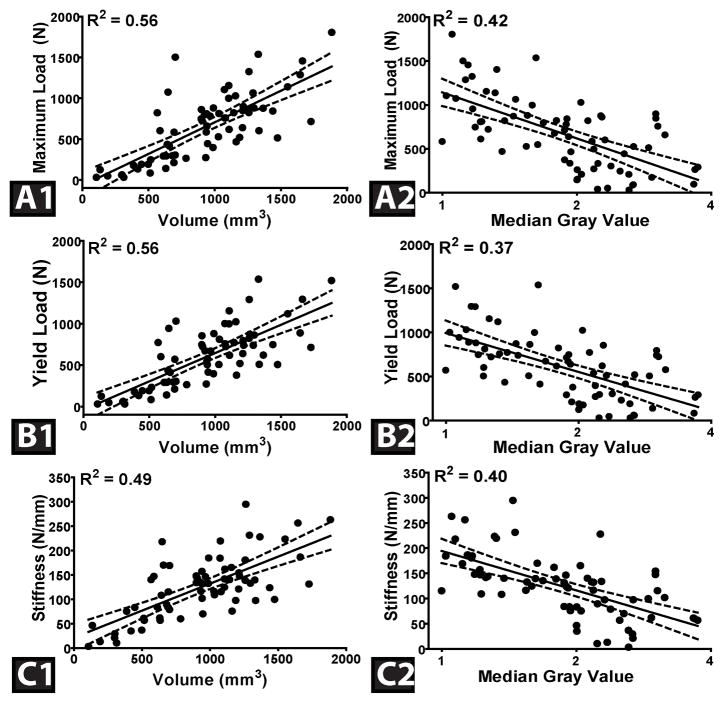
The volume of reconstructed, repaired, and untreated transected ligaments at both 15 and 52 week time points significantly predicted maximum failure load, yield load, and linear stiffness values; R2=0.56, 0.56, and 0.49, respectively (p≤0.001). The median grayscale value was also a significant predictor of maximum failure load, yield load, and linear stiffness, R2=0.42, 0.37, and 0.40, respectively (p<0.001) (Fig. 2). It should be noted that, during mechanical testing, all specimens failed mid-substance.
Fig. 2.
The graft structural properties for the 15 and 52 week specimens (A- Maximum Load, B-Yield Load, and C- Linear Stiffness) as a function of ligament volume (A1, A2 and A3) and the median grayscale value (B1, B2 and B3) in the linear regression models. Dashed lines represent 95% confidence interval.
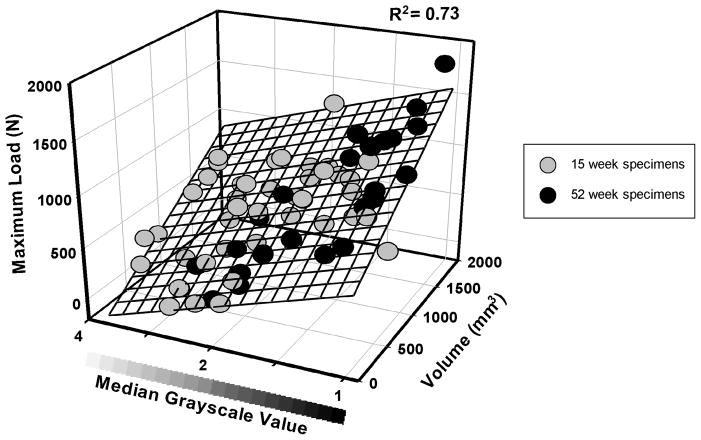
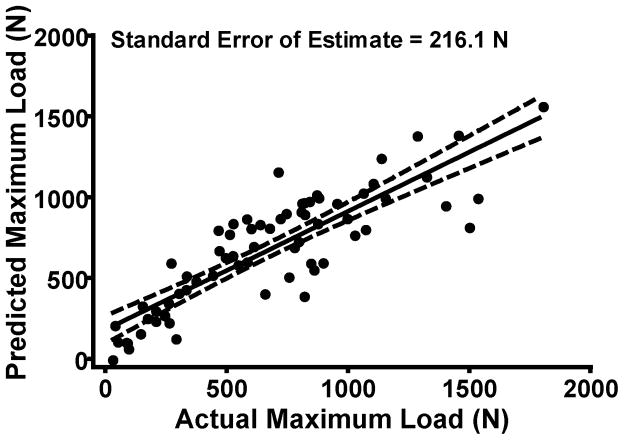
Using volume in conjunction with the median grayscale value, the multiple linear regression model predictions of maximum failure load, yield load, and linear stiffness for all specimens at both time points were improved; R2=0.73, 0.72 and 0.68, respectively (p≤0.001) (Table 1). Both volume and median grayscale value significantly contributed to the regression equations (both p≤0.001); with an increase in volume and/or a decrease in grayscale associated with higher structural properties of the reconstructed, repaired and transected ligaments (Fig. 3). Comparing the actual value of structural properties to the predicted value of the structural properties using the multiple regression model; the standard error of the prediction plane estimate was 216.1 N, 196.0 N and 36.1 N/mm for maximum load, yield load and linear stiffness, respectively (Table 1; Fig. 4).
Table 1.
Summary of the reconstructed ligament structural property prediction equations for both the 15 and 52 week time points as a function of volume (VOL) and median grayscale value (MGV)
| Dependant Variable (Y) | Predictor (X1, X2) | P value | R2 | Standard Error | Equation |
|---|---|---|---|---|---|
| Max Load (N) | VOL (mm3) | <0.001 | 0.73 | 216.1 | Max Load = 440.1 + 0.6*VOL − 370.1*MGV |
| MGV | <0.001 | ||||
| Yield Load (N) | VOL (mm3) | <0.001 | 0.72 | 196.0 | Yield Load = 343.8 + 0.6*VOL − 298.5* MGV |
| MGV | <0.001 | ||||
| Stiffness (N/mm) | VOL (mm3) | <0.001 | 0.68 | 36.1 | Stiffness = 141.0 + 0.1*VOL − 37.5* MGV |
| MGV | <0.001 |
Fig. 3.
The reconstructed, repaired and transected ligament prediction plane for maximum load as a function of volume and of median grayscale value. The ligaments at 52 weeks (black circles) had a significantly lower median grayscale value than ligaments at the 15 week time point (gray circles) (p≤0.001). Similar plots were found for the yield load and the linear stiffness prediction models.
Fig. 4.
Actual maximum load versus predicted Maximum Load for both 15 and 52 week time points, calculated using volume and of median grayscale value.
MRI-derived parameter differences between 15 and 52 week treatment groups
The mean of the MRI-derived reconstructed, repaired and transected ligament volumes at 15 weeks and 52 weeks were 944±380.7 mm3 and 908±435.2 mm3 respectively, though the difference was not significant (p=0.724). The mean of the median grayscale values of the reconstructed, repaired and transected ligaments at 15 weeks and 52 weeks were 1.06±0.48 and 0.61±0.44, respectively. The ligaments at 15 weeks had a significantly higher mean median grayscale value than ligaments at the 52 week time point (p≤0.001; Fig. 3).
Discussion
A quantitative method for accurately monitoring the post-operative healing of an ACL reconstruction or bio-enhanced primary ACL repair would be extremely valuable in both research and clinical settings. MRI is already widely available as a tool for non-invasive knee imaging and its ability to differentiate between joint structures11 and indirectly quantify graft maturation20 makes it an ideal technology to evaluate ligament and graft healing. In our study, T2* weighted MRI derived volumes and median grayscale values were significantly related to the magnitude of maximum failure load, yield load and linear stiffness of reconstructed grafts, repaired and untreated transected ligaments. Expanding on this, we found by combining the volume and median grayscale values of the reconstructed, repaired and transected ligaments, the predictions for the structural properties were improved when compared to each variable alone. This prediction method maybe advantageous for evaluating results non-invasively at early time points, thus removing the need for euthanasia and mechanical testing at those time points and reducing the number of animals required for a study. Furthermore, these findings may have clinical implications for tracking post-operative changes with graft healing in patient cohort studies and randomized controlled trials.
Our findings align with a previous study which found that intra-articular graft volume, when normalized to the MR T2-relaxation time of the graft, increased the correlations to graft structural properties over volume alone, after 6 weeks of healing in the caprine model.5 However, the prior study did not find a significant correlation between structural properties and T2 values as a separate quantifiable variable. This could be explained by the single 6 week time point investigated with this study, which would have limited T2 values to one phase of graft healing. By including an additional time point the current study encompasses more than one phase of the healing response and the associated range of grayscale values. With this time point included a significant relationship was found between the grayscale parameter and structural properties alone as well as combined with volume in a multiple linear regression. Furthermore, in previous studies, quantitative MRI grayscale parameters5,20 or clinician graded grayscale based scores3,9,13,16,18 were determined using 2-D mid substance MRI slices of a graft. The method presented herein analyzes the median grayscale value of the whole graft by reconstructing high resolution 3-D images of the graft volume. This could minimize inaccuracies associated with consistently locating a “mid-substance” slice with a 2-D method.
Traditionally, standard imaging techniques have been used to predict the strength of intact cadaveric ACLs.1,6,7 These methods rely on photographic technology to generate 3-D morphological models of the ACL. Results from these studies suggest that ACL volume could be used in a regression model to predict the structural properties of the native ACL.7 These standard imaging techniques provide a valuable method for predicting the structural properties of an intact ligament and may also be useful for the evaluation of the integrity of ACL grafts or repairs. However, these techniques require destruction of the joint to expose the ACL and obtain measurements and as a result could not be used with in vivo clinical applications. The regression results of the non-invasive MR technique in our study support the human cadaveric findings of volume being predictive of ACL structural properties. We found that two non-invasively obtained quantifiable variables, volume and median grayscale values, could both be used in a multiple regression analysis to characterize structural properties of a reconstructed, repaired and transected ligament. The significant contribution of the volume and grayscale parameters in the multiple regression equations indicates that the role of both independent variables should be considered in a predictive model. This was verified by the increased R2 values seen in the multiple regression predictions of structural properties compared to the single predictor R2 values (p≤0.0001). This signifies that the combination of volume and median grayscale value offers a more complete evaluation of graft integrity than either parameter alone. The relatively low standard errors of the predictions suggest that the measured volume and median grayscale values could be effective in future use of the regression prediction models following further validation.
In addition to the correlation between reconstructed ligament grayscale (signal intensity) and structural properties, a prior MR investigation also looked at signal-to-noise quotient (another MR parameter assessing grayscale) differences over time.20 Histological results from the graft healing process were used to indirectly confirm the remodeling process in parallel with signal-to-noise quotient changes.20 While the study conducted herein did not include histological evidence of the healing process, the grayscale differences observed between the 15 week and 52 week groups show a similar decreasing trend with time and could indicate healing and maturation of the graft or ligament on a tissue level. For the volume parameter, no difference was observed between the 15 week and 52 week groups.
There are study limitations that should be considered. First, the knees were imaged postmortem. In a clinical situation, in vivo artifacts due to blood flow may affect the results. Nonetheless, the non-invasive MR method utilized in the present study, which is based on graft volume and grayscale, shows considerable promise for use in vivo. Second, the grayscale variable used to represent signal intensity can vary depending on MR imaging parameters. We normalized the grayscale values to that of cortical bone within each image to minimize this concern. For this analysis, we did not separate out the group effects. It is possible that the CPC could affect the prediction. However, this possibility seems unlikely given the high coefficients of determination and that the addition of CPC did not affect the slope of the prediction of the structural properties relative to the MR graft volume.5 Finally, inherent differences between the reconstructed, repaired and transected ligament treatments could affect the relationship of the volume and grayscale parameters to structural properties. However, taking into account that the failure mechanism for all specimens was mid-substance and that the intra-articular portion of each ligament analyzed was consistent between the reconstructed, repaired or transected ligaments; it can be reasonably assumed that the MRI derived variables represented the same functional unit between specimens. Despite these limitations, we have shown that MRI derived morphology and grayscale can be used to predict structural properties of a reconstructed, repaired, and untreated transected ACL. We now plan to longitudinally validate this non-invasive MR based prediction method for documenting within-subject temporal changes relating to ACL strength and healing between treatment groups.
Acknowledgments
This publication was made possible by Grant Numbers RO1-AR056834, RO1-AR056834S1 and RO1-AR054099 from the NIAMS/NIH, National Football League Medical Charities, and the Lucy Lippitt Endowment. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS, NIH or NFL Charities. The MR images were acquired at the Brown MRI Research Facility (Providence RI). The authors gratefully acknowledge the assistance of Elise Magarian, Patrick Vavken, Carla Haslauer and Benedikt Proffen of Children’s Hospital Boston; Lynn Fanella, Erika Nixon and Edward Walsh of the Brown MRI Research Facility; and David Paller, Ryan Rich, and Sarath Koruprola of the Rhode Island Hospital Orthopaedic Foundation testing facility.
References
- 1.Chandrashekar N, Slauterbeck J, Hashemi J. Re: Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. Am J Sports Med. 2009;37(2):423. doi: 10.1177/0363546508330152. [DOI] [PubMed] [Google Scholar]
- 2.Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. Radiographics. 2009;29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa D, Melean P, Calvo R, et al. Magnetic Resonance Imaging Evaluation of the Integration and Maturation of Semitendinosus-Gracilis Graft in Anterior Cruciate Ligament Reconstruction Using Autologous Platelet Concentrate. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(10):1318–1325. doi: 10.1016/j.arthro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37(8):1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44(16):2843–2846. doi: 10.1016/j.jbiomech.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemi J, Chandrashekar N, Cowden C, Slauterbeck J. An alternative method of anthropometry of anterior cruciate ligament through 3-D digital image reconstruction. J Biomech. 2005;38(3):551–555. doi: 10.1016/j.jbiomech.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi J, Mansouri H, Chandrashekar N, Slauterbeck JR, Hardy DM, Beynnon BD. Age, sex, body anthropometry, and ACL size predict the structural properties of the human anterior cruciate ligament. J Orthop Res. 2011;29(7):993–1001. doi: 10.1002/jor.21245. [DOI] [PubMed] [Google Scholar]
- 8.Howell SM, Clark JA, Blasier RD. Serial magnetic resonance imaging of hamstring anterior cruciate ligament autografts during the first year of implantation. A preliminary study. Am J Sports Med. 1991;19(1):42–47. doi: 10.1177/036354659101900107. [DOI] [PubMed] [Google Scholar]
- 9.Howell SM, Knox KE, Farley TE, Taylor MA. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med. 1995;23(1):42–49. doi: 10.1177/036354659502300107. [DOI] [PubMed] [Google Scholar]
- 10.Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. Journal of Orthopaedic Research. 2011;29(7):1002–1007. doi: 10.1002/jor.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI from Picture to Proton. 2. Cambridge University Press; 2007. [Google Scholar]
- 12.Miller TT. MR imaging of the knee. Sports Med Arthrosc. 2009;17(1):56–67. doi: 10.1097/JSA.0b013e3181974353. [DOI] [PubMed] [Google Scholar]
- 13.Murakami Y, Sumen Y, Ochi M, Fujimoto E, Deie M, Ikuta Y. Appearance of anterior cruciate ligament autografts in their tibial bone tunnels on oblique axial MRI. Magnetic Resonance Imaging. 1999;17(5):679–687. doi: 10.1016/s0730-725x(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26(9 Suppl):S49–57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of Magnetic Resonance Imaging Findings in Anterior Cruciate Ligament Grafts With and Without Autologous Platelet-Derived Growth Factors. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26(1):50–57. doi: 10.1016/j.arthro.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Sansome M, Aprile F, Fusco R, Petrillo M, Siani A, Bracale U. A study on reference based time intensity curves quantification in DCE-MRI monitoring of Rectal Cancer. IFMBE Proceedings World Congress on Medical Physics and Biomedical Engineering. 2009;25(2):38–41. [Google Scholar]
- 18.Saupe N, White LM, Chiavaras MM, et al. Anterior Cruciate Ligament Reconstruction Grafts: MR Imaging Features at Long-term Follow-up—Correlation with Functional and Clinical Evaluation1. Radiology. 2008;249(2):581–590. doi: 10.1148/radiol.2492071651. [DOI] [PubMed] [Google Scholar]
- 19.Vavken P, Murray MM. The potential for primary repair of the ACL. Sports Med Arthrosc. 2011;19(1):44–49. doi: 10.1097/JSA.0b013e3182095e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29(6):751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]